Abstract
Background
Relapses are common in patients with multiple sclerosis (MS) even after the use of disease-modifying therapies. Repository corticotropin injection (RCI), plasmapheresis (PMP), and intravenous immunoglobulin (IVIg) may be utilized as alternative therapies in the management of MS relapse. There is a lack of health economic studies on these alternative therapies for the acute exacerbations of MS. The objective of this study was to estimate the cost per response of RCI compared with PMP or IVIg from the United States (US) commercial payer perspective.
Methods
Costs and response rates were sourced from published peer-reviewed observational studies. The cost of care included MS-related inpatient, outpatient, and medication costs. Treatment response was defined as no evidence of additional relapse treatment or procedure claims within 30 days after treatment. The cost per response for each treatment was calculated by dividing the total annual cost of care by the proportion of patients with resolved relapse for each treatment. The incremental cost per response ratio was calculated by dividing the difference in costs and the proportion of responses for RCI versus PMP or IVIg. One-way sensitivity analysis (OWSA) was conducted for both costs and response rates. All included costs were inflated to the 2019 US dollars.
Results
With a lower total annual cost of care and a higher response rate, RCI had a lower cost per response (US$141,970) compared with PMP or IVIg (US$253,331). RCI had a lower cost per response even when more stringent estimates for RCI were applied in the OWSA. The annual cost of care had a greater influence on the cost per response in the OWSA.
Conclusions
Based on the estimates from the real-world evidence, our economic evaluation suggests that RCI may have real-world clinical and economic benefits for patients with MS relapse who fail on corticosteroid therapy.
Keywords: cost per response, multiple sclerosis, relapse, repository corticotropin injection
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and demyelinating autoimmune disease of the central nervous system, primarily characterized as a relapsing-remitting disease.1,2 MS may result in severe relapses, resulting in poor recovery, and may attribute to a high-cost burden due to progressive disability. Further, relapses adversely impact a patient’s functional ability, social well-being, and overall health-related quality of life.2 Relapses are common and still occur in about 55.7% of patients in the United States (US) despite the use of disease-modifying therapies.3 Patients with MS may experience multiple relapses per year, with 20% experiencing ≥2 relapses per year.4
Current MS management guidelines recommend the use of corticosteroids as initial treatment for managing relapses.5 However, some of the patients may not tolerate or respond effectively to corticosteroids. Patients failing corticosteroids are at high risk for disease worsening and often require hospitalization for either immediate care or rehabilitation for management. Further, some patients have clear contraindications to high-dose corticosteroids. These patients then move to alternative therapies that are typically utilized to address refractory patients who are often considered corticosteroid failures.6
Second-line or alternative treatment options for the management of MS relapse include repository corticotropin injection (RCI; Acthar® Gel (Mallinckrodt Pharmaceuticals, Inc., Bedminster, NJ, USA)), plasmapheresis (PMP), and intravenous immunoglobulin (IVIg) therapy.7 RCI is a naturally sourced complex mixture of adrenocorticotropic hormone analogs and other pituitary peptides.8 RCI is the only therapy indicated for the treatment of acute exacerbations of MS in adults by the US Food and Drug Administration.8 Further, RCI has demonstrated effectiveness in accelerating the resolution of acute exacerbations of MS in controlled clinical trials.7,9 PMP is recommended as a second-line treatment for patients with steroid-resistant exacerbations in relapsing forms of MS by the American Academy of Neurology.10,11 However, the effectiveness of PMP in the treatment of MS remains inconclusive.12 IVIg is an off-label treatment occasionally used to treat relapses in patients who fail on corticosteroids, but it has limited supporting evidence for this indication.13,14
Considering the substantial societal impact of MS relapse,15 it is imperative to optimize patient care by identifying alternative treatments for patients who fail on corticosteroids. There is a lack of economic evidence on the alternative treatments for the management of acute exacerbations of MS; health economic evaluations are needed to compare the cost and effectiveness of these treatments. This study aimed to estimate the cost per response of alternative treatments for MS relapse from the US commercial payer perspective.
Methods
The goal of MS relapse treatment is to manage relapse by subsiding the related symptoms, improving the patient’s health, and reducing the use of healthcare services and associated economic burden. This analysis considered patients with a diagnosis of MS who experience a relapse and fail on corticosteroids as a first-line treatment. RCI and PMP or IVIg are available as alternative therapies for these patients. These treatments may be administered in an inpatient or outpatient setting based on demographics and clinical characteristics. A cost per response analysis was conducted for RCI and PMP or IVIg from the US commercial payer perspective using the data from peer-reviewed published studies over a time horizon of 12 months.
Clinical response rate
A weighted average response rate was obtained from two different administrative and claims databases – Humana Comprehensive Health Insights Database with a study period from January 1, 2008, to July 31, 201516 and HealthCore Integrated Research DatabaseSM, with a study period from January 1, 2006, to November 30, 201617 (Table 1). In both studies, an established claims-based methodology was used to define MS relapse.18 This claims-based algorithm for MS relapse was operationalized based on the care-seeking behavior of a patient experiencing a relapse. The patients were considered to have experienced an MS relapse if they qualified for one of these criteria: (1) an inpatient claim for MS diagnosis in the primary position at any time during hospitalization or (2) an outpatient claim for an MS diagnosis in the primary or secondary position followed by a medical or pharmacy claim for relapse treatment on or within 30 days after the outpatient visit. A 30-day timeframe, based on the National Multiple Sclerosis Society definition, was used as a marker to demarcate the relapse event as either an unresolved relapse or a new relapse event.19 If a subsequent claim for MS relapse was identified after 30 days from the first claim, the relapse was considered a new relapse. However, if a subsequent claim for inpatient or outpatient visits (with a pharmacy claim) was observed within 30 days of the first claim, the relapse was considered an unresolved relapse.16
Table 1.
Model parameters and inputs.
| Model input | RCI | PMP or IVIg | Reference | ||
|---|---|---|---|---|---|
| Base case | Range | Base case | Range | ||
| Total mean annual cost of care (2019 USD) | US$122,946 | US$107,462 to US$139,816 | US$126,412 | US$114,395 to US$139,816 | Gold, 201620 |
| Inpatient costs | US$3688 | US$3224–4194 | US$10,013 | US$9061–11,075 | |
| Outpatient costs | US$27,048 | US$23,642–30,760 | US$101,380 | US$91,743–112,130 | |
| Medication costs | US$92,210 | US$80,597–104,862 | US$15,019 | US$13,591–16,612 | |
| Weighted average response rate | 86.6% (n=377/435) | – | 49.9% (n=201/403) | – | |
| Humana Comprehensive Health Insights Database | – | 96.9% (n=189/195) | – | 45.9% (n=112/244) | Nazareth, 201916 |
| HealthCore Integrated Research Database | – | 78.3% (n=188/240) | – | 56.0% (n=89/159) | Nazareth, 201817 |
IVIg, intravenous immunoglobulin; PMP, plasmapheresis; RCI, repository corticotropin injection.
The response to treatment was defined as the resolution of the MS relapse. The treatment groups (RCI and PMP or IVIg) were assigned based on the first claim for the treatment on or after the first relapse date. Treatment was deemed effective in resolving a relapse if no further claim of relapse treatments or procedures was observed within 30 days.18
Cost inputs
The estimates for the adjusted annual cost of care were obtained from a retrospective study by Gold et al.20 evaluating the healthcare costs and resource utilization using Truven Health Analytics MarketScan® Commercial Claims and Encounters Database from July 1, 2007, to December 31, 2012. The analysis included 439 patients who experienced ≥2 MS exacerbations, of whom 213 received RCI, 50 received PMP, and 177 received IVIg (Table 1).20 One patient with a medical claim for both PMP and IVIg was excluded from the analysis.20 Patients received intravenous methylprednisolone for the initial relapse and were subsequently treated with RCI, PMP, or IVIg for the next relapse.20 Patients treated with PMP were combined with those treated with IVIg into a single group for the analysis (PMP or IVIg; n=226) given the small sample size of patients in the PMP group. Further, both PMP and IVIg are more intensive treatments that are administered in a hospital setting for acutely limited patients and thus were considered a single group. The cost of care comprised MS-related inpatient, outpatient (physician visit, emergency department, infusion center, or hospital outpatient department), and medication costs. The costs were adjusted for the following covariates: the presence of comorbid diabetes without complications, the number of relapses prior to the index date, year of index exacerbation, days between exacerbations, and healthcare utilization in the 6 months prior to the index exacerbation (number of hospitalizations, outpatient services, and medications). The outpatient services comprised claims for healthcare services that were provided in a hospital outpatient facility, emergency room, physician’s office, or other outpatient settings.21 All costs were inflated to 2019 US dollars utilizing the inflation rates from the medical Consumer Price Index for Medical Care from the US Bureau of Labor Statistics.22
Base case analysis
The cost per response for RCI and PMP or IVIg was estimated by dividing the total mean annual cost of care by the proportion of patients with resolved relapse for each treatment. The total mean annual cost of care was calculated as the total cost from the date of the subsequent, treated MS relapse (index date) and at least 30 days following the initial relapse that was previously treated with intravenous methylprednisolone. The costs were based on a 12-month period from the index date. Further, an incremental cost per response ratio was calculated by dividing the difference in total costs (incremental cost) by the difference in the proportion of patients with responses (incremental response).
Sensitivity analyses
One-way sensitivity analyses (OWSAs) were conducted to account for the uncertainty around model parameters. In the sensitivity analyses, variation in costs was evaluated using the 95% confidence interval (CI) derived from the publication by Gold et al.20 The response rates were varied using the estimates obtained from two different studies.16,17 The model was analyzed by varying each parameter individually to its corresponding upper or lower limit for the OWSA. For instance, to estimate the effect of uncertainty in the total cost of care on cost per response, the total annual cost of care was varied while keeping the response rate constant. Similarly, to estimate the effect of uncertainty in the response rates on cost per response, the response rate was varied while keeping the total annual cost of care constant.
Compliance with ethical guidelines
This study does not involve any human participants, human data, and/or human material. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
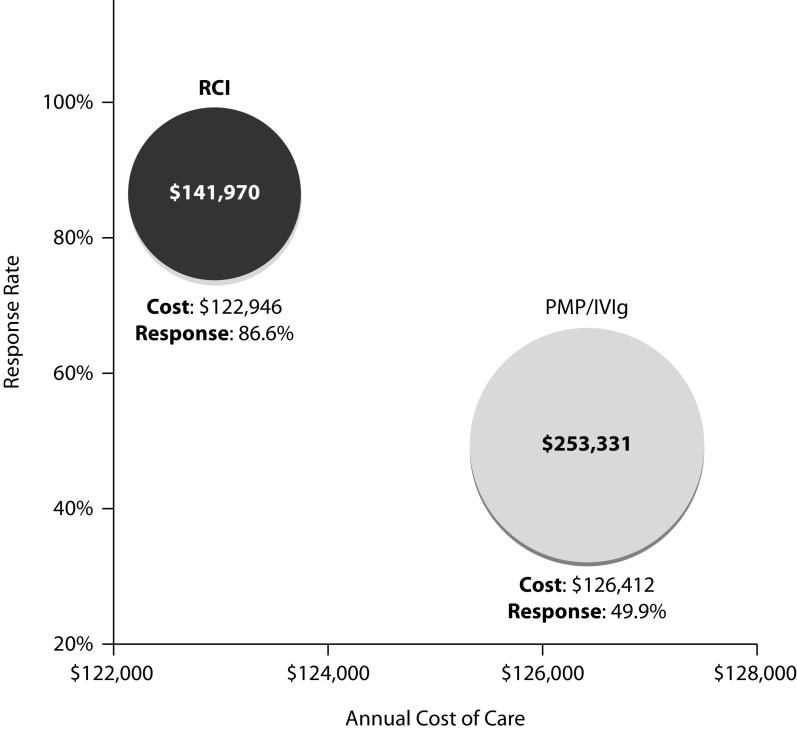
The mean 12-month healthcare cost for patients on RCI (US$122,946) was lower than that for patients on PMP or IVIg (US$126,412).20 The weighted mean response rates for patients on RCI were higher compared with PMP or IVIg (86.6% with RCI versus 49.9% with PMP or IVIg).16,17 The response rate with RCI was consistently greater than the response rate with PMP or IVIg reported by two independent retrospective studies (96.9% versus 45.9%, respectively, in Humana Comprehensive Health Insights Database and 78.3% versus 56.0%, respectively, in HealthCore Integrated Research Database). The base case annual cost per response for RCI is US$141,970 compared with US$253,331 for PMP or IVIg (Figure 1). With a lower mean annual cost of care and a higher response rate with RCI, the incremental cost per response ratio suggests that RCI is dominant over PMP or IVIg.
Figure 1.
Base case results for cost per response of RCI versus PMP or IVIg for acute exacerbation in MS. Costs in 2019 US dollars.
IVIg, intravenous immunoglobulin; MS, multiple sclerosis; PMP, plasmapheresis; RCI, repository corticotropin injection.
In the OWSA, the cost per response for RCI varied between US$126,879 and US$157,019, respectively, using the 96.9%16 and 78.3%17 values for the response rate (Table 2) and between US$124,090 and US$161,450, respectively, using the lower and upper limit of 95% CI for costs as reported by Gold et al.20 The annual cost of care had a greater influence on the overall cost per response than did the response rate in the OWSA. RCI was a dominant treatment strategy in the sensitivity analysis of the incremental cost per response ratio.
Table 2.
One-way sensitivity analysis results for cost per response of RCI versus PMP or IVIg
| Treatment | Parametera | Uncertainty in parameter | Cost per response | ||
|---|---|---|---|---|---|
| Lower estimate | Upper estimate | Lower bound | Upper bound | ||
| RCI | Total annual cost of care | US$107,462 | US$139,816 | US$124,090 | US$161,450 |
| response rate | 96.9% | 78.3% | US$126,879 | US$157,019 | |
| PMP or IVIg | Total annual cost of care | US$114,395 | US$139,816 | US$229,248 | US$280,192 |
| response rate | 56.0% | 45.9% | US$225,736 | US$275,407 | |
Discussion
MS relapses remain key drivers of considerable economic burden and increased disability, impacting patients’ health-related quality of life.2,23 MS relapses are primarily managed with high-dose corticosteroids, comprising either high-dose oral prednisone or intravenous methylprednisolone.5 However, some patients do not tolerate or respond effectively to corticosteroids and are at a high risk for worsening disease. Currently, there are no guidelines and consensus for the management of relapses in patients in whom treatment with corticosteroids fails. Alternative therapies, including RCI, PMP, and IVIg, are typically utilized to treat relapses in such refractory patients.6 RCI is the only treatment indicated for the treatment of acute exacerbations of MS in adults by the US Food and Drug Administration.8 However, there is a lack of economic evidence on the alternative treatments for the management of acute exacerbations in MS. To the best of our knowledge, this is the first economic evaluation assessing the trade-offs between the benefits and costs of these treatments.
The current analysis suggests that RCI is a dominant strategy versus PMP or IVIg. The goal of MS relapse treatment is to manage relapse by subsiding the related symptoms, improving the patient’s health, and reducing the use of healthcare services as well as the associated economic burden costs. Therefore, we estimated the cost per response of RCI and PMP or IVIg. The findings suggest that RCI has a 44.0% lower cost per response than that of PMP or IVIg, attributed to fewer outpatient services and hospitalizations.20 The cost per response of RCI is lower even when more stringent estimates for RCI are applied in the OWSA (29.6% with higher costs and 30.4% with a lower response rate of RCI). The total annual cost of care of RCI is the most influential factor identified in the OWSA. The medication costs in the PMP or IVIg group are lower than those in the RCI group; however, the outpatient costs in the PMP or IVIg group are higher compared to those in the RCI group. The lower medication costs in the PMP or IVIg group may be attributed to the hospital, office-based clinic, infusion center, or home administration of these procedures. Therefore, it is likely that medication costs associated with PMP or IVIg administration are being captured as services being rendered in the outpatient setting.
RCI has been shown to be effective and well tolerated in accelerating the resolution of acute exacerbations in MS in several studies.7,9 RCI can be self-administered, thus being convenient for the patient. In contrast, both PMP and IVIg are usually more intensive treatments administered in the hospital setting for acutely limited patients. Together, these clinical and economic data demonstrate the health economic value of RCI over PMP and IVIg. Treatments should be considered in the context of value, defined as the efficiency with which interventions deliver outcomes for their costs.24 Further, considering the substantial patient and societal burden of MS relapse,15 treatment decisions should be driven by factors including the judgment of physicians, the availability of resources, and patient preference. The current analysis did not include non-medical costs and indirect costs of treatment (e.g. caregiver costs or work productivity loss for patients and caregivers), which may lead to a potential underestimation of the value of RCI from a societal perspective.
The findings should be interpreted in light of the study limitations. First, the analysis was conducted from the US commercial payer perspective and may not be generalizable to other countries where recommendations for treatment of MS relapse might vary. Second, the findings cannot be generalized to other healthcare payers (e.g. Veteran Affairs, Medicaid, and Medicare) where rebates and healthcare plan discounts might be different than the commercial payer system. Third, costs reported within the commercial administrative claims database may not reflect discounts and/or rebates on the drug costs provided by the manufacturer. Future studies should consider different payer perspectives as well as discounts and/or rebates when assessing the clinical value and economic benefit of these treatments. The disparate and favorable lower costs apparent with RCI could be that this simpler therapy, albeit with greater specialty pharmacy costs, results in larger cost savings in medical care costs (including those of PMP or IVIg services). Fourth, the reasons for treatment selection from the retrospective pharmacy and medical claims databases cannot be determined. The professional judgment regarding the appropriateness of RCI for less severely affected patients as second-line therapy after corticosteroid failure may demonstrate lower costs in these events. The relapses in these claims databases were identified based on the patient’s treatment-seeking behavior; any treatment received in a non-healthcare setting was not addressed. Results from randomized controlled studies were not used as no direct head-to-head studies comparing alternative options for MS exacerbations were available. Fifth, unrestricted enrollment in these observational studies may underestimate unresolved relapses. The total annual cost of care did not consider other treatment-related characteristics such as convenience, compliance, or the safety profile of each therapy. Finally, in this analysis, RCI was not compared with PMP and IVIg separately as both PMP and IVIg are intensive treatments administered in the hospital setting. Further, the estimates for this study were obtained from published literature, in which PMP and IVIg were combined as a single group.20 We conducted sensitivity analyses to account for variation in costs and response rates; RCI had a favorable cost per response, consistent with the base case analysis.
Despite the limitations, this study adds to the emerging literature on alternative treatments for acute exacerbations of MS. Timely resolution of MS relapses with appropriate treatment may alleviate the patient’s burden. Our findings serve as a blueprint for clinical decision-making in providing clinical and economic benefits to patients, thereby reducing the financial impact on healthcare systems.
Conclusions
Based on the estimates from the published literature, our economic evaluation suggests that RCI may provide real-world clinical and economic benefits for patients with MS relapse who may not tolerate or respond effectively to corticosteroids. Future investigations should examine the effectiveness of RCI compared to PMP and IVIg from a societal perspective.
Acknowledgements
Falcon Research Group (North Potomac, MD, USA) provided medical writing and editorial assistance; these services were funded by Mallinckrodt Pharmaceuticals (Bedminster, NJ, USA).
Footnotes
Prior presentation: This study was presented, in part or full, at the 2019 Annual ECTRIMS Congress in Stockholm, Sweden on September 12, 2019.
Contributions: All authors contributed equally to the preparation of this review. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: All authors declare that they have no conflicts of interest. GJW and JN are employees of Mallinckrodt Pharmaceuticals and IC was paid consultant fees from at Mallinckrodt Pharmaceuticals for the duration of the project. SFH is a research collaborator and has received speaker honoraria, consulting agreements, and grant/research financial support from AbbVie, Acorda, Actelion, Adamas, Alkermes, Avanir, Bayer, Biogen Idec, Novartis, Osmotica, Mallinckrodt, Roche, Sanofi, Synthon, and Teva. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2020/11/dic.2020-9-4-COI.pdf
Funding declaration: This study was sponsored by Mallinckrodt Pharmaceuticals. The article processing charges and the open access fee for this publication were funded by Mallinckrodt Pharmaceuticals.
Correct attribution: Copyright © 2020 Wan GJ, Chopra I, Niewoehner J, Hunter SF. https://doi.org/10.7573/dic.2020-9-4. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Cook SD, Dhib-Jalbut S, Dowling P, et al. Use of magnetic resonance imaging as well as clinical disease activity in the clinical classification of multiple sclerosis and assessment of its course: a report from an International CMSC Consensus Conference, March 5–7, 2010. Int J MS Care. 2012;14(3):105–114. doi: 10.7224/1537-2073-14.3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Diseases Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269–285. doi: 10.1016/S1474-4422(18)30443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nazareth TA, Rava AR, Polyakov JL, et al. Relapse prevalence, symptoms, and health care engagement: patient insights from the multiple sclerosis in America 2017 survey. Mult Scler Relat Disord. 2018;26:219–234. doi: 10.1016/j.msard.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Mowry EM, Beheshtian A, Waubant E, et al. Quality of life in multiple sclerosis is associated with lesion burden and brain volume measures. Neurology. 2009;72(20):1760–1765. doi: 10.1212/WNL.0b013e3181a609f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Multiple Sclerosis Society. Treating MS. [Accessed August 7, 2019]. Multiple sclerosis web site. https://www.nationalmssociety.org/Treating-MS. Published 2018.
- 6.National Multiple Sclerosis Society. [Accessed August 13, 2020];Managing MS: relapse management. https://www.nationalmssociety.org/For-Professionals/Clinical-Care/Managing-MS/Relapse-Management. Published 2020. [Google Scholar]
- 7.Costello J, Njue A, Lyall M, et al. Efficacy, safety, and quality-of-life of treatments for acute relapses of multiple sclerosis: results from a literature review of randomized controlled trials. Degener Neurol Neuromuscul Dis. 2019;9:55–78. doi: 10.2147/DNND.S208815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mallinckrodt Pharmaceuticals. Acthar® Gel prescribing information. [Accessed December 18, 2019]. https://www.acthar.com/pdf/Acthar-PI.pdf. Published 1952, Revised March 2019.
- 9.Berkovich R. Treatment of acute relapses in multiple sclerosis. Neurotherapeutics. 2013;10(1):97–105. doi: 10.1007/s13311-012-0160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortese I, Chaudhry V, So YT, Cantor F, Cornblath DR, Rae-Grant A. Evidence-based guideline update: plasmapheresis in neurologic disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;76(3):294–300. doi: 10.1212/WNL.0b013e318207b1f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinshenker BG, O’Brien PC, Petterson TM, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46(6):878–886. doi: 10.1002/1531-8249(199912)46:6<878::aid-ana10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann HC, Hartung HP, Hetzel GR, Stuve O, Kieseier BC. Plasma exchange in neuroimmunological disorders: part 1: rationale and treatment of inflammatory central nervous system disorders. Arch Neurol. 2006;63(7):930–935. doi: 10.1001/archneur.63.7.930. [DOI] [PubMed] [Google Scholar]
- 13.Elovaara I, Kuusisto H, Wu X, Rinta S, Dastidar P, Reipert B. Intravenous immunoglobulins are a therapeutic option in the treatment of multiple sclerosis relapse. Clin Neuropharmacol. 2011;34(2):84–89. doi: 10.1097/WNF.0b013e31820a17f3. [DOI] [PubMed] [Google Scholar]
- 14.Sorensen PS, Haas J, Sellebjerg F, Olsson T, Ravnborg M, Group TS. IV immunoglobulins as add-on treatment to methylprednisolone for acute relapses in MS. Neurology. 2004;63(11):2028–2033. doi: 10.1212/01.WNL.0000145798.61383.39. [DOI] [PubMed] [Google Scholar]
- 15.Oleen-Burkey M, Castelli-Haley J, Lage MJ, Johnson KP. Burden of a multiple sclerosis relapse: the patient’s perspective. Patient. 2012;5(1):57–69. doi: 10.2165/11592160-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Nazareth T, Datar M, Yu TC. Treatment effectiveness for resolution of multiple sclerosis relapse in a US Health Plan Population. Neurol Ther. 2019;8(2):383–395. doi: 10.1007/s40120-019-00156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nazareth T, Zhang X, Yu T, et al. MS relapse treatments and relapse resolution: retrospective study results from a US Health Plan. Paper presented at: 7th Joint ECTRIMS2018; Berlin, Germany. [Google Scholar]
- 18.Chastek BJ, Oleen-Burkey M, Lopez-Bresnahan MV. Medical chart validation of an algorithm for identifying multiple sclerosis relapse in healthcare claims. J Med Econ. 2010;13(4):618–625. doi: 10.3111/13696998.2010.523670. [DOI] [PubMed] [Google Scholar]
- 19.National Multiple Sclerosis Society. Relapse management. [Accessed August 20, 2019]. https://www.nationalmssociety.org/For-Professionals/Clinical-Care/Managing-MS/Relapse-Management. Published 2017.
- 20.Gold LS, Suh K, Schepman PB, Damal K, Hansen RN. Healthcare costs and resource utilization in patients with multiple sclerosis relapses treated with H.P. Acthar Gel((R)) Adv Ther. 2016;33(8):1279–1292. doi: 10.1007/s12325-016-0363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Business Machines. IBM MarketScan Research Databases for life sciences researchers. [Accessed May 14, 2020]. https://www.ibm.com/downloads/cas/0NKLE57Y. Published 2019.
- 22.United States Bureau of Labor Statistics. Consumer Price Index. [Accessed August 12, 2019]. https://www.bls.gov/cpi/data.htm. Published 2019.
- 23.Gilden DM, Kubisiak J, Zbrozek AS. The economic burden of Medicare-eligible patients by multiple sclerosis type. Value Health. 2011;14(1):61–69. doi: 10.1016/j.jval.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 24.Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]