Abstract
Bioorthogonal site-specific chemical reaction to label biomolecules in vitro and in living cells is one of the most powerful and convenient tools in chemical biology. A reactive pairs frequently used for chemical conjugation are aldehydes/ketones with hydrazines/hydrazides/hydroxylamines. Although the reaction is generally specific for the two components, even in a cellular environment, the reaction is very slow under physiological conditions. Addition of a phosphate group at the ortho-position of an aromatic aldehyde increases the reaction rate by an order of magnitude and enhances the aqueous solubility of the reagent and the product. We have synthesized phosphate-substituted aldehyde synthetic models to study kinetics of their reactions with hydrazines and hydrazides that contain a fluorophore. This rapid bioorthogonal reaction should therefore be potentially a very useful reaction for routine site-specific chemical ligations to study and image complex cellular processes in biological systems.
Keywords: site-specific conjugation, kinetics, bioorthogonal reaction
Graphical abstract
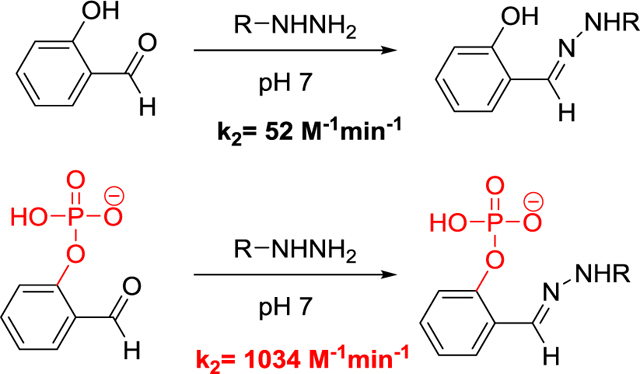
Site-specific conjugation of molecules in biological systems is a general method with many applications.1 Ideally, two molecules are joined in a reaction that occurs rapidly under physiologically compatible conditions without cross-reaction with other functional groups in the molecules or milleu. Multiple reactive pairs are in use, such as azide/phosphine, alkyne/azide, tetrazine/alkyne, and new bioorthogonal reactions continue to be developed.2 It has long been known that amino substituted heteroatoms, amines bonded to oxygen or nitrogen, react with aldehydes and ketones in aqueous solutions to form reversible but stable covalent bonds.3 Generally, hydrazines or hydroxylamines are used as nucleophiles, forming hydrazones or oximes, respectively, the carbonyl-containing molecule. The mechanism of this class of reactions has been very well studied.4 In particular, it is known that the rate of product formation as a function of pH is a bell-shaped curve. The rate normally peaks around pH 4, which is well below physiological pH. The reaction rate can be increased by addition of a nucleophilic catalyst such as aniline and derivatives of aniline.5 Although impressive rate enhancement can be achieved by substituted anilines,6 large excess of the catalyst is required to overcome the generally low equilibrium constants for imine formation in neutral aqueous solution.
Another approach to increasing the rate of hydrazone or oxime formation is to modify the structure of the reagent. Nucleophilicity of the hydrazine amine is dependent of the substitutents on the hydrazine.7 In very general terms, aromatic hydrazines are more reactive toward carbonyls than aliphatic hydrazines. Attaching electron withdrawing groups to the alpha nitrogen, such as in a hydrazide, decreases the reactivity of the functional group, while other groups, such as a beta-dialkylamine, can enhance the rate.
Less attention has been focused on enhancing the reaction by changing the structure of the electrophile. This approach is more limiting in the sense that naturally-occurring carbonyls, such as those generated in proteins, lipids and nucleic acids by reactive oxygen species, by definition have a fixed structure of the electrophile. Site-specific coupling is often performed with exogenous aldehydes or ketones and carbonyl-containing partner in the conjugation reaction, however modification of the carbonyl-containing partner can also be considered.
The reactivity of an aromatic aldehyde varies sharply with the structure of the molecule. This property has been recently exploited by Wang and Canary, who take advantage of the naturally occurring pyridoxal phosphate (PLP) as the source of the aldehyde for hydrazone ligation reactions.8 Both the phosphate and the phenol are known to participate in imine formation in PLP reactions in solution. When PLP is used as a ligation ligand, though, the phosphate is converted to a phosphoamidate, which is less likely to participate in the hydrazone forming reaction.
Recently, Cristalli and Kool showed that aniline derivatives with certain proton donating substituents ortho to the amine display enhanced catalytic activity.9 The best catalyst was possessed a phosphonate substituent, which has a pKa close to the pH of the reaction solution. They also examined a small series of substituted aromatic aldehydes with the new catalysts and noted that certain ortho substituents on the aldehyde can produce a synergistic effect on the reaction rate.
Results and Discussion
We have been interested in developing hydrazine-containing fluorophores that undergo a spectroscopic change upon hydrazone formation for use as probes in biological systems.10 Hydrazine-containing fluorophores were first synthesized and characterized later.13 These fluorophores do react with aromatic aldehydes at neutral pH, but the reaction is sluggish. In this work we show that the reaction can be accelerated by an order of magnitude by a phosphate substituent.
Model compounds (Figure 1) were used to assess the effect of an ortho phosphate on the kinetics of hydrazone formation between an aromatic aldehyde and an aromatic hydrazine. The aldehyde components were salicylaldehyde (1, SA) or 3-formyltyrosine (2, fY) and 2-formylphenyldihydrogen phosphate (3, SA-P). fY is of interest because it can be incorporated into proteins. Furthermore, it is much more water soluble than SA. Since formation of hydrazone makes a more hydrophobic product enhanced water solubility of the components of the model reaction is desirable.
Figure 1.
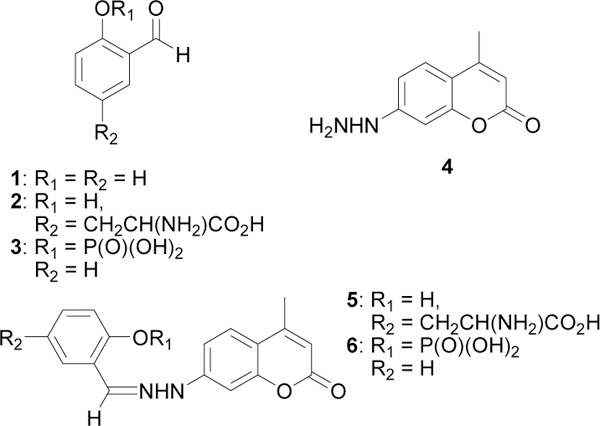
Structures of model aldehydes, hydrazine and hydrazones
The hydrazine, 7-hydrazino-4-methyl coumarin (4, CH), was selected as a model aromatic hydrazine because its absorption maximum at longer wavelength is more than the commonly used 2-hydrazinopyridine. It also has a larger extinction coefficient so it can be used as the limiting reagent while still providing a visible signal. In addition, we have shown previously that hydrazone formation affects the absorption and emission properties of the fluorophore and were thus assured of a signal to monitor kinetics.10
Kinetics of hydrazone formation was assessed at 25 °C using absorption difference spectroscopy as described in Supplementary Material. Figure 2 shows a kinetic trace at a single wavelength for the reaction of fY or SA-P with the same concentration of CH, forming hydrazones 5 or 6. Data were fit to a single pseudo-first order reaction to obtain peudo-first order rate constant. The kinetics for SA-P hydrazone formation at pH 4 and pH 5 was too fast to be measured on a standard spectrophotometer. Therefore these data were collected under pseudo-first order conditions using 2-fold lower concentrations of reagents. In order to compare all of the reactions, apparent second order rate constants were calculated (Table 1). A plot of these data is shown in Figure 3.
Figure 2.
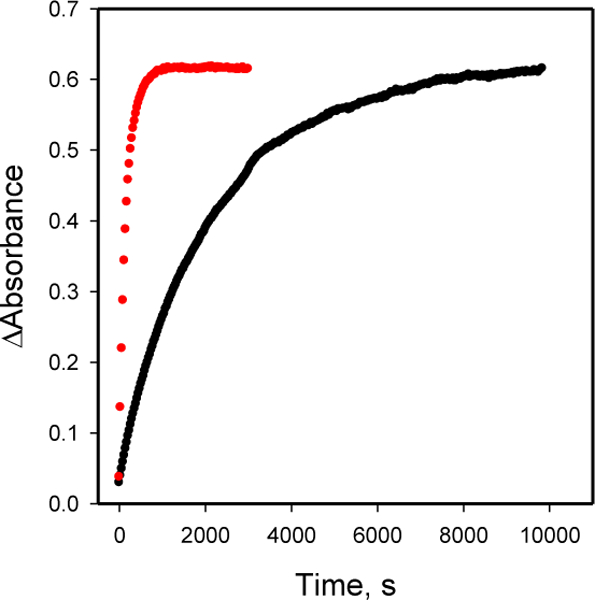
Formation of hydrazone 5 (black circles, •) or 6 (red circles,  ) at 25° C. The concentration of CH was 325 and the concentration of fY or SA-P was 32.5 μM.
) at 25° C. The concentration of CH was 325 and the concentration of fY or SA-P was 32.5 μM.
Table 1:
Apparent second order rate constants for hydrazone formation at 25 °C
| Apparent k2, M−1min−1 | Relative rate | ||
|---|---|---|---|
| CH+fY | CH+SA-P | ||
| pH 4 | 804 ± 14 | 3950 ±166 | 1.8 |
| pH 5 | 548 ± 2.5 | 3270 ± 260 | 5 |
| pH 6 | 196 ± 1.0 | 2790 ± 56 | 14 |
| pH 7 | 52 ± 0.4 | 1034 ± 73 | 20 |
| pH 8 | 50 ± 0.9 | 186 ± 1.2 | 3.8 |
| pH 9 | 41 ± 1.4 | 86 ± 0.5 | 2.1 |
| pH 10 | 40 ± 0.4 | 64 ± 0.2 | 1.6 |
Figure 3:
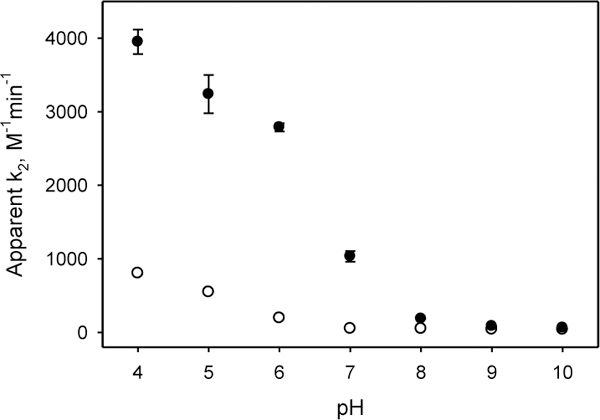
Apparent second order rate constants for hydrazone formation as a function of pH. Open circles, o: fY plus CH. Filled Circles, • : SA-P plus CH.
As expected, the rate of both the fY-CH and SA-P-CH reactions increases with decreasing pH11. The rate of the fY-CH reaction increases gradually with decreasing pH, but the rate if the SA-P-CH reaction increases suddenly at pH 7 and then more gradually as the pH decreases. Since the pKa of the phosphate is near 711, the 20-fold faster rate can be attributed to intramolecular acid catalysis by the phosphate. The difference in the apparent second order rate constants decreases with decreasing pH, which reflects the increasing influence of the protons in the buffer.
Hydrazides are much less reactive with aldehydes and ketones than aromatic hydrazines. Biological probes with hydrazides are frequently commercially available, so it was of interests to assess whether an ortho-phosphate would affect the rate of hydrazone formation. A commercially available coumarin hydrazide (7-diethylaminocoumarin-3-carbohydrazide 7, Figure 4) was allowed to react with fY or SA-P at pH 7 using the same concentrations employed for the CH reactions. A typical kinetic trace is shown in Figure 5.
Figure 4.
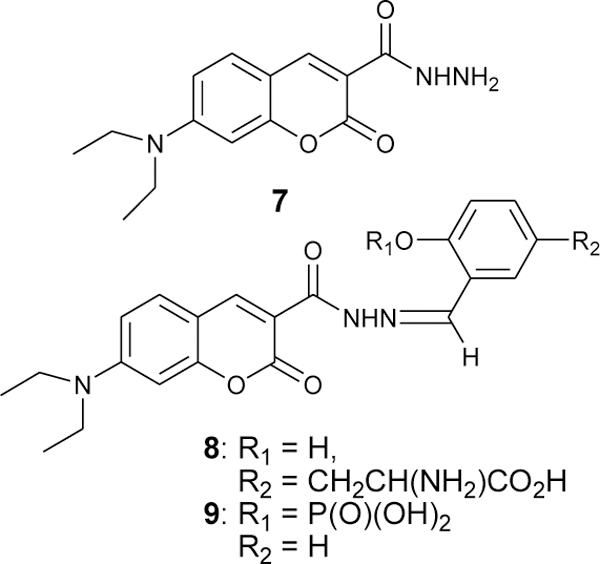
Structures of coumarin hydrazide (7) and hydrazones formed with fY (8) and SA-P (9).
Figure 5.
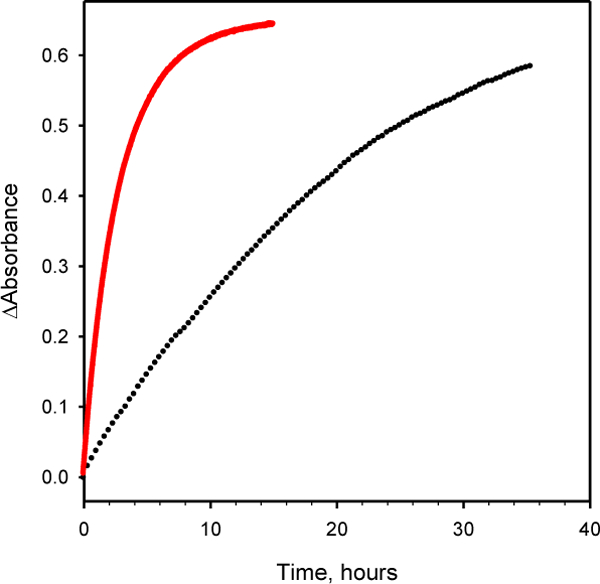
Formation of hydrazone 8 (black circles, •) or 9 (red circles, •) at 25° C. The concentration of coumarin hydrazide (7) was 325 μM and the concentration of fY or SA-P was 32.5 μM. Note the unit of the X-axis is hours.
The hydrazone forming reactions are much slower. The reaction between SA-P and coumarin hydrazide is essentially complete in about 12 hours. The reaction between fY and coumarin hydrazide, however, is not complete after 36 hours. Therefore, an ortho-phosphate increases the speed of the reaction with hydrazides as well as hydrazines at neutral pH. These data also serve to illustrate the large difference in reactivity between an aromatic hydrazine and a hydrazide. Under the same conditions, the hydrazine reaction is complete in less than 30 minutes, while the hydrazide reaction requires at least 12 hours to finish.
Conclusion
In conclusion, we show that directly appending a phosphate to an aromatic ring and positioning it adjacent to the aldehyde gives advantages in a hydrazone ligation reaction. First, the phosphate serves as an intramolecular general acid catalyst, which increases the rate of reaction over an order of magnitude compared to a phenol. Second, the phosphate group increases the aqueous solubility of the aldehyde and the hydrazone product. This should be particularly useful when relatively hydrophobic probes such as fluorophores are part of the ligation product.
Supplementary Material
Acknowledgment
We gratefully acknowledge NIH Grant R15 GM-102867 for financial support. Mr. Sorrentino thanks the Harpur College Dean’s office and the Starzak Award for additional support. The Regional NMR Facility (600 MHz instrument) at Binghamton University is supported by NSF (CHE-0922815).
Footnotes
Supporting Information
YES. 13C and 1H NMR spectra and experimental procedures.
References and Notes
- (1).Chattopadhaya S; Abu Bakar FB; Yao SQ, Expanding the Chemical Biologist’s Tool Kit: Chemical Labelling Strategies and Its Applications. Curr. Med. Chem. 2009, 16 (34), 4527–4543. [DOI] [PubMed] [Google Scholar]
- (2).King M; Wagner A, Developments in the Field of Bioorthogonal Bond Forming Reactions—Past and Present Trends. Bioconj. Chem. 2014, 25 (5), 825–839. [DOI] [PubMed] [Google Scholar]
- (3).Jencks WP, Mechanism of oxime and semicarbazone formation. J. Am. Chem. Soc. 1959, 81, 475–81. [Google Scholar]
- (4).(a)Jencks WP, Catalysis in Chemistry and Enzymology (McGraw-Hill Series in Advanced Chemistry). McGraw-Hill: 1969; p 644. [Google Scholar]; (b)Schmidt P; Zhou L; Tishinov K; Zimmermann K; Gillingham D, Dialdehydes Lead to Exceptionally Fast Bionconjugates at Neutral pH by Virtue of a Cyclic Intermediate. Angew Chemie. Int. Ed. 2014, 53, 10928–10931. [DOI] [PubMed] [Google Scholar]; (c)Schmidt P; Stress C; Gillingham D, Boronic acids faciliate rapid oxime condensations at neutral pH. Chem. Sci. 2015, 6, 329–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d)Bandyopadhyay A; Gao J Iminoboronate Formation Leads to Fast and Reversible Conjugation Chemistry of α-Nucleophiles at Neutral pH. Chem. Eur. J. Chemistry. 2015, 21, 14748–14752 [DOI] [PMC free article] [PubMed] [Google Scholar]; (e)Cal PMSD, Vicente JB, Pires E, Coelho AV, Veiros LF, Cordeiro C, Gois PMP Iminoboronates: a new strategy for reversible protein modification. J. Am. Chem. Soc. 2012, 134, 10299–10305. [DOI] [PubMed] [Google Scholar]
- (5).Dirksen A; Dawson PE, Rapid Oxime and Hydrazone Ligations with Aromatic Aldehydes for Biomolecular Labeling. Bioconj. Chem. 2008, 19 (12), 2543–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Wendeler M; Grinberg L; Wang X; Dawson PE; Baca M, Enhanced Catalysis of Oxime-Based Bioconjugations by Substituted Anilines. Bioconj. Chem. 2014, 25 (1), 93–101. [DOI] [PubMed] [Google Scholar]
- (7).Kool ET; Crisalli P; Chan KM, Fast Alpha Nucleophiles: Structures that Undergo Rapid Hydrazone/Oxime Formation at Neutral pH. Org. Lett. 2014, 16 (5), 1454–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Wang X; Canary JW, Rapid Catalyst-Free Hydrazone Ligation: Protein-Pyridoxal Phosphoramides. Bioconj. Chem. 2012, 23 (12), 2329–2334. [DOI] [PubMed] [Google Scholar]
- (9).Crisalli P; Kool ET, Importance of ortho Proton Donors in Catalysis of Hydrazone Formation. Org. Lett. 2013, 15 (7), 1646–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a)Dilek Ö; Bane SL, Synthesis of boron dipyrromethene fluorescent probes for bioorthogonal labeling. Tet. Lett. 2008, 49 (8), 1413–1416; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b)Banerjee A; Panosian TD; Mukherjee K; Ravindra R; Gal S; Sackett DL; Bane S, Site-specific orthogonal labeling of the carboxy terminus of alpha-tubulin. ACS Chem. Biol. 2010, 5 (8), 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Bender ML; Lawlor JM, Isotopic and Kinetic Studies of the Mechanism of Hydrolysis of Salicyl Phosphate. Intramolecular General Acid Catalysis. Journal of the American Chemical Society 1963, 85 (19), 3010–3017. [Google Scholar]
- (12).Silverberg JL; Dillon LJ; Vemishetti P, A simple, rapid and efficient protocol for the selective phosphorylation of phenols with dibenzyl phosphite. Tet. Lett. 1996. 27(6) 771–774. [Google Scholar]
- (13).Synthesis of Compound 5: Coumarin hydrazine2 (100 mg, 0.44 mmol) was added to dibenzyl-SA-P (0.162 g, 0.44 mmol) in methanol. The reaction was stirred at room temperature for 2 hrs. Completion of the reaction was monitored by TLC. The precipitate was filtered and washed with excess methanol and dried. Yellow product was obtained (130 mg, Yield 55%).1H NMR (d-DMSO): δ ppm 2.36 (s, 3H), 5.17 (s, 2H), 7.26 (s, 2H), 5.20 (s, 2H ), 6.07 (s, 1H), 6.99–7.05 (m, 2H), 7.25–7.39 (m, 14H), 7.62 (d, 1H), 8.23 (s, 1H), 11.09 (s, 1H).13C NMR (d-DMSO): δ ppm 18.0, 69.6, 97.9, 109.3, 109.5, 111.8, 120.6, 125.6, 125.9, 126.5, 126.6, 127.9, 128.5, 128.6, 129.7, 133.7, 135.5, 135.6, 147.7, 147.8, 148.3, 158.4, 155.1, 160.4.Synthesis of Compound 6 (deprotection): Compound 5 was added to 1:1 (TFA: CH2O2) and stirred at room temperature for overnight. Solvent was removed to yield actual product (60 mg, Yield 100%). 1H NMR (d-DMSO): δ ppm 2.35 (s, 3H), 6.05 (s, 1H), 6.95–7.06 (m, 2H), 7.14–7.39 (m, 3H ), 7.60 (d, 1H ), 8.01 (d, 1H), 8.26 (s, 1H), 11.09 (s, 1H). 13C NMR (d-DMSO): δ ppm 18.0, 97.8, 109.3, 111.6, 120.9, 124.4, 125.4, 126.5, 126.7, 129.6, 135.1, 148.6, 149.4, 149.5, 153.6, 155.2, 160.5. MS (ESI) M+ 373.08 found 374.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


