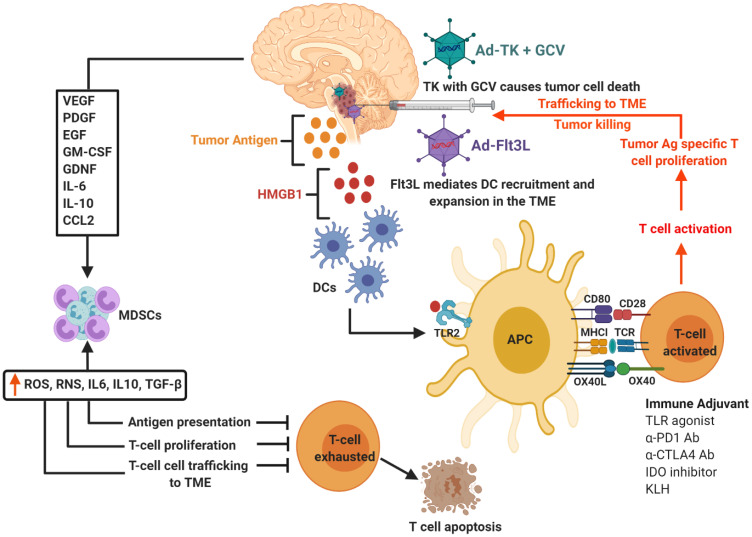
Figure 1. Schematics of TK/Flt3L based immunostimulatory gene therapy and underlying anti-DIPG immune mechanism.
Tumor cells transfected with Ad-Flt3L express Flt3L protein enter systemic circulation. In the bone marrow (BM), Flt3L induces the expansion of dendritic cells (DCs), followed by their recruitment and accumulation in the tumor microenvironment (TME). Ganciclovir, which is a prodrug (GCV) is administered systemically. Tumor cells transfected with Ad-TK express TK protein capable of converting GCV to GCV-monophosphate (GCVp), which is further phosphorylated to GCV-diphosphate (GCVpp) by cellular kinase guanylate kinase and to the active antimetabolite GCV-triphosphate (GCVppp) by cellular nucleoside diphosphokinase. GCVppp, is incorporated into the replicating DNA of tumor cells, resulting in DNA replication termination and cell death. This also leads to the concomitant release of damage associated molecular patterns (DAMPs), i.e., HMBG1, Calreticulin, and ATP from dying tumor cells. Recruited DCs uptake the DIPG tumor Ag released from the dying cells. HMGB1 binds to TLR2/4, which facilitates the production of cytokines and tumor antigen cross-presentation. The DCs loaded with tumor antigens migrate to the cervical draining lymph node (dLN) where they present tumor antigens (Ag) to naive T cells, priming tumor specific anti-glioma effector T cells. Primed effector T cells enter the bloodstream from dLN and migrate towards the TME and kill residual tumor cells. Cytokines (VEGF, PDGF, LIF, GDNF, IL-6, IL-10, CCL2) released by glioma cells supporting differentiation and expansion of immune suppressive immature myeloid cells (MDSCs). To block effective anti-tumor immune responses, MDSCs are recruited to the tumor microenvironment and circulate back to lymphoid organs. The differentiation, maturation, activation, and proliferation of T cells are disrupted by these MDSCs, ultimately leading to T cell exhaustion and death.