Abstract
In this study, CD34+/CD31− progenitor cells were isolated from the stromal vascular fraction (SVF) of adipose tissue using magnetic activated cell sorting. The endothelial differentiation capability of these cells in vitro was evaluated by culturing them in vascular endothelial growth factor (VEGF) induced medium for 14 days. Viability, proliferation, differentiation and tube formation of these cells were evaluated. Cell viability study revealed that both undifferentiated and endothelial differentiated cells remained healthy for 14 days. However, the proliferation rate was higher in undifferentiated cells compared to endothelial differentiated ones. Upregulation of endothelial characteristic genes (Von Willebrand Factor (vWF) and VE Cadherin) was observed in 2D culture. However, PECAM (CD31) was only found to be upregulated after the cells had formed tube-like structures in 3D Matrigel culture. These results indicate that adipose derived CD34+/CD31− cells when cultured in VEGF induced medium, are capable differentiation into endothelial-like lineages. Tube formation of the cells started 3h after seeding the cells on Matrigel and formed more stable and connected network 24 h post seeding in presence of VEGF.
Keywords: Stromal vascular fraction, CD34+ cells, endothelial differentiation, adipose tissue
1. Introduction
Vascularization is essential to supply cells with nutrients and oxygen in engineered tissue constructs after implantation [1]. Recently it has been suggested that post-natal vasculogenesis is influenced by endothelial progenitor cells (EPCs). Although their exact phenotype is unknown they express the CD34 surface marker and can be identified in bone marrow and peripheral blood. As they can home to tissue repair sites and differentiate into mature endothelial cells [2] they have emerged as an attractive source for regenerative medicine applications. Asahara et al [3] observed successful in vitro endothelial differentiation of isolated CD34+ from peripheral blood using magnetic beads. Others have demonstrated multilineage differentiation potential into endothelial cells and cardiomyocytes in a rat myocardial infarction model [4]. These cells have also been described as pericytes due to their pericyte like properties by co expression of typical pericyte markers including NG2, PDGFRβ, CD105 and CD 90 [5]. Multi-lineage differentiation potentials have also been observed in adipose derived mesenchymal stem cells [6–8]. Additionally, previous studies have reported evidence of pericytes and vascular smooth muscle cells in the stromal vascular fraction of adipose tissue [9]. Sengenes et al., reported that some adipose derived stem/progenitor cells express the CD34+ surface marker and are negative for the endothelial cell marker CD31 [10]. This suggests that adipose tissue may house progenitors for both vasculogenesis and tissue formation. For example, an in vivo study recently demonstrated the healing potential of femoral fractures in nude rats after CD34+ cell transplantation [10]. In a very recent study Hertweck and his group tested the impact of CD34+ cells (purified from circulating blood) in collagen scaffolds as monoculture and as co‐ culture together with human osteoblasts on bone formation in nude mice [11]. They observed better bone healing for monoculture of CD34+ cells as well as co‐ culture of CD34+/osteoblasts compared to monoculture of human osteoblasts. It is reported that when CD34+ cells, fraction of adipose stromal cells are seeded on a semi solid surface such as Matrigel, vWF is over expressed [12].These studies have shown the importance of CD34+ cells in tissue regeneration and their contribution to endothelial differentiation.
The present study was aimed to investigate if CD34+/CD31− cells isolated from adipose tissue will acquire an endothelial cell phenotype when induced by VEGF in vitro and have the ability for tube formation. To characterize endothelial differentiation of CD34+/CD31− cells a study of viability, proliferation and differentiation in VEGF induced medium was conducted over 14 days. Cell viability and proliferation were analyzed using LIVE/DEAD® staining and PicoGreen® DNA quantification assay. Gene expression (CD31, vWF and VE Cadherin) was assessed by qRT-PCR and surface marker expression was analyzed using immunofluorescence staining. Furthermore, tube formation of CD34+/CD31− cells were evaluated by seeding the cell on Matrigel. The results of this study showed successful differentiation of CD34+/CD31− cells, into an endothelial-like lineage after culture in VEGF containing inducing media for up to 14 days in vitro.
2. Materials and Methods
2.1. Isolation and culture of CD34+ /CD31− cells:
To obtain human CD34+/CD31− cells, surgically discarded adipose tissues were obtained from patients who underwent an elective adipose tissue removal process (e.g., panniculectomy) at the Pennsylvania State University (Hershey, PA) with patient’s consent and approval from the Institutional Review Board (IRB protocol # 00004972). The obtained tissue samples were minced and rinsed vigorously multiple times with Hanks Balanced Salt Solution (HBSS) until excess blood was removed. Minced adipose tissue was digested with 0.2% of collagenase (Gibco, MD) and incubated at 37 °C on a shaker for 2 h. The collagenase-digested tissue was centrifuged for 10 min at 2,000 rpm and the pellet was obtained. The pellet was then suspended in red blood cell (RBC) lysis buffer and filtered through 100 μm and 40 μm filters to remove large tissue particles and centrifuged. The pellet was re-suspended in HBSS buffer and layered on Ficoll-Paque reagent for density gradient centrifugation for 30 min at 2,000 rpm, and the white from layer (middle) band was collected, denoted as the SVF. We first depleted CD31 positive cells from the SVF using microbeads (Milteyni Biotec, CA). CD31 depleted cells were labelled with CD34 microbeads and sorted using AutoMACs Pro (Milteyni Biotec, CA). Isolated CD34+ cells were tested for purity by flow cytometry [13]. Briefly, the CD34+ cells were centrifuged to obtain a cell pellet and fixed with BD Cytofix (BD Biosciences PharMingen, Franklin Lakes, NJ) for 10–15 minutes, washed and re-suspended in BD perm wash buffer for 10 minutes. CD34 cells were then re-suspended in perm/wash buffer with primary antibody, CD34-FITC, CD31-PE (BD Biosciences, Franklin Lakes, NJ) and incubated at room temperature for 30 minutes and washed. Labeled cells were acquired by a LSR-II flow cytometer (BD Biosciences, Franklin Lakes, NJ) and data analyzed using FlowJo (Ashland, OR) software.
2.2. Endothelial differentiation of CD34+/CD31− cells:
To assess the endothelial differentiation potential of CD34 cells from 5 different patients at passage 1, they were grown to 80% confluency in 24-well plates, and cultured in basal DMEM-F12 medium supplemented with 50 ng/mL VEGF (Sigma) and 2% FBS for 14 days. CD34 cells cultured without VEGF media were used as a negative control. Human Umbilical Vein Endothelial Cells (HUVEC) from Lonza served as a positive control and were maintained in EGM-2 medium (Lonza). The cells were incubated at 37°C in 5% CO2 and medium was changed every 3 days.
2.3. Cell viability and proliferation:
LIVE/DEAD® staining (Cell viability, Invitrogen – using Olympus fluorescence microscope) was performed at 7 and 14 days to assess cell viability. Total DNA content was used to determine the cell count of each as previously described [14]. Proteinase K of 0.5 mL (Sigma-Aldrich) at a concentration of 0.5 mg/mL was added to each well, and plates were incubated at 56°C overnight for enzymatic lysis of cells and DNA release. Aliquots (50 mL) were mixed with equal volumes of 0.1 g/mL PicoGreen® dye solution (Invitrogen) in 96-well plates. Samples were then excited at 480 nm with an emission wave length of 520 nm using a plate reader (SpectraMax M5). Total DNA concentration was compared to a standard curve generated from serial dilutions of CD34+/CD31− cells to calculate the number of the cells in each well. Samples prepared without cells served as blank controls.
2.4. Matrigel tube formation assay:
CD34+/ CD31− cells cultured for 2 weeks in endothelial differentiation medium and growth medium were plated on top of Matrigel substrate (at density of 25,000 cells/well on 150 μL Matrigel, Corning® in a 48 well plate) and incubated at 37°C in a 5% CO2 for up to 24 h. Phase contrast microscopy was used to observe the formation of cord like structures.
2.5. Immunofluorescence staining:
After 14 days in differentiation medium, immunofluorescence staining was performed on induced and un-induced cells to detect the expression of CD31, VE-Cadherin and vWF. Cells were fixed in 4% paraformaldehyde for 10 min at room temperature, rinsed with PBS twice, blocked with 1% bovine serum albumin (BSA) for 1 h at 37° C, and incubated with AlexaFluor-488-conjugated Mouse anti-CD31 (Abcam), Rabbit anti-VE Cadherin (Abcam) and Rabbit vWF (Abcam) antibodies at 4° C overnight, followed by washing in PBS three times. AlexaFluor-555-conjugated goat anti-rabbit secondary antibody (ThemoFisher Scientific) was then added to VE-Cadherin and vWF stained samples and incubated for a further 1 h. Cells were then counterstained with DAPI (Sigma) and observed under a confocal microscope.
2.6. Real time quantitative PCR:
Total RNA was extracted from cells as previously described [15]. Total RNA was converted to cDNA using Verso cDNA Synthesis Kit (ThermoFisher Scientific). qRT-PCR was performed using PowerUp™ SYBR® Green Master Mix (ThermoFisher Scientific) and primers for CD31, vWF and VE-Cadherin to quantify endothelial target gene expression of CD34+/CD31− cells cultured in growth medium (GM) and endothelial differentiation medium up to 14 days. Reactions were performed with a QuantStudio 3 PCR system (Thermo Fisher Scientific). The sequences of PCR primers (forward and backward, 5’–3’) from Integrated DNA Technologies were as follows: CD31: 5-ACTGGACAAGAAAGAGGCCATCCA-3 and 5-TCCTTCTGGATGGTGAAGTTGGCT −3 [16], VE Cadherin: 5-ATGCACCTGTGTTTGAGAAAAGC-3 and 5-CCTGGATGCGGTATGAGACTT-3[17], vWF: 5-AACGGAAGTCCATGGTTCTG-3 and 5-CCCCATTGAAGGCATACTCC-3[1].
Samples were normalized (ΔCt) against the house keeping gene 18S rRNA and the -ΔΔCt value of the genes in the differentiated cells compared to non-differentiated group was calculated using the -ΔΔCt method as described in supporting information.
2.7. Statistical analysis:
Statistical analysis of results was performed using GraphPad Prism. Data was analyzed for statistically significant differences with two-way ANOVA, p<0.05. Fisher’s LSD test was used for post comparisons.
3. Results
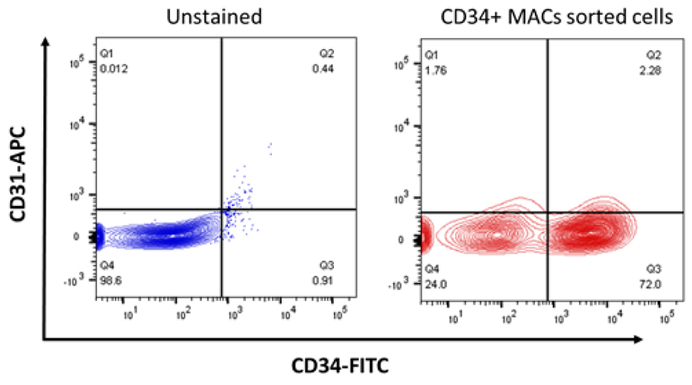
CD34+/CD31− cells were isolated from SVF based on surface markers utilizing magnetic activated cell sorting. Fig. 1 indicates isolation of these cells by density gradient centrifugation and by flow cytometry. The percentage of CD34+ cells is 50–60% within the SVF isolated from fat tissue.
Fig. 1.
CD34+/CD31− cells were isolated from the adipose tissue using MACS microbeads and purity was analyzed by Flow cytometry (CD34-FITC and CD31-APC).
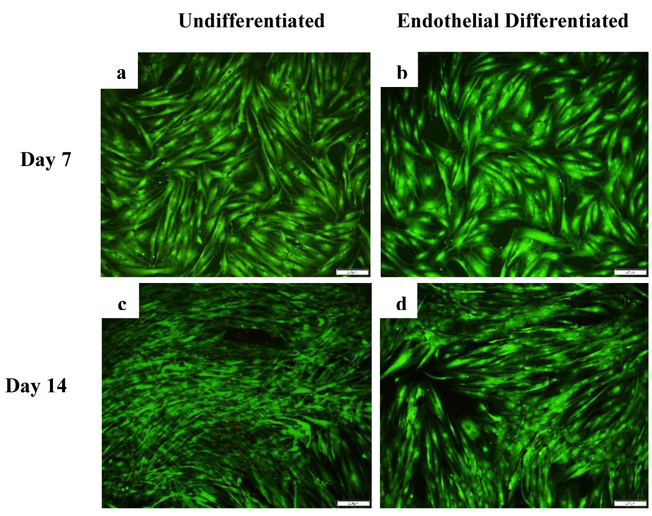
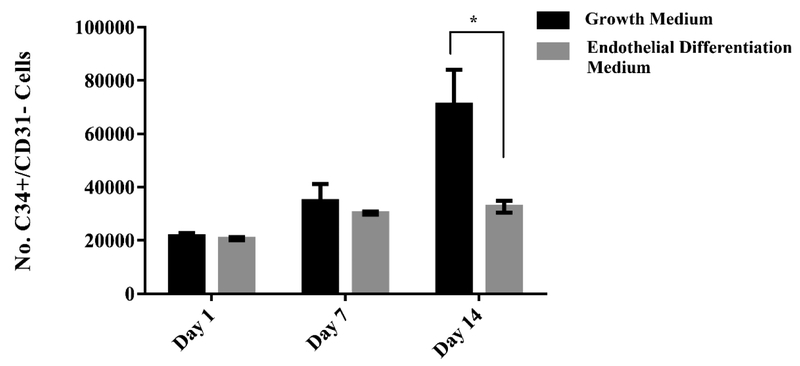
Viability of CD34+/CD31− cells was visualized with LIVE/DEAD® staining over 14 days of culture in growth medium and endothelial differentiation medium. Representative images of the cells at day 7 and 14 can be seen with live cells in green and dead cells in red (Fig. 2). More dead cells were observed in growth medium compared to endothelial differentiation medium after 14 days. PicoGreen® total DNA quantification was used to determine the number of the cells at day 1, 7 and 14 as shown in Fig.3. DNA content analysis indicates that proliferation rate is higher in undifferentiated cells compared to endothelial differentiated (ED) ones. A significant difference was observed between the number of the cells in undifferentiated group (3.3 fold increase) and endothelial differentiated group (1.5 fold increase) over 14 days. Images from LIVE/DEAD® staining support the data generated from DNA content analysis that indicate the undifferentiated cells proliferated more than endothelial differentiate cells.
Fig. 2.
LIVE/DEAD® staining assessment over 14 days. LIVE/DEAD® assay stains live cells green and dead cells red. Representative overlay images of cells in growth medium (a &c) and endothelial differentiation medium (b& d) at days 7 and 14 respectively.
Fig. 3.
Cell proliferation in undifferentiated and differentiated CD34+/CD31− cells in 14 day culture period. Total number of cells was determined using PicoGreen® DNA quantification assay. Error bars represent the standard error of the mean, for experimental and technical triplicates. * p<0.05
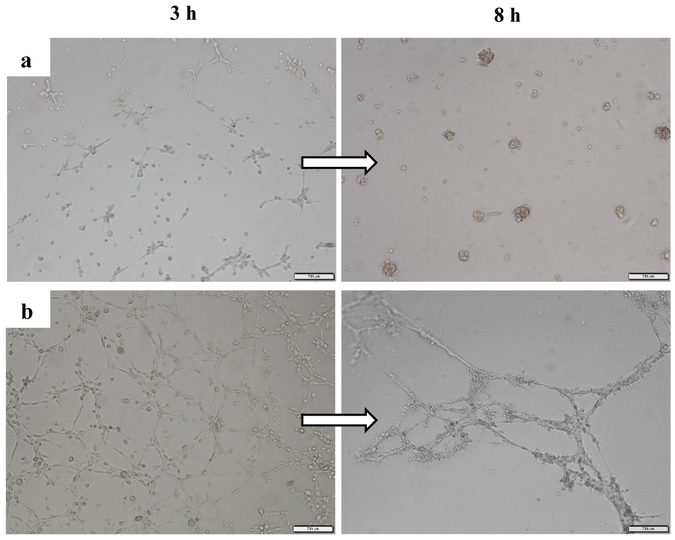
After 14 days of exposure to endothelial differentiation medium, CD34+ cells started to form tube like structure 3 h after seeding on Matrigel and more stable cord like structure was observed after 8 h. Conversely, undifferentiated cells formed aggregates and showed no tube-like after 8 h (Fig. 4).
Fig. 4.
Bright field images of tube formation of a) non-differentiated and b) Endothelial differentiated CD34+/CD31− cells on Matrigel 3h and 8h after seeding.
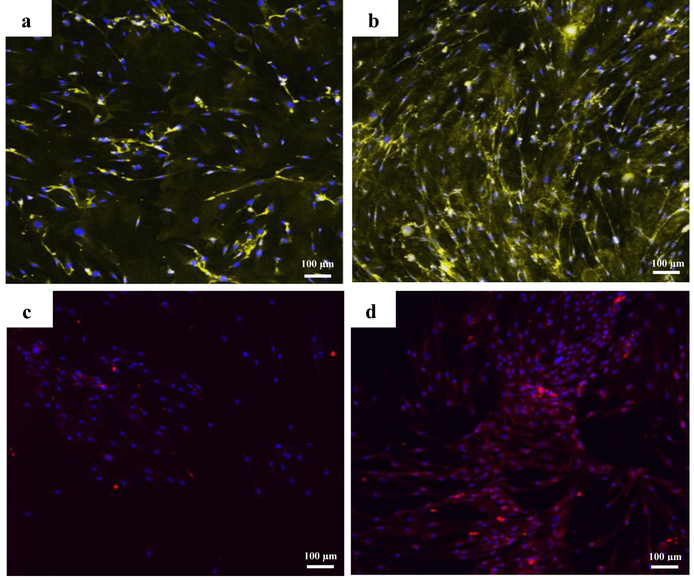
Expression of vWF, VE Cadherin and CD31 proteins was studied using immunofluorescence staining technique on both differentiated and undifferentiated cells at day 14 in culture. In 2D culture, CD34+/CD31− cells showed increased expression for vWF and VE Cadherin in endothelial differentiation media compared to the growth media condition (Fig. 5).
Fig. 5.
Immunofluorescence staining against vWF (yellow) on a) non differentiated cells and, b) endothelial differentiated cells and against VE Cadherin (red) on c) non differentiated cells and, d) endothelial differentiated cells after 14 days in 2D culture. The nuclei were counterstained with DAPI.
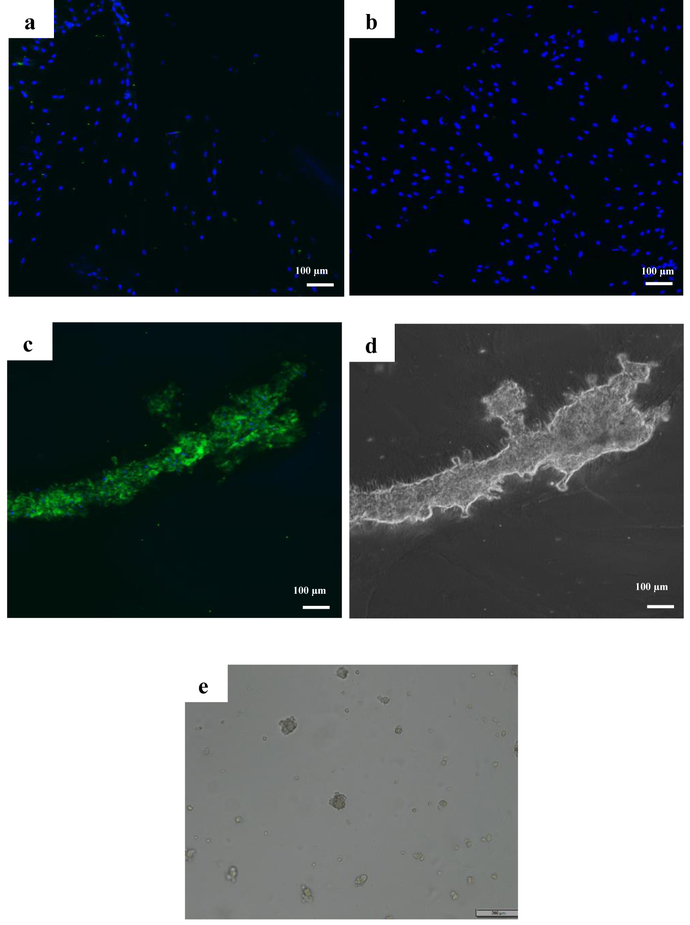
None of the 2D culture groups showed positive staining for CD31 surface marker regardless of media condition (Fig. 6 a&b). However, CD31 expression (5 fold upregulation) was observed in endothelial differentiated tubes 24 h after seeding on Matrigel as can be seen in Fig. 6 c&d. Undifferentiated cells however detached and formed aggregation 24 h after seeding on Matrigel (Fig. 6 e).
Fig. 6.
Images of immunofluorescence staining against CD31 for a) growth medium and b) Endothelial differentiation medium cells after 14 days in 2D culture, c) on endothelial differentiated cells on Matrigel after 24 h. d) bright field image of endothelial differentiated cells forming tube on Matrigel after 24 h. e) bright field image of cells in growth medium on Matrigel after 24 h.
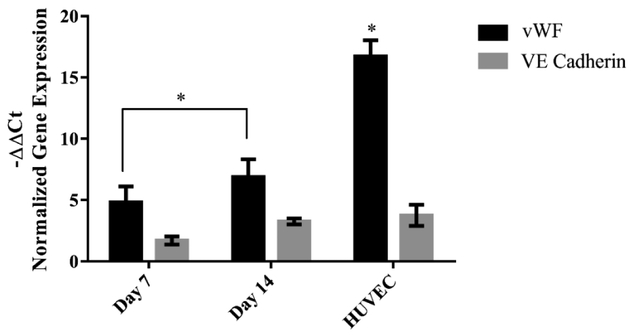
Gene expression analysis by qRT-PCR demonstrated upregulation of VE Cadherin, CD31 and vWF genes in differentiated cells when normalized to that of undifferentiated group. Fig. 7 shows expression of vWF and VE Cadherin on CD34+/CD31− cells as well as the positive control (HUVEC cells). No significant difference was observed in expression of VE Cadherin between differentiated cells and HUVEC cells (positive control).
Fig. 7.
Expression of vWF and VE Cadherin genes in endothelial differentiated cells compared to undifferentiated conditions using qRT-PCR. Gene expression was normalized comparing target gene to 18s housekeeping gene. HUVEC cells were used as positive control. Error bars represent the standard error of the mean for experimental and technical duplicates. Significant difference was observed in vWF between HUVEC and Endothelial differentiation groups. * p<0.05
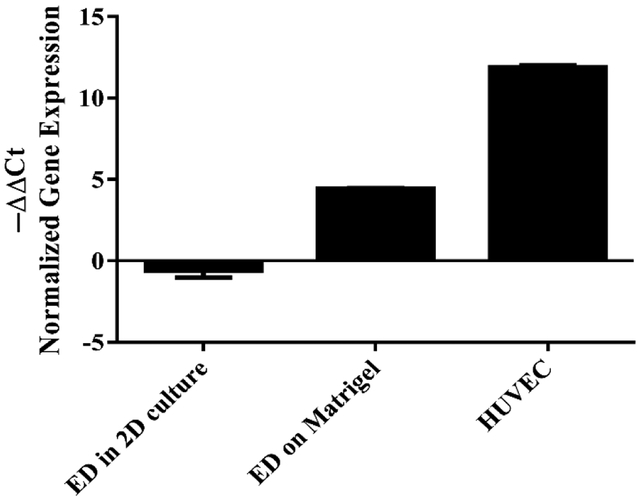
CD31 gene expression was observed after seeding the cells on Matrigel while it did not show any upregulation in 2D (Fig. 8).
Fig. 8.
Expression of CD31 gene in differentiated cells in 2D and on Matrigel after 24 h compared to undifferentiated conditions using qRT-PCR. Gene expression was normalized comparing target gene to 18s housekeeping gene. HUVEC cells were used as positive control. Error bars represent the standard error of the mean for experimental and technical duplicates. Significant difference was observed between the groups. * p<0.05.
4. Discussion
Neovascularization is crucial for cell survival, formation of new tissue and maintaining tissue functionality in tissue engineering [18–20]. Therefore, finding a proper source of progenitor cells capable of endothelial differentiation is of substantial importance in tissue engineering for the vascularization of large volume complex tissues [2]. Mature endothelial cells have shown successful blood vessel development and capillary networks formation within the tissue constructs. However, the source and availability of mature endothelial cells as well as their proliferative potential is limited [21, 22]. Endothelial progenitor cells are an alternative source for vascularization in damaged tissues. Asahara et al., in 1997 proposed that CD34+ cells can be isolated from peripheral blood and bone marrow as endothelial precursor cells and also can differentiate into endothelial cells in vitro [3]. Miranville et al., later demonstrated the presence of CD34+/CD31− cells within the SVF of human adipose tissue (AT) [12]. They demonstrated that CD34+/CD31− cells can differentiate into endothelial cells in presence of endothelial cell growth supplement. Moreover, they observed increase in the blood flow and the capillary density in ischemic hindlimb when they injected these cells into the mouse [12].
These cells with the capability of vascularization in damaged tissues can be isolated in abundance unlike other mesenchymal stem cells such as bone marrow stem cells which makes them advantages for vascularization in tissue engineering [23, 24]. Suga et al investigated the differentiation potential of CD34+/CD31− with and without growth factors in comparison with CD34−/CD31− cells in vitro and they observed capillary network formation of these cell when seeded on Matrigel [25]. VEGF is one of the growth factors that have been used for inducing endothelial differentiation in vitro. Oswald et al., first reported that VEGF induction at a concentration of 50 ng/mL for 7 days induces endothelial differentiation of MSCs and they observed major characteristics of mature endothelial-like expression of vWF, VEGF receptors 1 and 2 (FLT-1 and KDR), VE cadherin, and VCAM-1[26].
The fact that acquisition of fat from is a rapid and easy procedure, and CD34+/CD31− cells exist there prompted us to explore whether these cells show endothelial markers and tube formations in VEGF induced condition as observed in bone marrow stem cells [25] when cultured in vitro. Wang et al., observed that endothelial differentiation of mesenchymal stem cells reaches a plateau at day 14 in presence of VEGF [27]. Levenberg et al., also reported expression of CD31 reaching a maximum at 13–15 days. [28]. Therefore, based on literature suggesting two weeks as the optimal time for acquiring endothelial cells, CD34+/CD31− cells were cultured in VEGF media for up to 14 days.
Cell proliferation and differentiation have an inverse relationship, as cells go through differentiation their rate of proliferation decreases. Precursor cells continue division before acquiring a fully differentiated state and cells usually exit from the division cycle when they are fully differentiated which explains lower proliferation rate in differentiated cells compared to non-differentiated ones. The reason for more dead cells in growth medium is since they proliferated more than differentiated cells over 14 days they passed the confluency point which leads to cell death and that happens late in the log phase of growth. Cell death occurs when cultures become overcrowded which could be caused by lack of nutrients and environmental temperature above or below the tolerance band for the cells [29, 30].
qRT-PCR results revealed 5 fold and 7.5 fold increase in vWF after 7 and 14 days respectively. VE Cadherin also showed 2 and 4 fold upregulation after 7 and 14 days which was close to that of HUVEC (positive control). This in vitro observation is consistent with previous work by others that demonstrated expression of endothelial markers in VEGF induced medium. Zhang et al., reported that human BMSCs after induction with VEGF for 14 days resulted in a twofold increase in vWF [31]. Chen et al., also observed a six fold increase in vWF, VE cadherin, and VEGFR2 expression in bone marrow stem cells with VEGF and EGF treatment [32]. CD31 expression however was not observed in VEGF induced cells on 2D culture and only expressed after seeing on Matrigel. A similar result was observed during endothelial differentiation of mesenchymal stem cells after 10 days in EGM-2 medium by Portalska et al. One possible explanation could be that there is a positive feedback loop between the ECM stimulation and CD31 expression in MSC [22]. Other studies also investigated the involvement of PECAM-1 (CD31) in vessel formation of endothelial cells [33]. Matsumura et al., observed a tight correlation between the expression of CD31, tube formation and F actin alignment of HUVECs on Matrigel [34]. This could explain the expression of CD31 on CD34+/CD31− cells after seeding on Matrigel. In their study tube formation were prevented by adhesion-blocking mAb to CD31 both in vivo and in vitro. Tube formation assay is a simple rapid and reliable assay in vitro to screen for various factors that promote endothelial differentiation. Matrigel, a basement membrane extract has been used extensively as a cell culture substratum for three-dimensional cultures to promote endothelial differentiation [35, 36]. Cells form tubes by first attaching to the matrix, migration toward each other and then aligning in a tube-like structure. Synthesis of proteins including collagen and proteases are required in this process [37, 21]. Our tube formation results show that in presence of an angiogenic factor (50 ng/ml VEGF) the cells attach, align and form tubes with the appearance of a network-like lumen area. Formation of tubular-like network is more profound in VEGF induced cells compared to non-differentiated CD34+/CD31− cells. Undifferentiated cells form some alignments but that structure is not stable and the cells detach from the substrate within 24 h. Suga et al observed very similar network formation 6h after seeding their CD34+ cells on in growth factors (−) [38]. In another study Oliver et al observed tube formation after seeding the cells on Matrigel without growth factors, however the cells were all adipose derived stem cells and were not sorted for CD34+/CD31− cells in which the presence of CD31+ cells could have affected capillary network formation despite the lack of growth factors [39].
Immunofluorescence staining and qRT-PCR results show that CD34+/CD31− cells isolated from SVF show endothelial phenotype as indicated by CD31, VE Cadherin and vWF expression when cultured in VEGF induced medium. These results are in agreement with those of hMSCs differentiated into endothelial cells in vitro. Oswald et al., observed positive vWF staining on human bone marrow stem cells after 7 days in 50 ng/mL culture medium[26]. Expression of vWF protein was also observed in another study with Miao et al., when differentiating human bone marrow stem cells in presence of VEGF for 7 days [40]. Wang et al., observed expression of CD31 on tube like cells on Matrigel 3 days after induction[27]. However, the ASC whole fraction which contain CD34+/CD31− cells don’t always respond to VEGF stimulation in vitro. One explanation could be that expression of the CD34 antigen which plays an important role in endothelial differentiation of the cells is not stable in vitro culture and it is lost during expansion or freezing/thawing process[41]. Boquest et al., observed down regulation of adipose derived stem cell surface phenotypes, specifically CD34 marker expression during culture in vitro which can negatively impact endothelial differentiation process of adipose derived cells [42]. Although the ability CD34+/CD31− cells make them an alternative source for cell therapy applications some factors such as health status [43–45] and age of the donors could impact their differentiation potential [46, 39].
Taken together this study shows differentiation of CD34+/CD31− cells into an endothelial cell-like lineage when cultured in VEGF containing media. The ease of isolation from adipose derived SVF and the ability of CD34+/CD31− cells to form EC-like population makes them attractive candidates for the development of vascularized engineered tissues as well as augment vessel growth in ischemic tissue.
Conclusion
In the present study we examined endothelial differentiation of adipose tissue derived CD34+/CD31− cells in vitro. These cells were isolated from SVF of adipose tissue using magnetic activated cell sorting technique. The results showed that these cells successfully differentiate into endothelial-like cells in vitro by acquiring several characteristics of the endothelial cell phenotype when induced with VEGF over 14 days in culture. These features include tube formation in response to Matrigel; upregulation and expression of vWF, VE Cadherin and CD31 genes and proteins after 14 days in VEGF induced medium. Both undifferentiated group and differentiated cells remained healthy and viable over 14 days however differentiated cells proliferated less compared to non-differentiated group. As these cells can be easily harvested from patients in a simple, minimally invasive lipoaspiration procedure and can be expanded ex vivo, they are suitable for autologous cell therapy.
Supplementary Material
Acknowledgment
Research reported in this article was supported by National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number (RDE024790A). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References:
- 1.Wang N, Wang S-J, Zhang R, Zhang T-C, Zhang C-L, Mao L-B et al. Vascular endothelial growth factor stimulates endothelial differentiation from mesenchymal stem cells via Rho/myocardin-related transcription factor-A signaling pathway. International Journal of Biochemistry and Cell Biology. 2013;45(7):1447–56. [DOI] [PubMed] [Google Scholar]
- 2.Rouwkema J, Khademhosseini A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends in Biotechnology. 2016;34(9):733–45. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Murohara T, Sullivan A, Silver M, Rien van der Z, Li T et al. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science. 1997;275(5302):964–7. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. CIRCULATION. 2006;113(10):1311–25. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Luo Y, Hui H, Cai T, Huang H, Yang F et al. CD146 coordinates brain endothelial cell–pericyte communication for blood–brain barrier development. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(36):E7622–E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frese L, Dijkman PE, Hoerstrup SP. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfusion Medicine and Hemotherapy. 2016;43(4):268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindner U, Kramer J, Rohwedel J, Schlenke P. Mesenchymal Stem or Stromal Cells: Toward a Better Understanding of Their Biology? Transfusion Medicine and Hemotherapy. 2010;37(2):75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baer PC, Geiger H. Adipose-Derived Mesenchymal Stromal/Stem Cells: Tissue Localization, Characterization, and Heterogeneity. Stem Cells International. 2012;2012:812693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimble JM, Katz AJ, Bunnell BA. Adipose-Derived Stem Cells for Regenerative Medicine. Circulation Research. 2007;100(9):1249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coralie S, Alexandra M, Marie M, Sandra dB, Rudi B, Anne B. Chemotaxis and Differentiation of Human Adipose Tissue CD34+/CD31− Progenitor Cells: Role of Stromal Derived Factor-1 Released by Adipose Tissue Capillary Endothelial Cells. STEM CELLS. 2007;25(9):2269–76. [DOI] [PubMed] [Google Scholar]
- 11.Hertweck J, Ritz U, Götz H, Schottel PC, Rommens PM, Hofmann A. CD34+ cells seeded in collagen scaffolds promote bone formation in a mouse calvarial defect model. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2018;106(4):1505–16. [DOI] [PubMed] [Google Scholar]
- 12.Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. CIRCULATION. 2004;110(3):349–55. [DOI] [PubMed] [Google Scholar]
- 13.Yang JJ, Ii M, Kamei N, Alev C, Kwon SM, Kawamoto A et al. CD34(+) Cells Represent Highly Functional Endothelial Progenitor Cells in Murine Bone Marrow. PLOS ONE. 2011;6(5):e20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q, Cen L, Yin S, Chen L, Liu G, Chang J et al. A comparative study of proliferation and osteogenic differentiation of adipose-derived stem cells on akermanite and β-TCP ceramics. Biomaterials. 2008;29(36):4792–9. [DOI] [PubMed] [Google Scholar]
- 15.Zanetti AS, McCandless GT, Chan JY, Gimble JM, Hayes DJ. Characterization of novel akermanite:poly‐ ϵ-caprolactone scaffolds for human adipose-derived stem cells bone tissue engineering. Journal of Tissue Engineering and Regenerative Medicine. 2015;9(4):389–404. [DOI] [PubMed] [Google Scholar]
- 16.Lian X, Bao X, Al-Ahmad A, Liu J, Wu Y, Dong W et al. Efficient Differentiation of Human Pluripotent Stem Cells to Endothelial Progenitors via Small-Molecule Activation of WNT Signaling. Stem Cell Reports. 2014;3(5):804–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behr B, Tang C, Germann G, Longaker MT, Quarto N. Locally Applied Vascular Endothelial Growth Factor A Increases the Osteogenic Healing Capacity of Human Adipose-Derived Stem Cells by Promoting Osteogenic and Endothelial Differentiation. STEM CELLS. 2011;29(2):286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zonari A, Novikoff S, Electo NRP, Breyner NM, Gomes DA, Martins A et al. Endothelial Differentiation of Human Stem Cells Seeded onto Electrospun Polyhydroxybutyrate/Polyhydroxybutyrate-Co-Hydroxyvalerate Fiber Mesh. PLOS ONE. 2012;7(4):e35422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unger RE, Sartoris A, Peters K, Motta A, Migliaresi C, Kunkel M et al. Tissue-like self-assembly in cocultures of endothelial cells and osteoblasts and the formation of microcapillary-like structures on three-dimensional porous biomaterials. Biomaterials. 2007;28(27):3965–76. [DOI] [PubMed] [Google Scholar]
- 20.Fang TD, Salim A, Xia W, Nacamuli RP, Guccione S, Song HM et al. Angiogenesis Is Required for Successful Bone Induction During Distraction Osteogenesis. Journal of Bone and Mineral Research. 2005;20(7):1114–24. [DOI] [PubMed] [Google Scholar]
- 21.Peters EB. Endothelial Progenitor Cells for the Vascularization of Engineered Tissues. Tissue Engineering Part B: Reviews. 2018;24(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portalska KK, Leferink AM, Groen N, Fernandes HAM, Moroni L, van Blitterswijk C et al. Endothelial Differentiation of Mesenchymal Stromal Cells. PLoS ONE. 2012;7(10):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P, Moudgill N, Hager E, Tarola N, DiMatteo C, McIlhenny S et al. Endothelial Differentiation of Adipose-Derived Stem Cells from Elderly Patients with Cardiovascular Disease. Stem Cells and Development. 2011;20(6):977–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dao TT-T, Vu NB, Phi LT, Ha Thi-Ngan L, Phan NK, Ta VT et al. Human adipose-derived mesenchymal stem cell could participate in angiogenesis in a mouse model of acute hindlimb ischemia. Biomedical Research and Therapy. 2016;3(8):770–9. [Google Scholar]
- 25.Suga H, Matsumoto D, Eto H, Inoue K, Aoi N, Kato H et al. Functional Implications of CD34 Expression in Human Adipose–Derived Stem/Progenitor Cells. Stem Cells and Development. 2009;18(8):1201–10. [DOI] [PubMed] [Google Scholar]
- 26.Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M et al. Mesenchymal Stem Cells Can Be Differentiated Into Endothelial Cells In Vitro. STEM CELLS. 2004;22(3):377–84. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Li Y, Yang M, Zou Y, Liu H, Liang Z et al. Efficient Differentiation of Bone Marrow Mesenchymal Stem Cells into Endothelial Cells in Vitro. European Journal of Vascular & Endovascular Surgery. 2018;55(2):257–65. [DOI] [PubMed] [Google Scholar]
- 28.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial Cells Derived from Human Embryonic Stem Cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(7):4391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Heuvel S Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression AU - Ruijtenberg, Suzan. Cell Cycle. 2016;15(2):196–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper GM. The Cell - A Molecular Approach 2nd Edition Sunderland (MA): Sinauer Associates; 2000. [Google Scholar]
- 31.Zhang G, Zhang F, Zhou J, Fan Q, Zheng Z, Liu X et al. Arterial–venous endothelial cell fate is related to vascular endothelial growth factor and Notch status during human bone mesenchymal stem cell differentiation. FEBS Letters. 2008;582(19):2957–64. [DOI] [PubMed] [Google Scholar]
- 32.Chen M-Y, Lie P-C, Li Z-L, Wei X. Endothelial differentiation of Wharton’s jelly–derived mesenchymal stem cells in comparison with bone marrow–derived mesenchymal stem cells. Experimental Hematology. 2009;37(5):629–40. [DOI] [PubMed] [Google Scholar]
- 33.Cao G, O’Brien CD, Zhou Z, Sanders SM, Greenbaum JN, Makrigiannakis A et al. Involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. American Journal of Physiology - Cell Physiology. 2002;282(5):1181–90. [DOI] [PubMed] [Google Scholar]
- 34.Matsumura T, Wolff K, Petzelbauer P. Endothelial cell tube formation depends on cadherin 5 and CD31 interactions with filamentous actin. The Journal of Immunology. 1997;158(7):3408–16. [PubMed] [Google Scholar]
- 35.Kleinman HK, Arnaoutova I. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nature Protocols. 2010;5(4):628–35. [DOI] [PubMed] [Google Scholar]
- 36.Kleinman HK, Martin GR. Matrigel: Basement membrane matrix with biological activity. Seminars in Cancer Biology. 2005;15(5):378–86. [DOI] [PubMed] [Google Scholar]
- 37.Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. ANGIOGENESIS. 2009;12(3):267–74. [DOI] [PubMed] [Google Scholar]
- 38.PE B. Endothelial Progenitor Cells for the Vascularization of Engineered Tissues. Tissue Engineering Part B: Reviews. 2018;24(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliva-Olivera W, Coín-Aragüez L, Lhamyani S, Salas J, Gentile A-M, Romero-Zerbo S-Y et al. Differences in the neovascular potential of thymus versus subcutaneous adipose-derived stem cells from patients with myocardial ischaemia. Journal of Tissue Engineering and Regenerative Medicine. 2018;12(3):e1772–e84. [DOI] [PubMed] [Google Scholar]
- 40.Zongning M, Jun J, Lei C, Jianzhong Z, Wei H, Jidong Z et al. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biology International. 2006;30(9):681–7. [DOI] [PubMed] [Google Scholar]
- 41.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R et al. A Population of Multipotent CD34-Positive Adipose Stromal Cells Share Pericyte and Mesenchymal Surface Markers, Reside in a Periendothelial Location, and Stabilize Endothelial Networks. Circulation Research. 2008;102(1):77–85. [DOI] [PubMed] [Google Scholar]
- 42.Boquest AC, Shahdadfar A, Frønsdal K, Sigurjonsson O, Tunheim SH, Collas P et al. Isolation and Transcription Profiling of Purified Uncultured Human Stromal Stem Cells: Alteration of Gene Expression after In Vitro Cell Culture. Molecular Biology of the Cell. 2005;16(3):1131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oñate B, Vilahur G, Ferrer-Lorente R, Ybarra J, Díez-Caballero A, Ballesta-López C et al. The subcutaneous adipose tissue reservoir of functionally active stem cells is reduced in obese patients. The FASEB Journal. 2012;26(10):4327–36. [DOI] [PubMed] [Google Scholar]
- 44.Oliva-Olivera W, Lhamyani S, Coín-Aragüez L, Castellano-Castillo D, Alcaide-Torres J, Yubero-Serrano EM et al. Neovascular deterioration, impaired NADPH oxidase and inflammatory cytokine expression in adipose-derived multipotent cells from subjects with metabolic syndrome. Metabolism. 2017;71:132–43. [DOI] [PubMed] [Google Scholar]
- 45.Oliva-Olivera W, Moreno-Indias I, Coín-Aragüez L, Lhamyani S, Alcaide Torres J, Fernández-Veledo S et al. Different response to hypoxia of adipose-derived multipotent cells from obese subjects with and without metabolic syndrome. PloS one. 2017;12(11):e0188324-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Efimenko A, Dzhoyashvili N, Kalinina N, Kochegura T, Akchurin R, Tkachuk V et al. Adipose-derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential. Stem Cells Transl Med. 2014;3(1):32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.