Abstract
We designed a 16-week scaffolded student-scientist curriculum using inquiry-based research experiences integrated with professional development activities. This curriculum was implemented to teach undergraduate students enrolled in an introduction to biology course about enzyme activity, biochemical reactions, and alcohol fermentation. While working through the curriculum, students completed the entire scientific process by planning experiments, maintaining laboratory journals, analyzing and interpreting data, peer-reviewing research proposals, and producing and presenting a poster. The overall outcome was for students to complete a multiweek, collaborative, student-scientist project using Saccharomyces cerevisiae as the model organism. Student learning outcomes were evaluated using formative assessments (post-Research on the Integrated Science Curriculum survey and peer- and self-reflection worksheets) and summative assessments (pre/post assessments and assignment grades). Results indicated that more than 50% of the students scored 70% or higher on the collaborative student-scientist project, demonstrated several self-reported learning gains in scientific concepts and skills, and reported they would recommend this laboratory course to their peers. By providing the opportunity for students to carry out the entire scientific process, this curriculum enhanced their technical, analytical, and communication skills.
INTRODUCTION
The Vision and Change in Undergraduate Biology Education: A Call to Action report (1) increases awareness for biology faculty to adopt creative student-centered curricula that use the scientific process and have measurable learning outcomes, thus enabling science majors from diverse backgrounds to demonstrate their analytical, experimental, technical, and communication skills. Introductory biology courses are essential for undergraduate students, serving as foundation and gateway courses for science majors or general education courses for nonscience majors. Integrating inquiry-based research provides students the opportunity to explore the scientific process and learn core biological concepts, fostering critical thinking and enhancing communication skills, which encourage science majors to become scientists and nonscience majors to become science-literate citizens (1). Engaging in research experiences allows students to work collaboratively as research teams while promoting independent learning and assimilation into the “STEM culture” (2).
Early exposure to research creates an atmosphere in which students can experience what it is like to be a scientist by “making observations, formulating questions, gathering evidence in a reproducible manner, making scientific claims based on evidence and existing scientific knowledge, communicating results, and revising the explanation or revisiting the experiment based on feedback and critique from the community” (3). It is recommended to scaffold inquiry-based instruction, because all students do not enter college with the same prior knowledge about the scientific process. Scaffolding the curriculum in an introductory science course provides students with repeated opportunities to learn and practice research skills. Students thus gain a deeper knowledge of the concepts while increasing their confidence in conducting the scientific process (4). The skills acquired while performing inquiry-based research prepare students for conducting research projects in the future (5). Moreover, Winkelmann et al. find that multiweek research-inspired chemistry laboratory modules increase students’ confidence in their ability to perform inquiry-based activities and produce positive outcomes comparable with those of authentic research experiences (6).
Results from a meta-analysis of peer-reviewed, published articles discussing undergraduate inquiry-based laboratory experiences reveal that the infusion of inquiry-based curricula has a positive effect on student learning gains; however, 56% of these published studies are for upper-level biology courses designed for majors (7). Even though many colleges and universities are integrating inquiry-based learning (IBL) into their laboratory curriculum, there is a need to develop and distribute inquiry-based curricula for introductory biology laboratory courses (7). In this article, we describe a scaffolded student-scientist curriculum designed for an introductory biology laboratory course that integrates several core competencies applied to biology practice recommended in the Vision and Change report (1) and activities performed by scientists (i.e., research proposals, peer-review, and poster preparation and presentation).
Intended audience and prerequisite student knowledge
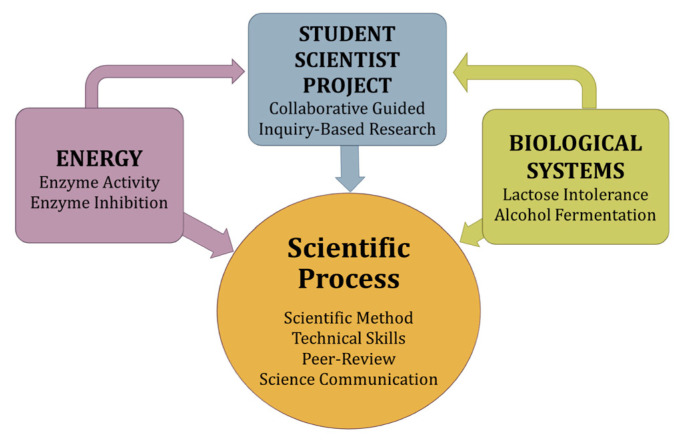
This course is intended for first-year biology, chemistry, and pharmaceutical science majors who plan to complete upper-level biology courses. This laboratory course teaches the scientific process and basic laboratory techniques, such as how to use a micropipette and compound microscope, and describes the interactions between enzyme activity, biochemical reactions, and alcohol fermentation (Fig. 1). This inquiry-based research curriculum also includes professional development activities that allow students to participate in exercises simulating the peer-review process and poster presentation competition (Table 1). Prerequisite science courses are not required because the curriculum is designed to accommodate students with a range of prior knowledge of biological concepts and experience practicing the scientific process, operating technical equipment, and communicating science. Furthermore, this course can be modified to teach general biology courses to nonscience majors.
FIGURE 1.
The big ideas reiterated during student-scientist curricula.
TABLE 1.
Student learning objective and performance assessments.
| Student Learning Objectives | Performance Assessments |
|---|---|
| 1. Use Microsoft Excel to analyze data | Short Course Tutorials: HHMI Microsoft Excel Data Analysis Assignment; Intro to Statistics and Data Analysis Worksheet; Student-Scientist Project |
| 2. Properly operate a micropipette | Lactase Activity Assay; Enzyme Inhibition and Cell Viability Assay; Student-Scientist Project |
| 3. Properly operate a compound microscope | Microscopy and Cell Viability Assignment; Student-Scientist Project |
| 4. Interpret data | Intro to Statistics and Data Analysis Worksheet; Lab Journals; Mini-Posters and Student-Scientist Project Poster |
| 5. Document inquiry-based research in a laboratory journal | Lab Journals |
| 6. Prepare research proposals for hypothesis-driven inquiry-based research experiments | Lactose Intolerance Research Proposal; Enzyme Inhibition Research Proposal; Student-Scientist Project Research Proposal |
| 7. Create poster to communicate scientific findings | Mini-Posters and Student-Scientist Project Poster |
| 8. Design and conduct inquiry-based experiments using the scientific process | Student-Scientist Project |
Learning time
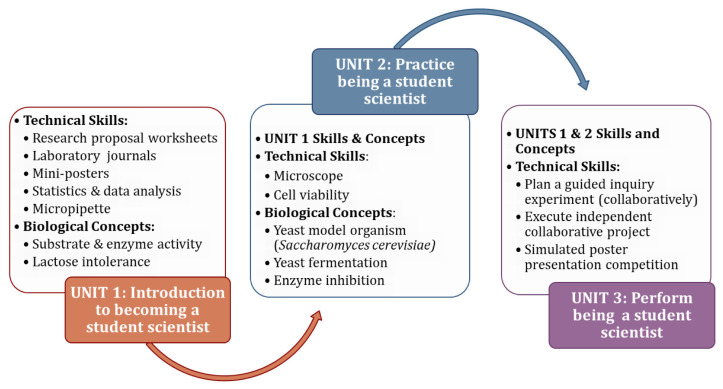
The student-scientist curriculum is designed to be assimilated into an introductory biology course that meets weekly for a two-hour laboratory session and three 50-minute lectures over a full semester (16 weeks). This curriculum is scaffolded into three units to allow students to learn and practice the laboratory, technical, and communication skills needed to complete a collaborative student-scientist project (SSP) at the end of the semester (Fig. 2). During Units 1 and 2, the laboratory sessions begin with a 20-minute lecture to introduce or revisit biological concepts and techniques, followed by the hands-on inquiry-based laboratory activities. Unit 3 is devoted entirely to student projects wherein they apply material from Units 1 and 2.
FIGURE 2.
The student scientist curriculum scaffold into three units.
Unit 1 (weeks 1 to 5) introduces students to becoming a student-scientist (Fig. 2).
Week 1
Students are introduced to the student-scientist curriculum requirements and laboratory safety.
Week 2
Students are introduced to basic statistics, data analysis, and graph interpretation. Students are then given 2 weeks to complete a two-part data analysis assignment. Part 1 consists of completing the Teaching Statistics and Math Using Spreadsheet Tutorials and Galápagos Finches (https://www.hhmi.org/biointeractive/spreadsheet-data-analysis-tutorials) (8), followed by a five-question assignment administered on Blackboard. Part 2 is an Introduction to Statistics and Data Analysis worksheet designed to allow students to practice analyzing data while learning about lactose intolerance (Appendix 2). While completing this Introduction to Statistics and Data Analysis worksheet, students practice using the Quick Cals website (www.Graphpad.com) to perform and interpret t-tests.
Week 3
Students are taught to use the scientific process including research proposal and experimental designs. Each student is tasked with using their biology textbook, published peer-reviewed literature and laboratory assignment handouts provided on Blackboard to complete a research proposal template over a 1-week period. In addition to completing their individual research proposals (Appendix 4), students are given a pre-lab assignment which consists of watching a 5-minute video on micropipetting (www.youtube.com/watch?v=NgosWmRjjAo), reading a handout on how to properly operate a micropipette, and completing a five-question assignment administered on Blackboard.
Week 4
Students revisit operating a micropipette, learn the biological concepts associated with enzyme activity, biochemical reactions, and lactose intolerance, practice detailing their experiment in a 20-page composition notebook (Appendix 5), and conduct a lactase activity assay. Each group of four students collaborates to measure the lactase activity from each sample. Each student records their group data in their individual journal. One member from each group inputs their data into a Microsoft Excel spreadsheet to create a larger dataset for the class to analyze and interpret. Students are given 2 weeks to analyze the data and complete their individual lab journal assignment.
Week 5
Students are introduced to their first professional development activity. They learn the criteria for preparing an effective poster to communicate science. In groups of four students, they model the experience of serving on a poster competition review committee using a mini-poster evaluation form (Appendices 6 and 7) to critique, score, and rank two mini-posters. This activity allows the students to view mini-posters and become familiar with criteria to create an informative collaborative mini-poster. Each group of four students collaborates to create a mini-poster using a template to communicate their findings from the lactase activity assay study (Appendix 8).
Unit 2 (weeks 6 and 7) allows students to practice being student-scientists while learning about enzyme inhibition, alcohol fermentation, and cell viability.
Week 6
Students are assigned a pre-lab assignment consisting of watching a 13-minute video on compound microscopy (www.youtube.com/watch?v=P6Iyo_ODtP4), viewing a 6-minute video on wet mount and microscope focus (www.youtube.com/watch?v=FoqhPoZnsmY), and reading a handout on how to properly operate a compound microscope, followed by completing an eight-question assignment administered on Blackboard. Students complete an Altering Enzyme Activity on Yeast Cells, Cell Viability & Microscopy Laboratory worksheet to practice operating a light compound microscope, creating a wet mount, and explaining the inhibition enzyme activity on Saccharomyces cerevisiae using hydrochloric acid (Appendix 10).
Week 7
Students are assigned a pre-laboratory assignment to prepare their individual research proposal (Appendix 11). During the laboratory session, they investigate alcohol fermentation and enzyme inhibition using a modified version of a “Which Beer is Best?” (9) IBL activity, with Saccharomyces cerevisiae as the model organism. During the “Which Beer is Best” IBL activity (9), groups of four students work collaboratively to measure alcohol fermentation rates, cell viability, and pH data. Each student records their group data in their individual journal. One member from each group inputs the data into a Microsoft Excel spreadsheet to create a larger dataset for the class to analyze and interpret. Students are given 2 weeks to analyze the data and complete their individual lab journal assignment. Students collaborate within their group to create a mini-poster detailing the findings from the “Which Beer is Best?” study (Appendix 12).
Unit 3 (weeks 8 to 16) allows students to practice being student-scientists by collaborating in teams of four to complete an SSP.
Week 8
Students collaboratively design an SSP research proposal (Appendix 13). They apply protocols, research techniques, and biological concepts provided in Units 1 and 2 to prepare a collaborative research proposal detailing their experimental design.
Week 9
Students participate in a mock double-blind research proposal peer-review panel as their second professional development activity (Table 2). During this activity each team is required to use the grading rubric to evaluate, critique, and provide written feedback of SSP research proposals drafted by another student group (Appendix 13). The written feedback is intended to be used to improve the quality of the research proposal as well as enhance the students’ knowledge of alcohol fermentation and cell viability.
TABLE 2.
Description of assignments and activities used in the study.
| Assignments and Activities | Description |
|---|---|
| Teaching statistics and math using spreadsheet tutorials and Galápagos finches | Electronic learning activity using Microsoft Excel spreadsheets to organize data; use functions to calculate the mean, standard deviation, and standard error of the mean; and create bar graphs with error bars (https://www.hhmi.org/biointeractive/spreadsheet-data-analysis-tutorials) |
| Introduction to statistics and data analysis worksheet | Introduces the concepts of enzyme activity, biochemical reactions and the effect of ethnicity on lactose intolerance. Uses secondary lactose intolerance data for artificial patients with different ethnic backgrounds to practice using both Microsoft Excel to organize and analyze the data and create a bar graph with error bars and Quick Cals website (www.Graphpad.com) to execute t-tests to compare the lactose intolerance levels. Two open-ended questions were included to guide students with their data interpretation. |
| Readiness assessments | Essentially pop quizzes administered at the beginning a of laboratory session to assess students’ retention of information. Each assessment contained 3 to 4 questions with an assortment of formats including open-ended questions, crossword puzzles, multiple-choice, and matching. |
| Research proposals | Microsoft Word templates containing 21 open-ended questions guided students in designing their research. Questions focused on background information regarding the research topic, hypothesis, experimental design, data collection and analysis, and anticipated outcomes. Research proposals were individual assignments during Units 1 and 2, but a group assignment during Unit 3. |
| Posters | Group assignment using a pre-designed Microsoft PowerPoint template to create mini-posters (8.5″ × 11″) to communicate research results at the end of Units 1 and 2. Traditional-sized posters (36″ × 24″) were printed at the end of Unit 3. |
| Lab journals | Uses a 20-page composition notebook to log details of the research, peer and self-reflections, raw data, data analysis, data interpretations, graphs, pictures, concept maps, and any other relevant information. Lab journals were collected at the end of each unit for an individual grade. New journals were provided to each student prior to starting the Lactase Assay – Lactose Intolerance IBL activity (Unit 1) – and Alcohol Fermentation Assay and Cell Viability Assay – “Which beer is best” IBL activity (Unit 2). A 40-page composition notebook was given to each student prior to executing the SSP (Unit 3). |
| Pre-laboratory video assignments | Watching a video demonstration operating laboratory equipment, micropipettes (Unit 1) and compound microscopy (Unit 2), accompanied by an electronic assignment consisting of multiple-choice, order ranking, or matching questions, administered via Blackboard one week prior to operating the equipment in lab. |
| Student-scientist project | Designed to apply the knowledge and skills acquired during Units 1 and 2 to an experiment focusing on enzyme activity, alcohol fermentation, and cell viability. Working collaboratively as a research team of four students, each group was required to choose a substrate or inhibitor, collect data consecutively over a 3-week period, and present their findings as a final poster presentation. The SSP grade was an average of the scores received on the research proposal, laboratory journals, and final poster presentation. |
| Mock poster competition review committee | Prior to preparing their first mini-poster, students simulated serving on an undergraduate poster presentation competition committee. The purpose of becoming members of scientific organizations and attending scientific meetings was discussed. Each review committee consisted of at least three students and was given a “Mini-Poster Evaluation Form” to critique, score, and rank posters. During the follow-up class discussion, each committee justified their scores while becoming familiar with the expectations for effectively creating a poster. |
| Mock research proposal peer-review panel | Students emulated serving on a blind peer-review panel to demonstrate their proficiency in designing and critiquing research. Research proposal grading rubrics were used as the evaluation criteria. Each team was required to provide written feedback and a funding recommendation. The proposal with the highest score was “funded” with five extra credit points. |
| Poster competition | Students dressed professionally and, as a group, presented their SSP poster to their peers, instructors, and other STEM faculty in a setting mimicking a poster competition at a scientific meeting. The research team with the highest final poster presentation score won the competition. |
Week 10
Students submit the final research proposal to the instructor. The instructor uses a grading rubric to score each research proposal and “funds” the research proposal with the highest score five extra credit points.
Weeks 10 to 12
Students measure alcohol fermentation rates and observe the cell viability of S. cerevisiae. Each student records the data and details of their study in their individual lab journals.
Weeks 13 and 14
Students collaborate as a team to create a high-quality and informative poster detailing the findings from their SSP (Appendix 15).
Week 15
Students submit their journals detailing their SSP for grading and present their SSP poster as a group to their peers, instructors, and other STEM faculty in a setting that models a poster competition at a scientific meeting. The instructor and graduate assistant use a grading rubric to score each poster. The research team with the highest poster presentation score wins the competition.
Learning objectives
Upon completion of student-scientist curricula, students will be able to:
Use Microsoft Excel to analyze data and create graphs
Properly operate a micropipette
Properly operate a compound microscope
Interpret data
Document inquiry-based research in a laboratory journal
Prepare research proposals for hypothesis-driven inquiry-based research experiments
Create posters to communicate their scientific findings
Exhibit and collaboratively present a student-scientist poster to peers and STEM faculty
Design and conduct inquiry-based experiments using the scientific process
PROCEDURE
Materials
A detailed list of reagents, including the per-student numbers and faculty instructions for each inquiry-based activity, is provided in the supplemental materials.
Student instructions
The student-scientist curriculum consists of several assessments such as assignments with grading rubrics, professional development activities, and peer and self-reflection worksheets (supplemental materials).
The curriculum is designed for students to work in small research teams of four students. Students are provided background documents, assignments, and other essential resources. After completing the mini-posters in Units 1 and 2 and the SSP poster, they are asked to complete a peer and self-reflection worksheet to evaluate their contribution and that of their team members. Students are given a new 20-page composition notebook to document their research findings at the beginning of Units 1 and 2 and a 40-page composition notebook at the beginning of Unit 3.
Faculty instructions
The student-scientist curriculum provides a 16-week hands-on inquiry-based research experience. Students begin the scaffolded curriculum during week 2 of the semester. The laboratory instructor introduces the course requirements to the students on the first day of class and discloses that this is a team-taught course with a postdoctoral fellow as the primary laboratory instructor, one graduate teaching assistant (TA), and four undergraduate TAs. Students are informed that this laboratory course is designed to encourage them to become student-scientists by carrying out an independent SSP and that the benefits of participating in the course make them more competitive for summer internship opportunities. Blackboard is used as a tool to disseminate all course materials. A GroupMe chat (https://groupme.com) is used to increase the interaction with the laboratory teaching team and perhaps enhance student learning (10). For the instructors, GroupMe is used as a supplement to Blackboard to provide students instant access to all the instructors, outside of traditional office hours, to ask questions and receive feedback regarding their laboratory assignments. A laboratory technician assists the laboratory teaching team with preparing solutions, setting up the lab materials, and ordering laboratory supplies. The laboratory teaching team meets weekly before the laboratory sessions during Units 1 and 2 and as needed during Unit 3. During these weekly meetings, the laboratory teaching team discusses the overview of the laboratory activity, learning objectives, expectations for the teaching team and students, and any other concerns regarding the course, including course feedback from the TAs.
Strategies for determining student learning
The student-scientist curriculum is designed for students to learn about enzyme activity, enzyme inhibition, and alcohol fermentation; perform the scientific process; analyze and interpret data; and communicate scientific findings in written and oral formats. Student learning is assessed using a combination of individual and group assignments accompanied by specific grading rubrics, pre/post assessment, Post-Course Research on the Integrated Science Curriculum (RISC) survey (11), and peer and self-reflection worksheets. A detailed description of each performance assessment is provided in Table 2. The pre-assessment is administered on the first day of the class and a week prior to the SSP poster presentations. The RISC survey is administered the same day as the post-assessment at the end of the semester. Students were provided a grading rubric prior to beginning each assessment. Both students and faculty used the same grading rubric during peer reviews and faculty evaluations of course assessments, respectively. Student grades were based solely on the faculty evaluations. Peer-review scores were not included in the assessment grades but were used to assist research teams with improving their work prior to submitting their assessment for a grade from the instructor.
Safety issues
The student-scientist curriculum is designed to comply with the American Society of Microbiology Guidelines for Biosafety in Teaching Laboratories (12). Students use personal protective equipment (safety goggles, lab coats, closed-toed shoes, and gloves) to work with S. cerevisiae, a biosafety level 1 organism (12), and chemical reagents. Students are lectured and trained about the laboratory safety requirements during the first laboratory session.
DISCUSSION
Field testing
The student-scientist curriculum was implemented in two laboratory sections during each of the fall 2016 and spring 2017 semesters (n = 65). Each laboratory section was limited to a maximum enrollment of 24 students. Early-college high school students and undergraduates randomly self-selected to enroll in the laboratory sections embedded with the student-scientist paradigm curriculum (Table 3). Pre/post assessments, course assignments, RISC post-course survey (11), and peer and self-reflection worksheets were used to evaluate the curriculum outcomes. Statistical tests were conducted using Systat Sigma Plot 12.5. Normalized Change Scores and effect size were calculated using Microsoft Excel.
TABLE 3.
Demographic characteristics of the student participants in the study.
| Demographic Characteristics | n | % |
|---|---|---|
| Gender | ||
| Female | 41 | 63 |
| Male | 24 | 37 |
| Academic classification | ||
| Early college high school | 2 | 3 |
| First-year freshmana | 31 | 48 |
| Freshmanb | 7 | 11 |
| Sophomore | 19 | 29 |
| Junior | 6 | 9 |
| Ethnicity | ||
| Black or African American | 54 | 83 |
| Hispanic or Latino | 4 | 6 |
| Other | 3 | 5 |
| Prefer not to say | 4 | 6 |
| Generation status | ||
| First-generation | 12 | 23 |
| Continuing-generation | 24 | 46 |
| Professional scientist in family | 9 | 17 |
| Academic major | ||
| Biology | 35 | 54 |
| Chemistry | 7 | 11 |
| Pharmaceutical Science | 16 | 25 |
| Non-STEM majorc | 5 | 8 |
| Undecided | 2 | 3 |
| High school Biology courses completed | ||
| Regular Biology only | 32 | 62 |
| Honors Biology only | 3 | 6 |
| Regular Biology & AP Biology | 4 | 8 |
| Honors Biology & AP Biology | 1 | 2 |
First-year freshmen are students who enrolled in college during fall 2016.
Freshmen who attended college prior to fall 2016 but did not have enough college credits to be classified as a sophomore.
Non-STEM majors consist of criminal justice, nursing, physical education, psychology, and social work majors.
Evidence of student learning
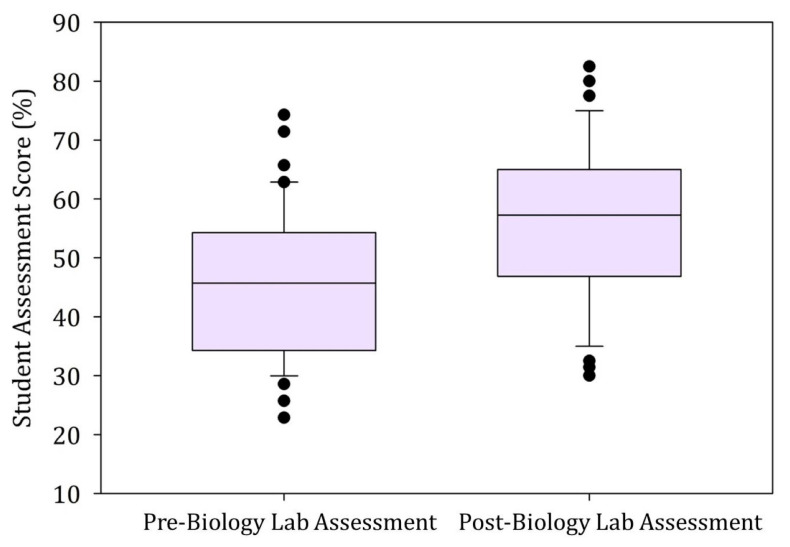
The pre/post-assessment scores were used to calculate normalized change scores to measure the learning gains (13). The effect size r (Cohen’s d for repeated measures) was calculated to provide evidence regarding the magnitude of the curriculum on the student learning gains (14, 15). The normalized change scores and the effect sizes for pre/post-assessment total scores indicated the student-scientist curriculum enhanced students’ ability to practice science and had a large effect on student’s learning gains (Table 4). Results from a paired-sample t-test (α = 0.05) revealed the post-assessment scores [M = 56.6%, standard deviation (SD) = 14.1%] were significantly higher than the pre-assessment scores [M = 45.4%; SD = 12.2%, t (56) = 6.465, p < 0.001] (Fig. 3). Post-assessment scores for experimental design, data analysis, reliable scientific literature, and calculations skills increased significantly when compared with the pre-assessment scores (Table 4).
TABLE 4.
Mean pre/post assessment skills scores, normalized gains and effect size (n = 56).
| Pre-Assessment Score | Post-Assessment Score | Gain | Effect Size | p value | Alpha | |
|---|---|---|---|---|---|---|
| Laboratory assessment | 45.5 | 55.58 | 0.17 | 0.78 | <0.001 | 1.00 |
| Scientific methoda | 53.4 | 55.6 | −0.13 | 0.09 | 0.567 | — |
| Experimental design | 42.2 | 50 | 0.10 | 0.41 | <0.001 | 0.774 |
| Data analysis | 37.6 | 63.2 | 0.37 | 1.27 | <0.001 | 1.00 |
| Reliable scientific literature | 49.6 | 59.9 | 0.15 | 0.47 | <0.001 | 0.824 |
| Technical skills | 55.6 | 54.2 | −0.08 | 0.06 | 0.726 | 0.064 |
| Calculation skillsa | 26.8 | 56.9 | 0.35 | 1.01 | <0.001 | — |
Indicates the normality test (Shapiro-Wilk) failed; pre/post assessment scores represent the median value.
FIGURE 3.
Distribution of the pre/post laboratory total assessment percentage scores (n = 56). The assessment consisted of 36 multiple-choice questions that focused on the scientific method, experimental design, data analysis, reliable scientific resources, technical skills, and calculation skills. It was administered at the beginning and end of each semester. A score of 100% represents a perfect score.
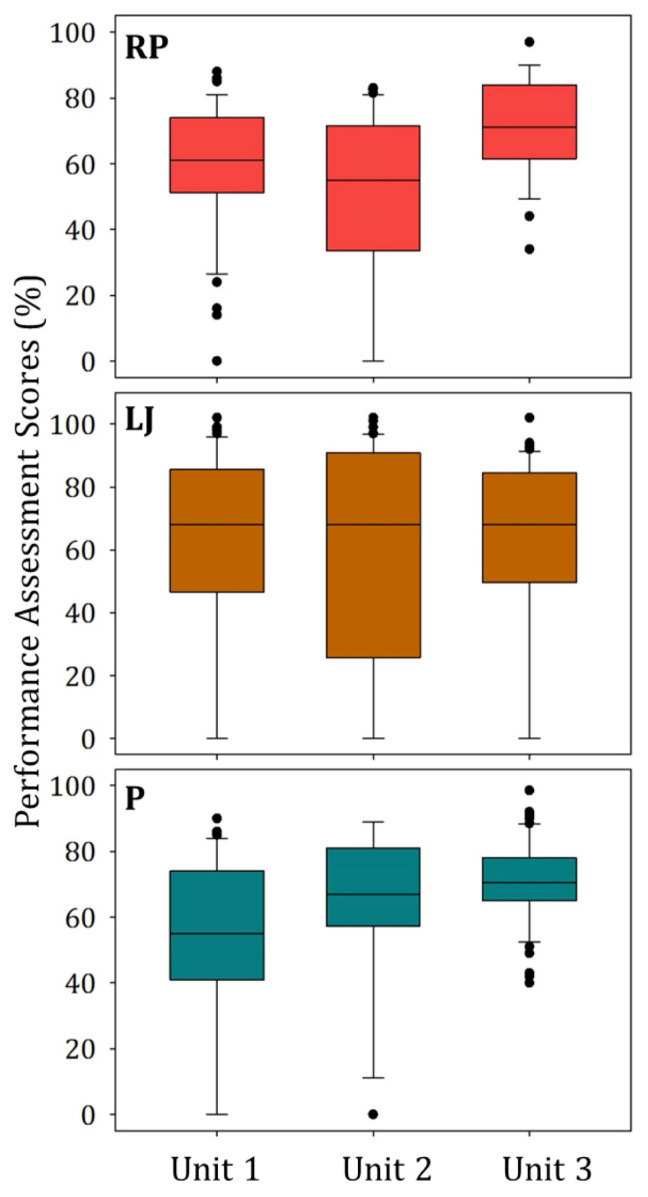
A total of 18 distinct collaborative SSPs were completed during the fall 2016 and spring 2017 semesters. Table 5 provides the performance scores for the various scaffolded inquiry-based laboratory curriculum assessments. More than 50% of the students earned a score of > 70% for their overall laboratory grade, SSP, and SSP poster. The distribution of the research proposal, lab journal, and poster (mini-posters and SSP poster) scores varied across the three units (Fig. 4). The maximum and minimum scores in Table 5 are diverse within the various assessments because some students used the grading rubric criteria to complete the assignments while other students submitted incomplete assignments.
TABLE 5.
Student-scientist laboratory curriculum performance scores.
| n | Mean | Median | Std. Dev. | Std. Error | Max | Min | |
|---|---|---|---|---|---|---|---|
| Overall lab grades | 65 | 69 | 70 | 14.2 | 1.76 | 93 | 39 |
| Student-scientist projecta | 65 | 68 | 71 | 12.4 | 1.53 | 92 | 38 |
| SSP poster presentation score | 64 | 70 | 71 | 13.0 | 1.62 | 99 | 40 |
| Research proposalsb | 63 | 58 | 64 | 20.0 | 2.52 | 86 | 8 |
| Lab journalsb | 61 | 64 | 69 | 25.5 | 3.27 | 101 | 10 |
| Mini-postersb | 64 | 58 | 60 | 20.2 | 2.53 | 89 | 14 |
Student-Scientist Project (SSP) score is the average of the group research proposal, lab journal, and SSP poster presentation.
Represents the mean score for the assignments completed during Units 1 and 2
FIGURE 4.
Student performance assessment percentage scores for the research proposal (RP), laboratory journals (LJ), and posters (P) during each unit (n = 65). An opportunity for extra credit points is provided for each assessment, and a score greater than 100% therefore represents a perfect score.
The research proposal scores were lower during the first two units than during the third unit, suggesting the research proposal grades improved when students collaborated to design their experiments and participated in a peer-review process. These findings were consistent with the student feedback stating that serving on the mock proposal peer-review panels (75%) and receiving constructive feedback (68%) was beneficial. A repeated measures ANOVA revealed there was not a statistically significant difference between the mean lab journal grades across each unit [F(2,64) = 0.536, p = 0.586], suggesting student performance in documenting the experiment remained constant throughout the semester. While serving on the mock poster competition review committee, students judged the posters very harshly, demonstrating that they were capable of detecting grammatical and spelling errors, inconsistent information, and formatting issues. Student feedback supports that participating in the mock poster competition committee was useful (96%) and increased their confidence in constructing an “A” quality mini-poster (74%). However, when they produced their own mini-posters, students made similar mistakes. An increase in the poster scores for each unit suggests that participating in the mock poster competition review committee and repetitively creating posters sharpened students’ ability to create an effective poster to communicate their findings (Fig. 4). This was further supported by the repeated measures ANOVA results, which indicated mean poster grades increased significantly [F(2,64) = 12.2, p < 0.001] as the semester progressed. Post-hoc tests using the Holm-Sidak Method revealed that mean ± SEM mini-poster grades in Unit 2 (62.9 ± 3.2%) were significantly (p < 0.009) higher than in those in Unit 1 (51.6 ± 3.2%). The mean poster grades in Unit 3 (70.4 ± 1.6%) were significantly (p < 0.043) higher than those in Unit 2 (62.9 ± 3.2%) and Unit l (51.6 ± 3.2%, p < 0.001) (Appendices 16 to 18).
Several self-reported student learning gains were measured using the RISC post-course survey (Tables 6 and 7). Course elements with the highest percentage consisted of “becoming responsible for the part of a project” (72%), “working on a problem in which the students have some input into the research process and/or what is being studied” (74%), “working on a project entirely of student’s own design” (73%), “working in small groups or teams” (70%), “learning that the use of disciplinary knowledge needs to be accurate and fair” (70%), “collecting data” (74%), and “analyzing data” (74%). “Understanding of how scientists work on real problems” (71%) had the highest beneficial learning gain. The mean scores for the self-reported student learning gains from the student-scientist curriculum were comparable with the self-reported learning gain scores from other research-driven courses (16).
TABLE 6.
Self-reported learning gains from specific tasks of the student-scientist laboratory curriculum.
| n | % Indicating High Gaina | Median | Mean | Standard Deviation | Standard Error | |
|---|---|---|---|---|---|---|
| Presenting my science work in posters | 60 | 60% | 4.0 | 3.7 | 1.0 | 0.13 |
| Critiquing the work of other students | 59 | 56% | 4.0 | 3.7 | 1.0 | 0.13 |
| Working with students who major (or probably intend to major) in other disciplines or fields of study | 58 | 62% | 4.0 | 3.8 | 1.0 | 0.13 |
| Working on defining a problem and refining the definition while solving the problem | 59 | 61% | 4.0 | 3.6 | 1.1 | 0.14 |
| Maintaining lab notebooks | 60 | 60% | 4.0 | 3.8 | 1.0 | 0.13 |
| Attempting a complete understanding of a complex problem | 60 | 62% | 4.0 | 3.7 | 1.1 | 0.14 |
| Becoming responsible for a part of a project | 60 | 72% | 4.0 | 4.0 | 0.9 | 0.12 |
| Working together with other students as a whole class | 60 | 65% | 4.0 | 3.9 | 1.0 | 0.13 |
| Engaging in experimental learning in the course | 60 | 67% | 4.0 | 3.8 | 1.0 | 0.13 |
| Working on a lab or problem in which only the instructor knows the outcome | 56 | 61% | 4.0 | 3.7 | 1.0 | 0.13 |
| Working on at least one problem that is assigned and structured by the instructor | 60 | 67% | 4.0 | 3.8 | 1.0 | 0.13 |
| Working on a problem in which the students have some input into the research process and/or what is being studied | 61 | 74% | 4.0 | 4.0 | 1.0 | 0.12 |
| Working on a project or problem entirely of student’s own design | 59 | 73% | 4.0 | 3.9 | 0.9 | 0.11 |
| Working in small groups or teams | 60 | 70% | 4.0 | 3.8 | 1.0 | 0.13 |
| Learning that the use of disciplinary knowledge needs to be accurate and fair | 57 | 70% | 4.0 | 4.0 | 0.9 | 0.12 |
| Reading scientific journal articles | 59 | 53% | 4.0 | 3.6 | 1.1 | 0.14 |
| Collecting data | 61 | 74% | 4.0 | 4.0 | 1.0 | 0.13 |
| Analyzing data | 61 | 74% | 4.0 | 4.0 | 1.1 | 0.14 |
| Learning that disciplines may approach problems in different and sometimes conflicting ways | 59 | 66% | 4.0 | 3.8 | 1.0 | 0.14 |
“High Gain” is the aggregate of “4-Large Gain” and “5-Very Large Gain.” Likert Scale: 1 = No Gain/Very Small Gain to 5 = Very Large Gain.
TABLE 7.
Self-reported perceived learning gains from the student-scientist laboratory curriculum.
| n | % Indicating High Gaina | Median | Mean | Standard Deviation | Standard Error | |
|---|---|---|---|---|---|---|
| Clarification of a career path | 59 | 53% | 4.0 | 3.4 | 1.1 | 0.14 |
| Skill in the interpretation of results | 59 | 61% | 4.0 | 3.7 | 1.0 | 0.13 |
| Tolerance for obstacles faced in the research process | 59 | 66% | 4.0 | 3.7 | 0.9 | 0.12 |
| Readiness for more demanding research | 59 | 63% | 4.0 | 3.7 | 1.0 | 0.13 |
| Understanding how knowledge is constructed | 58 | 66% | 4.0 | 3.7 | 1.0 | 0.13 |
| Understanding of the research process in your field | 59 | 64% | 4.0 | 3.8 | 0.9 | 0.12 |
| Ability to integrate theory and practice | 59 | 63% | 4.0 | 3.8 | 1.0 | 0.13 |
| Understanding of how scientists work on real problems | 59 | 71% | 4.0 | 3.9 | 1.0 | 0.13 |
| Understanding that scientific assertions require supporting evidence | 59 | 63% | 4.0 | 3.8 | 1.0 | 0.13 |
| Ability to analyze data and other information | 59 | 63% | 4.0 | 3.8 | 0.9 | 0.11 |
| Understanding science | 59 | 64% | 4.0 | 3.8 | 0.9 | 0.12 |
| Learning ethical conduct in your field | 56 | 63% | 4.0 | 3.8 | 0.8 | 0.10 |
| Learning laboratory techniques | 59 | 64% | 4.0 | 3.9 | 0.9 | 0.11 |
| Ability to read and understand primary literature | 57 | 61% | 4.0 | 3.6 | 1.1 | 0.15 |
| Skill in how to give an effective oral presentation | 59 | 63% | 4.0 | 3.7 | 1.1 | 0.14 |
| Skill in science writing | 59 | 63% | 4.0 | 3.7 | 1.0 | 0.13 |
| Self-confidence | 57 | 58% | 4.0 | 3.7 | 1.1 | 0.14 |
| Understanding of how scientists think | 59 | 64% | 4.0 | 3.8 | 1.1 | 0.14 |
| Learning to work independently | 59 | 59% | 4.0 | 3.7 | 1.0 | 0.13 |
| Becoming part of a learning community | 58 | 62% | 4.0 | 3.9 | 1.0 | 0.13 |
“High Gain” is the aggregate of “4-Large Gain” and “5-Very Large Gain.” Likert Scale: 1 = No Gain/Very Small Gain to 5 = Very Large Gain.
Students’ evaluation of the student-scientist curriculum
Students were asked to provide feedback about experiences with the student-scientist curriculum. Student expectations and overall thoughts about the curriculum were obtained using the RISC post-course survey (11). Survey results revealed the student-scientist curriculum met student expectations (Table 8). Student participants agreed that “this course challenged me to think critically and in new ways about the subject matter (85%) and “taking this course has motivated me to pursue additional courses in this field” (52%). Overall, a large percentage of students agreed that, “considering content design and structure, this course was excellent” (74%), “considering the syllabus and objectives, the organization of the course was excellent” (76%), and “considering course content and objectives, the instructors were effective” (87%). Additionally, peer and self-reflection worksheets following each unit and professional development activities were completed and disclosed mixed viewpoints (Appendix 19) about the student-scientist curriculum.
TABLE 8.
Student expectations of the student-scientist laboratory curriculum (n = 62).
| % Agreeing | Median | Mean | Standard Deviation | Standard Error | |
|---|---|---|---|---|---|
| This course taught me what I wanted to know about the subject matter. | 68% | 4.0 | 3.9 | 0.9 | 0.11 |
| This course challenged me to think critically and in new ways about the subject matter. | 85% | 4.0 | 4.2 | 0.8 | 0.11 |
| Taking this course has motivated me to pursue a career in the sciences. | 58% | 4.0 | 3.7 | 1.0 | 0.12 |
| Taking this course has motivated me to pursue additional courses in this field. | 52% | 4.0 | 3.4 | 1.1 | 0.14 |
| This course helped motivate me to attend graduate/professional school. | 68% | 4.0 | 3.8 | 1.0 | 0.12 |
Likert Scale: 1 = No Gain/Very Small Gain to 5 = Very Large Gain
Possible modifications
This student-scientist curriculum is designed to offer students from diverse backgrounds and educational levels the experience of a research environment early in their collegiate training. The curriculum can be adapted for AP biology classes, early-college high school students, and undergraduate science nonscience majors. This semester-long curriculum can be extended to a year-long curriculum. The first semester can focus on Units 1 and 2 to provide more time to practice scientific process and written communication skills. The second semester can be dedicated to completing an extended student-scientist project. Furthermore, the student-scientist curriculum can be modified to teach other biological concepts by inserting different inquiry-based activities.
CONCLUSION
Our findings suggest implementing the student-scientist curriculum in an introductory biology laboratory course provides an opportunity for students from diverse backgrounds to learn and practice the entire scientific process. Even though the mean performance scores are below average, the normal change scores, effect sizes, and student self-reported learning gains indicate this scaffolded inquiry-based curriculum is effective in providing first-year undergraduate science majors with the basic skills to conduct research and perform professional development activities. This curriculum is intense for first-year students with limited experience performing the scientific process; however, it challenges students to “think critically and in new ways about the subject matter.” The student-scientist curriculum is most effective when employed by supportive, encouraging, and optimistic instructors, because their positive energy will motivate students to achieve the goal of designing, performing, and communicating a collaborative student-scientist project. We recognize that these research projects are not “authentic” in terms of contributing new knowledge to the scientific community, but the research experiences are authentic to our student population.
SUPPLEMENTAL MATERIALS
ACKNOWLEDGMENTS
We acknowledge HHMI Precollege and Undergraduate Science Education Program (Grant # 52007553). Undergraduate TAs Jaimun Agnihotry, Jasmine Perry, Romny Imbert, and Luz Plumey are recognized for their hard work and dedication. We thank David Lopatto and his research team for giving permission to use survey items from the RISC survey (Research on the Integrated Science Curriculum; Lopatto, 2011). In addition, we thank Dawayne Whittington of Strategic Evaluations, Inc. (Durham, NC) for his team’s support with data collection and a subset of the analyses presented in the manuscript. Additional items on the survey we used were adopted from the College Biology Self-Efficacy Instrument for Non-Majors, Baldwin et al., 1999. A special thank you goes to Aditi Pai, professor of Biology, Spelman College, for reviewing the manuscript and providing helpful feedback. A portion of this research is supported by National Science Foundation grant #1346567 for the Advancing Competencies in Experimentation – Biology (ACE–Bio) Network. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation or HHMI. The authors declare that there are no conflicts of interest.
Footnotes
Supplemental materials available at http://asmscience.org/jmbe
REFERENCES
- 1.American Association for the Advancement of Science. Vision and Change in Undergraduate Biology Education: a Call to Action. [Online.] 2011. http://www.visionandchange.org/VC_report.pdf.
- 2.National Academies of Sciences, Engineering, and Medicine. Undergraduate research experiences for STEM students: successes, challenges, and opportunities. National Academies Press; Washington, DC: 2017. [DOI] [Google Scholar]
- 3.Weaver GC, Russell CB, Wink DJ. Inquiry-based and research-based laboratory pedagogies in undergraduate science. Nature Chem Biol. 2008;4:577–580. doi: 10.1038/nchembio1008-577. [DOI] [PubMed] [Google Scholar]
- 4.D’Costa A, Schlueter M. Scaffolded instruction improves student understanding of the scientific method & experimental design. Am Biol Teach. 2013;75(1):18–28. doi: 10.1525/abt.2013.75.1.6. [DOI] [Google Scholar]
- 5.Bell RL, Smetana L, Binns I. Simplifying inquiry instruction. Sci Teach. 2005;72:30–33. [Google Scholar]
- 6.Winkelmann K, Baloga M, Marcinkowski T, Giannoulis C, Anquandah G, Cohen P. Improving students’ inquiry skills and self-efficacy through research-inspired modules in the general chemistry laboratory. J Chem Educ. 2015;92:247–255. doi: 10.1021/ed500218d. [DOI] [Google Scholar]
- 7.Beck C, Butler A, Burke da Silva K. Promoting inquiry-based teaching in laboratory courses: are we meeting the grade? CBE Life Sci Educ. 2014;13:444–452. doi: 10.1187/cbe.13-12-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard Hughes Medical Institute BioInteractive. Teaching statistics and math using spreadsheet tutorials and Galápagos finches, on HHMI BioInteractive. 2015. https://www.hhmi.org/biointeractive/spreadsheet-data-analysis-tutorial.
- 9.Townsley WW. Which beer is best? Teacher’s guide. Carolina Biological Supply Company; Burlington, NC.: 2000. [Google Scholar]
- 10.Umbach PD, Wawrzynski MR. Faculty do matter: the role of college faculty in student learning and engagement. Res Higher Educ. 2005;46:153–184. doi: 10.1007/s11162-004-1598-1. [DOI] [Google Scholar]
- 11.Grinnell College. Research on the Integrated Science Curriculum (RISC) survey. Grinnell College; 2009. https://www.grinnell.edu/academics/centers-programs/ctla/assessment/risc. [Google Scholar]
- 12.American Society for Microbiology. ASM Guidelines for Biosafety in Teaching Laboratories. American Society for Microbiology; Washington, DC: 2019. [Google Scholar]
- 13.Marx JD, Cummings K. Normalized change. Am J Phys. 2007;75:87–91. doi: 10.1119/1.2372468. [DOI] [Google Scholar]
- 14.Dunlap WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol Meth. 1996;1:170–177. doi: 10.1037/1082-989X.1.2.170. [DOI] [Google Scholar]
- 15.Andrews TM, Leonard MJ, Colgrove CA, Kalinowski ST. Active learning not associated with student learning in a random sample of college biology courses. CBE Life Sci Educ. 2011;10:394–405. doi: 10.1187/cbe.11-07-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keiler KC, Jackson KL, Jaworski L, Lopatto D, Ades SE. Teaching broader impacts of science with undergraduate research. PLOS Biol. 2017;15(3):e2001318. doi: 10.1371/journal.pbio.2001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.