Graphical Abstract
Keywords: Cardiomyocyte, Anticancer drugs, Embryonic stem cells, High throughput, Human-induced pluripotent stem cells, Toxicity
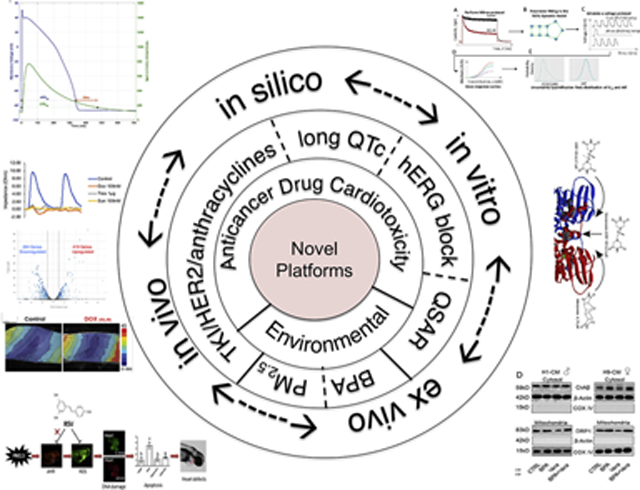
Within the 21st century, technological innovations such as miniaturization, high-speed data acquisition and analyses, have inspired widespread applications within the biological sciences. Nowhere has this been more apparent than in the areas of pharmacology and toxicology with emergence of high-throughput toxicity screening for drugs and environmental chemicals. Because of these obvious advances, we collected representative and specific examples of important scientific approaches applied in quantification of cardiac toxicity of both drugs and environmental agents in this Virtual Special Issue of Toxicology and Applied Pharmacology entitled: Emerging Platform Technologies for Cardiac Pharmacology and Toxicology. Thus, in the spirit of recognition of the persistent need to better understand cardiotoxicity, we organized a special issue to solicit articles that explore and validate new platforms for cardiotoxicity testing including in silico prediction, zebrafish assays, human induced pluripotent stems cells differentiated into cardiomyocytes (hiPSC-CMs) and full heart slices. This Editorial provides highlights of some of the special contributions made by each of these papers (Graphical Abstract).
1. Anticancer drug cardiotoxicity
Drug-induced cardiotoxicity is a major cause of market withdrawal of new drug candidates (Onakpoya et al., 2016). The lack of high-throughput platforms using human heart tissues/organs that are functionally and structurally viable for more than 24 h is a limiting factor in reliable cardiotoxicity testing. Thus, there is an urgent need to develop reliable, high-throughput systems for culturing human heart cells/tissues/organs under physiologic conditions for testing drugs for inherent toxicity. At present, only immature fetal cardiac cells (i.e., human-induced pluripotent stem cell-derived cardiomyocytes, hiPS-CMs; and human stem cell-derived cardiomyocytes, hES-CMs) are used to test both therapeutic effectiveness while also screening for drug toxicity, as these are the only human cardiac cells that can be cultured for prolonged time periods – a condition necessary to test for both drug efficacy and toxicity. Of course, a single cell type cannot replicate the complex phenotype of a 3-D heart that contains multiple cell types with their intimate interconnections. Additionally, the effects of drugs need to be discerned in adult cardiomyocytes, which have different and mature characteristics, including sensitivity (toxicity) responses, compared with fetal cardiomyocytes (Robertson et al., 2013). Therefore, it is clear there remains an urgent need for development of novel platforms for cardiotoxicity testing.
Recent pharmaceutical industry surveys indicate that preclinical and clinical safety remains a major cause of drug attrition and that cardiovascular toxicity represents the most frequent drug reaction, accounting for approximately 40% of all drugs being withdrawn due to safety concerns (Fermini et al., 2018). The estimated average cost of development of one drug is around $500 million U.S. dollars, and the early discovery of cardiotoxicity, prior to clinical trials, will save not only money and time, but human lives. To this end, the U.S. Food and Drug Administration (FDA) has publicly acknowledged the advantages of the cardiac tissue culture system in their ‘Cardiotoxicity Workshop’ summary and are now collaborating with researchers to rapidly develop this technology further (Pang et al., 2019).
Three articles of this VSI provide elegant examples of the ingenious ways that the power of in silico computing can be harnessed in the study of cardiotoxicity. In an article from Merck & Co., Inc., Morissette et al. combine an in silico proarrhythmic risk assay with a translational pharmacokinetic/pharmacodynamic (tPKPD) model to improve the predictive capacity of the QTc interval prolongation in vivo (Morissette et al., 2020). This study provides a new platform for in silico prediction of the cardiotoxicity of new compounds that certainly has the advantage as an alternative to performing in vivo testing (e.g., reduce animal use). Similarly, the in silico study by Hasinoff et al. uses computer modeling of the quantitative structure activity relationship (QSAR) to predict the exact binding sites of the cardioprotective drug, dexrazoxane and its derivatives, to DNA enzyme topoisomerase II (Hasinoff et al., 2020). Dexrazoxane protects from doxorubicin-induced cardiotoxicity presumably by preventing doxorubicin from activating topoisomerase II. In addition, this QSAR study shows that a series of bisdioxopiperazine analogs of dexrazoxane and the bisimide, mitindomide, are highly correlated with the ability of these compounds to catalytically inhibit the decatenation activity of topoisomerase II. In addition, this QSAR study defines the structural features of the dexrazoxane analogs that contribute to the binding and inhibition of topoisomerase II. Interestingly, this study further demonstrates a high homology in the amino acid sequence surrounding the dexrazoxane binding site in invertebrates and yeast topoisomerase II isoforms. This study is another example of creative computer modeling that enlightens the ways to identify the mechanisms of cardiotoxicity, and hence, ways to intervene to prevent cardiotoxicity. Moreover, the study described by Ridder et al. is a cross-industry collaboration to collect hERG data on 28 drugs with known torsadogenic risk using a standardized experimental protocol (Ridder et al., 2020). They performed a Bayesian Hierarchical Modeling (BHM) approach to assess the hERG blocking potency of these drugs by quantifying both the inter- and intra-site variability. These modeling and simulation studies are also done to evaluate protocol-dependent changes in hERG potency estimates. This new methodology estimates hERG blocking potency specific to a given voltage protocol, and thus, this systematic study indicates that we should be using different safety margin thresholds when in a different context for prediction of hERG activity. Collectively, these three studies reveal the power that in silico computing holds as an investigative tool – whether used for modeling or for predictive analysis using a data source continuum from in vitro to in vivo – for understanding the nature of drug-induced cardiotoxicity and developing new ways to prevent it.
Finally, there is an exciting study provided by Miller et al., wherein the authors introduce the ‘cardiac heart slice’ as a novel platform for cardiotoxicity testing (Miller et al., 2020). In this paper, the authors have validated the capacity of the heart slice model in detection of the selective toxicity of 3 different classes of anticancer drugs (i.e., TKI, HER2 and anthracyclines), which cannot be discerned in hiPS-CMs due to limitations of cellular complexity. For example, similar to hiPS-CMs, heart slices (both porcine and human) recapitulate the expected toxicity of doxorubicin (anthracycline) and trastuzumab (HER2); however, heart slices detect sunitinib-induced (TKI) cardiotoxicity and confirm its mechanism of action within a clinically-relevant concentration range of 0.1–1 μM – a sensitivity- and target-driven effect not found in hiPS-CMs. Thus, this study represents a notable advance that addresses a critical need by using the real-world complexity of the heart yet still has issues such as a requirement of specialized technology and logistics/access (porcine or healthy donor human hearts may not be readily available for all labs) – still high hurdles to overcome.
2. Environmental chemicals and cardiotoxicity
The field of “Environmental Cardiology” was established late last century but our troubles with environmental pollution are far from over (Dockery et al., 1993). In fact, exposure to ambient air pollution has emerged as one of the leading causes of death world-wide with the World Health Organization (WHO) estimating air pollution causes 7 million premature deaths or about 1 in 8 global deaths (http://www.who.int/mediacentre/factsheets/fs317/en/). With increased urbanization, the disease burden of air pollution is likely to increase, and although exposure to polluted environments increases the risk of many diseases, extensive epidemiological studies indicate that 60–70% of the premature mortality attributed to air pollution exposure are cardiovascular deaths (Pope 3rd et al., 2015). Thus 2 studies in this VSI are dedicated to environmental causes of cardiovascular toxicity. Although zebrafish have been used in chemical toxicity screens for decades, the study by Ren et al. uses zebrafish embryos to model the cardiac developmental toxicity of exposure to fine particle matter (PM2.5) that is independent of aryl hydrocarbon receptor (AhR) activation and to show a protective effect of resveratrol treatment, a natural polyphenolic compound commonly found in food and drinks (wine) with potent antioxidant effects (Ren et al., 2020). This study supports the use of non-mammalian vertebrate models in vivo to both test for developmental cardiotoxicity of chemicals but also for elucidating mechanism(s) of action. Another interesting study by Cheng et al. uses human embryonic stem cells differentiated into cardiomyocytes (hES-CMs) to demonstrate potential sex-dependent differences in response to low levels of bisphenol A (BPA) (Cheng et al., 2020). Exposures to the endocrine disruptor BPA are linked to sex-dependent cardiac hypertrophy as reported by the “Consortium Linking Academic and Regulatory Insights on BPA Toxicity” (CLARITY-BPA) project. This study provides molecular insight through a complex interplay of BPA with mitochondrial fission via modulation of calcineurin-DRP1 and hypertrophy signaling pathways. Because BPA is a ubiquitous environmental chemical, this study has important implications for cardiac development with low level BPA exposures but also represents a unique model that undoubtedly will require validation in future studies.
Overall, this VSI provides a glimpse into the rapidly emerging and novel platforms used for cardiotoxicity testing that ultimately hold promise for more reliable and sensitive drug and chemical screening via implementation of technological advances coupled with investigator ingenuity and perseverance – that remain the intellectual fuel to power future discoveries.
Acknowledgements
The authors thank Larry H. Lash, Ph.D., Editor, Toxicology and Applied Pharmacology, and leadership and administrative staff at Elsevier for supporting this VSI.
Funding
This work was supported by the National Institutes of Health (HL147921, ES023716, GM127607, HL122676, HL120163).
Footnotes
Declaration of Competing Interest
TMAM, holds equities in Tenaya Therapeutics. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration or the American Heart Association.
References
- Cheng W, Yang S, Li X, Liang F, Zhou R, Wang H, Feng Y, Wang Y, 2020. Low doses of BPA induced abnormal mitochondrial fission and hypertrophy in human embryonic stem cell-derived cardiomyocytes via the calcineurin-DRP1 signaling pathway: a comparison between XX and XY cardiomyocytes. Toxicol. Appl. Pharmacol 388, 114850. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA III, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG Jr., Speizer FE, 1993. An association between air pollution and mortality in six U.S. cities. N.Engl.J.Med 329, 1753–1759. [DOI] [PubMed] [Google Scholar]
- Fermini B, Coyne ST, Coyne KP, 2018. Clinical trials in a dish: a perspective on the coming revolution in drug development. SLAS Discov. 23, 765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasinoff BB, Patel D, Wu X, 2020. A QSAR study that compares the ability of bisdioxopiperazine analogs of the doxorubicin cardioprotective agent dexrazoxane (ICRF-187) to protect myocytes with DNA topoisomerase II inhibition. Toxicol. Appl. Pharmacol 399, 115038 http://www.who.int/mediacentre/factsheets/fs317/en/. [DOI] [PubMed] [Google Scholar]
- Miller JM, Meki MH, Ou Q, George SA, Gams A, Abouleisa RRE, Tang XL, Ahern BM, Giridharan GA, El-Baz A, Hill BG, Satin J, Conklin DJ, Moslehi J, Bolli R, Ribeiro AJS, Efimov IR, Mohamed TMA, 2020. Heart slice culture system reliably demonstrates clinical drug-related cardiotoxicity. Toxicol. Appl. Pharmacol 406, 115213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette P, Polak S, Chain A, Zhai J, Imredy JP, Wildey MJ, Travis J,Fitzgerald K, Fanelli P, Passini E, Rodriguez B, Sannajust F, Regan C, 2020. Combining an in silico proarrhythmic risk assay with a tPKPD model to predict QTc interval prolongation in the anesthetized Guinea pig assay. Toxicol. Appl. Pharmacol 390, 114883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onakpoya IJ, Heneghan CJ, Aronson JK, 2016. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. BioMed. Central Med 14, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang L, Sager P, Yang X, Shi H, Sannajust F, Brock M, Wu JC, Abi-Gerges N, Lyn-Cook B, Berridge BR, Stockbridge N, 2019. Workshop report: FDA workshop on improving cardiotoxicity assessment with human-relevant platforms. Circ. Res 125, 855–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA 3rd, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, Krewski D, Brook RD, 2015. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ. Res 116, 108–115. [DOI] [PubMed] [Google Scholar]
- Ren F, Huang Y, Tao Y, Ji C, Aniagu S, Jiang Y, Chen T, 2020. Resveratrol protects against PM2.5-induced heart defects in zebrafish embryos as an antioxidant rather than as an AHR antagonist. Toxicol. Appl. Pharmacol 398, 115029. [DOI] [PubMed] [Google Scholar]
- Ridder BJ, Leishman DJ, Bridgland-Taylor M, Samieegohar M, Han X, Wu WW, Randolph A, Tran P, Sheng J, Danker T, Lindqvist A, Konrad D, Hebeisen S, Polonchuk L, Gissinger E, Renganathan M, Koci B, Wei H, Fan J, Levesque P, Kwagh J, Imredy J, Zhai J, Rogers M, Humphries E, Kirby R, Stoelzle-Feix S, Brinkwirth N, Rotordam MG, Becker N, Friis S, Rapedius M, Goetze TA, Strassmaier T, Okeyo G, Kramer J, Kuryshev Y, Wu C, Himmel H, Mirams GR, Strauss DG, Bardenet R, Li Z, 2020. A systematic strategy for estimating hERG block potency and its implications in a new cardiac safety paradigm. Toxicol. Appl. Pharmacol 394, 114961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C, Tran DD, George SC, 2013. Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells 31, 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]


