Abstract
Plants perceive various external and internal signals to self-modulate biological processes through members of the receptor-like kinase (RLK) family, among which Catharanthus roseus receptor-like kinase 1-like (CrRLK1L) proteins with their ligands, rapid alkalinization factor (RALF) peptides, have attracted considerable interest. FERONIA (FER), a CrRLK1L member, was initially reported to act as a major plant cell growth modulator in distinct tissues. Subsequently, the RALF–FER pathway was confirmed to function as an essential regulator of plant stress responses, including but not limited to immune responses. Furthermore, the RALF–FER pathway modulates immune responses and cell growth in a context-specific manner, and the vital roles of this pathway are beginning to be appreciated in crop species. The recent remarkable advances in understanding the functions and molecular mechanisms of the RALF–FER pathway have also raised many interesting questions that need to be answered in the future. This review mainly focuses on the roles of FER and other CrRLK1L members in modulating immune responses in the context of cell growth in response to their RALF peptide ligands and presents a brief outlook for future research.
Keywords: FER receptor kinase, RALF peptide, immune responses, cell expansion, cell wall
Plants use receptor-like kinases to perceive and respond to various growth and stress signals. This review discusses the vital roles of the receptor kinase FERONIA in regulating plant growth and immune responses in response to its ligands, RALF peptides, in Arabidopsis and crop species. It also raises interesting questions that need to be answered in the future.
Introduction
Plants must survive and reproduce under different environmental conditions despite being immobile. Thus, sensing external environmental signals and being able to respond accordingly are extremely important for plant growth and survival. Receptor-like kinases (RLKs), the largest single-transmembrane receptor family in plants, are employed to handle external signals. More than 600 RLKs have been recognized in Arabidopsis thaliana (hereafter referred to as Arabidopsis), and different RLKs contribute to multiple aspects of plant life (Shiu and Bleecker, 2001; Lehti-Shiu et al., 2009; Dievart et al., 2020). Catharanthus roseus receptor-like kinase 1-like (CrRLK1L) proteins are a subfamily of RLKs, and they structurally contain two malectin-like domains (MLDs) in their extracellular domain (ECD), a transmembrane domain (TMD) and an intracellular kinase domain. The first CrRLK1L member, CrRLK1, was cloned in 1996 (Schulze-Muth et al., 1996), and the function of CrRLK1L has been comprehensively studied in Arabidopsis over the past decade. Among CrRLK1Ls in Arabidopsis, 15 of 17 have been studied in detail, revealing their functions (Table 1). Recently, the roles of CrRLK1L proteins in regulating agronomic traits have begun to be appreciated in crop species (Table 1). In general, CrRLK1L proteins play versatile roles in plant growth and development, reproduction, hormone signaling, stress responses, immunity (Escobar-Restrepo et al., 2007; Guo et al., 2009, 2018; Duan et al., 2010, 2014; Yu et al., 2012, 2014; Haruta et al., 2014; Li et al., 2015, 2016a; Mao et al., 2015; Chen et al., 2016; Du et al., 2016; Ge et al., 2017, 2019; Mecchia et al., 2017; Stegmann et al., 2017; Chakravorty et al., 2018; Feng et al., 2018; Franck et al., 2018; Gonneau et al., 2018; Hansen et al., 2019; Wang et al., 2020a; Zhou and Zhang, 2020), energy production and RNA metabolism (Xu et al., 2019; Zhu et al., 2020a; Wang et al., 2020b).
Table 1.
Overview of CrRLK1L Family Members from Plants.
| Name | Gene ID | Growth and development | Biotic stress and abiotic stress | Ligands |
|---|---|---|---|---|
| AtFER | AT3G51550 | Vegetative growth (Guo et al., 2009); root growth and cell wall integrity (Haruta et al., 2014; Du et al., 2016; Stegmann et al., 2017); root hair development (Duan et al., 2010; Zhu et al., 2020a); leaf epidermis cell morphogenesis (Li et al., 2015); pollen tube growth and fertilization (Haruta et al., 2014; Stegmann et al., 2017); flowering time (Wang et al., 2020a); seed size and seed set (Yu et al., 2014; Li et al., 2016a); C/N balance (Xu et al., 2019); mRNA alternative splicing (Wang et al., 2020a, 2020b); nitric oxide accumulation (Duan et al., 2020); stomatal movement (Yu and Assmann, 2018) | Positively regulates plant resistance to bacteria and fungus (Keinath et al., 2010; Kessler et al., 2010; Stegmann et al., 2017; Guo et al., 2018; Xiao et al., 2019). Negatively regulates resistance to Fusarium oxysporum (Masachis et al., 2016). Hypersensitive to cold, heat, and salt stress; hyposensitive to osmotic stress (Yu et al., 2012; Chen et al., 2016; Feng et al., 2019) | RALF1, RALF17, RALF23, RALF32, RALF33; F-RALF |
| AtANX1 | AT3G04690 | Pollen tube growth and integrity (Boisson-Dernier et al., 2009; Miyazaki et al., 2009; Ge et al., 2017; Gonneau et al., 2018; Feng et al., 2019); cell wall integrity in tip-growing cells (Boisson-Dernier et al., 2013; Franck et al., 2018) | Negatively regulate PTI and ETI (Mang et al., 2017) | RALF4, RALF19, RALF34 |
| AtANX2 | AT5G28680 | |||
| AtBUPS1 | AT4G39110 | Pollen tube growth and integrity (Feng et al., 2019; Ge et al., 2019). | ||
| AtBUPS2 | AT2G21480 | |||
| AtTHE1 | AT5G54380 | Cell wall synthesis and cell expansion (Hématy et al., 2007; Merz et al., 2017; Van der Does et al., 2017); cell wall integrity (Gonneau et al., 2018) | Positively regulates plant resistance to fungus Botrytis cinerea (Qu et al., 2017) | RALF34 |
| AtHERK1 | AT3G46290 | Plant growth and cell elongation (Guo et al., 2009) | ||
| AtHERK2 | AT1G30570 | |||
| AtERU/CAP1 | AT5G61350 | Pollen tube growth; root development (Schoenaers et al., 2018) | ||
| AtCVY1 | AT2G39360 | Cell elongation and morphogenesis (Gachomo et al., 2014) | ||
| AtANJ | AT5G59700 | Pollen tube reception (Galindo-Trigo et al., 2020) | ||
| AtMDS1 | AT5G38990 | Metal ion stress response (Richter et al., 2018) | ||
| AtMDS2 | AT5G39000 | |||
| AtMDS3 | AT5G39020 | |||
| AtMDS4 | AT5G39030 | |||
| OsFLR1(DRUS1) | Os03g21540 | FLR1 (DRUS1) and FLR2 (DRUS2) regulate rice growth, development, and reproduction (Li et al., 2016a; Pu et al., 2017) | Rice immunity (Yang et al., 2020) | |
| OsFLR2(DRUS2) | Os01g56330 | |||
| OsFLR9(RUPO) | Os06g03610 | Pollen tube growth and integrity; K+ homeostasis (Liu et al., 2016a) | ||
| OsFLR11 | Os10g39010 | Rice immunity (Yang et al., 2020) | ||
| OsFLR13 | Os03g17300 | Rice reproduction (Yang et al., 2020) | Rice immunity (Yang et al., 2020) | |
| CpRLK1 | AB920609 | Reproduction (Hirano et al., 2015) | ||
| MpTHE | KU758861 | Cell wall integrity (Honkanen et al., 2016) | ||
| MpFER | BAF79940 | Cell wall integrity; tip growth (Westermann et al., 2019) | ||
| FaMRLK47 | 13 568.1 | Fruit ripening and quality formation (Jia et al., 2017a) | ||
| PbrCrRLK1L3 | Pbr004347.1 | Pollen tube rupture (Kou et al., 2017) | ||
| PbrCrRLK1L26 | Pbr028472.1 | Pollen tube growth (Kou et al., 2017) | ||
| MdFERL1 | MDP0000445374 | Fruit ripening (Jia et al., 2017b) | ||
| MdFERL6 | MDP0000465341 |
Rapid alkalinization factor (RALF) peptides, as ligands of CrRLK1L receptors, constitute an evolutionarily conserved peptide family, with at least 37 RALF members in Arabidopsis. Most of the reported RALF members induce rapid alkalinization of the extracellular compartment of plant cells and regulate a series of developmental and physiological responses in plants. The functions of RALF peptides in different plant species have been comprehensively reviewed recently (Blackburn et al., 2020). Based on the currently available genomic data, the oldest CrRLK1L genes are found in charophytes, and the gene family gradually expanded before monocots diverged from dicots. The average numbers of CrRLK1L proteins per species are 22 and 30 in monocots and dicots, respectively, indicating the CrRLK1L gene family has not expanded since monocots diverged (Dievart et al., 2020). Although the oldest RALF genes are found in Physcomitrella patens, a non-flowering plant, the evolutionary history of RALF peptides is more complicated than that of the CrRLK1L gene family, as RALF genes are also found outside of the plant kingdom (Campbell and Turner, 2017). This review focuses on the roles of the RALF–FER pathway in immune responses in the context of cell growth in plants. We briefly provide an overview of the functions and components of the RALF–FER pathway, introduce RALF signals and their receptors outside of cells, and lastly discuss the factors involved in the roles of the RALF–FER pathway in immunity and their crosstalk in the cytoplasm.
An Overview of the Functions and Components of the RALF–FER Pathway
Arabidopsis FER is the best characterized CrRLK1L member (Haruta et al., 2014; Li et al., 2016b; Liao et al., 2017; Stegmann et al., 2017; Xiao et al., 2019; Duan et al., 2020). FER was first cloned by the Grossniklaus research group during a screen of double-fertilization regulators (i.e., pollen tube reception genes) (Escobar-Restrepo et al., 2007). FER has recently emerged as a potential target for crop improvement and protection because of its versatile, fundamental, and tissue-specific roles in plant growth, immune responses, crop yield control, and abiotic responses. FER modulates growth and stress responses, H+-ATPase activity, calcium accumulation, reactive oxygen species (ROS) burst, and cell wall integrity (CWI) in a variety of cell types in response to its RALF ligands (Table 1). LORELEI (LRE) is mainly expressed in synergid cells of the ovule, lre and fer mutants, and the lre fer double mutants that display the same defects in pollen tube reception, namely pollen tube overgrowth and sperm release defects, indicating that LRE requires FER for pollen tube reception (Capron et al., 2008; Liu et al., 2016b). LORELEI-like-GPI-anchored protein 1 (LLG1) is the closest homolog of LRE based on amino acid sequences. It is mainly expressed in vegetative tissues, with no deficiency in pollen tube reception observed in LLG1 mutants (Li et al., 2015). Li et al. (2015), showing that LLG1 interacts with the RALF1 peptide and the ECD of FER to regulate cell growth in vegetative tissues and acts as the co-receptor of the RALF1 peptide. Recently, RALF4/1–LLG2/3 has been shown to participate in pollen tube growth via ANXUR1/2 (ANX1/2) and BUDDHAPAPER SEAL1/2 (BUPS1/2) CrRLK1L members (Feng et al., 2019; Ge et al., 2019). Additionally, RALF34 has been identified as a ligand of THESEUS1 (THE1), which is a homolog of FER and a modulator of CWI (Gonneau et al., 2018). The leucine-rich repeat (LRR) extensin (LRXs) family of proteins functions to survey CWI. High-throughput screens of proteins interacting with LRXs indicated that RALF peptides interact with LRXs, suggesting that LRXs have roles in RALF-regulated pollen tube growth (Covey et al., 2010). Mecchia et al. (2017) further confirmed this finding and showed that RALF4/19 interacts with LRXs to regulate pollen tube integrity and growth (Moussu et al., 2020).
Several interesting studies have reported the structural basis of RALF binding to its LLG/LRX-CrRLK1L receptor complex. Using mass spectrometry-based footprinting methods and covalent crosslinking experiments in vitro, Liu et al. (2018) revealed the ECD and juxtamembrane regions of FER linked to the highly conserved C terminus of AtRALF1. Furthermore, Xiao et al. (2019), using X-ray crystallography, showed that LLG2 directly binds the N terminus of RALF23 to nucleate the assembly of RALF23–LLG2–FER heterocomplexes. Detailed structural data show that the conserved YISY motif of RALF23 forms extensive interactions with the loops of LLG2 through a combination of hydrophobic and polar contacts. However, the X-ray structural data do not provide detailed information on how the C terminus of RALF23 interacts with the FER ECD. Additionally, the crystal structures of the LRX2–RALF4 and LRX8–RALF4 complexes reveal that disulfide-bond-stabilized loops in folded AtRALF4 peptides are a major factor in binding the LRR domain of LRX proteins, which is required to control pollen tube growth (Moussu et al., 2020). After recognizing the RALF1 peptide, FER recruits and interacts with RPM1-induced protein kinase (RIPK), a receptor-like cytoplasmic kinase (RLCK), to inhibit cell growth (Du et al., 2016). In the cytoplasm, the RALF1–FER complex also uses the small G-protein pathway to regulate auxin responses and root hair growth (Duan et al., 2010) as well as abscisic acid (ABA) responses (Yu et al., 2012; Chen et al., 2016). FER also monitors the carbon/nitrogen (C/N) balance by interacting with ATL6 (Xu et al., 2019), regulates mRNA alternative splicing by phosphorylating the RNA-binding protein glycine-rich protein 7 (GRP7) to modulate plant fitness and flowering time (Wang et al., 2020a, 2020b) and promotes protein synthesis and polar cell growth by phosphorylating eIF4E1 (Zhu et al., 2020a). The RALF1–FER complex uses a “shortcut” mechanism to directly regulate gene expression by phosphorylating a DNA-binding protein, namely ErbB3-binding protein 1 (EBP1) (Li et al., 2018). The RALF–FER complex also interferes with jasmonic acid (JA) signaling and flg22-induced complex formation between the immune receptor kinase FLAGELLIN-SENSING 2 (FLS2) and its co-receptor BRASSINOSTEROID INSENSITIVE 1-ASSOCIATED RECEPTOR KINASE 1 (BAK1) (Shen et al., 2017; Stegmann et al., 2017; Guo et al., 2018; Xiao et al., 2019).
Ligands and Co-receptors/Receptors Involved in RALF–CrRLK1L Signaling
The initial function of the RALF peptide was derived from the rapid alkalinization of tobacco (Nicotiana tabacum) extracellular media and the activation of mitogen-activated protein kinase (MAPK) kinase (Pearce et al., 2001). In plants, most mature RALF peptides share conserved domains, namely the YISY motif in the N terminus and four cysteine residues near the C terminus (referred to as the RGC(5N)C motif) (Campbell and Turner, 2017). As mentioned above, the C and N terminus of RALF interact with the FER–LLGs receptor complex and LRXs. The history and functions of RALF peptides in plants have been comprehensively reviewed elsewhere (Blackburn et al., 2020). Here, we mainly review the roles of RALF and its co-/receptors, including FER and other CrRLK1L receptor kinases, in immune responses.
The Grossniklaus and Panstruga research groups reported the role of FER in fungal and bacterial triggered immune responses, first linking FER with immune responses (Keinath et al., 2010; Kessler et al., 2010). In search of the ligand of FER, Haruta et al. (2014) first reported that AtRALF1 peptides bind to the FER ECD and initiate FER kinase activation, which inhibits H+-ATPase activity and proton transport, thus suppressing root elongation. RALF peptides are expressed as precursors that need to be cleaved by proteases in order to produce a mature RALF peptide that can execute its function. Besides the AtRALF1 precursors, nine other related AtRALF precursors, including AtRALF22 and AtRALF23, are predicted to be cleaved by site-1 protease (AtS1P), a plant subtilisin-like serine protease (Srivastava et al., 2009; Stegmann et al., 2017). During a screening of the modifiers of bak1–5 immunity response mutants, Stegmann et al. (2017) found that AtS1P cleaves endogenous AtRALF precursors, such as RALF23, to inhibit plant immunity. The RALF23–FER complex suppresses flg22-induced ROS burst, as well as immune responses, by destabilizing the complex formation of EFR and FLS2 with their co-receptor BAK1 (Stegmann et al., 2017). The authors proposed that FER acts as a RALF-regulated scaffold that modulates the assembly of the receptor kinase complex. Notably, this study also suggests that there is an opposite role in the immune response between S1P cleaved and non-S1P cleaved RALF peptides. Thus, the roles of the predicted S1P cleaved RALF peptides in plant growth and development merit further investigation. In the future, additional studies are needed to identify new proteases that produce mature RALF peptides. The RALF co-receptor, LLG1, also regulates innate immunity via its association with and modulation of FLS2 receptor kinase (Shen et al., 2017; Xiao et al., 2019).
Accumulating evidence suggests that RALF peptides are conserved across the plant kingdom as well as outside of it. Potential RALF peptides have been isolated from fungal and bacterial genomes (Masachis et al., 2016; Thynne et al., 2017). For example, F-RALF from Fusarium oxysporum was determined to be a functional peptide that induces the alkalinization and inhibition of root growth as well as AtRALF1 (Masachis et al., 2016). Additionally, it promotes fungal virulence and suppresses plant immune responses through the FER receptor kinase (Masachis et al., 2016). Moreover, F. oxysporum infection was found to involve JA signaling (Thatcher et al., 2009), and AtRALF23, the closest homolog of F-RALF (Masachis et al., 2016), has been shown to stabilize the basic helix-loop-helix transcription factor MYC2 and promote JA signaling via FER (Guo et al., 2018). Thus, it will be interesting to determine whether F-RALF from F. oxysporum modulates JA signaling via FER. Plant and non-plant RALFs possess similar sequences in both their C- and N-terminal regions, but both have numerous sequence polymorphisms in their middle domains, which has been reported to contribute to RALF–LRXs affinity, as Moussu et al. (2020) found that pH and disulfide-bond-stabilized RALFs and LRXs are important for RALF–LRXs affinity. As plant immune responses are always coupled with pH (Kesten et al., 2019) and ROS changes, there is the potential for microorganisms to trigger pH and ROS changes to modulate RALF–LRXs and RALF–FER affinity and further regulate plant immune responses. The evolutionary history of RALFs and whether plant FER receptor complexes can differentiate plant RALFs from non-plant RALFs still requires further investigation.
RALF-Mediated pH Changes Regulate Plant Growth and Immunity
The cell wall maintains plant cell morphology and is the first barrier between plant cells and their environment. Thus, the cell wall acts as a dynamic barrier against the invasion of pathogens. Therefore, the maintenance of CWI is necessary for plant survival and cell growth and development. To promote plant cell growth, the cell wall must be remodeled to allow cell expansion. Cell wall acidification triggers cell wall loosening, thus allowing plant cell expansion, as cell wall-loosening enzymes are activated by low pH conditions (Cosgrove, 2015). As mentioned above, the RALF1–FER pathway phosphorylates the proton pump (e.g., AHA2), leading to transient alkalinization of the extracellular matrix and inhibiting primary root cell elongation (Haruta et al., 2014). Except for pollen-specific RALF4, all RALF peptides tested so far have the ability to promote extracellular alkalinization (Morato do Canto et al., 2014). Alkalinization is proposed to be the mechanism through which RALF peptides regulate cell growth. However, RALF peptides have different roles in cell growth in different tissues and cell types, and additional studies are needed to understand the roles of RALF in modulating cell growth via extracellular alkalinization.
Extracellular pH is a key factor controlling the virulence of pathogens. Several studies have reported that the dynamic phosphorylation of AHAs can rapidly regulate proton pump activity and cause apoplastic pH changes, which are involved in the response to invasion by pathogenic microorganisms (Masachis et al., 2016; Yu et al., 2019). RALF-induced pH alterations may also regulate pathogenicity. Specifically, F. oxysporum secretes RALF-like (F-RALF) peptides that mimic plant RALFs, activate FER kinase, and inhibit AHA activity, thus inducing the alkalinization of apoplasts, which activates the orthologous MAPK FMK1 kinase and promotes virulence in fungi (Masachis et al., 2016). Conversely, the rhizosphere-associated bacterium Pseudomonas decreases the environmental pH to suppress plant immune responses (Yu et al., 2019). Stegmann et al. (2017) has reported that RALF-induced extracellular alkalinization is not required for the inhibition of the plant immune response by RALFs. Thus, RALF triggers extracellular alkalinization in context-specific manners, which involve both cell growth and immunity. One possible explanation is that different microorganisms have different apoplastic pH preferences, which have diverse roles in processes such as parasitism and/or infection. Accordingly, how the RALF–FER pathway modulates immune responses and cell growth by triggering pH changes in a context-dependent manner requires further investigation.
Cell Walls Act as Dynamic Barriers, Affecting Plant Growth and Pathogen Invasion
The cell wall is actively remodeled and reinforced around sites of pathogen invasion. Most plant microorganisms seek to break through cell walls to access intracellular nutrients. Thus, most pathogenic microorganisms interact with cell walls (Underwood, 2012; Franck et al., 2018). Studies have demonstrated that plants treated with cell wall biosynthesis inhibitors or cell wall mutants show a series of immune responses such as defense-related gene expression, ROS production, and defense-related hormone accumulation (Hamann, 2015). Not surprisingly, the RALF–CrRLK1L complex also participates in the invasion of pathogens. The ECD of CrRLK1L, which contains two MLDs, is homologous to a carbohydrate-binding domain. Plant cell walls are rich in hemicellulose, pectin, and other complex carbohydrates. Thus, CrRLK1L was hypothesized to bind and survey cell wall perturbations. However, CrRLK1L MLDs lack residues important for carbohydrate-rich ligand binding (Du et al., 2018). This characteristic pattern of the CrRLK1L domain was established early in plant evolution. Studies in both seed and non-seed plants suggest that there is functional conservation in the role of CrRLK1L as a cell wall sensor (Franck et al., 2018). THE1 was the first CrRLK1L member identified to monitor cell wall status and repress growth by inhibiting cellulose synthesis in the context of cell wall perturbations (Hématy et al., 2007; Merz et al., 2017; Van der Does et al., 2017). THE1 negatively regulates cell growth in the presence of cell wall perturbations. Furthermore, THE1 acts upstream of the plasma membrane-localized mechanosensitive Ca2+ channel MCA1 and the RLK FEI2 to perceive cell wall damage. THE1, FEI2, and MCA1 regulate CWI, further inducing the expression of host defense peptides that promote pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) (Engelsdorf et al., 2018). Additionally, THE1 recognizes cell wall modifications caused by fungal-induced necrosis and plays positive regulatory roles in the resistance of plants to the necrotrophic fungus Botrytis cinerea via the GEF4 pathway (Qu et al., 2017). RALF34 acts as a ligand of THE1, and the RALF34–THE1 pathway has been reported to participate in the regulation of CWI, which is coordinated by RALF–FER signaling (Gonneau et al., 2018). Furthermore, RALF4, RALF19, and RALF34, as ligands of ANX-BUPS, also play essential roles in maintaining CWI in tip-growing cells (Ge et al., 2017). Moreover, LLG2 and LLG3 have been identified to act as co-receptors of RALF4/19 and to maintain pollen tube CWI in cooperation with ANX1/2 and BUPS1/2 (Feng et al., 2019; Ge et al., 2019).
Cell wall-anchored LRXs, as RALF-binding proteins, consist of an N-terminal LRR domain and a C-terminal extension domain attached to the cell wall (Ringli, 2010; Draeger et al., 2015; Doblas et al., 2018). A recent crystallography study revealed that disulfide-bond-stabilized loops in AtRALF4folded are a major determinant of LRX binding affinity, which is required to control pollen tube growth (Moussu et al., 2020). A previous study has reported that pH alterations modulate the interaction of RALF4folded with LRX8 and LLGs, with LRX proteins consistently recognizing RALF4folded with higher affinity (Moussu et al., 2020). No simultaneous interactions were observed among RALF4folded, LRX proteins, and LLGs or CrRLK1Ls, suggesting that RALF peptides trigger two parallel, converging, but mechanistically distinct signaling pathways to modulate CWI (Moussu et al., 2020). Moreover, ANX1 and ANX2 negatively regulate PTI and effector-triggered immunity (ETI) by associating with the bacterial flg22 receptors FLS2/BAK1 and nucleotide-binding domain leucine-rich repeat (NLR) protein RPS2, respectively (Mang et al., 2017). Bacterial recognition by ANX1/2 regulates plant physiological processes, such as CWI and apoplastic pH changes, but this requires further investigation. Finally, the ECD of FER associates with pectin, which is a component of the cell wall, to protect cell walls from damage induced by salinity (Feng et al., 2018). Therefore, FER senses cell wall pectin polymers to monitor CWI and regulates the formation of the leaf epidermis cell shape (Li et al., 2015; Lin et al., 2018). As previously discussed, CWI influences pathogen recognition by plants, and pectin may be an immune signal that is sensed by FER. Furthermore, RALF–FER signaling coordinates immune receptor complex assembly and dynamics to respond to cell wall changes. However, whether FER or other CrRLK1L members connect the plasma membrane to the cell wall to coordinate receptor complex assembly and dynamics merits further investigation.
FER has been described to be a molecular component shared between pollen tube reception and powdery mildew infection, which involves communication between tip-growing hyphae and plant cell walls (Kessler et al., 2010). The RALF23–LLG2–FER complex has been reported to be involved in immune responses (Xiao et al., 2019), as has been demonstrated for the F-RALF–FER complex (Masachis et al., 2016). FER positively regulates CWI and polar growth. However, THE1, despite its functional homology with FER, appears to negatively regulate cell growth in the presence of cell wall perturbations. This also coincides with the roles of FER and THE1 in immune responses.
Compared with FER, the immune-related functions of ANX1/2 have been studied more intensely, revealing that ANX1/2 negatively regulates PTI and ETI by blocking the formation of receptor FLS2/BAK1 and destabilizing RPS2, respectively. By contrast, as reported by Stegmann et al. (2017), FER has positive roles in the formation of PRR complexes that FER promotes the formation of FLS2 and BAK1 to respond to bacterial pathogens until sensing RALFs (e.g., RALF23). RALF23–FER also influences the phosphorylation of MYC2, which directly controls JA signaling. Additionally, apoplastic pH changes induced by FER seem to be involved in F. oxysporum parasitism. However, almost all plant RALFs can trigger such pH changes. The S1P protease may be a key factor regulating RALF–FER signaling in immune responses, such that mature RALF23 cleaved by S1P suppresses the PTI response, whereas non-S1P-cleaved RALFs promote the PTI response. Thus, the relationship among RALF23–FER, apoplastic pH, and S1P proteases needs to be further studied with the aim of developing a context-dependent view. FER also regulates immune responses in a developmental stage-dependent manner. For example, FER mutant seedlings were resistant to Pseudomonas syringae pv. tomato DC3000 (Pst DC3000) growth (Keinath et al., 2010), whereas 4- to 5-week-old FER mutants were hypersensitive to this pathogen (Stegmann et al., 2017; Guo et al., 2018). Complicating matters, RALF peptides have different or even opposite roles toward FER in a context-dependent manner. For example, RALF1 and RALF23 activate FER signaling during root growth, whereas RALF23 represses FER signaling during PTI responses. Thus, the mechanisms of CrRLK1Ls' malectin-like domain and LRXs' cell wall fusion domain in sensing different RALFs and/or cell wall perturbations to modulate various signaling endpoints remain mostly enigmatic.
ROS, Ca2+, NO, and mRNA Local Translation Modulate Polar Cell Growth by the RALF–FER Network
Once plant pathogens approach the host plant, cell wall-sensing mechanisms are activated and PTI or ETI responses are triggered. PTI responses are usually accompanied by MAPK signaling, ROS bursts, transcriptional activation of defense-related genes, and accumulation of callose (Nürnberger et al., 2004), whereas the ETI response is a more rapid and robust form of the PTI response that is typically associated with a localized cell death reaction known as the hypersensitive response (Jones and Dangl, 2006). During these processes, plant cells tightly control the cell wall composition and modifications. They also modulate their signaling components with spatial and temporal specificity. The RALF–FER pathway is involved in the manipulation of its signaling components in terms of this context specificity to regulate polar cell growth and immune responses. FER plays an essential role in ROS production. FER regulates root hair polar growth by positively regulating auxin-triggered ROS production. FER also positively regulats flg22-induced ROS production during immune responses, whereas FER negatively regulates ROS levels in guard cells in relation to ABA (Yu et al., 2012). ROS are ubiquitous and can be divided into two types based on their location, namely apoplastic ROS and intracellular ROS (Qi et al., 2017). Apoplastic ROS have more important roles in plant interactions with pathogens, which are primarily regulated by NADPH oxidases, cell wall peroxidases, or amine oxidases (Kadota et al., 2015). NADPH oxidases, also known as respiratory burst oxidase homologs (RBOHs), consist of ten members from RBOHA to RBOHJ (Baxter et al., 2014). Among these, RBOHC, RBOHD, RBOHF, RBOHH, and RBOHJ have been found to be regulated by FER and related proteins (Franck et al., 2018). The FER–LLG1–Rop-guanine nucleotide exchange factor (RopGEF)–RAC/ROP complex recruits and positively regulates RBOHC-dependent ROS production (Duan et al., 2010, 2014; Li et al., 2015). THE1 acts upstream of RBOHD and RBOHF in response to cell wall damage, triggering ROS production and immune responses (Sagi and Fluhr, 2006; Ogasawara et al., 2008; Denness et al., 2011). ANX1 and ANX2 modulate RBOHH and RBOHJ, maintain ROS production, and regulate CWI during pollen tube growth (Boisson-Dernier et al., 2013). Thus, the RALF–CrRLK1L complex uses ROS as important signaling molecules, affecting polar cell growth and stress responses. Unfortunately, how the RALF–CrRLK1L complex regulates ROS production remains unknown. RAC and Rho guanosine triphosphatases of plants (ROPs) may act as potential links during this process. RAC/ROP (referred to collectively as ROP) activates cellular responses by switching the inactive guanosine diphosphate-bound state to the active GRP-bound state. ROP is a conserved molecule that regulates cell polarity (Fu et al., 2001; Molendijk et al., 2001). ROP functions as an essential downstream factor during FER-regulated auxin and ABA signaling by regulating ROS levels in plants (see RALF–FER Regulates Hormone Signaling Crosstalk for more details). ROP2 has been reported to interact with the ECD of RBOHD, which is likely to regulate ROS levels (Li et al., 2015). Recently, PBL13 (a homolog of RIPK) has been reported to interact with RBOHD to regulate NADPH oxidase-induced ROS production (Lee et al., 2020). Thus, further studies are needed to determine whether CrRLK1L/FER uses the ROP (e.g., ROP2) pathway to directly modulate ROS production, thus affecting cell polar growth and immune responses.
The Ca2+ signature is also critical for RALF–CrRLK1L-mediated polar cell growth, and it always works with the ROS synergism. For example, synergid-derived ROS mediates pollen tube rupture in a Ca2+-dependent manner (Duan et al., 2014) in a process that involves FER, LER, and NORTIA (NTA). NTA is a member of the MLO family and contains a calmodulin domain for binding Ca2+. However, the gene or genes encoding Ca2+ channels that are involved in RALF–CrRLK1L signaling have not yet been identified.
Recently, Zhu et al. (2020a) found that polar-localized RALF1–FER interacts with eIF4E1 (an early translation factor) and forms a complex with the translation machinery in the tips of growing root hairs. RALF1, FER and eIF4E1 show polar localization in root hairs, providing spatial control of localized protein synthesis and the determination of polarized cell growth (Zhu et al., 2020a). Phosphorylated eIF4E1 increases mRNA affinity, and thus, regulates the translation of mRNAs to affect the synthesis of several polar cell growth-related proteins, such as ROP2, RIPK, and RSL4, in a spatiotemporal manner. Both ROPs and ROS generated by ROPs are known to regulate myriad biological processes, such as polarized cell growth (Zhu et al., 2020b). In animals, plants, and fungi, there are distinct examples of cells that exhibit polarized growth, including growth of neuronal axons, pollen tubes, root hairs, and fungal hyphae (Drubin and Nelson, 1996; Hepler et al., 2001). During the rapid uniaxial extension of cells, rapid protein synthesis (and cell wall components for plants) and accumulation with spatial precision in the tip growth region are indispensable.
Recently, FER was reported to modulate nitric oxide (NO) levels, which resulted in the nitrosation of cysteines in the chemoattractant LURE1 and XIUQIU peptides, thereby blocking their secretion and interaction with their RLK receptors, respectively, and in turn suppressing pollen tube attraction (Duan et al., 2020). Thus, ROS, Ca2+, NO, and mRNA local translation are all involved in the regulation of polarized cell growth by RALF–FER. However, it is not clear whether these processes are also involved in the invasion of pathogens (e.g., the polarized growth of fungal hyphae cells into plant cell walls). We assume that the RALF–FER pathway spatiotemporally controls ROS, Ca2+, and NO levels and mRNA local translation, which leads to the rapid production and accumulation of defense-associated components near the region of pathogen invasion. Additionally, this process is indispensable for activating appropriate immune responses, such as changes in cell wall composition and modifications. Indeed, during pollen tube reception, polar localization of FER is necessary for NTA to have polarized accumulation and be redistributed at the filiform apparatus, enabling pollen tubes to penetrate synergid cells.
RALF–FER Regulates Hormone Signaling Crosstalk
RALF–FER signaling has multiple roles in crosstalk with several hormone pathways, regulating key processes such as plant growth and immunity. FER mediates RALF signaling interfaced with auxin (Duan et al., 2010; Li et al., 2015; Barbez et al., 2017), brassinosteroid (BR) (Guo et al., 2009; Deslauriers and Larsen, 2010), ethylene (Mao et al., 2015), ABA (Yu et al., 2012; Chen et al., 2016), and JA signaling (Guo et al., 2018). Liao et al. (2017) have reviewed how FER mediates crosstalk with different hormones to regulate cell growth and stress responses. Here, we concentrate on the latest advances in FER-mediated plant immune responses. Pathogens secrete a large number of effectors and phytotoxins during infection, and among these, coronatine (COR) originates from Pst DC3000 and mimics JA-isoleucine, thus promoting JA signaling and leading to host disease susceptibility (Xin and He, 2013). FER offsets excessive JA signaling by phosphorylating and destabilizing MYC2, which plays a key role in JA signaling. However, RALF23 mediates JA signaling by stabilizing MYC2 via FER, which negatively contributes to plant immune responses to bacterial pathogens (Guo et al., 2018). Unfortunately, it is still unknown how membrane-localized FER interacts with and phosphorylates MYC2 in the nucleus and how RALF23 inhibits the phosphorylation of MYC2 by FER.
FER is a key modulator of ethylene responsiveness in Arabidopsis (Deslauriers and Larsen, 2010) and negatively modulates the production of S-adenosylmethionine (SAM) via SAM1 and SAM2, which partially block the ethylene biosynthesis pathway (Mao et al., 2015). Besides regulating plant growth, ethylene signaling also modulates plant innate immunity, which is regulated by PAMP signaling, Ca2+signaling, ROS production, NO signaling and MAPK signaling (Vidhyasekaran, 2015). In particular, ethylene signaling was found to contribute to ROS production triggered by flg22 in Arabidopsis, as the accumulation of the FLS2 receptor relies on ethylene signaling (Mersmann et al., 2010), suggesting that RALF–FER signaling participates in ROS production and ethylene signaling to affect immune responses.
Stomata offer entry points into plant tissues for plant pathogens (Mersmann et al., 2010). Coincidentally, ABA signaling is a key modulator of stomatal closure. The accumulation of ABA in guard cells induces stomatal closure under biotic or abiotic stresses (Assmann, 2003), which reduces entry for plant pathogens (Melotto et al., 2008). The crosstalk of the RALF–FER pathway with ABA signaling, whereby FER mediates RALF1 and interacts with the downstream partners of GEF1/4/10–ROP11–ABI2, further inhibits ABA signaling, whereas ABI2 interacts with FER and dephosphorylates FER to provide negative feedback on the FER–GEF1/4/10–ROP11 cascade (Yu et al., 2012; Chen et al., 2016). Additionally, FER regulates salt stress responses via directly interacting with AGB1, the β subunit of a G-protein that positively regulates ABA-mediated regulation of stomatal movements. AtRALF1 inhibits stomatal opening and promotes stomatal closure (Yu and Assmann, 2018). Taken together, the RALF–FER pathway may interact with ABA signaling to modulate abiotic stress responses and plant growth and may further regulate immune responses via stomatal closure.
Hormone signaling crosstalk also occurs in other plant species. MdFERL6 and MdFERL1, two FER-like receptors in apple, interact with MdSAMS. In apples, SAMs suppress ethylene production, which delays apple fruit ripening (Jia et al., 2017b). SlFERL1, a FER-like receptor in tomato, also modulates ripening via ethylene signaling (Jia et al., 2017b). Meanwhile, FaMRLK47, a FER-like receptor in strawberry, interacts with FaABI1, a negative regulator of ABA signaling, thus modulating strawberry ripening and fruit quality (Jia et al., 2017a).
Downstream Immunity-Related Factors in the RALF–FER Pathway
FER mediates RALF signaling from membrane to nucleus, recruiting a series of downstream partner proteins. RIPK, a plasma membrane-associated RLCK involved in P. syringae invasion (Liu et al., 2011), can directly interact with FER. FER phosphorylates RIPK in a RALF1-dependent manner, leading to apoplastic alkalinization, which cooperatively suppresses primary root growth (Du et al., 2016). Additionally, RIPK has been shown to phosphorylate RPM1-INTERACTING PROTEIN 4 (RIN4), which perceives P. syringae effectors AvrB and AvrRpm1. Furthermore, phosphorylated RIN4 is sensed by the NLR protein RPM1 (Mackey et al., 2002), and the degraded RIN4 perceives AvrRpt2, which is sensed by another NLR protein, RPS2, thus triggering immune responses (Axtell and Staskawicz, 2003; Mackey et al., 2003). It may be productive to determine whether the RALF–FER–RIPK pathway regulates plant immunity via RIN4 and RPM1/RPS2. Notably, ANX1 interacts with RPM1 and RPS2, thus negatively regulating immunity (Mang et al., 2017).
Increasing evidence suggests that RALF1–FER signaling plays dual roles in energy metabolism and immunity by recruiting different functional proteins. Cytosolic glyceraldehyde-3-phosphate dehydrogenase (GAPDH, GAPC1, and GAPC2) was identified to be involved in FER signaling by modulating energy metabolism (Yang et al., 2015). Additionally, ATL6, an E3 ubiquitin ligase, directly interacts with FER, destabilizing 14-3-3 proteins, which modulate the C/N balance and signaling (Xu et al., 2019). FER-like receptors 1 and 2 (FLR1 and FLR2) from rice are also involved in plant height, branching and tillering as well as male gametophyte development (Li et al., 2016a) and negatively regulate immunity against rice blast. FLR2 negatively modulates fungus-triggered ROS associated with rice blast. The mutation of FLR2 and FLR11 increases disease resistance against rice blast without reducing the growth of rice plants (Yang et al., 2020).
The mechanisms by which RALF1–FER signaling controls gene expression are being revealed. Li et al. (2018) found that RALF1–FER signaling uses a “shortcut” mechanism to directly regulate gene expression. RALF–FER signaling promotes the mRNA translation of EBP1. FER directly phosphorylates the cytoplasmic nuclear shuttle protein EBP1, leading to the accumulation of EBP1 in the nucleus and the expression of binding genes (Li et al., 2018). Conversely, EBP1 has feedback along the RALF1–FER pathway via transcription–translation feedback loops to regulate cell growth. Interestingly, EBP1 is also involved in the regulation of plant immunity, as demonstrated by RALF1 more severely impairing flg22-triggered ROS burst in ebp1 mutant plants compared with wild-type plants. As discussed above, FER interacts with the transcription factor MYC2 to modulate JA signaling. At the RNA level, besides its role in the RALF1–FER–elF4E module, which is involved in mRNA translation, FER also directly interacts with and phosphorylates GRP7, thus enhancing its mRNA-binding ability in a RALF1-dependent manner and regulating mRNA alternative splicing to affect plant stress responses (Wang et al., 2020b). FER regulates flowering time via the mRNA alternative splicing of key flowering genes, such as FLOWERING LOCUS C (FLC) and its homolog MADS AFFECTING FLOWERING (MAF) (Wang et al., 2020a). Notably, GRP7 is an essential component of the FLS2-EFR complex. P. syringae type III-secreted effector HopU1 hijacks GRP7, decreasing the expression of FLS2 and EFR, thereby suppressing ROS burst (Nicaise et al., 2013). It is reasonable to assume that the RALF1–FER complex modulates both cell growth and immune responses partly via GRP7. Meanwhile, there are many other downstream signaling partners of the RALF–FER pathway that need to be explored, which might benefit from the elucidation of the RALF–FER cascade.
Prospects of RALF–FER Signaling
Taken together, these findings indicate that CrRLK1L originated in early non-seed plants, where it was probably involved in monitoring CWI and modulating plant cell growth and stress responses under environmental changes. Following the discovery of FER receptor kinase, FER has become the most widely studied member of the CrRLK1L family. FER has recently emerged as a potential target for crop improvement and protection because of its versatile, fundamental, and tissue-specific roles in plant growth and development, crop yield control, multiple biotic stress and abiotic stress responses, energy production, and RNA metabolism (Figure 1). Despite recent advances in understanding the functions and molecular mechanisms of the RALF–CrRLK1L network, many questions remain unanswered.
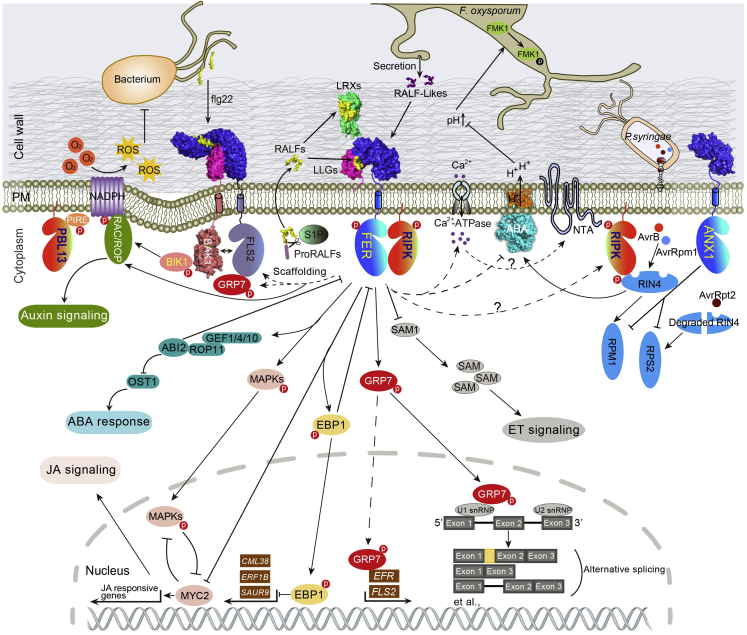
Figure 1.
Network of RALF–FER Signaling in Plant Immunity.
FER and LLGs respond to RALF23, which is cleaved by S1P, and modulate plant immunity, whereas LRXs mediate RALF signaling to maintain cell growth. RALF23-FER-LLGs destabilize the formation of the FLS2–BAK1 complex. Fusarium oxysporum secretes F-RALF peptides that hijack the RALF–FER pathway, leading to the phosphorylation of F. oxysporum MAPK FMK1 and potentially facilitating host infection, together with the suppression of PDF1.2 expression. FER modulates auxin, abscisic acid (ABA), ethylene (ET), Ca2+, reactive oxygen species (ROS), MAPK phosphorylation, and pH to affect cell growth and immunity. FER interacts with and phosphorylates RIPK, modulating AHA2 activity. RIPK phosphorylates RIN4, which is detected by RPM1 and RPS2, triggering immune responses. ANX1 also inhibits ETI responses by inhibiting RPM1 and RPS2. PBL13, a homolog of RIPK, interacts with RBOHD to regulate NADPH oxidase-induced ROS production. FER mediates RALF1-induced mRNA alternative splicing via GRP7, which also interacts with FLS2, EFR, and their transcripts. Positive regulatory actions are indicated by arrows, negative regulatory actions are indicated by bars, physical interactions between different proteins are indicated by solid lines and potential physical interactions are indicated by dashedotted lines. Question marks denote mechanisms that remain to be empirically demonstrated.
The number of non-plant genomes that encode RALF-like peptides is largely unknown, and the evolutionary history of RALF peptides is also unclear. It is likely that non-plant species acquired RALF peptide-encoding genes through horizontal gene transfer from host plants during their long-term coevolution. The detailed mechanisms through which CrRLK1Ls, LLGs, and LRXs combine and sense different RALF peptides to respond to distinct environmental changes (e.g., pH, ROS and pathogen invasion) are unknown. More importantly, how different RALF peptides activate (e.g., RALF1 or RALF23 for FER in cell growth regulation) and suppress (e.g., RALF23 for FER in immunity) CrRLK1L signaling in a cell type-specific manner remains enigmatic. Furthermore, RALF–FER signaling can have opposite effects in different cell types. For example, RALF1–FER inhibits primary root cell growth but promotes root hair cell growth. Further studies of this mechanism may provide fruitful means to maximize yields and minimize agricultural losses. RALF–FER signaling contributes to root elongation, flowering and reproduction, which is an appropriate entry point to increase crop yields. Furthermore, RALF–FER signaling seems to be a common strategy for the invasion of microorganisms. Therefore, understanding the features of RALF–FER/CrRLK1L signaling is an effective and meaningful way to improve agronomically important crop traits.
Funding
Research on this subject in the authors' laboratory is supported by grants from the National Natural Science Foundation of China (NSFC-31871396, 31571444, 31400232) and the Young Elite Scientist Sponsorship Program by CAST (YESS20160001).
Acknowledgments
We thank the past and present members of the Yu Laboratory for their contributions in establishing the RALF–FER network. We also apologize to colleagues whose work could not be cited due to the limited space of this review. No conflicts of interest are declared.
Published: June 11, 2020
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and IPPE, CAS.
References
- Assmann S.M. OPEN STOMATA1 opens the door to ABA signaling in Arabidopsis guard cells. Trends Plant Sci. 2003;8:151–153. doi: 10.1016/S1360-1385(03)00052-9. [DOI] [PubMed] [Google Scholar]
- Axtell M.J., Staskawicz B.J. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell. 2003;112:369–377. doi: 10.1016/s0092-8674(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Barbez E., Dünser K., Gaidora A., Lendl T., Busch W. Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U S A. 2017;114:E4884–E4893. doi: 10.1073/pnas.1613499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter A., Mittler R., Suzuki N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014;65:1229–1240. doi: 10.1093/jxb/ert375. [DOI] [PubMed] [Google Scholar]
- Blackburn M.R., Haruta M., Moura D.S. Twenty years of progress in physiological and biochemical investigation of RALF peptides. Plant Physiol. 2020;182:1657–1666. doi: 10.1104/pp.19.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A., Lituiev D.S., Nestorova A., Franck C.M., Thirugnanarajah S., Grossniklaus U. ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol. 2013;11:e1001719. doi: 10.1371/journal.pbio.1001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A., Roy S., Kritsas K., Grobei M.A., Jaciubek M., Schroeder J.I., Grossniklaus U. Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development. 2009;136:3279–3288. doi: 10.1242/dev.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty D., Yu Y., Assmann S.M. A kinase-dead version of FERONIA receptor-like kinase has dose-dependent impacts on rosette morphology and RALF1-mediated stomatal movements. FEBS Lett. 2018;592:3429–3437. doi: 10.1002/1873-3468.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L., Turner S.R. A comprehensive analysis of RALF proteins in green plants suggests there are two distinct functional groups. Front. Plant Sci. 2017;8:37. doi: 10.3389/fpls.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron A., Gourgues M., Neiva L.S., Faure J.-E., Berger F., Pagnussat G., Krishnan A., Alvarez-Mejia C., Vielle-Calzada J.P., Lee Y.R. Maternal control of male-gamete delivery in Arabidopsis involves a putative GPI-anchored protein encoded by the LORELEI gene. Plant Cell. 2008;20:3038–3049. doi: 10.1105/tpc.108.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Yu F., Liu Y., Du C., Li X., Zhu S., Wang X., Lan W., Rodriguez P.L., Liu X. FERONIA interacts with ABI2-type phosphatases to facilitate signaling cross-talk between abscisic acid and RALF peptide in Arabidopsis. Proc. Natl. Acad. Sci. U S A. 2016;113:E5519–E5527. doi: 10.1073/pnas.1608449113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D.J. Plant expansins: diversity and interactions with plant cell walls. Curr. Opin. Plant Biol. 2015;25:162–172. doi: 10.1016/j.pbi.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey P.A., Subbaiah C.C., Parsons R.L., Pearce G., Lay F.T., Anderson M.A., Ryan C.A., Bedinger P.A. A pollen-specific RALF from tomato that regulates pollen tube elongation. Plant Physiol. 2010;153:703–715. doi: 10.1104/pp.110.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denness L., McKenna J.F., Segonzac C., Wormit A., Madhou P., Bennett M., Mansfield J., Zipfel C., Hamann T. Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol. 2011;156:1364–1374. doi: 10.1104/pp.111.175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers S.D., Larsen P.B. FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol. Plant. 2010;3:626–640. doi: 10.1093/mp/ssq015. [DOI] [PubMed] [Google Scholar]
- Dievart A., Gottin C., Perin C., Ranwez V., Chantret N. Origin and diversity of plant receptor-like kinases. Annu. Rev. Plant Biol. 2020;71:131–156. doi: 10.1146/annurev-arplant-073019-025927. [DOI] [PubMed] [Google Scholar]
- Doblas V.G., Gonneau M., Höfte H. Cell wall integrity signaling in plants: malectin-domain kinases and lessons from other kingdoms. Cell Surf. 2018;3:1–11. doi: 10.1016/j.tcsw.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draeger C., Fabrice T.N., Gineau E., Mouille G., Kuhn B.M., Moller I., Abdou M.-T., Frey B., Pauly M., Bacic A. Arabidopsis leucine-rich repeat extensin (LRX) proteins modify cell wall composition and influence plant growth. BMC Plant Biol. 2015;15:155. doi: 10.1186/s12870-015-0548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C., Li X., Chen J., Chen W., Li B., Li C., Wang L., Li J., Zhao X., Lin J. Receptor kinase complex transmits RALF peptide signal to inhibit root growth in Arabidopsis. Proc. Natl. Acad. Sci. U S A. 2016;113:E8326–E8334. doi: 10.1073/pnas.1609626113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S., Qu L.J., Xiao J. Crystal structures of the extracellular domains of the CrRLK1L receptor-like kinases ANXUR1 and ANXUR2. Protein Sci. 2018;27:886–892. doi: 10.1002/pro.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q., Kita D., Johnson E.A., Aggarwal M., Gates L., Wu H.M., Cheung A.Y. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat. Commun. 2014;5:3129. doi: 10.1038/ncomms4129. [DOI] [PubMed] [Google Scholar]
- Duan Q., Kita D., Li C., Cheung A.Y., Wu H.M. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl. Acad. Sci. U S A. 2010;107:17821–17826. doi: 10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q., Liu M.-C.J., Kita D., Jordan S.S., Yeh F.-L.J., Yvon R., Carpenter H., Federico A.N., Garcia-Valencia L.E., Eyles S.J. FERONIA controls pectin- and nitric oxide-mediated male-female interaction. Nature. 2020;579:561–566. doi: 10.1038/s41586-020-2106-2. [DOI] [PubMed] [Google Scholar]
- Drubin D., Nelson W. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Engelsdorf T., Gigli-Bisceglia N., Veerabagu M., McKenna J.F., Vaahtera L., Augstein F., Van der Does D., Zipfel C., Hamann T. The plant cell wall integrity maintenance and immune signaling systems cooperate to control stress responses in Arabidopsis thaliana. Sci. Signal. 2018;11:eaao3070. doi: 10.1126/scisignal.aao3070. [DOI] [PubMed] [Google Scholar]
- Escobar-Restrepo J.M., Huck N., Kessler S., Gagliardini V., Gheyselinck J., Yang W.C., Grossniklaus U. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- Feng H., Liu C., Fu R., Zhang M., Li H., Shen L., Wei Q., Sun X., Xu L., Ni B. LORELEI-LIKE GPI-ANCHORED PROTEINS 2/3 regulate pollen tube growth as chaperones and coreceptors for ANXUR/BUPS receptor kinases in Arabidopsis. Mol. Plant. 2019;12:1612–1623. doi: 10.1016/j.molp.2019.09.004. [DOI] [PubMed] [Google Scholar]
- Feng W., Kita D., Peaucelle A., Cartwright H.N., Doan V., Duan Q., Liu M.C., Maman J., Steinhorst L., Schmitz-Thom I. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 2018;28:666–675.e5. doi: 10.1016/j.cub.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck C.M., Westermann J., Boisson-Dernier A. Plant malectin-like receptor kinases: from cell wall integrity to immunity and beyond. Annu. Rev. Plant Biol. 2018;69:301–328. doi: 10.1146/annurev-arplant-042817-040557. [DOI] [PubMed] [Google Scholar]
- Fu Y., Wu G., Yang Z. Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J. Cell Biol. 2001;152:1019–1032. doi: 10.1083/jcb.152.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachomo E.W., Jno Baptiste L., Kefela T., Saidel W.M., Kotchon S.O. The Arabidopsis CURVY1 (CVY1) gene encoding a novel receptor-like protein kinase regulates cell morphogenesis, flowering time and seed production. BMC plant biology. 2014;14:221. doi: 10.1186/s12870-014-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Trigo S., Blanco-Touriñán N., DeFalco T.A., Wells E.S., Gray J.E., Zipfel C., Smith L.M. CrRLK1L receptor-like kinases HERK1 and ANJEA are female determinants of pollen tube reception. EMBO Rep. 2020;21:e48466. doi: 10.15252/embr.201948466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z., Bergonci T., Zhao Y., Zou Y., Du S., Liu M.C., Luo X., Ruan H., García-Valencia L.E., Zhong S. Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science. 2017;358:1596–1600. doi: 10.1126/science.aao3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z., Zhao Y., Liu M.C., Zhou L.Z., Wang L., Zhong S., Hou S., Jiang J., Liu T., Huang Q. LLG2/3 Are co-receptors in BUPS/ANX-RALF signaling to regulate Arabidopsis pollen tube integrity. Curr. Biol. 2019;29:3256–3265.e5. doi: 10.1016/j.cub.2019.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonneau M., Desprez T., Martin M., Doblas V.G., Bacete L., Miart F., Sormani R., Hematy K., Renou J., Landrein B. Receptor kinase THESEUS1 is a rapid alkalinization factor 34 receptor in Arabidopsis. Curr. Biol. 2018;28:2452–2458.e4. doi: 10.1016/j.cub.2018.05.075. [DOI] [PubMed] [Google Scholar]
- Guo H., Li L., Ye H., Yu X., Algreen A., Yin Y. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U S A. 2009;106:7648–7653. doi: 10.1073/pnas.0812346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Nolan T.M., Song G., Liu S., Xie Z., Chen J., Schnable P.S., Walley J.W., Yin Y. FERONIA receptor kinase contributes to plant immunity by suppressing jasmonic acid signaling in Arabidopsis thaliana. Curr. Biol. 2018;28:3316–3324.e6. doi: 10.1016/j.cub.2018.07.078. [DOI] [PubMed] [Google Scholar]
- Hamann T. The plant cell wall integrity maintenance mechanism-concepts for organization and mode of action. Plant Cell Physiol. 2015;56:215–223. doi: 10.1093/pcp/pcu164. [DOI] [PubMed] [Google Scholar]
- Hansen R.L., Guo H., Yin Y., Lee Y.J. FERONIA mutation induces high levels of chloroplast-localized Arabidopsides which are involved in root growth. Plant J. 2019;97:341–351. doi: 10.1111/tpj.14123. [DOI] [PubMed] [Google Scholar]
- Haruta M., Sabat G., Stecker K., Minkoff B.B., Sussman M.R. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science. 2014;343:408–411. doi: 10.1126/science.1244454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler P.K., Vidali L., Cheung A. Polarized cell growth in higher plants. Annu. Rev. Cell Dev. Biol. 2001;17:159–187. doi: 10.1146/annurev.cellbio.17.1.159. [DOI] [PubMed] [Google Scholar]
- Hématy K., Sado P.E., Van Tuinen A., Rochange S., Desnos T., Balzergue S., Pelletier S., Renou J.P., Höfte H. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol. 2007;17:922–931. doi: 10.1016/j.cub.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Hirano N., Marukawa Y., Abe J., Hashiba S., Ichikawa M., Tanabe Y., Ito M., Nishii I., Tsuchikane Y., Sekimoto H. A receptor-like kinase, related to cell wall sensor of higher plants, is required for sexual reproduction in the unicellular Charophycean alga, Closterium peracerosum-strigosum-littorale complex. Plant Cell Physiol. 2015;56:1456–1462. doi: 10.1093/pcp/pcv065. [DOI] [PubMed] [Google Scholar]
- Honkanen S., Jones V.A.S., Morieri G., Champion C., Hetherington A.J., Kelly S., Proust H., Saint-Marcoux D., Prescott H., Dolan L. The mechanism forming the cell surface of tip-growing rooting cells is conserved among land plants. Curr. Biol. 2016;26:3238–3244. doi: 10.1016/j.cub.2016.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia M., Ding N., Zhang Q., Xing S., Wei L., Zhao Y., Du P., Mao W., Li J., Li B. A FERONIA-like receptor kinase regulates strawberry (Fragaria × ananassa) fruit ripening and quality formation. Front. Plant Sci. 2017;8:1099. doi: 10.3389/fpls.2017.01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia M., Du P., Ding N., Zhang Q., Xing S., Wei L., Zhao Y., Mao W., Li J., Li B. Two FERONIA-like receptor kinases regulate apple fruit ripening by modulating ethylene production. Front. Plant Sci. 2017;8:1406. doi: 10.3389/fpls.2017.01406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kadota Y., Shirasu K., Zipfel C. Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol. 2015;56:1472–1480. doi: 10.1093/pcp/pcv063. [DOI] [PubMed] [Google Scholar]
- Keinath N.F., Kierszniowska S., Lorek J., Bourdais G., Kessler S.A., Shimosato-Asano H., Grossniklaus U., Schulze W.X., Robatzek S., Panstruga R. PAMP (pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. J. Biol. Chem. 2010;285:39140–39149. doi: 10.1074/jbc.M110.160531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S.A., Shimosato-Asano H., Keinath N.F., Wuest S.E., Ingram G., Panstruga R., Grossniklaus U. Conserved molecular components for pollen tube reception and fungal invasion. Science. 2010;330:968–971. doi: 10.1126/science.1195211. [DOI] [PubMed] [Google Scholar]
- Kesten C., Gámez-Arjona F.M., Menna A., Scholl S., Dora S., Huerta A.I., Huang H.Y., Tintor N., Kinoshita T., Rep M. Pathogen-induced pH changes regulate the growth-defense balance in plants. EMBO J. 2019;38:e101822. doi: 10.15252/embj.2019101822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou X., Qi K., Qiao X., Yin H., Liu X., Zhang S., Wu J. Evolution, expression analysis, and functional verification of Catharanthus roseus RLK1-like kinase (CrRLK1L) family proteins in pear (Pyrus bretchneideri) Genomics. 2017;109:290–301. doi: 10.1016/j.ygeno.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Lee D., Lal N.K., Lin Z.D., Ma S., Liu J., Castro B., Toruno T., Dinesh-Kumar S.P., Coaker G. Regulation of reactive oxygen species during plant immunity through phosphorylation and ubiquitination of RBOHD. Nat. Commun. 2020;11:1838. doi: 10.1038/s41467-020-15601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehti-Shiu M.D., Zou C., Hanada K., Shiu S.H. Evolutionary history and stress regulation of plant receptor-like kinase/pelle genes. Plant Physiol. 2009;150:12–26. doi: 10.1104/pp.108.134353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Liu X., Qiang X., Li X., Li X., Zhu S., Wang L., Wang Y., Liao H., Luan S. EBP1 nuclear accumulation negatively feeds back on FERONIA-mediated RALF1 signaling. PLoS Biol. 2018;16:e2006340. doi: 10.1371/journal.pbio.2006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Wang L., Cui Y., He L., Qi Y., Zhang J., Lin J., Liao H., Lin Q., Yang T. Two FERONIA-like receptor (FLR) genes are required to maintain architecture, fertility, and seed yield in rice. Mol. Breed. 2016;36:151. [Google Scholar]
- Li C., Wu H.M., Cheung A.Y. FERONIA and her pals: functions and mechanisms. Plant Physiol. 2016;171:2379–2392. doi: 10.1104/pp.16.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Yeh F.L., Cheung A.Y., Duan Q., Kita D., Liu M.C., Maman J., Luu E.J., Wu B.W., Gates L. Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife. 2015;4:e06587. doi: 10.7554/eLife.06587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H., Tang R., Zhang X., Luan S., Yu F. FERONIA receptor kinase at the crossroads of hormone signaling and stress responses. Plant Cell Physiol. 2017;58:1143–1150. doi: 10.1093/pcp/pcx048. [DOI] [PubMed] [Google Scholar]
- Lin W., Tang W., Anderson C.T., Yang Z. FERONIA’s sensing of cell wall pectin activates ROP GTPase signaling in Arabidopsis. bioRxiv. 2018 doi: 10.1101/269647. [DOI] [Google Scholar]
- Liu J., Elmore J.M., Lin Z.J., Coaker G. A receptor-like cytoplasmic kinase phosphorylates the host target RIN4, leading to the activation of a plant innate immune receptor. Cell Host Microbe. 2011;9:137–146. doi: 10.1016/j.chom.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Zheng C., Kuang B., Wei L., Yan L., Wang T. Receptor-like kinase RUPO interacts with potassium transporters to regulate pollen tube growth and integrity in rice. PLoS Genet. 2016;12:e1006085. doi: 10.1371/journal.pgen.1006085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Haruta M., Minkoff B.B., Sussman M.R. Probing a plant plasma membrane receptor kinase's three-dimensional structure using mass spectrometry-based protein footprinting. Biochemistry. 2018;57:5159–5168. doi: 10.1021/acs.biochem.8b00471. [DOI] [PubMed] [Google Scholar]
- Liu X., Castro C., Wang Y., Noble J., Ponvert N., Bundy M., Hoel C., Shpak E., Palanivelu R. The role of LORELEI in pollen tube reception at the interface of the synergid cell and pollen tube requires the modified eight-cysteine motif and the receptor-like kinase FERONIA. Plant Cell. 2016;28:1035–1052. doi: 10.1105/tpc.15.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey D., Belkhadir Y., Alonso J.M., Ecker J.R., Dangl J.L. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell. 2003;112:379–389. doi: 10.1016/s0092-8674(03)00040-0. [DOI] [PubMed] [Google Scholar]
- Mackey D., Holt B.F., Wiig A., Dangl J.L. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell. 2002;108:743–754. doi: 10.1016/s0092-8674(02)00661-x. [DOI] [PubMed] [Google Scholar]
- Mang H., Feng B., Hu Z., Boisson-Dernier A., Franck C.M., Meng X., Huang Y., Zhou J., Xu G., Wang T. Differential regulation of two-tiered plant immunity and sexual reproduction by ANXUR receptor-like kinases. Plant Cell. 2017;29:3140–3156. doi: 10.1105/tpc.17.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D., Yu F., Li J., Van de Poel B., Tan D., Li J., Liu Y., Li X., Dong M., Chen L. FERONIA receptor kinase interacts with S-adenosylmethionine synthetase and suppresses S-adenosylmethionine production and ethylene biosynthesis in Arabidopsis. Plant Cell Environ. 2015;38:2566–2574. doi: 10.1111/pce.12570. [DOI] [PubMed] [Google Scholar]
- Masachis S., Segorbe D., Turra D., Leon-Ruiz M., Furst U., El Ghalid M., Leonard G., Lopez-Berges M.S., Richards T.A., Felix G. A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat. Microbiol. 2016;1:16043. doi: 10.1038/nmicrobiol.2016.43. [DOI] [PubMed] [Google Scholar]
- Mecchia M.A., Santos-Fernandez G., Duss N.N., Somoza S.C., Boisson-Dernier A., Gagliardini V., Martínez-Bernardini A., Fabrice T.N., Ringli C., Muschietti J.P. RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science. 2017;358:1600–1603. doi: 10.1126/science.aao5467. [DOI] [PubMed] [Google Scholar]
- Melotto M., Underwood W., He S.Y. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 2008;46:101–122. doi: 10.1146/annurev.phyto.121107.104959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersmann S., Bourdais G., Rietz S., Robatzek S. Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol. 2010;154:391–400. doi: 10.1104/pp.110.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz D., Richter J., Gonneau M., Sanchez-Rodriguez C., Eder T., Sormani R., Martin M., Hématy K., Höfte H., Hauser M.T. T-DNA alleles of the receptor kinase THESEUS1 with opposing effects on cell wall integrity signaling. J. Exp. Bot. 2017;68:4583–4593. doi: 10.1093/jxb/erx263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Murata T., Sakurai-Ozato N., Kubo M., Demura T., Fukuda H., Hasebe M. ANXUR1 and 2, sister genes to FERONIA/SIRENE, are male factors for coordinated fertilization. Curr. Biol. 2009;19:1327–1331. doi: 10.1016/j.cub.2009.06.064. [DOI] [PubMed] [Google Scholar]
- Molendijk A.J., Bischoff F., Rajendrakumar C.S., Friml J., Braun M., Gilroy S., Palme K. Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 2001;20:2779–2788. doi: 10.1093/emboj/20.11.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morato do Canto A., Ceciliato P.H., Ribeiro B., Ortiz Morea F.A., Franco Garcia A.A., Silva-Filho M.C., Moura D.S. Biological activity of nine recombinant AtRALF peptides: implications for their perception and function in Arabidopsis. Plant Physiol. Biochem. 2014;75:45–54. doi: 10.1016/j.plaphy.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Moussu S., Broyart C., Santos-Fernandez G., Augustin S., Wehrle S., Grossniklaus U., Santiago J. Structural basis for recognition of RALF peptides by LRX proteins during pollen tube growth. Proc. Natl. Acad. Sci. U S A. 2020;117:7494–7503. doi: 10.1073/pnas.2000100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise V., Joe A., Jeong B.R., Korneli C., Boutrot F., Westedt I., Staiger D., Alfano J.R., Zipfel C. Pseudomonas HopU1 modulates plant immune receptor levels by blocking the interaction of their mRNAs with GRP7. EMBO J. 2013;32:701–712. doi: 10.1038/emboj.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nürnberger T., Brunner F., Kemmerling B., Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunological Rev. 2004;198:249–266. doi: 10.1111/j.0105-2896.2004.0119.x. [DOI] [PubMed] [Google Scholar]
- Ogasawara Y., Kaya H., Hiraoka G., Yumoto F., Kimura S., Kadota Y., Hishinuma H., Senzaki E., Yamagoe S., Nagata K. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J. Biol. Chem. 2008;283:8885–8892. doi: 10.1074/jbc.M708106200. [DOI] [PubMed] [Google Scholar]
- Pearce G., Moura D.S., Stratmann J., Ryan C.A. RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc. Natl. Acad. Sci. U S A. 2001;98:12843–12847. doi: 10.1073/pnas.201416998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu C.X., Han Y.F., Zhu S., Song F.Y., Zhao Y., Wang C.Y., Zhang Y.C., Yang Q., Wang J., Bu S.L. The rice receptor-like kinases DWARF AND RUNTISH SPIKELET1 and 2 repress cell death and affect sugar utilization during reproductive development. Plant Cell. 2017;29:70–89. doi: 10.1105/tpc.16.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J., Wang J., Gong Z., Zhou J.M. Apoplastic ROS signaling in plant immunity. Curr. Opin. Plant Biol. 2017;38:92–100. doi: 10.1016/j.pbi.2017.04.022. [DOI] [PubMed] [Google Scholar]
- Qu S., Zhang X., Song Y., Lin J., Shan X. THESEUS1 positively modulates plant defense responses against Botrytis cinerea through GUANINE EXCHANGE FACTOR4 signaling. J. Integr. Plant Biol. 2017;59:797–804. doi: 10.1111/jipb.12565. [DOI] [PubMed] [Google Scholar]
- Richter J., Watson J.M., Stasnik P., Borowska M., Neuhold J., Berger M., Stolt-Bergner P., Schoft V., Hauser M.T. Multiplex mutagenesis of four clustered CrRLK1L with CRISPR/Cas9 exposes their growth regulatory roles in response to metal ions. Sci. Rep. 2018;8:12182. doi: 10.1038/s41598-018-30711-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringli C. The hydroxyproline-rich glycoprotein domain of the Arabidopsis LRX1 requires Tyr for function but not for insolubilization in the cell wall. Plant J. 2010;63:662–669. doi: 10.1111/j.1365-313X.2010.04270.x. [DOI] [PubMed] [Google Scholar]
- Sagi M., Fluhr R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006;141:336–340. doi: 10.1104/pp.106.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenaers S., Balcerowicz D., Breen G., Hill K., Zdanio M., Mouille G., Holman T.J., Oh J., Wilson M.H., Nikonorova N. The auxin-regulated CrRLK1L kinase ERULUS controls cell wall composition during root hair tip growth. Curr. Biol. 2018;28:722–732.e6. doi: 10.1016/j.cub.2018.01.050. [DOI] [PubMed] [Google Scholar]
- Schulze-Muth P., Irmler S., Schröder G., Schröder J. Novel type of receptor-like protein kinase from a higher plant (Catharanthus roseus). cDNA, gene, intramolecular autophosphorylation, and identification of a threonine important for auto- and substrate phosphorylation. J. Biol. Chem. 1996;271:26684–26689. doi: 10.1074/jbc.271.43.26684. [DOI] [PubMed] [Google Scholar]
- Shen Q., Bourdais G., Pan H., Robatzek S., Tang D. Arabidopsis glycosylphosphatidylinositol-anchored protein LLG1 associates with and modulates FLS2 to regulate innate immunity. Proc. Natl. Acad. Sci. U S A. 2017;114:5749–5754. doi: 10.1073/pnas.1614468114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu S.H., Bleecker A.B. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. U S A. 2001;98:10763–10768. doi: 10.1073/pnas.181141598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R., Liu J.X., Guo H., Yin Y., Howell S.H. Regulation and processing of a plant peptide hormone, AtRALF23, in Arabidopsis. Plant J. 2009;59:930–939. doi: 10.1111/j.1365-313X.2009.03926.x. [DOI] [PubMed] [Google Scholar]
- Stegmann M., Monaghan J., Smakowska-Luzan E., Rovenich H., Lehner A., Holton N., Belkhadir Y., Zipfel C. The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science. 2017;355:287–289. doi: 10.1126/science.aal2541. [DOI] [PubMed] [Google Scholar]
- Thatcher L.F., Manners J.M., Kazan K. Fusarium oxysporum hijacks COI1-mediated jasmonate signaling to promote disease development in Arabidopsis. Plant J. 2009;58:927–939. doi: 10.1111/j.1365-313X.2009.03831.x. [DOI] [PubMed] [Google Scholar]
- Thynne E., Saur I.M.L., Simbaqueba J., Ogilvie H.A., Gonzalez-Cendales Y., Mead O., Taranto A., Catanzariti A.M., McDonald M.C., Schwessinger B. Fungal phytopathogens encode functional homologues of plant rapid alkalinization factor (RALF) peptides. Mol. Plant Pathol. 2017;18:811–824. doi: 10.1111/mpp.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood W. The plant cell wall: a dynamic barrier against pathogen invasion. Front. Plant Sci. 2012;3:85. doi: 10.3389/fpls.2012.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Does D., Boutrot F., Engelsdorf T., Rhodes J., McKenna J.F., Vernhettes S., Koevoets I., Tintor N., Veerabagu M., Miedes E. The Arabidopsis leucine-rich repeat receptor kinase MIK2/LRR-KISS connects cell wall integrity sensing, root growth and response to abiotic and biotic stresses. PLoS Genet. 2017;13:e1006832. doi: 10.1371/journal.pgen.1006832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidhyasekaran P. Plant Hormone Signaling Systems in Plant Innate Immunity. Vol. 2. Springer; Dordrecht: 2015. Ethylene signaling system in plant innate immunity; pp. 195–244. (Signaling and Communication in Plants). [Google Scholar]
- Wang L., Yang T., Lin Q., Wang B., Li X., Luan S., Yu F. Receptor kinase FERONIA regulates flowering time in Arabidopsis. BMC Plant Biol. 2020;20:26. doi: 10.1186/s12870-019-2223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Yang T., Wang B., Lin Q., Zhu S., Li C., Ma Y., Tang J., Xing J., Li X. RALF1-FERONIA complex impacts splicing dynamics to modulate stress responses and growth in plants. Sci. Adv. 2020;6:eaaz1622. doi: 10.1126/sciadv.aaz1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann J., Streubel S., Franck C.M., Lentz R., Dolan L., Boisson-Dernier A. An evolutionarily conserved receptor-like kinases signaling module controls cell wall integrity during tip growth. Curr. Biol. 2019;29:3899–3908.e3. doi: 10.1016/j.cub.2019.09.069. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Stegmann M., Han Z., DeFalco T.A., Parys K., Xu L., Belkhadir Y., Zipfel C., Chai J. Mechanisms of RALF peptide perception by a heterotypic receptor complex. Nature. 2019;572:270–274. doi: 10.1038/s41586-019-1409-7. [DOI] [PubMed] [Google Scholar]
- Xin X.F., He S.Y. Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annu. Rev. Phytopathol. 2013;51:473–498. doi: 10.1146/annurev-phyto-082712-102321. [DOI] [PubMed] [Google Scholar]
- Xu G., Chen W., Song L., Chen Q., Zhang H., Liao H., Zhao G., Lin F., Zhou H., Yu F. FERONIA phosphorylates E3 ubiquitin ligase ATL6 to modulate the stability of 14-3-3 proteins in response to the carbon/nitrogen ratio. J. Exp. Bot. 2019;70:6375–6388. doi: 10.1093/jxb/erz378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Wang L., Li C., Liu Y., Zhu S., Qi Y., Liu X., Lin Q., Luan S., Yu F. Receptor protein kinase FERONIA controls leaf starch accumulation by interacting with glyceraldehyde-3-phosphate dehydrogenase. Biochem. Biophys. Res. Commun. 2015;465:77–82. doi: 10.1016/j.bbrc.2015.07.132. [DOI] [PubMed] [Google Scholar]
- Yang Z., Xing J., Wang L., Liu Y., Qu J., Tan Y., Fu X., Lin Q., Deng H., Yu F. Mutations of two FERONIA-like receptor genes enhance rice blast resistance without growth penalty. J. Exp. Bot. 2020;71:2112–2126. doi: 10.1093/jxb/erz541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Li J., Huang Y., Liu L., Li D., Chen L., Luan S. FERONIA receptor kinase controls seed size in Arabidopsis thaliana. Mol. Plant. 2014;7:920–922. doi: 10.1093/mp/ssu010. [DOI] [PubMed] [Google Scholar]
- Yu F., Qian L., Nibau C., Duan Q., Kita D., Levasseur K., Li X., Lu C., Li H., Hou C. FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc. Natl. Acad. Sci. U S A. 2012;109:14693–14698. doi: 10.1073/pnas.1212547109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Liu Y., Tichelaar R., Savant N., Lagendijk E., van Kuijk S.J., Stringlis I.A., van Dijken A.J., Pieterse C.M., Bakker P.A. Rhizosphere-associated Pseudomonas suppress local root immune responses by gluconic acid-mediated lowering of environmental pH. Curr. Biol. 2019;29:3913–3920.e4. doi: 10.1016/j.cub.2019.09.015. [DOI] [PubMed] [Google Scholar]
- Yu Y., Assmann S.M. Inter-relationships between the heterotrimeric Gβ subunit AGB1, the receptor-like kinase FERONIA, and RALF1 in salinity response. Plant Cell Environ. 2018;41:2475–2489. doi: 10.1111/pce.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Zhang Y. Plant immunity: danger perception and signaling. Cell. 2020;181:30491–30498. doi: 10.1016/j.cell.2020.04.028. [DOI] [PubMed] [Google Scholar]
- Zhu S., Estévez J.M., Liao H., Zhu Y., Yang T., Li C., Wang Y., Li L., Liu X., Pacheco J.M. The RALF1-FERONIA complex phosphorylates eIF4E1 to promote protein synthesis and polar root hair growth. Mol. Plant. 2020;13:30431–30439. doi: 10.1016/j.molp.2019.12.014. [DOI] [PubMed] [Google Scholar]
- Zhu S., Martínez Pacheco J., Estevez J.M., Yu F. Autocrine regulation of root hair size by the RALF-FERONIA-RSL4 signaling pathway. New Phytol. 2020;227:45–49. doi: 10.1111/nph.16497. [DOI] [PubMed] [Google Scholar]