Abstract
High temperature activates the transcription factor PHYTOCHROME-INTERACTING FACTOR4 (PIF4) to stimulate auxin signaling, which causes hypocotyl elongation and leaf hyponasty (thermomorphogenesis). HOOKLESS1 (HLS1) is a recently reported positive regulator of thermomorphogenesis, but the molecular mechanisms by which HLS1 regulates thermomorphogenesis remain unknown. In this study, we initially compared PIF4- and/or HLS1-dependent differential gene expression (DEG) upon high-temperature treatment. We found that a large number of genes are coregulated by PIF4 and HLS1, especially genes involved in plant growth or defense responses. Moreover, we found that HLS1 interacts with PIF4 to form a regulatory module and that, among the HLS1-PIF4-coregulated genes, 27.7% are direct targets of PIF4. We also identified 870 differentially alternatively spliced genes (DASGs) in wild-type plants under high temperature. Interestingly, more than half of these DASG events (52.4%) are dependent on both HLS1 and PIF4, and the spliceosome-defective mutant plantsexhibit a hyposensitive response to high temperature, indicating that DASGs are required for thermomorphogenesis. Further comparative analyses showed that the HLS1/PIF4-coregulated DEGs and DASGs exhibit almost no overlap, suggesting that high temperature triggers two distinct strategies to control plant responses and thermomorphogenesis. Taken together, these results demonstrate that the HLS1-PIF4 module precisely controls both transcriptional and posttranscriptional regulation during plant thermomorphogenesis.
Key words: PIF4, HLS1, transcription, alternative splicing, thermomorphogenesis
This study reveals that PIF4 and HOOKLESS1 coregulate a proportion of differential gene expression and alternative splicing events during thermomorphogenesis in Arabidopsis.
Introduction
As sessile organisms, plants have evolved a series of elegant developmental programs for adapting to high ambient temperatures. In Arabidopsis thaliana, for example, high ambient temperature stimulates hypocotyl elongation in seedlings and promotes petiole elongation and leaf upward growth in adult plants (Gray et al., 1998). These architectural changes are collectively termed thermomorphogenesis. It has been experimentally demonstrated that thermomorphogenesis helps plants to decrease their leaf surface temperature when they are in a high-temperature environment (Crawford et al., 2012). The major regulator for the establishment of thermomorphogenesis is the basic helix-loop-helix transcription factor PHYTOCHROME-INTERACTING FACTOR4 (PIF4) (Koini et al., 2009). It was reported recently that high temperature promotes phytochrome dark reversion to desensitize phytochrome signaling, which results in the activation of PIF4 (Jung et al., 2016, Legris et al., 2016). PIF4 then directly binds to several auxin biosynthesis- or signaling-related gene promoters to stimulate auxin responses and promote cell elongation (Franklin et al., 2011, Sun et al., 2012, Huai et al., 2018). Among these PIF4 targets, YUCCA8 encodes a flavin monooxygenase that catalyzes indole-3-acetic acid production from the biosynthetic precursor indole-3-pyruvic acid (Sun et al., 2012).
In addition to PIF4, HOOKLESS1 (HLS1, At4g37580) also participates in plant thermomorphogenesis (Jin and Zhu, 2019). HLS1 was originally identified through forward genetic screening for ethylene-insensitive mutants (Lehman et al., 1996). Etiolated hls1 mutants do not exhibit exaggerated apical hooks when grown under ethylene treatment (Guzman and Ecker, 1990). It was shown that ethylene induces HLS1 transcription to mediate the asymmetric auxin distribution between the concave and convex sides of apical hook regions (Li et al., 2004, Vandenbussche et al., 2010, An et al., 2012). Although HLS1 was cloned more than two decades ago, the biochemical nature of the HLS1 protein is still a mystery. The HLS1 protein sequence is similar to that of N-acetyltransferase (Lehman et al., 1996); however, HLS1 does not show acetyltransferase activity, at least in in vitro experiments (Liao et al., 2016). In addition to its pivotal role in apical hook development, HLS1 is assumed to present multiple other functions. HLS1 physically interacts with mediator subunit 18 (MED18) and associates with the WRKY33 promoter to regulate the plant defense response, suggesting that HLS1 could be a transcriptional modulator (Liao et al., 2016). HLS1 is also reported to be involved in flowering time control and sucrose signaling via unknown mechanisms (Li et al., 2004, Ohto et al., 2006). We recently demonstrated that hls1 mutants exhibit hyposensitivity to high-temperature-triggered cell elongation and transcriptomic changes and proposed that HLS1 acts as another positive regulator during thermomorphogenesis (Jin and Zhu, 2019), but how HLS1 exerts its function is not clear.
Although transcriptional regulation is an efficient way to rapidly trigger dynamic mRNA changes in the presence of external stimuli, alternative pre-mRNA splicing provides an additional layer of regulation modulating global protein complexity. For example, red light stimulates both transcriptome and alternative splicing events through red light photoreceptor phytochromes (Shikata et al., 2014). The regulatory mechanisms underlying pre-mRNA splicing are conserved in eukaryotes. The large spliceosome complex recognizes adjacent alternative sites to execute its splicing function (Wan, 2018). Serine/arginine-rich proteins selectively bind cis-elements in pre-mRNA and then repress or enhance spliceosome recruitment to cause alternative splicing (Kornblihtt et al., 2013). Accurate alternative splicing has been implicated in a wide range of plant physiological responses. The mutation of the spliceosome component SKIP (SNW/Ski-interacting protein) results in genome-wide pre-mRNA splicing dysregulation, which in turn causes circadian clock defects and abnormal responses to salt stress (Wang et al., 2012, Feng et al., 2015). It has been reported that high temperature triggers alternative splicing in certain genes (Lee et al., 2013, Pose et al., 2013, James et al., 2018), but a genome-wide survey has yet to be performed, particularly in the context of PIF4 central regulators.
In this study, we investigated both transcriptome (transcriptional regulation) and alternative splicing events (posttranscriptional regulation) under high-temperature treatment and compared HLS1- and/or PIF4-dependent regulation. We first revealed that a majority of either transcriptional or posttranscriptional alterations are coregulated by both HLS1 and PIF4. Furthermore, HLS1 physically interacts with PIF4 and occupies PIF4-targeted gene promoters to form a regulatory module. Second, we found that the differentially expressed genes (DEGs) coregulated by HLS1 and PIF4 show very little overlap with differentially alternatively spliced genes (DASGs), which suggests that high temperature triggers two distinct mechanisms through the same HLS1-PIF4 module to mediate thermomorphogenesis in Arabidopsis.
Results
HLS1 and PIF4 Coregulate the High-Temperature-Responsive Transcriptome
Both HLS1 and PIF4 are positive regulators for establishing thermomorphogenesis; however, their mutual relationships are still unknown. To reveal their individual contributions during thermomorphogenesis, we took advantage of an RNA sequencing (RNA-seq) approach to investigate the global transcriptomic changes upon high-temperature treatment of the wild-type (Col-0) and the pif4-2 or hls1-1 mutants (Figure 1). The strategy was to compare mRNA expression levels and/or alternative splicing events in different genetic backgrounds to reveal their functional hierarchy. More than 95% of the sequencing reads for each sample were mapped to the Arabidopsis genome (TAIR 10), which indicated that the RNA-seq results were reliable (Supplemental Table 1). High-temperature treatment resulted in thousands of DEGs in different plant materials (1740 in wild-type plants, 1710 in hls1-1 mutants, and 913 in pif4-2 mutants) (Figure 2A and Supplemental Table 2). It was noteworthy that, among the 1740 DEGs identified in the wild-type plants, 1501 (86.3%) were PIF4 dependent, while 806 (46.3%) were HLS1 dependent (Figure 2A and Supplemental Table 3). This result further confirmed that PIF4 is a key component of plant thermomorphogenesis, even at the global transcriptome level.
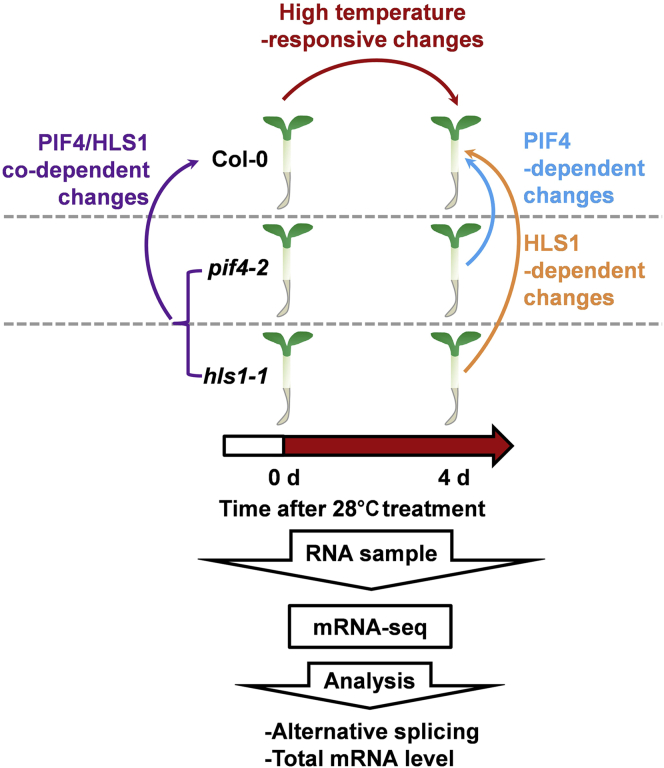
Figure 1.
Illustration of the Experimental Design.
Flowchart for the analysis of the high-temperature-responsive transcriptome and alternative splicing events in Col-0 and hls1-1 or pif4-2 mutants. RNA samples were extracted from seedlings that were initially grown at 22°C under 70 μmol m−2 s−1 of white light for 3 days and then transferred to 28°C (as 28°C treatment) or maintained at 22°C (as 22°C treatment) for an additional 4 days.
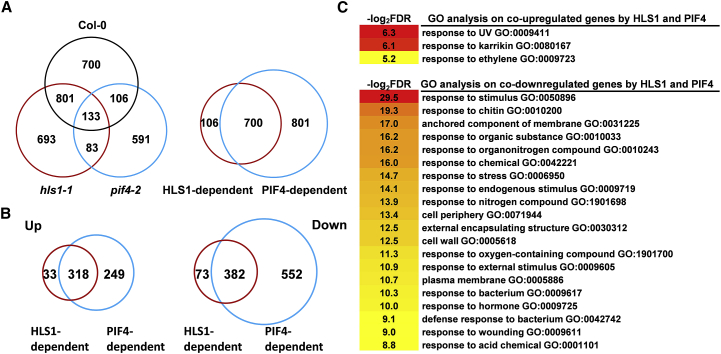
Figure 2.
DEGs under High-Temperature Treatment.
(A) Venn diagrams showing the differentially expressed gene (DEG) numbers in various genotypes (left) and the HLS1-dependent and/or PIF4-dependent gene numbers (right).
(B) Venn diagrams showing differentially upregulated or downregulated gene numbers.
(C) GO enrichment analysis of DEGs coregulated by HLS1 and PIF4.
Next, we tried to determine the relationships between PIF4-dependent and HLS1-dependent DEGs. Interestingly, 86.8% of the HLS1-dependent DEGs were also dependent on PIF4 (Figure 2A and Supplemental Table 3). Further detailed analysis showed that among the 351 HLS1-dependent upregulated genes, 318 were coregulated by both PIF4 and HLS1, while 382 of the HLS1-dependent downregulated genes were also PIF4 dependent (Figure 2B and Supplemental Table 3). We randomly selected three genes from the 318 PIF4-HLS1 co-upregulated gene list and detected their expression levels through qRT–PCR to verify the RNA-seq results. All of these genes were significantly induced in Col-0 but were either not induced or showed attenuated induction in pif4-2 or hls1-1 mutants (Supplemental Figure 1), which confirmed our RNA-seq results. The gene ontology (GO) results demonstrated that the HLS1/PIF4-codependent upregulated genes were mostly enriched in the functional categories of the response to karrikin or ethylene, whereas the downregulated genes mostly belonged to categories related to defense responses (Figure 2C). It has been shown that PIF4 mediates the repression of plant immunity under high temperature to balance the growth-defense trade-off (Gangappa et al., 2017). Our results also illustrated this scenario and further indicated that HLS1 performs its function in a PIF4-dependent manner to synergistically control plant thermomorphogenesis.
HLS1 Directly Regulates YUC8 Expression
Because we identified 700 genes that were coregulated by HLS1 and PIF4 in response to high temperature (Supplemental Table 3), we further asked how many of these genes were directly targeted by the transcription factor PIF4. Chromatin immunoprecipitation sequencing (ChIP-seq) assays have revealed more than 4200 PIF4 target genes (Oh et al., 2012); therefore, we mapped our DEG results with PIF4 ChIP-seq data and found that 194 genes were PIF4 targets (Figure 3A). Considering transcriptional cascades, 194 out of 700 (27.7%) is a high ratio, indicating that HLS1-PIF4 directly controls the majority of transcription. GO analysis showed that most of these genes were enriched in functional categories related to growth responses, such as the response to stimulus, response to light, or response to hormone signaling (Figure 3B).
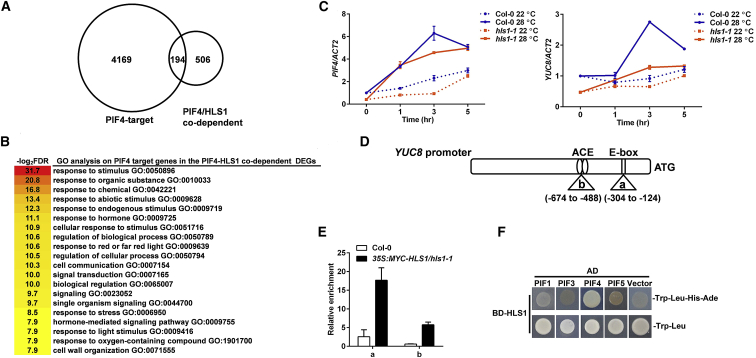
Figure 3.
HLS1 Localizes at PIF4-Binding Sites.
(A) Overlap between PIF4-binding sites (PIF4-target) and PIF4/HLS1-codependent DEGs (PIF4/HLS1 codependent).
(B) GO enrichment analysis of PIF4 target genes among the HLS1-PIF4-codependent DEGs.
(C) Relative expression of PIF4 (left) or YUC8 (right) in Col-0 and hls1-1 mutants at 22°C or 28°C at various time points. Values are means ± SD; n = 3.
(D) Diagram of the YUC8 promoter structure. Two PIF4-binding sites are indicated with the letters “a” and “b.” The numbers indicate the distance (bp) to the start codon (ATG).
(E) ChIP–PCR assay showing the in vivo binding of HLS1 with the YUC8 promoter.
(F) Yeast two-hybrid assay showing the HLS1-PIF4 interaction.
Then, we performed a series of experiments to test whether HLS1 directly regulates PIF4 target gene expression. Among PIF4 targets, YUC8 is well known for its fundamental role in auxin biosynthesis. High temperature induced PIF4 expression in both wild-type and hls1-1 plants, suggesting that the expression of PIF4 itself is not dependent on HLS1. However, the upregulation of YUC8 only occurred in the wild type and not in the hls1-1 mutants (Figure 3C). This result indicated that the induction of YUC8 was dependent on HLS1. There are two PIF4-binding sites in the YUC8 promoter region that mediate YUC8 transcriptional upregulation (Huai et al., 2018) (Figure 3D). We thus performed ChIP–PCR to directly test whether HLS1 binds to the PIF4-binding sites in the YUC8 promoter. Our results showed that HLS1 associated with both PIF4-binding sites and showed a stronger affinity for the E-box region (Figure 3E).
The findings that HLS1 coregulates the high-temperature-responsive transcriptome with PIF4 (Figure 2) and directly binds to PIF4 target genes prompted us to speculate that HLS1 might physically interact with PIF4. We rapidly tested the interactions between HLS1 and all four PIF transcription factors (PIF1/3/4/5) in a yeast two-hybrid system and found that HLS1 strongly interacted with PIF4 (Figure 3F). A luciferase complementation imaging assay further demonstrated that HLS1 interacted with PIF4, although HLS1 also interacted with PIF3 and PIF5 in planta (Supplemental Figure 3). Considering these findings together, we conclude that HLS1 interacts with PIF4 and co-occupies PIF4 binding regions to modulate the high-temperature-responsive transcriptome.
HLS1-PIF4 Module Controls Alternative Splicing of mRNAs
DEGs represent the transcriptional response upon high-temperature treatment, and posttranscriptional regulation provides a second layer of regulation before mRNA maturation. Alternative splicing events occur in almost all physiological responses; however, little is known about the role of alternative splicing at a genome-wide scale during thermomorphogenesis. We thus took advantage of our RNA-seq data to further examine alternative spicing events. In the wild-type, 870 genes (corresponding to 1206 splicing events) were DASGs, while in the hls1-1 and pif4-2 mutants, there were 932 and 696 DASGs, respectively (Figure 4A and Supplemental Table 4). Different types of alternative splicing events were distributed unequally. Alternative 3′ splicing site events (A3′SS) were the most abundant category, while mutually exclusive exon events (MXE) were among the least abundant (Supplemental Figure 2).
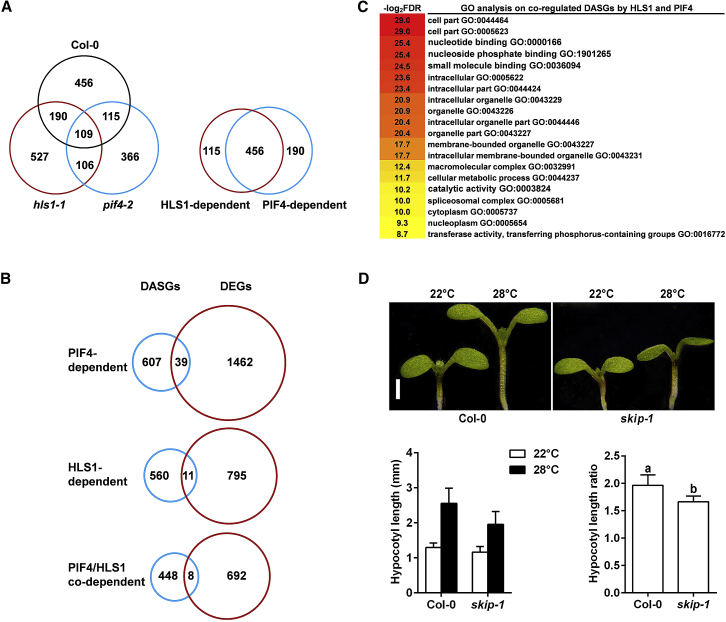
Figure 4.
DASGs under High-Temperature Treatment.
(A) Venn diagrams showing the differentially alternatively spliced gene numbers in different genotypes (left) and the overlap between HLS1-dependent and PIF4-dependent DASG numbers (right).
(B) Overlap between PIF4-dependent (top), HLS1-dependent (middle), and PIF4/HLS1 codependent (bottom) DASGs and DEGs.
(C) GO enrichment analysis of DASGs coregulated by HLS1 and PIF4.
(D) Hypocotyl length phenotypes in Col-0 and skip-1 mutants. Representative images (top), quantification results (left bottom) and the relative hypocotyl length ratio (28°C vs 22°C) (bottom right corner) are shown. Values are means ± SD; n = 30. Significant differences between the hypocotyl length ratios in Col-0 and skip-1 mutants are indicated by the letter “a” or “b” as determined by Tukey's least significant difference (LSD) test (P ≤ 0.01). Scale bar corresponds to 1 mm.
We further examined PIF4- or HLS1-dependent DASGs and found that PIF4 controlled 646 DASGs, while HLS1 governed 571 DASGs (Figure 4A and Supplemental Table 5). Among the 571 HLS1-dependent DASGs, 456 (79.9%) were also dependent on PIF4 (Figure 4A and Supplemental Table 5). This result demonstrated that, similar to the HLS-PIF4-codependent DEGs, HLS1 and PIF4 also synergistically controlled pre-mRNA splicing. RT–PCR was carried out for two arbitrarily selected genes (intron retention type) to verify this result (Supplemental Figure 4). The high-temperature-triggered intron retention events were clearer in Col-0 (Supplemental Figure 4B and 4D); however, neither pif4-2 nor hls1-1 showed an increase in intron retention after high-temperature treatment (Supplemental Figure 4B and 4D). Interestingly, the PIF4- and/or HLS1-dependent DASGs presented less overlap with their corresponding DEGs (Figure 4B), which suggested that high ambient temperature triggered both transcriptional and posttranscriptional programs to regulate the plant growth response. Consistent with this hypothesis, GO analysis also showed that HLS1-PIF4-coregulated DASGs enriched in various functions, including nucleotide binding, organelle behavior, and even the spliceosomal complex (Figure 4C).
To substantiate the biological relevance of alternative splicing in thermomorphogenesis, we used a spliceosome-defective mutant (skip-1) to observe the thermomorphogenesis phenotype. It has been reported that skip-1 mutation results in genome-wide pre-mRNA splicing dysregulation. We also confirmed that high-temperature-responsive intron retention events were attenuated in skip-1 mutants (Supplemental Figure 4). skip-1 mutants displayed a reduced hypocotyl elongation response under high temperature (Figure 4D), suggesting that alternative splicing is necessary for thermomorphogenesis.
HLS1-PIF4 Act in the Same Regulatory Module
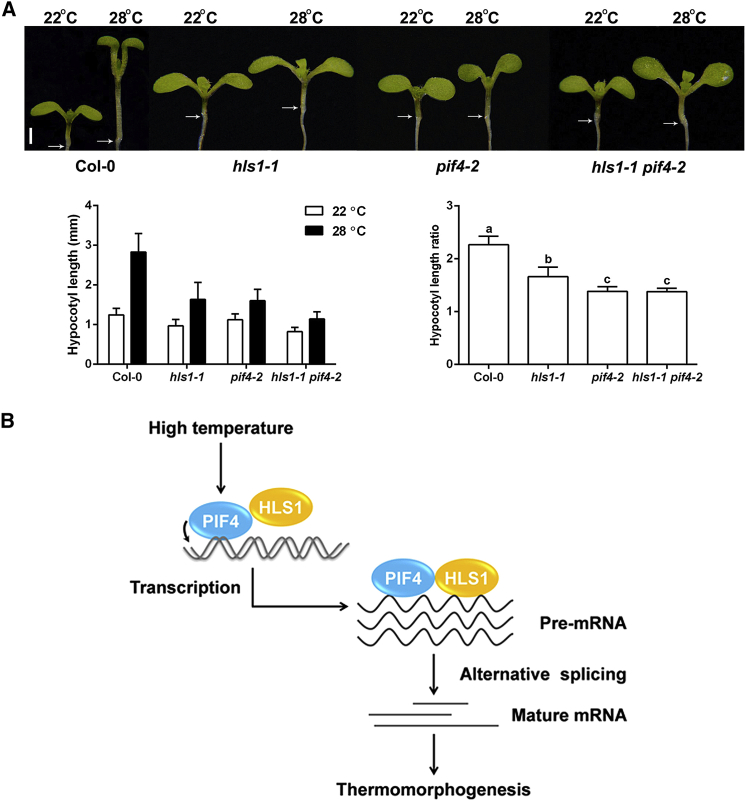
Both HLS1 and PIF4 act as positive regulators during thermomorphogenesis. To characterize their genetic relationships, we generated hsl1-1 pif4-2 double mutants and observed their thermomorphogenesis phenotypes compared with those of their parental lines. We found that hls1-1 mutants exhibited partial insensitivity to high temperature, and pif4-2 mutants displayed stronger insensitivity (Figure 5A). hls1-1 pif4-2 double mutants resembled the pif4-2 single mutants (Figure 5A), further suggesting that HLS1 exerts its function through PIF4. These phenotypic observations were consistent with our DEG and DASG results, showing that HLS1 controls fewer genes than does PIF4 under high temperature.
Figure 5.
HLS1 Acts Synergistically with PIF4 during Thermomorphogenesis.
(A) Hypocotyl length phenotypes in various mutant materials. Representative images (top), quantification results (left bottom) and the relative hypocotyl length ratio (28°C vs 22°C) (bottom right corner) are shown. Values are means ± SD; n = 30. Significant differences between the hypocotyl length ratios are indicated with the letters “a” to “c” as determined by Tukey's LSD test (P ≤ 0.01). Scale bar corresponds to 1 mm.
(B) A working model of HLS1-PIF4 co-action during plant thermomorphogenesis. High temperature activates the transcription factor PIF4, which interacts with HLS1 to regulate both transcription and pre-mRNA splicing to precisely control mature mRNA biogenesis and thermomorphogenesis.
Discussion
As sessile organisms, plants cannot escape their environmental conditions and have to modulate their growth and developmental programs to adapt to harsh environments. For example, under high ambient temperature, plants elongate their leaf petioles or hypocotyls to relieve high leaf temperatures. The transcription factor PIF4 is the dominant regulator that promotes cell elongation through the induction of auxin biosynthesis- and signaling-related gene expression (Quint et al., 2016). PIF4 also negatively regulates the plant immune response to balance the growth-defense trade-off (Gangappa et al., 2017). We recently reported that HLS1 is a positive regulator of plant thermomorphogenesis (Jin and Zhu, 2019). However, the molecular basis of HLS1 underlying thermomorphogenesis is still not completely understood.
In this study, we simultaneously compared the HLS1-PIF4-dependent transcriptome and alternative splicing events and found that HLS1 acts synergistically with PIF4 to modulate both transcriptional and posttranscriptional regulation (Figure 5B). On the basis of the genome-wide analysis, we report that, during thermomorphogenesis, HLS1 functions through PIF4 (Figures 2 and 4). We also show that HLS1 physically interacts with PIF4 and associates with the same PIF4-binding regions in the YUC8 promoter (Figure 3E and 3F and Supplemental Figure 3). These results suggest that HLS1 modulates the global transcriptome through its co-action with PIF4. A case study has demonstrated that HLS1 interacts with MED18 to regulate WRKY33 expression during the plant defense response (Liao et al., 2016). It is plausible to test whether the mediator complex is involved in thermomorphogenesis in the future. HLS1 also shows sequence similarities to N-acetyltransferase (Lehman et al., 1996); however, HLS1 proteins expressed from Escherichia coli (E. coli) cells do not exhibit acetyltransferase activity (Liao et al., 2016). Interestingly, a recent study showed that histone deacetylation is required for thermomorphogenesis. High temperature triggers histone deacetylation in YUC8 loci through the HISTONE DEACETYLASE 9-interacting protein POWERDRESS (Tasset et al., 2018). Given the crucial roles of histone (de)acetylation during thermomorphogenesis, we suspect that the HLS1-PIF4 interaction could recruit an unknown histone (de)acetylase to alter histone acetylation levels. It is also necessary to re-examine HLS1 acetyltransferase activity by purifying the HLS1 protein from a eukaryotic protein expression system to avoid the loss of necessary protein modifications when expressed in E. coli cells.
In addition to the transcriptome, the HLS1-PIF4 module governs alternative splicing regulation under high-temperature treatment (Figure 4A). We detected hundreds of HLS1-PIF4-coregulated DASGs and further showed that spliceosome mutants displayed attenuated thermomorphogenesis-related phenotypes (Figure 4D). In mammalian cells, the transcription factor FOXP3 interacts with heterogeneous nuclear ribonucleoproteins (hnRNPs) to inhibit hnRNP binding with target pre-mRNAs for the regulation of alternative splicing (Du et al., 2018). We assume that HLS1-PIF4 might interact with splicing-related proteins to regulate alternative splicing. In Arabidopsis, 395 putative splicing-related proteins were identified through a computational approach (Wang and Brendel, 2004), and systematic one-by-one protein–protein interaction tests will be performed to reveal the underlying mechanisms.
Interestingly, the identified high-temperature-responsive DEGs and DASGs were completely divergent (Figure 4B), which is reminiscent of the results of a previous light-responsive RNA-seq study (Shikata et al., 2014). Red light also triggers divergent transcriptional and posttranscriptional regulation through phytochromes. Because phytochromes act as both photoreceptors and thermosensors (Jin and Zhu, 2019), although PIF4 has only been clearly shown to be a necessary component of light signaling, we assume that light and high-temperature stimuli impinge on a conserved signaling module to precisely control protein dynamics. The role of HLS1 in red light signaling is thus worthy of exploration in future studies. A very recent study suggested that HLS1 forms oligomers in the nucleus as its active state, whereas light-activated phytochrome B (phyB) directly interacts with HLS1 to achieve a photoresponsive deoligomerization status to inhibit HLS1 activity and further trigger apical hook opening (Lyu et al., 2019). It is worth examining whether phyB also alters HLS1 oligomer status under high temperature and, more importantly, whether PIF4-HLS1 interactions occur in the same oligomer complex.
Finally, we show that the phenotypes of hls1-1 pif4-2 double mutants resemble the pif4-2 phenotypes, which further strengthens our hypothesis that HLS1 coordinates with PIF4 to regulate thermomorphogenesis (Figure 5A). As a crucial component of plant thermomorphogenesis, PIF4 controls a variety of high-temperature responses, including cell elongation, stomatal development, flowering, and defense (Kumar et al., 2012, Quint et al., 2016, Gangappa et al., 2017, Lau et al., 2018). In this study, we mainly focused on cell elongation as a phenotypic output and did not examine other thermoresponsive phenotypes. It is possible that PIF4 interacts with proteins other than HLS1 to precisely control other signaling activities.
Methods
Plant Materials and Growth Conditions
All lines used in this study were from the Columbia (Col-0) background. The seeds of hls1-1 (Lehman et al., 1996), pif4-2 (Ma et al., 2016), skip-1 (Wang et al., 2012), and 35S:MYC-HLS1/hls1-1 (Jin and Zhu, 2019) have been described previously. hls1-1 pif4-2 double mutants were generated through genetic crossing. Seeds were sterilized with 10% sodium hypochlorite and 0.1% Triton X-100 for 5 min, rinsed five times with sterile water and then placed on Murashige and Skoog (MS) medium plates (4.4 g/l MS salt, 1.5% sucrose [pH 5.8], 1% agar) for phenotypic observation. After stratification at 4°C for 3 days, seedlings were initially grown at 22°C for 3 days and then transferred to 28°C for an additional 4 days. The photoperiod conditions were 16 h light (70 μmol m−2 s−1 white light)/8 h dark. Hypocotyls were imaged under a stereomicroscope and then measured using ImageJ software (http://rsbweb.nih.gov/ij/).
RNA Extraction and RNA-Seq Analysis
The raw RNA-seq data for Col-0 and the hls1-1 mutants have been reported (Jin and Zhu, 2019) and deposited at the Genome Sequence Archive at the BIG Data Center (http://bigd.big.ac.cn/gsa/) (accession number CRA001128). In this study, we performed RNA-seq analysis again for the pif4-2 mutants with identical treatment and analysis methods. Two independent biological replicates were included. pif4-2 seedlings were initially grown at 22°C under 70 μmol m−2 s−1 of white light for 3 days and then transferred to 28°C (28°C treatment) or kept at 22°C (22°C treatment) for an additional 4 days. Total RNA was extracted according to the PureLink RNA Mini Kit (Invitrogen) protocol. Further RNA quality checks, library construction and sequencing were performed in GENEWIZ (Suzhou, China). In brief, the quantity and quality of the total RNA of each sample were determined by using an Agilent Bioanalyzer 2100. One microgram of total RNA with an RIN above 7 was used for subsequent library preparation. Next-generation sequencing library preparations were constructed according to the manufacturer's protocol for the NEBNext Ultra RNA Library Prep Kit for Illumina. Poly(A) mRNA isolation was performed using the NEBNext Poly(A) mRNA Magnetic Isolation Module. mRNA fragmentation and priming were performed using NEBNext First Strand Synthesis Reaction Buffer and NEBNext Random Primers. First-strand cDNA was synthesized using ProtoScript II Reverse Transcriptase, and second-strand cDNA was synthesized using Second Strand Synthesis Enzyme Mix. The purified double-stranded cDNA was then treated with End Prep Enzyme Mix to repair both ends and add a dA-tail in one reaction, followed by TA ligation to add adaptors to both ends. The size selection of adaptor-ligated DNA was then performed using AxyPrep Mag PCR Clean-up, and fragments of ∼360 bp (with an approximate insert size of 300 bp) were recovered. The PCR products were cleaned up, validated, and quantified using a Qubit 2.0 Fluorometer. Then, libraries with different indices were multiplexed and loaded into an Illumina HiSeq instrument according to the manufacturer's instructions. Sequencing was carried out using a 2 × 150-bp paired end configuration; image analysis and base calling were conducted with HiSeq Control Software (HCS) + OLB + GAPipeline-1.6 (Illumina) in the HiSeq instrument. Genes with a fold change >2 (28°C vs 22°C) and q < 0.05 were defined as DEGs.
Differential Alternative Splicing Analysis
We extracted, quantified, and compared alternative splicing events from the RNA-seq data using the ASprofile program (version 1.0) (Florea et al., 2013), in which a GTF transcript file created by Cufflinks was used as the input. Then, we analyzed differential alternative splicing events using rMATS software (Park et al., 2013, Shen et al., 2014). The alternative splicing events included A5′SS, A3′SS, MXE, intron retention, and exon skipping. DASGs were defined according to a false discovery rate ≤ 0.05 and inclusion level difference > 0.02 and were then statistically analyzed.
GO Enrichment Analyses
The functional enrichment of DEGs and DASGs were analyzed at AgriGO (http://systemsbiology.cau.edu.cn/agriGOv2/index.php) (Du et al., 2010).
qRT–PCR Assay and RT–PCR
To detect PIF4 or YUCCA8 (YUC8) expression, 4-day-old seedlings grown at 22°C under continuous white light conditions (40 μmol m−2 s−1) were transferred to 28°C or were continually maintained at 22°C for various lengths of time. After RNA extraction and reverse transcription, the relative expression levels of PIF4 or YUC8 were determined through qRT–PCR (primers are listed in Supplemental Table 6). To verify the DEG results from the RNA-seq datasheet, RNA from Col-0, pif4-2, or hls1-1 plants grown under the same growth conditions described in the RNA-seq section was extracted with TRIzol Reagent (Invitrogen). After reverse transcription, qPCR was performed to determine relative expression levels (primers are listed in Supplemental Table 6). For the verification of DASGs, RNA was extracted from Col-0, pif4-2, hls1-1, or skip-1 plants grown under the same growth conditions described in the RNA-seq section. After reverse transcription, the RT–PCR products (30 cycles) were directly loaded onto agarose gels to separate different bands (primers are listed in Supplemental Table 6).
ChIP–PCR
ChIP–PCR was performed as described previously (Zhang et al., 2014). Three-gram samples of young leaves from 2-week-old Col-0 or 35S:MYC-HLS1/hls1-1 plants were subjected to crosslinking in 1% formaldehyde, and chromatin was isolated according to a standard protocol. An anti-Myc antibody (Abcam) was added to the sonicated chromatin, followed by incubation overnight to precipitate bound DNA fragments. After immobilization using Protein A-Agarose (Santa Cruz), bound DNA was eluted to detect relative enrichment levels through normalization against the input controls. The primers used for ChIP–PCR were as follows: YUC8 (a)-F: ACAACTCTAAGCAATCATAC, YUC8 (a)-R: ATTTGTTTGGAGGTTACAATG; YUC8 (b)-F: GGAATGGGTTTGATGTGGAATTC and YUC8 (b)-R: AGTGATGGAATTAGGGAGCAAC.
Yeast Two-Hybrid Assay
For the yeast two-hybrid assay, the coding sequence of HLS1 was cloned into the pGBKT7 vector as bait, while constructs of the individual coding sequences of PIF1/3/4/5 in the pGADT7 vector were used as prey. Each bait–prey plasmid pair was cotransformed into the yeast AH109 strain according to the Yeast Protocols Handbook (Clontech). Protein–protein interactions were identified from the yeast transformants streaked on SD/-Trp-Leu-His-Ade dropout plates.
Luciferase Complementation Imaging Assay
The coding sequences of HLS1 and PIF1/PIF3/PIF4/PIF5 were PCR amplified and cloned into the pCAMBIA1390-nLUC and pCAMBIA1390-cLUC vectors, respectively. The plasmids were first transformed into Agrobacterium tumefaciens strain GV3101. Then, the indicated A. tumefaciens cultures were combined and infiltrated into Nicotiana benthamiana leaves according to a standard protocol (Sun et al., 2017).
Statistical Analysis
Tukey's least significant difference (LSD) test was performed with STATISTICA software. Regarding the parameter settings, a 99% confidence interval was used for evaluating differences and p values.
Funding
This work was supported by the National Natural Science Foundation of China (31970256), the Fok Ying Tong Education Foundation (161023), the Fundamental Research Funds for the Central Universities (lzujbky-2019-kb05), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author Contributions
H.J. performed RNA-seq and generated hls1 pif4 double mutants. J.L. carried out RT–PCR and observed phenotypes. All authors analyzed the data. Z.Z. wrote the paper.
Acknowledgments
We thank Dr. Ligeng Ma for the skip-1 seeds, Dr. Hongtao Liu for the pif4-2 seeds, and Dr. Shan Lu for the pCAMBIA1390 vectors. No conflict of interest declared.
Published: February 19, 2020
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and IPPE, CAS.
Supplemental Information is available at Plant Communications Online.
Accession Numbers
The RNA-seq data for pif4-2 reported in this work are deposited at the BIG Data Center (http://bigd.big.ac.cn/gsa/) under accession number CRA001535.
Supplemental Information
References
- An F., Zhang X., Zhu Z., Ji Y., He W., Jiang Z., Li M., Guo H. Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell Res. 2012;22:915–927. doi: 10.1038/cr.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A.J., McLachlan D.H., Hetherington A.M., Franklin K.A. High temperature exposure increases plant cooling capacity. Curr. Biol. 2012;22:R396–R397. doi: 10.1016/j.cub.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Du Z., Zhou X., Ling Y., Zhang Z., Su Z. agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010;38:64–70. doi: 10.1093/nar/gkq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Wang Q., Ziegler S.F., Zhou B. FOXP3 interacts with hnRNPF to modulate pre-mRNA alternative splicing. J. Biol. Chem. 2018;293:10235–10244. doi: 10.1074/jbc.RA117.001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Li J., Gao Z., Lu Y., Yu J., Zheng Q., Yan S., Zhang W., He H., Ma L. SKIP confers osmotic tolerance during salt stress by controlling alternative gene splicing in Arabidopsis. Mol. Plant. 2015;8:1038–1052. doi: 10.1016/j.molp.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Florea L., Song L., Salzberg S.L. Thousands of exon skipping events differentiate among splicing patterns in sixteen human tissues. F1000Res. 2013;2:188. doi: 10.12688/f1000research.2-188.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K.A., Lee S.H., Patel D., Kumar S.V., Spartz A.K., Gu C., Ye S., Yu P., Breen G., Cohen J.D. Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc. Natl. Acad. Sci. U S A. 2011;108:20231–20235. doi: 10.1073/pnas.1110682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangappa S.N., Berriri S., Kumar S.V. PIF4 coordinates thermosensory growth and immunity in Arabidopsis. Curr. Biol. 2017;27:243–249. doi: 10.1016/j.cub.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W.M., Ostin A., Sandberg G., Romano C.P., Estelle M. High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc. Natl. Acad. Sci. U S A. 1998;95:7197–7202. doi: 10.1073/pnas.95.12.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman P., Ecker J.R. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huai J., Zhang X., Li J., Ma T., Zha P., Jing Y., Lin R. SEUSS and PIF4 coordinately regulate light and temperature signaling pathways to control plant growth. Mol. Plant. 2018;11:928–942. doi: 10.1016/j.molp.2018.04.005. [DOI] [PubMed] [Google Scholar]
- James A.B., Calixto C.P.G., Tzioutziou N.A., Guo W., Zhang R., Simpson C.G., Jiang W., Nimmo G.A., Brown J.W.S., Nimmo H.G. How does temperature affect splicing events? Isoform switching of splicing factors regulates splicing of LATE ELONGATED HYPOCOTYL (LHY) Plant Cell Environ. 2018;41:1539–1550. doi: 10.1111/pce.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Zhu Z. HOOKLESS1 is a positive regulator in Arabidopsis thermomorphogenesis. Sci. China Life Sci. 2019;62:423–425. doi: 10.1007/s11427-018-9418-2. [DOI] [PubMed] [Google Scholar]
- Jin H., Zhu Z. Dark, light and temperature: key players in plant morphogenesis. Plant Physiol. 2019;180:1793–1802. doi: 10.1104/pp.19.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.H., Domijan M., Klose C., Biswas S., Ezer D., Gao M., Khattak A.K., Box M.S., Charoensawan V., Cortijo S. Phytochromes function as thermosensors in Arabidopsis. Science. 2016;354:886–889. doi: 10.1126/science.aaf6005. [DOI] [PubMed] [Google Scholar]
- Koini M.A., Alvey L., Allen T., Tilley C.A., Harberd N.P., Whitelam G.C., Franklin K.A. High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr. Biol. 2009;19:408–413. doi: 10.1016/j.cub.2009.01.046. [DOI] [PubMed] [Google Scholar]
- Kornblihtt A.R., Schor I.E., Allo M., Dujardin G., Petrillo E., Munoz M.J. Alternative splicing: a pivotal step between eukaryotic transcription and translation. Nat. Rev. Mol. Cell Biol. 2013;14:153–165. doi: 10.1038/nrm3525. [DOI] [PubMed] [Google Scholar]
- Kumar S.V., Lucyshyn D., Jaeger K.E., Alos E., Alvey E., Harberd N.P., Wigge P.A. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature. 2012;484:242–245. doi: 10.1038/nature10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O.S., Song Z., Zhou Z., Davies K.A., Chang J., Yang X., Wang S., Lucyshyn D., Tay I.H.Z., Wigge P.A. Direct control of SPEECHLESS by PIF4 in the high-temperature response of stomatal development. Curr. Biol. 2018;28:1273–1280.e3. doi: 10.1016/j.cub.2018.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Ryu H.S., Chung K.S., Pose D., Kim S., Schmid M., Ahn J.H. Regulation of temperature-responsive flowering by MADS-box transcription factor repressors. Science. 2013;342:628–632. doi: 10.1126/science.1241097. [DOI] [PubMed] [Google Scholar]
- Legris M., Klose C., Burgie E.S., Rojas C.C., Neme M., Hiltbrunner A., Wigge P.A., Schafer E., Vierstra R.D., Casal J.J. Phytochrome B integrates light and temperature signals in Arabidopsis. Science. 2016;354:897–900. doi: 10.1126/science.aaf5656. [DOI] [PubMed] [Google Scholar]
- Lehman A., Black R., Ecker J.R. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- Li H., Johnson P., Stepanova A., Alonso J.M., Ecker J.R. Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev. Cell. 2004;7:193–204. doi: 10.1016/j.devcel.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Liao C.J., Lai Z., Lee S., Yun D.J., Mengiste T. Arabidopsis HOOKLESS1 regulates responses to pathogens and abscisic acid through interaction with MED18 and acetylation of WRKY33 and ABI5 chromatin. Plant Cell. 2016;28:1662–1681. doi: 10.1105/tpc.16.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu M., Shi H., Li Y., Kuang K., Yang Z., Li J., Chen D., Li Y., Kou X., Zhong S. Oligomerization and photo-deoligomerization of HOOKLESS1 controls plant differential cell growth. Dev. Cell. 2019;51:78–88.e3. doi: 10.1016/j.devcel.2019.08.007. [DOI] [PubMed] [Google Scholar]
- Ma D., Li X., Guo Y., Chu J., Fang S., Yan C., Noel J.P., Liu H. Cryptochrome 1 interacts with PIF4 to regulate high temperature-mediated hypocotyl elongation in response to blue light. Proc. Natl. Acad. Sci. U S A. 2016;113:224–229. doi: 10.1073/pnas.1511437113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E., Zhu J.Y., Wang Z.Y. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 2012;14:802–809. doi: 10.1038/ncb2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto M.A., Hayashi S., Sawa S., Hashimoto-Ohta A., Nakamura K. Involvement of HLS1 in sugar and auxin signaling in Arabidopsis leaves. Plant Cell Physiol. 2006;47:1603–1611. doi: 10.1093/pcp/pcl027. [DOI] [PubMed] [Google Scholar]
- Park J.W., Tokheim C., Shen S., Xing Y. Identifying differential alternative splicing events from RNA sequencing data using RNASeq-MATS. Methods Mol. Biol. 2013;1038:171–179. doi: 10.1007/978-1-62703-514-9_10. [DOI] [PubMed] [Google Scholar]
- Pose D., Verhage L., Ott F., Yant L., Mathieu J., Angenent G.C., Immink R.G., Schmid M. Temperature-dependent regulation of flowering by antagonistic FLM variants. Nature. 2013;503:414–417. doi: 10.1038/nature12633. [DOI] [PubMed] [Google Scholar]
- Quint M., Delker C., Franklin K.A., Wigge P.A., Halliday K.J., van Zanten M. Molecular and genetic control of plant thermomorphogenesis. Nat. Plants. 2016;2:15190. doi: 10.1038/nplants.2015.190. [DOI] [PubMed] [Google Scholar]
- Shen S., Park J.W., Lu Z.X., Lin L., Henry M.D., Wu Y.N., Zhou Q., Xing Y. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. U S A. 2014;111:E5593–E5601. doi: 10.1073/pnas.1419161111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata H., Hanada K., Ushijima T., Nakashima M., Suzuki Y., Matsushita T. Phytochrome controls alternative splicing to mediate light responses in Arabidopsis. Proc. Natl. Acad. Sci. U S A. 2014;111:18781–18786. doi: 10.1073/pnas.1407147112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Qi L., Li Y., Chu J., Li C. PIF4-mediated activation of YUCCA8 expression integrates temperature into the auxin pathway in regulating Arabidopsis hypocotyl growth. PLoS Genet. 2012;8:e1002594. doi: 10.1371/journal.pgen.1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Zheng Y., Zhu Z. Luciferase complementation imaging assay in Nicotiana benthamiana leaves for transiently determining protein-protein interaction dynamics. J. Vis. Exp. 2017 doi: 10.3791/56641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasset C., Singh Yadav A., Sureshkumar S., Singh R., van der Woude L., Nekrasov M., Tremethick D., van Zanten M., Balasubramanian S. POWERDRESS-mediated histone deacetylation is essential for thermomorphogenesis in Arabidopsis thaliana. PLoS Genet. 2018;14:e1007280. doi: 10.1371/journal.pgen.1007280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F., Petrasek J., Zadnikova P., Hoyerova K., Pesek B., Raz V., Swarup R., Bennett M., Zazimalova E., Benkova E. The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development. 2010;137:597–606. doi: 10.1242/dev.040790. [DOI] [PubMed] [Google Scholar]
- Wan R. A key component of gene expression, revealed. Science. 2018;362:904. doi: 10.1126/science.aav6875. [DOI] [PubMed] [Google Scholar]
- Wang B.B., Brendel V. The ASRG database: identification and survey of Arabidopsis thaliana genes involved in pre-mRNA splicing. Genome Biol. 2004;5:R102. doi: 10.1186/gb-2004-5-12-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wu F., Xie Q., Wang H., Wang Y., Yue Y., Gahura O., Ma S., Liu L., Cao Y. SKIP is a component of the spliceosome linking alternative splicing and the circadian clock in Arabidopsis. Plant Cell. 2012;24:3278–3295. doi: 10.1105/tpc.112.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhu Z., An F., Hao D., Li P., Song J., Yi C., Guo H. Jasmonate-activated MYC2 represses ETHYLENE INSENSITIVE3 activity to antagonize ethylene-promoted apical hook formation in Arabidopsis. Plant Cell. 2014;26:1105–1117. doi: 10.1105/tpc.113.122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.