Abstract
Plants have developed various mechanisms for avoiding pathogen invasion, including resistance (R) genes. Most R genes encode nucleotide-binding domain and leucine-rich repeat containing proteins (NLRs). Here, we report the isolation of three new bacterial blight R genes in rice, Xa1-2, Xa14, and Xa31(t), which were allelic to Xa1 and encoded atypical NLRs with unique central tandem repeats (CTRs). We also found that Xa31(t) was the same gene as Xa1-2. Although Xa1-2 and Xa14 conferred different resistance spectra, their performance could be attenuated by iTALEs, as has previously been reported for Xa1. XA1, XA1-2, XA14, and non-resistant RGAF differed mainly in the substructure of the leucine-rich repeat domain. They all contained unique CTRs and belonged to the CTR-NLRs, which existed only in Gramineae. We also found that interactions among these genes led to differing resistance performance. In conclusion, our results uncover a unique locus in rice consisting of at least three multiple alleles (Xa1, Xa1-2, and Xa14) that encode CTR-NLRs and confer resistance to Xanthomonas oryzae pv. oryzae (Xoo).
Keywords: multiple alleles, NLR, resistance, Xanthomonas oryzae pv. oryzae, iTALE, CTR
This study shows that Xa1-2, Xa14, and Xa31(t) are allelic to Xa1 and that Xa31(t) is the same gene as Xa1-2. Like Xa1 resistance, the resistance conferred by Xa1-2 and Xa14 can be attenuated by iTALEs, but their resistance spectra are different. Xa1, Xa1-2, and Xa14 encode CTR-NLRs, which are only found in Gramineae, and complex interactions among them lead to differing resistance performance.
Introduction
Plant diseases cause serious losses of biomass each year worldwide, leading to reduced crop yields and economic distress for farmers. Over time, plants have developed different survival mechanisms, such as resistance (R) genes. Most reported R genes encode nucleotide-binding domain and leucine-rich repeat containing proteins (NLRs) (Mermigka et al., 2020), which are typically classified into two groups based on the structure of their N-terminal sequences. The TNL (TIR-NLR) group contains a Toll/interleukin receptor (TIR) domain, whereas the CNL (CC-NLR) group contains a coiled-coil (CC) motif (van Wersch et al., 2020). The Arabidopsis genome has approximately 150 NLR-encoding genes, two-thirds of them encode TNLs and one-third encodes CNLs (Meyers et al., 2003). TNLs are absent from the genomes of many monocots (Jacob et al., 2013). In the rice genome, for instance, most of the 500+ NLR genes encode CNLs, and none encode TNLs (Meyers et al., 2005). However, recent studies have reported that many NLRs have additional integrated domains, such as the WRKY, kinase, and BED finger domains (Le Roux et al., 2015; Kroj et al., 2016; Bailey et al., 2018). Yr7, Yr5, and YrSP are recently cloned yellow rust resistance genes from hexaploid wheat (Triticum aestivum), all of which encode BED-domain-containing NLRs (BED-NLRs) (Marchal et al., 2018).
Many NLRs play important roles in rice defense, especially against fungi and insects. In interactions with Magnaporthe oryzae, 22 of the 26 isolated R genes encode NLRs (Fukuoka et al., 2014; Xu et al., 2014; Chen et al., 2015; Ma et al., 2015; Deng et al., 2017). Another nine NLR-encoding R genes have been isolated during rice–planthopper interactions (Du et al., 2009; Zhao et al., 2016). However, the interactions between rice and Xanthomonas oryzae pv. oryzae (Xoo), a pathogenic bacterium that causes bacterial blight, seem to be unique (Zhang and Wang, 2013). To date, 11 bacterial blight R genes have been isolated: Xa1, Xa3/Xa26, Xa4, xa5, Xa10, xa13, Xa21, Xa23, xa25, Xa27, and xa41(t) (Yoshimura et al., 1998; Zhang and Wang, 2013; Tian et al., 2014; Hutin et al., 2015; Wang et al., 2015; Hu et al., 2017). These genes encode several different types of proteins that are involved in various aspects of rice-Xoo interactions (Zhang and Wang, 2013). Surprisingly, except for three receptor-like kinase-encoding bacterial blight R genes, the remaining eight genes are all related to transcription activator-like effectors (TALEs), which are secreted by Xanthomonas and Ralstonia solanacearum (Song et al., 1995; Sun et al., 2004; Zhang and Wang, 2013; Ji et al., 2016; Hu et al., 2017). TALEs can be translocated into plant cells via the type III secretion system (T3SS). Then, they directly bind to effector binding elements (EBEs), also called upregulated by TALE (UPT) boxes, in host genomic DNA by their central repeat region and promote gene expression (Boch et al., 2009; Romer et al., 2009; Antony et al., 2010). Three Xoo TALEs, AvrXa27, AvrXa10, and AvrXa23, bind to the EBEs of rice executor R genes Xa27, Xa10, and Xa23 (Gu et al., 2005; Tian et al., 2014; Wang et al., 2015). Mutations in the EBEs of xa13, xa25, and xa41(t) retard interactions with Xoo TALEs PthXo1, PthXo2, AvrXa7, and Tal5 (Chu et al., 2006; Yang et al., 2006; Liu et al., 2011; Yuan et al., 2011; Hutin et al., 2015; Zhou et al., 2015). The recessive gene xa5 encodes TFIIAγ5V39E and attenuates the TALE-induced expression of rice genes (Gu et al., 2009; Yuan et al., 2016). The most interesting gene is Xa1, a BED-NLR, which recognizes multiple different TALEs and confers resistance (Yoshimura et al., 1998; Ji et al., 2016; Marchal et al., 2018). It has been reported that many full-length TALEs can trigger Xa1-mediated resistance. Meanwhile, interfering TAL effectors (iTALEs), in which Their N-terminals and transcription activation domains contain some deletions, can interfere with Xa1-mediated resistance. iTALEs exist in many Xoo and Xoc strains and may be responsible for the current narrow spectrum of Xa1 (Ji et al., 2016).
Previously, Xa2, Xa12, Xa14, Xa-25, Xa31(t), and Xa38 were reported to be localized to the region near Xa1 and to confer race-specific resistance to Xoo (Ogawa et al., 1978; Gao et al., 2005; He et al., 2006; Cheema et al., 2008; Wang et al., 2009; Bao et al., 2010; Ellur et al., 2016). Furthermore, the uncloned Xanthomonas oryzae pv. oryzicola (Xoc) R gene, Xo1, which recognizes TALEs in an activation-domain-independent manner and can be suppressed by truncated TALEs, has also been mapped to the Xa1 region (Read et al., 2016; Triplett et al., 2016). A recent report found two BED-NLR-encoding genes at the Xo1 locus, including one that was highly similar to Xa1 (Read et al., 2020). However, the relationships among these genes remain unclear.
Here, we report the isolation of three new allelic bacterial blight R genes, Xa1-2, Xa14, and Xa31(t), which are allelic to the previously isolated Xa1. Furthermore, we show that Xa1, Xa1-2, Xa14, and Xa31(t) all encode NLRs with unique central tandem repeats (CTRs), and that their homologs form a new class of NLR found only in Gramineae. We also demonstrate that interactions among Xa1, Xa1-2, and Xa14 play complex roles in rice disease resistance to Xoo.
Results
Xa14 Is Allelic to Xa1
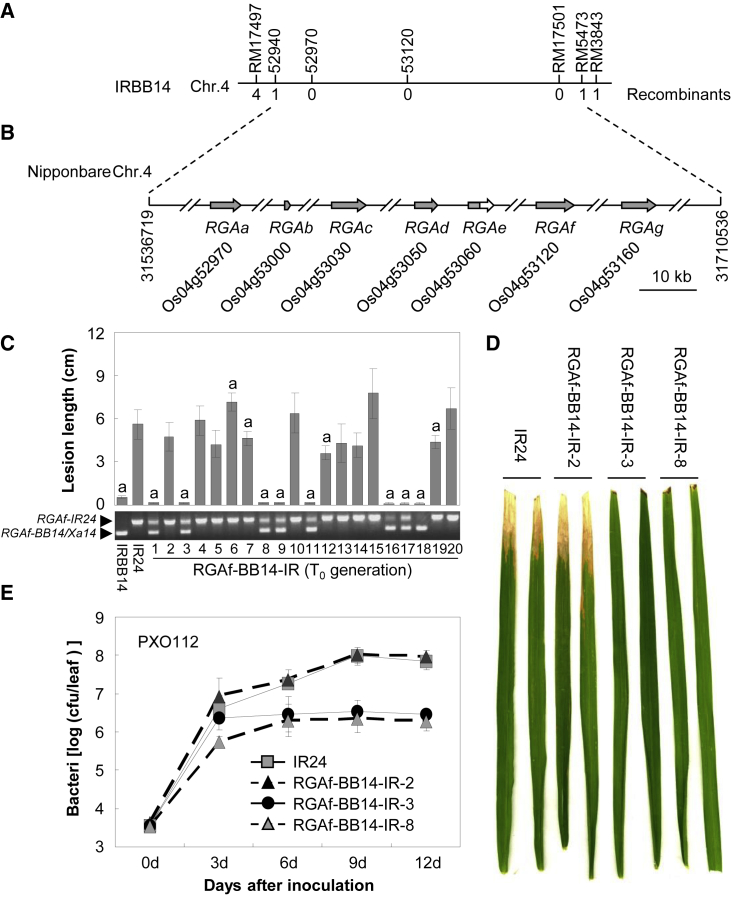
Previously, the Xa14 gene was mapped to a 300-kb region between markers HZR970-8 and HZR988-1 on chromosome 4 (Bao et al., 2010). To isolate Xa14, we inoculated an F2 population of 599 individuals derived from a cross between rice cultivars IRBB14 and IR24, which carry and lack Xa14, respectively, with the Xoo strain PXO112, which triggers Xa14-mediated resistance in rice. After lesion length analysis, Xa14 was further mapped between markers 52940 and RM5473 on chromosome 4 (Figure 1A). According to the reference sequence of the rice cultivar Nipponbare, there were 20 genes in this region, seven of which were predicted to encode NLRs (Figure 1B). Here, these genes were renamed R-gene analogs RGAa (Os04g52970), RGAb (Os04g53000), RGAc (Os04g53030), RGAd (Os04g53050), RGAe (Os04g53060), RGAf (Os04g53120), and RGAg (Os04g53160). RGAb and RGAe encoded proteins that contained only the leucine-rich repeat (LRR) domain. The other five genes encoded intact NLRs (Figure 1B). RGAa, RGAc, and RGAd encoded non-BED-NLRs, whereas RGAf and RGAg encoded BED-NLRs (Supplemental Figure 1A). Interestingly, both RGAf and RGAg were homologous to the isolated bacterial blight R gene Xa1 (Supplemental Figure 1B). The genomic sequence of RGAf showed 84.7% identity to that of Xa1, whereas the sequence of RGAg showed only 59.8% identity (Supplemental Figure 1C). The homology of the 1-kb upstream sequences between RGAf and Xa1 was 99.3%, but no significant homology was found in this region between RGAg and Xa1. These results suggest that RGAf was allelic to Xa1 and that RGAg was a paralog of Xa1.
Figure 1.
Mapping and Homology-Based Cloning of Xa14.
(A) Genetic map of the Xa14 locus.
(B) Physical map and candidate genes of the Xa14 locus in the corresponding region of Nipponbare. Arrows indicate RGA genes. The gray arrow/box indicates the coding region. The white arrow indicates the non-coding region.
(C) Lesion length analysis of RGAf-BB14-IR T0 plants after inoculation with the Xoo strain PXO112. Significant differences were detected between IR24 and transgenic plants or IRBB14 at P < 0.01 (indicated with “a”).
(D) Leaves of RGAf-BB14-IR lines inoculated with the Xoo strain PXO112.
(E) Bacterial growth ratio in RGAf-BB14-IR lines after inoculation with the Xoo strain PXO112. cfu, colony-forming units.
Data are presented as mean ± SD.
Further analysis showed that both RGAg-BB14 and RGAg-IR24, the alleles of RGAg in IRBB14 and IR24, respectively, encoded truncated proteins. A single base insertion (A) at position 4068 was found in the genomic sequence of RGAg-BB14, leading to a frame shift and a subsequent stop codon. In RGAg-IR24, the truncation was caused by the deletion of 3945 “T” (Supplemental Figure 2A). RGAf-IR24, the allele of RGAf in IR24, also encoded a truncated protein due to a 5845 “C” deletion in the genomic sequence (Supplemental Figure 2B). Only RGAf-BB14 encoded an intact NLR, and it showed 89.2% and 87.4% sequence identity to Xa1 and RGAf, respectively (Supplemental Figure 2B). Thus, RGAf-BB14 was the most likely Xa14 candidate gene.
To test whether RGAf-BB14 was Xa14, we transformed a native promoter-driven RGAf-BB14 gene into the cultivar IR24. After transformation, 55 independent T0 plants were generated and named RGAf-BB14-IR plants. These plants were inoculated with the Xoo strain PXO112 at the booting stage. All 26 positive plants showed significantly higher resistance compared with the negative plants and wild-type plants (Figure 1C, Supplemental Figure 3, and Supplemental Table 1). To further confirm the resistance, we inoculated three different T1 lines derived from positive PXO112-resistance T0 plants. Similarly, these positive T1 plants were significantly more resistant than negative plantsand wild-type plants (Supplemental Figure 4). Similar results were obtained with transgenic Zhonghua11 (ZH11) and Mudanjiang8 (MDJ8) cultivars, both of which lack Xa14 (Supplemental Figures 5 and 6; Supplemental Tables 2 and 3). These results strongly suggest that RGAf-BB14 was Xa14.
Figure 6.
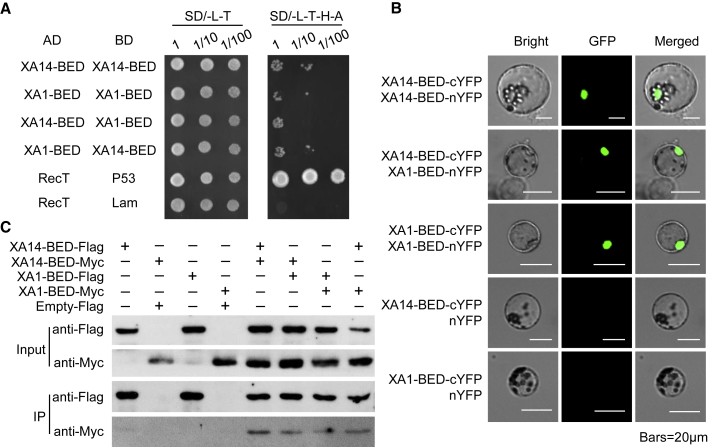
XA14-BED and XA1-BED Interact with Themselves and One Another.
(A) XA14-BED and XA1-BED interact with themselves and one another in yeast. RecT and P53 were used as positive controls, and RecT and Lam were used as negative controls.
(B) XA14-BED and XA1-BED interact with themselves and one another in rice protoplasts. cYFP, C-terminal portion of YFP; nYFP, N-terminal portion of YFP.
(C) Interactions between XA1-BED and XA14-BED are confirmed in a co-immunoprecipitation assay. IP, immunoprecipitation.
In addition, bacterial growth ratios were analyzed using two positive and one negative RGAf-BB14-IR T1 lines (Figure 1D). Bacterial growth in RGAf-BB14-IR-3 and RGAf-BB14-IR-8 was only 2.4%–21.2% of the wild-type plants at 6–12 days after infection, whereas bacterial growth in RGAf-BB14-IR-2 was 103.2%–138.9% of the wild type (Figure 1E). These results indicate that the resistance mediated by Xa14 was associated with reduced bacterial growth in plants.
Xa1-2 and Xa31(t) Are Also Allelic to Xa14 and Xa1
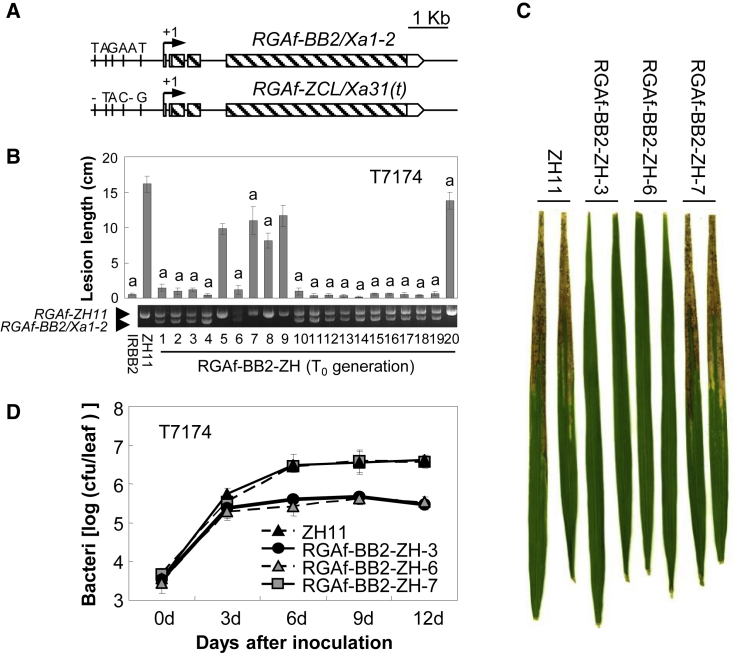
It has been reported that Xa2 and Xa31(t) are also localized to a region near Xa1 or Xa14 and that both confer race-specific resistance to Xoo (He et al., 2006; Wang et al., 2009). Therefore, Xa14 and Xa1 alleles in donors IRBB2 and Zhachanglong (ZCL) (referred to as RGAf-BB2 and RGAf-ZCL, respectively, hereafter) were likely Xa2 and Xa31(t) candidate genes. Surprisingly, the genomic sequences of RGAf-BB2 and RGAf-ZCL were completely identical. Only five polymorphisms, nucleotide deletions or substitutions, were identified more than 500 bp upstream of the predicted transcription start site in each gene (Figure 2A). Compared with the RGAf genes from cultivars MDJ8 and ZH11, designated RGAf-MDJ8 and RGAf-ZH11, respectively, the genomic sequence of RGAf-BB2 (or RGAf-ZCL) was more similar to those from Xa1 and Xa14 (Supplemental Figure 7A). The main difference between RGAf-BB2 and Xa1 was a 279-bp deletion in the LRR-encoding region, the remaining sequence of RGAf-BB2 showed 99.9% identity to that of Xa1 (Supplemental Figure 7B). Compared with Xa14, a low-homologous region existed in RGAf-BB2 but the two remaining regions showed 99.0% and 89.8% identity to the corresponding regions in Xa14 (Supplemental Figure 7B). These results indicate that RGAf-BB2 and RGAf-ZCL may confer Xoo resistance.
Figure 2.
Isolation of Xa1-2 and Xa31(t).
(A) Comparison between RGAf-BB2/Xa1-2 and RGAf-ZCL/Xa31(t) gene structures. Oblique lines indicate exons. Arrows indicate transcription start sites.
(B) Lesion length analysis of RGAf-BB2-ZH transgenic plants (T0 generation) after inoculation with the Xoo strain T7174. Significant differences were detected between ZH11 and transgenic plants or IRBB2 at P < 0.01 (indicated with “a”).
(C) Leaves of RGAf-BB2-ZH lines inoculated with the Xoo strain T7174.
(D) Bacterial growth ratio in RGAf-BB2-ZH lines after inoculation with the Xoo strain T7174. cfu, colony-forming units.
Data are represented as mean ± SD.
To investigate this possibility, we transformed a native promoter-driven RGAf-BB2 gene into cultivars ZH11 and MDJ8. The transgenic plants were named RGAf-BB2-ZH and RGAf-BB2-MDJ, respectively. Xa2 has previously been reported to confer resistance to the Xoo strain T7147, consistently, the IRBB2 plants obtained in this study showed resistance to T7174 (He et al., 2006). Additionally, in 30 RGAf-BB2-ZH T0 plants, 21 positive plants were highly resistant to T7174 compared with negative plants and wild-type plants (Figure 2B). The resistance was confirmed in three RGAf-BB2-ZH T1 lines (Supplemental Figure 8) and similar results were obtained with RGAf-BB2-MDJ plants (Supplemental Figure 9A and 9B). Furthermore, bacterial growth and lesion length were significantly reduced at 6–12 days in two positive T1 lines (Figure 2C and 2D). Although these results strongly suggest that RGAf-BB2 was the Xa2 gene, we named it Xa1-2 to avoid confusion.
Xa31(t) has previously been reported to mediate resistance to the Chinese Xoo strain OS105. Considering the sequence identity between RGAf-BB2 and RGAf-ZCL, IRBB2 plants were inoculated with OS105 to determine whether Xa1-2 could confer resistance. Indeed, IRBB2 plants were highly resistant to OS105 (Supplemental Figure 10A), and resistance was also present in RGAf-BB2-ZH-3 and RGAf-BB2-ZH-6 plants (Supplemental Figure 10B). These results strongly indicate that Xa31(t) and Xa1-2 were the same gene.
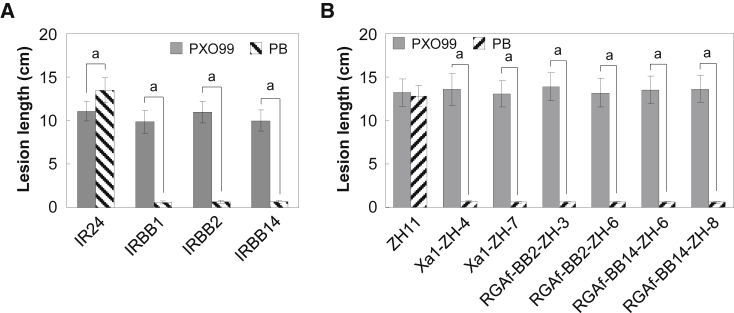
In addition, IRBB1 and IRBB14 plants were also resistant to OS105 (Supplemental Figure 10A). Transgenic plants carrying Xa1 in a ZH11 background (referred to as Xa1-ZH plants hereafter) and RGAf-BB14-ZH plants also showed OS105 resistance (Supplemental Figure 10B). Moreover, all of the Xa1-ZH, RGAf-BB2-ZH, and RGAf-BB14-ZH plants showed resistance to the Xoo strain T7174 (Supplemental Figures 10B, 11, and 12). RGAf-BB14-ZH and Xa1-ZH plants were resistant to T7133 whereas RGAf-BB2-ZH plants were as susceptible as ZH11 (Supplemental Figure 10B), and neither Xa1 nor Xa1-2 conferred resistance to PXO112 and PXO280 (Supplemental Figure 10A and 10B). These results indicate that Xa1, Xa1-2, and Xa14 each had a unique resistance spectrum.
iTALEs Suppress Xa1-2- and Xa14-Mediated Resistance
Xa1 has previously been reported to confer Xoo resistance by recognizing multiple full-length TALEs, and this resistance can be attenuated by some iTALEs, such as Tal3a and Tal3b in PXO99 (Ji et al., 2016). To determine whether Xa1-2 and Xa14 function in a similar way, we inoculated plants carrying these genes with PXO99 and PB, a mutant in which both Tal3a and Tal3b are knocked out (Ji et al., 2016). After inoculation with PXO99, IRBB2 and IRBB14 plants were as susceptible as IRBB1 and IR24 plants. By contrast, PB-inoculated IRBB2 and IRBB14 plants showed significant resistance, similar to that observed for the IRBB1 plants (Figure 3A). Moreover, similar results were obtained with transgenic plants that carried Xa1-2 and Xa14. In contrast to the PXO99 inoculation, the PB inoculation rendered RGAf-BB2-ZH and RGAf-BB14-ZH plants more resistant, similar to that observed for Xa1-ZH plants (Figure 3B and Supplemental Figure 13). These results strongly suggest that the resistance mediated by Xa1-2 and Xa14 could also be suppressed by iTALEs.
Figure 3.
Xa1-2- and Xa14-Mediated Resistance Can Be Attenuated by iTALEs.
(A) Lesion length analysis of IR24, IRBB1, IRBB2, and IRBB14 plants after inoculation.
(B) Lesion length analysis of ZH11 and transgenic lines carrying Xa1, Xa1-2, and Xa14 after inoculation.
All the plants were inoculated with Xoo strains PXO99 and PB, a mutant in which both iTALEs are knocked out. The letter “a” indicates a significant difference between the two treatments at P < 0.01.
The LRR Domains in XA1, XA1-2, XA14, and RGAF Proteins Show Different Substructures
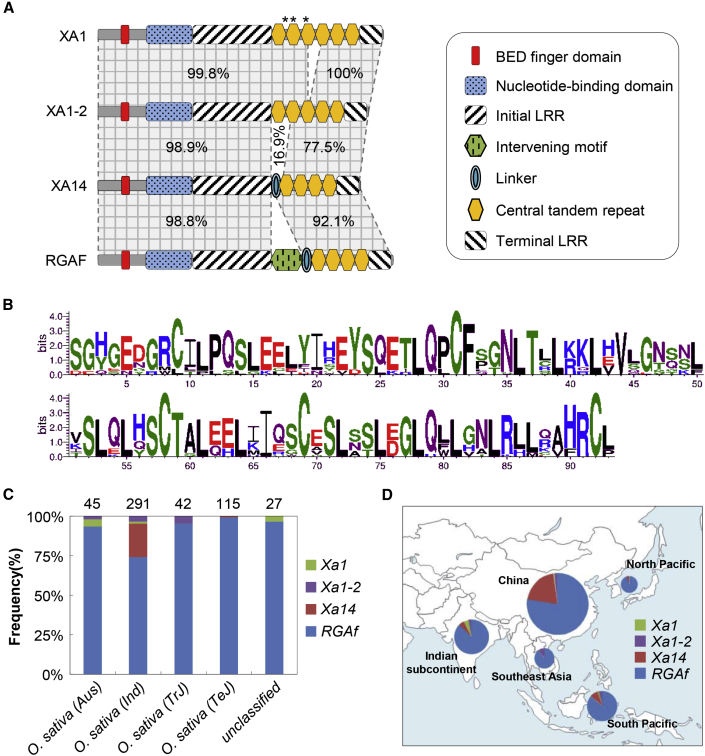
Because some deletions/insertions were found in the genomic sequences of Xa1, Xa1-2, Xa14, and RGAf, their protein structures were analyzed to identify motifs that were essential for resistance. The N-terminal sequences of the four proteins were highly conserved, and all consisted of a BED finger domain and a nucleotide-binding (NB) domain (Figure 4A). Interestingly, the LRR domains of the four proteins all contained nearly perfect tandem repeats, named CTRs here. Each CTR in the four proteins was composed of 93 highly conserved amino acids (Figure 4B) but the repeat numbers varied from four to six (Figure 4A). XA1 was found to have six repeats, R1 to R6. Compared with XA1, a deletion of a whole repeat was found in XA1-2 (Figure 4A; Supplemental Figures 14 and 15), and XA14 and RGAF both contained only four repeats (Figure 4A). In addition to the repeats, all proteins contained homologous regions in the N and C termini of the LRR domains, designated here the Initial and Terminal LRR, respectively (Figure 4A). XA14 contained an additional motif between the Initial LRR and CTRs, designated the Linker, that was not present in XA1 and XA1-2 (Figure 4A and Supplemental Figure 16). RGAF contained an identical Linker compared with XA14 but a unique motif not found in the other three R proteins; this motif was designated the intervening motif (Figure 4A and Supplemental Figure 17). Further analysis showed that the intervening motif was also present in RGAF-IR24, RGAF-MDJ8, and RGAF-ZH11, which were encoded by other non-resistant alleles (Supplemental Figure 18). Very recently, a study described the CTRs of XA1, RGAF, and CGS-Xo111 (Read et al., 2020). In this work, the CTRs were described as beginning at a different amino acid residue. According to our results, they belonged to the Initial LRR and were separated by the Linker or intervening motif in XA14 and RGAF. Our CTR classification was based on substructural comparisons among the four proteins and is thus more conducive to application.
Figure 4.
Different Substructures and Distributions of XA1, XA1-2, XA14, and RGAF.
(A) Domains and motifs in XA1, XA1-2, XA14, and RGAF. Percentages indicate sequence identity. Stars indicate missense mutations.
(B) Conserved amino acid residues in the CTRs of the four proteins shown by WebLogos.
(C) Ratios of Xa1, Xa1-2, Xa14, and RGAf alleles in the rice variety collection. Numbers above the columns indicate the total accession numbers of the corresponding subpopulation.
(D) Geographic distribution of Xa1, Xa1-2, Xa14, and RGAf haplotypes in tropical and temperate regions in Asia. Relative frequencies of the haplotypes in each area are represented by the colored sectors of the pie chart. The size of the pie chart is proportional to the number of varieties.
To understand the geographic distributions and relationships among different alleles, we analyzed the frequency of each allele in a collection of 520 rice cultivars using PCR markers. Most accessions were of the RGAf type, followed by the Xa14 type, which was especially common in indica rice. Both the Xa1 and Xa1-2 types were rare (Figure 4C and Supplemental Table 4) but their frequencies varied among different geographic areas. Interestingly, approximately one-fifth of the accessions from China were of the Xa14 type. In addition, several accessions from the Indian subcontinent and South Pacific area were of the Xa14 type (Figure 4D, Supplemental Figure 19, and Supplemental Table 4). The Xa1 type had a relatively higher frequency in the United States and the Indian subcontinent than other areas, and the Xa1-2 type was more frequently found in the United States and Southeast Asia. Also, in some areas such as Africa and Western Europe, all accessions were of the RGAf type (Figure 4D, Supplemental Figure 19, and Supplemental Table 4). These results suggest that these alleles were selected differentially in different regions, a phenomenon that may be partly related to the different geographic distributions of various Xoo strains.
XA1, XA1-2, XA14, and RGAF Belong to a Unique NLR Class Specific to Gramineae
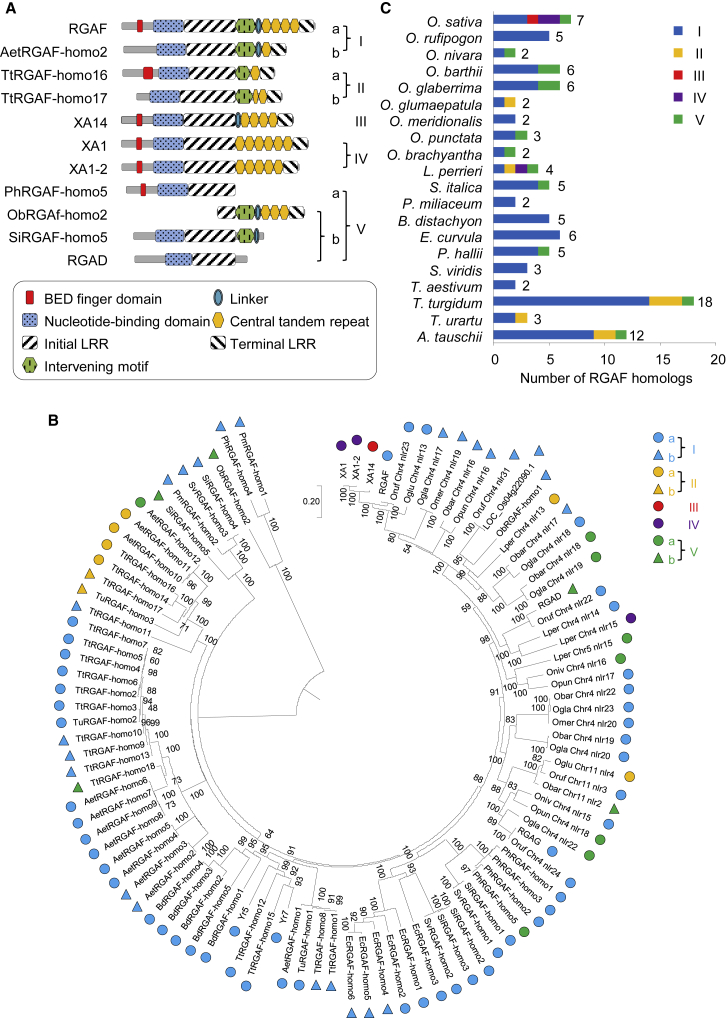
To understand the evolutionary history of XA1, XA1-2, XA14, and RGAF, we searched for homologous proteins using protein BLAST at NCBI and the MSU Rice Genome Annotation Project (RGAP) Database, as well as sequences obtained from relevant articles. In Nipponbare, only RGAG, RGAD, and LOC_Os04g22090.1 were classified as homologs. Other RGA proteins at the Xa1 locus failed to show high enough homology to RGAF (Supplemental Figure 20). Orthologs of the RGA proteins in IR24, IRBB1, and IRBB14 were not included, as they were highly conserved relative to corresponding proteins in Nipponbare and were not representative, with the exception of XA1, XA1-2, and XA14. We ultimately obtained 100 homologs, including RGAF. Surprisingly, all homologs were from Gramineae (Supplemental Table 5).
The homologs could be classified into five groups based on full-length protein-based phylogeny and substructure analysis (Figure 5A and 5B; Supplemental Table 6). Three-fourths of them belonged to group I, which contained all motifs, including Initial LRR, intervening motif, Linker, CTRs and Terminal LRR, in their LLR regions, such as RGAF (Figure 5A–5C). NLRs encoded by yellow rust resistance genes Yr5 and Yr7 in Triticum aestivum also belonged to group I (Figure 5B). Group II was composed of eight homologs without the Linker in their LRR domains. Among the eight group II homologs, six came from Triticum turgidum, Triticum urartu, and Aegilops tauschii, which are genome donors of common wheat, one from Oryza glumaepatula, a kind of wild rice, and one from Leersia perrieri, the nearest outgroup of genus Oryza (Figure 5B and 5C). XA14, which did not contain the intervening motif, was the only protein in group III (Figure 5A and 5B). XA1 and XA1-2 belonged to group IV, whose members contained neither the Linker nor the intervening motif in the LRR region (Figure 5A). One homolog from L. perrieri was also included in this group (Figure 5B). Group V was composed of different types of truncated proteins from various species (Figure 5A–5C). Notably, almost all members of groups II, III, and IV appeared later than group I members in the same clade, suggesting that they may have arisen from group I homologs as a result of motif deletions (Figure 5B).
Figure 5.
RGAF Homologs Existed only in Gramineae.
RGAF homologs could be clustered into five groups, denoted I–V. Subgroups are indicated by a and b.
(A) Classification of RGAF homologs.
(B) Full-length protein-based phylogeny of RGAF homologs identified by BLAST as described in Methods. Numbers on the interior branches indicate the bootstrap support values (%; only values greater than 50% are shown) from 1000 replicates.
(C) Number of RGAF-homolog in each group in different Gramineae species. Numbers on the right of the columns indicate numbers of RGAF homologs.
Notably, the BED finger domain in RGAF, XA1, XA1-2, and XA14 was missing from approximately one-third of the RGAF homologs. Thus, groups I, II, and V could be further divided into two subgroups, a and b, based on the presence or absence of the BED finger domain (Figure 5A and 5B; Supplemental Table 6). Except for members from group V, all other homologs contained the Initial LRR, Terminal LRR, and CTRs, which varied in number from one to nine units, and showed high levels of sequence conservation in CTRs (Supplemental Table 7). These results suggest that the substructure of LRR is essential and conserved during evolution. Thus, all CTR-containing homologs could be classified as CTR-NLRs. Together, these results clearly demonstrate that CTR-NLRs constitute a unique class of NLRs in Gramineae.
Interactions among Xa1, Xa1-2, and Xa14 Lead to Differences in Rice Disease Resistance
Dimerization or oligomerization through the TIR or CC domain has been observed in some NLRs (Bernoux et al., 2011; Maekawa et al., 2011; Deng et al., 2017; Wang et al., 2019), however, whether BED-NLRs, such as XA1, XA1-2, and XA14, interact with themselves or with one another remain elusive. In yeast, the BED finger domain of XA1 and XA14, designated XA1-BED and XA14-BED, respectively, could interact with themselves or with one another (Figure 6A and Supplemental Figure 21A). However, these interactions were weak and could be disrupted by the NB domain (Supplemental Figure 21B–21D). Similar interactions were found in rice protoplasts, and all interactions appeared to occur in the nuclei (Figure 6B). To further confirm these results, we expressed XA1-BED and XA14-BED labeled with 3×FLAG or 9×Myc in tobacco leaves for co-immunoprecipitation. The results show that XA1-BED and XA14-BED could form both homo- and heterocomplexes (Figure 6C). Given that the BED finger domain in XA1-2 (XA1-2-BED) is identical to that in XA1-BED, similar interactions may exist among XA1-BED, XA1-2-BED, and XA14-BED.
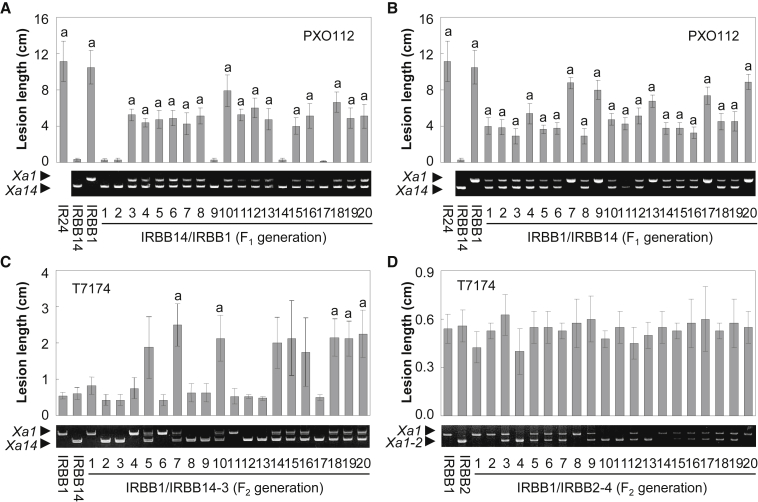
Further analysis using hybrids derived from IRBB1, IRBB2, and IRBB14 showed that these interactions could impede rice disease resistance. The IRBB14/IRBB1 hybrid was more susceptible to the Xoo strain PXO112 compared with the IRBB14 parent (Figure 7A). Similar results were obtained with the reciprocal hybrid IRBB1/IRBB14 (Figure 7B) and the F2 plants derived from both the IRBB14/IRBB1 and IRBB1/IRBB14 crosses (Supplemental Figure 22). Next, the IRBB1/IRBB14 F2 individuals were inoculated with T7174, to which both IRBB1 and IRBB14 plants were resistant. Surprisingly, the hybrid showed greater susceptibility than either IRBB1 or IRBB14 plants (Figure 7C). Similar results were obtained with T7174-inoculated IRBB2/IRBB14 F2 plants (Supplemental Figure 23). By contrast, the IRBB1/IRBB2 hybrid exhibited the same level of resistance as IRBB1 and IRBB2 when inoculated with T7174 (Figure 7D). These results suggest that interactions between XA14 and XA1 or XA1-2 impaired resistance, whereas the interaction between XA1 and XA1-2 did not.
Figure 7.
Interactions among Xa1, Xa1-2, and Xa14 Lead to Differences in Rice Resistance.
(A) Lesion length analysis in IRBB14/IRBB1 F1 hybrids after PXO112 inoculation.
(B) Lesion length analysis in IRBB1/IRBB14 F1 hybrids after PXO112 inoculation.
(C) Lesion length analysis in IRBB1/IRBB14-3 F2 plants after T7174 inoculation.
(D) Lesion length analysis in IRBB1/IRBB2-4 F2 plants after T7174 inoculation.
Data are presented as mean ± SD. The letter “a” indicates a significant difference compared with IRBB14 at P < 0.01.
Discussion
Allelic variation is very important for the evolution of functional genes in plants. Several formerly identified genes were subsequently found to be alleles of the same gene, this phenomenon is especially common among resistance genes. In rice, many blast resistance genes are allelic. For example, Pik, Pikm, Pik-p, Pi1, and Pike are allelic to one another, Pi2 is allelic to Piz-t, and Pid3 is allelic to Pi25 (Zhang and Wang, 2016). There are also multiple alleles of Brown planthopper (BPH) R genes. After BPH9 was cloned, another eight BPH genes were found to be allelic to it (Zhao et al., 2016). Here, we showed that in rice–Xoo interactions, Xa1, Xa1-2, and Xa14 are allelic, and Xa31(t) is the same as Xa1-2. There are still other uncloned R genes located near this locus, such as Xa12, Xa-25, Xa38, and Xo1 (Ogawa et al., 1978; Gao et al., 2005; Cheema et al., 2008; Ellur et al., 2016; Triplett et al., 2016). Although it is difficult to confirm that these are also alleles due to the lack of related germplasms, our results show that variations in this gene play important roles in Xoo resistance in rice.
Interestingly, similar to Xa1, Xa1-2- and Xa14-mediated resistance can be attenuated by iTALEs, and it is likely that endogenous iTALEs in some Xoo strains make them compatible with plants that carry Xa1, Xa1-2, or Xa14. However, whether there are direct physical interactions between iTALEs (or TALEs) and XA1 or XA1-like NLRs remains unclear, and our ongoing experiments suggest that iTALEs may only partially contribute to the different resistance spectra of Xa1, Xa1-2, and Xa14 (data not shown). This is consistent with a previous report that some iTALEs also exist in many IRBB1-incompatible Xoo strains and fail to suppress Xa1-mediated resistance (Ji et al., 2016). TALE-encoding genes contain a large portion of repetitive sequence and are multi-copied in many Xoo strains, therefore, the full characterization of all TALE-encoding genes in different Xoo genomes will facilitate future studies.
The different LRR domain substructures of XA1, XA1-2, XA14, and RGAF homologs may be the main reason for the differences in Xoo resistance. Sequences that encode the intervening motif were present in many non-resistant alleles, such as RGAf, RGAf-IR24, RGAf-MDJ8, and RGAf-ZH11. It seems that the intervening motif may affect the function of other motifs and lead to susceptibility. The Linker, however, may associate with other motifs during the Xoo–rice interaction, as Xa14 exhibited a different resistance spectrum compared with Xa1 and Xa1-2. It is likely that the Linker makes the conformation of XA14 slightly different from those of XA1 and XA1-2 and leads to changes in the recognition of potential interacting proteins, perhaps including some TALEs. However, the Linker and intervening motifs were reported here for the first time, and further studies are required to fully characterize their functions in resistance.
It is also interesting that XA1, XA1-2, XA14, RGAF, and their homologs all contain CTRs in the LRR region. The CTRs were well conserved in sequence but varied in numbers even within the same species. This may be the result of adaptation and evolution, generating new genes to cope with different pathogens. The molecules or sequences with which CTRs can interact remain completely unknown and present an interesting question for future investigation.
The Phylogenetic analysis of RGAF homologs demonstrated that XA1, XA1-2, XA14, and RGAF clustered in the same clade and appeared later than many other homologs (Figure 5B). Thus, it is likely that RGAf first originated from an ancestral gene in a wild rice variety and Xa14 then originated from RGAf, as suggested by the deletion of the intervening motif-encoding sequence. Xa1 and Xa1-2 may have arisen from Xa14, as suggested by the deletion of the Linker-encoding sequence. However, it is difficult to determine whether Xa1 or Xa1-2 is the ancestral gene, as the gain and loss of a CTR-encoding sequence may occur at a similar frequency. Moreover, the possibility that other paralogs were involved in the evolution of these genes cannot be excluded. A recent report showed that the Xo1 locus has expanded and contains 14 predicted NLRs in the rice cultivar Carolina Gold Select (Read et al., 2020). Further investigation of the exact number and content of NLR-encoding genes at this locus in IRBB1, IRBB2, IRBB14, and other rice cultivars will contribute to our understanding of the evolution of these genes.
Xa1, Xa1-2, and Xa14 encode atypical NLRs that contain the BED finger domain, and we found that the BED finger domains of XA1, XA1-2, and XA14 could interact with themselves or with one another. Although there were other possibilities, such as their full-length proteins being unable to interact with one another or interactions with other RGAs or BED-containing proteins, the varied resistance of the progenies of crosses among IRBB1, IRBB2, and IRBB14 nonetheless provided insight into the complex relationships among Xa1, Xa1-2, and Xa14. One-fifth of rice cultivars in China carry the Xa14 gene whereas cultivars from other areas are more likely to contain Xa1 or Xa1-2. The results presented here may be helpful for further applications of NLRs, such as Xa1, Xa1-2, and Xa14, especially in hybrid rice breeding and production.
In addition, BED fingers were previously found to be DNA-binding domains in many transposases and chromatin-boundary-element-binding proteins (Aravind, 2000). Although it has been described as an integrated decoy domain, evidence is still needed for the cloned BED-NLRs. The BED domains in XA1, XA1-2, XA14, and even RGAF were highly conserved and were thus less likely to be decoys than LRR domains. Also, it seems unreasonable that these BED-NLRs contained two decoy domains separated by an NB domain, as most confirmed decoys are located at the C terminus of NLRs (Cesari et al., 2013; Le Roux et al., 2015). Although the mechanisms by which the BED domain functions in the defense process are largely unknown, the atypical architecture of BED-NLRs strongly suggests that they will provide new insights into plant immunity.
Methods
Plasmid Construction and Rice Transformation
Each of the fragments containing native promoter-driven Xa14, Xa1, or Xa1-2 was obtained using restriction endonuclease digestion and ligation from three PCR products using IRBB14, IRBB1, and IRBB2 genomic DNA, respectively, as templates. PCRs were performed using the Xa2-7F-2/Xa14-19R, Xa2-12F-2/Xa2-14R, and Xa2-8F-K/Xa14-8R primers (Supplemental Table 8). The PCR products were digested by BamHI/HindIII, BamHI/SacI, and BamHI/KpnI, respectively, and ligated one after another into a modified pC1301 vector, in which the SacI site had been deleted.
All constructs were delivered separately into the Agrobacterium tumefaciens strain EHA105. Rice transformation was then performed using the calli of rice variety IR24, Zhonghua 11, or Mudanjiang 8 by the Agrobacterium-mediated method (Lin and Zhang, 2005).
Pathogen Inoculation
The Xanthomonas oryzae pv. oryzae strains used in this study included Japanese races T7174, T7147, and T7133, Chinese strains OS105 and FuJ23, Philippine strains PXO99, PXO112, and PXO280, and PB, which is an engineered mutant generated from PXO99 (Ji et al., 2016). All strains were cultured on potato-based medium for 2 days at 28°C. Rice plants were inoculated with Xoo strains at the booting (panicle development) stage by the leaf-clipping method (Chen et al., 2002). Lesion lengths were measured 14 days after inoculation. All inoculations were repeated at least twice with similar results, and only one replicate per inoculation is presented.
WebLogos
WebLogos showing the conserved amino acid residues of the CTRs were generated with WebLogo 3 (https://weblogo.berkeley.edu/logo.cgi) (Crooks et al., 2004). CTR sequences are presented in Supplemental Figures 14–17.
Identification of RGAF Homologs
Most homologs were identified by protein BLAST at NCBI (http://blast.ncbi.nlm.nih.gov) using the RGAF sequence as a query. Accessions with a Max Score ≥800, a Query Cover ≥50%, an E-value ≤0.01, and an Identity ≥40% were classified as RGAF homologs. Homologs from the RGAP database (http://rice.plantbiology.msu.edu) and relevant articles were then identified manually using protein BLAST with the same thresholds (Supplemental Table 5).
Phylogenetic Trees
Phylogenetic trees were constructed using the MEGA 5.2.1 software (Tamura et al., 2011). The trees obtained by different methods, such as maximum likelihood, neighbor joining (NJ), and maximum parsimony, were similar, therefore, only the NJ trees with 1000 bootstrap replicates are shown. The full-length protein sequences of the RGAF homologs were used for Figure 7B. Full-length genomic sequences were used for Supplemental Figure 1B and 7A.
Allele Analysis in the Rice Germplasm Collection
A population of 520 rice cultivar accessions, including indica subspecies (indica, Aus) and japonica rice (temperate japonica, tropical japonica), from micro-core germplasm resources was planted in an experimental field at the Huazhong Agricultural University in the summer of 2019 (Xie et al., 2015). Due to a lack of sequence information for this locus in all cultivars, the allele analysis was based on the assumption that all the sequences were homologous to that of Nipponbare, including members and the number of RGA genes. RGAf allele types were identified by PCR using two pairs of gene-specific primers, Xa14-6F/Xa14-6R and Xa2-19F/Xa2-14R (Supplemental Table 8). The analysis was performed using sterilized water and the genomic DNA of Nipponbare, IRBB1, IRBB2, and IRBB14 as negative and positive controls.
Yeast Two-Hybrid Assays
Fragments containing different domains of Xa14 or Xa1 were amplified from the cDNAs of IRBB14 or IRBB1, respectively, using gene-specific primers (Supplemental Table 8). The products were then inserted into the pGBKT7 and pGADT7-Rec (Clontech) vectors by restriction endonuclease digestion and ligation. The paired constructs were co-transformed into the yeast strain AH109. Co-transformants were plated on synthetic medium without leucine, tryptophan, uracil, and histidine and incubated for 3 days at 28°C. The plasmids pGBKT7-53/pGADT7-RecT and pGBKT7-Lam/pGADT7-RecT were used as positive and negative controls, respectively. Each yeast two-hybrid assay was repeated at least twice with similar results, and only one replicate is presented.
Bimolecular Fluorescence Complementation Assay in Rice Protoplasts
Fragments encoding the BED domain of XA14 and XA1 were amplified using gene-specific primers and ligated into vectors pSPYCE(M) and pSPYNE173, respectively (Supplemental Table 8) (Waadt et al., 2008). Protoplasts were isolated from rice Oc suspension culture and transformed using a polyethylene glycol method as described by Shen et al. (2017). After incubation for 16–22 h at 30°C, the protoplasts were observed under a confocal microscope (FV1200; Olympus). Spectral settings for the detection of GFP fluorescence were excitation at 488 nm and detection at 500–525 nm.
Co-immunoprecipitation
Fragments containing the BED domain of XA14 or XA1 were amplified using gene-specific primers and ligated into the pU1031-9myc vector or the pU1301-3FLAG vector (Supplemental Table 8) (Yuan et al., 2016). The constructs were then introduced into the A. tumefaciens strain GV3101. Agrobacterium-mediated transformation was performed by infiltration into Nicotiana benthamiana leaves using needleless syringes. Proteins were extracted from N. benthamiana leaves, incubated with anti-FLAG beads (Sigma-Aldrich, St. Louis, USA), and washed as described previously (Ma et al., 2017). Western blot analyses were performed using anti-FLAG and anti-Myc antibodies (ABclonal, Wuhan, China). Co-immunoprecipitation analysis was repeated twice with similar results, and only one replicate is presented.
Statistical Analysis
Significant differences between control and treated samples were analyzed by pairwise t-test using Microsoft Office Excel (Microsoft, Redmond, WA, USA).
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant nos. 31821005, 31772145, and 31200912) and the China Scholarship Council (file no. 201908420054).
Author Contributions
Conceptualization, H.Z. and S.W.; Methodology, B.Z., H.Z., and S.W.; Investigation, B.Z., F.L., H.Z., and S.W.; Writing – Original Draft, B.Z. and H.Z.; Writing – Review & Editing, H.Z. and S.W.; Funding Acquisition, H.Z. and S.W.; Resources, J.X. and X.L.; Supervision, M.Y. and Y.O.
Acknowledgments
We thank Dr. Kaijun Zhao (Chinese Academy of Agricultural Sciences) and Prof. Gongyou Chen (Shanghai Jiao Tong University) for kindly providing rice seeds and the Xoo strain PB. The authors declare that they have no conflicts of interest.
Published: June 20, 2020
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and IPPE, CAS.
Supplemental Information is available at Plant Communications Online.
Contributor Information
Haitao Zhang, Email: zht@hubu.edu.cn.
Shiping Wang, Email: swang@mail.hzau.edu.cn.
Accession Numbers
Sequence data from this article can be found in the GenBank data libraries under the following accession numbers: MN794307 for Xa1, MN794308 for Xa1-2, MN794309 for Xa14, and MN794310 for Xa31(t).
Supplemental Information
References
- Antony G., Zhou J., Huang S., Li T., Liu B., White F., Yang B. Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. Plant Cell. 2010;22:3864–3876. doi: 10.1105/tpc.110.078964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind L. The BED finger, a novel DNA-binding domain in chromatin-boundary-element-binding proteins and transposases. Trends Biochem. Sci. 2000;25:421–423. doi: 10.1016/s0968-0004(00)01620-0. [DOI] [PubMed] [Google Scholar]
- Bailey P.C., Schudoma C., Jackson W., Baggs E., Dagdas G., Haerty W., Moscou M., Krasileva K.V. Dominant integration locus drives continuous diversification of plant immune receptors with exogenous domain fusions. Genome Biol. 2018;19:23. doi: 10.1186/s13059-018-1392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S., Tan M., Lin X. Genetic mapping of a bacterial blight resistance gene Xa14 in rice. Acta Agronom. Sinica. 2010;36:422–427. [Google Scholar]
- Bernoux M., Ve T., Williams S., Warren C., Hatters D., Valkov E., Zhang X., Ellis J.G., Kobe B., Dodds P.N. Structural and functional analysis of a plant resistance protein TIR domain reveals interfaces for self-association, signaling, and autoregulation. Cell Host Microbe. 2011;9:200–211. doi: 10.1016/j.chom.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch J., Scholze H., Schornack S., Landgraf A., Hahn S., Kay S., Lahaye T., Nickstadt A., Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- Cesari S., Thilliez G., Ribot C., Chalvon V., Michel C., Jauneau A., Rivas S., Alaux L., Kanzaki H., Okuyama Y. The rice resistance protein pair RGA4/RGA5 recognizes the Magnaporthe oryzae effectors AVR-Pia and AVR1-CO39 by direct binding. Plant Cell. 2013;25:1463–1481. doi: 10.1105/tpc.112.107201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema K.K., Grewal N.K., Vikal Y., Sharma R., Lore J.S., Das A., Bhatia D., Mahajan R., Gupta V., Bharaj T.S. A novel bacterial blight resistance gene from Oryza nivara mapped to 38 kb region on chromosome 4L and transferred to Oryza sativa L. Genet. Res. (Camb.) 2008;90:397–407. doi: 10.1017/S0016672308009786. [DOI] [PubMed] [Google Scholar]
- Chen H., Wang S., Zhang Q. New gene for bacterial blight resistance in rice located on chromosome 12 identified from minghui 63, an elite restorer line. Phytopathology. 2002;92:750–754. doi: 10.1094/PHYTO.2002.92.7.750. [DOI] [PubMed] [Google Scholar]
- Chen J., Peng P., Tian J., He Y., Zhang L., Liu Z., Yin D., Zhang Z. Pike, a rice blast resistance allele consisting of two adjacent NBS-LRR genes, was identified as a novel allele at the Pik locus. Mol. Breed. 2015;35:1–15. [Google Scholar]
- Chu Z., Yuan M., Yao J., Ge X., Yuan B., Xu C., Li X., Fu B., Li Z., Bennetzen J.L. Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev. 2006;20:1250–1255. doi: 10.1101/gad.1416306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks G.E., Hon G., Chandonia J.M., Brenner S.E. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Zhai K., Xie Z., Yang D., Zhu X., Liu J., Wang X., Qin P., Yang Y., Zhang G. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science. 2017;355:962–965. doi: 10.1126/science.aai8898. [DOI] [PubMed] [Google Scholar]
- Du B., Zhang W., Liu B., Hu J., Wei Z., Shi Z., He R., Zhu L., Chen R., Han B. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc. Natl. Acad. Sci. U S A. 2009;106:22163–22168. doi: 10.1073/pnas.0912139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellur R.K., Khanna A., S G.K., Bhowmick P.K., Vinod K.K., Nagarajan M., Mondal K.K., Singh N.K., Singh K., Prabhu K.V. Marker-aided incorporation of Xa38, a novel bacterial blight resistance gene, in PB1121 and comparison of its resistance spectrum with xa13 + Xa21. Sci. Rep. 2016;6:29188. doi: 10.1038/srep29188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka S., Yamamoto S.-I., Mizobuchi R., Yamanouchi U., Ono K., Kitazawa N., Yasuda N., Fujita Y., Thi Thanh Nguyen T., Koizumi S. Multiple functional polymorphisms in a single disease resistance gene in rice enhance durable resistance to blast. Sci. Rep. 2014;4:4550. [Google Scholar]
- Gao D.Y., Liu A.M., Zhou Y.H., Cheng Y.J., Xiang Y.H., Sun L.H., Zhai W.X. Molecular mapping of a bacterial blight resistance gene Xa-25 in rice. Yi Chuan Xue Bao. 2005;32:183–188. [PubMed] [Google Scholar]
- Gu K., Tian D., Qiu C., Yin Z. Transcription activator-like type III effector AvrXa27 depends on OsTFIIAγ5 for the activation of Xa27 transcription in rice that triggers disease resistance to Xanthomonas oryzae pv. oryzae. Mol. Plant Pathol. 2009;10:829–835. doi: 10.1111/j.1364-3703.2009.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu K., Yang B., Tian D., Wu L., Wang D., Sreekala C., Yang F., Chu Z., Wang G.L., White F.F. R gene expression induced by a type-III effector triggers disease resistance in rice. Nature. 2005;435:1122–1125. doi: 10.1038/nature03630. [DOI] [PubMed] [Google Scholar]
- He Q., Li D., Zhu Y., Tan M., Zhang D., Lin X. Fine mapping of Xa2, a bacterial blight resistance gene in rice. Mol. Breed. 2006;17:1–6. [Google Scholar]
- Hu K., Cao J., Zhang J., Xia F., Ke Y., Zhang H., Xie W., Liu H., Cui Y., Cao Y. Improvement of multiple agronomic traits by a disease resistance gene via cell wall reinforcement. Nat. Plants. 2017;3:17009. doi: 10.1038/nplants.2017.9. [DOI] [PubMed] [Google Scholar]
- Hutin M., Sabot F., Ghesquiere A., Koebnik R., Szurek B. A knowledge-based molecular screen uncovers a broad-spectrum OsSWEET14 resistance allele to bacterial blight from wild rice. Plant J. 2015;84:694–703. doi: 10.1111/tpj.13042. [DOI] [PubMed] [Google Scholar]
- Jacob F., Vernaldi S., Maekawa T. Evolution and conservation of plant NLR functions. Front Immunol. 2013;4:297. doi: 10.3389/fimmu.2013.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z., Ji C., Liu B., Zou L., Chen G., Yang B. Interfering TAL effectors of Xanthomonas oryzae neutralize R-gene-mediated plant disease resistance. Nat. Commun. 2016;7:13435. doi: 10.1038/ncomms13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroj T., Chanclud E., Michel-Romiti C., Grand X., Morel J.B. Integration of decoy domains derived from protein targets of pathogen effectors into plant immune receptors is widespread. New Phytol. 2016;210:618–626. doi: 10.1111/nph.13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux C., Huet G., Jauneau A., Camborde L., Tremousaygue D., Kraut A., Zhou B., Levaillant M., Adachi H., Yoshioka H. A receptor pair with an integrated decoy converts pathogen disabling of transcription factors to immunity. Cell. 2015;161:1074–1088. doi: 10.1016/j.cell.2015.04.025. [DOI] [PubMed] [Google Scholar]
- Lin Y., Zhang Q. Optimising the tissue culture conditions for high efficiency transformation of indica rice. Plant Cell Rep. 2005;23:540–547. doi: 10.1007/s00299-004-0843-6. [DOI] [PubMed] [Google Scholar]
- Liu Q., Yuan M., Zhou Y., Li X., Xiao J., Wang S. A paralog of the MtN3/saliva family recessively confers race-specific resistance to Xanthomonas oryzae in rice. Plant Cell Environ. 2011;34:1958–1969. doi: 10.1111/j.1365-3040.2011.02391.x. [DOI] [PubMed] [Google Scholar]
- Ma H., Chen J., Zhang Z., Ma L., Yang Z., Zhang Q., Li X., Xiao J., Wang S. MAPK kinase 10.2 promotes disease resistance and drought tolerance by activating different MAPKs in rice. Plant J. 2017;92:557–570. doi: 10.1111/tpj.13674. [DOI] [PubMed] [Google Scholar]
- Ma J., Lei C., Xu X., Hao K., Wang J., Cheng Z., Ma X., Zhou K., Zhang X., Guo X. Pi64, encoding a novel CC-NBS-LRR protein, confers resistance to leaf and neck blast in rice. Mol. Plant Microbe Interact. 2015;28:558–568. doi: 10.1094/MPMI-11-14-0367-R. [DOI] [PubMed] [Google Scholar]
- Maekawa T., Cheng W., Spiridon L.N., Toller A., Lukasik E., Saijo Y., Liu P., Shen Q.H., Micluta M.A., Somssich I.E. Coiled-coil domain-dependent homodimerization of intracellular barley immune receptors defines a minimal functional module for triggering cell death. Cell Host Microbe. 2011;9:187–199. doi: 10.1016/j.chom.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Marchal C., Zhang J., Zhang P., Fenwick P., Steuernagel B., Adamski N.M., Boyd L., McIntosh R., Wulff B.B.H., Berry S. BED-domain-containing immune receptors confer diverse resistance spectra to yellow rust. Nat. Plants. 2018;4:662–668. doi: 10.1038/s41477-018-0236-4. [DOI] [PubMed] [Google Scholar]
- Mermigka G., Amprazi M., Mentzelopoulou A., Amartolou A., Sarris P.F. Plant and animal innate immunity complexes: fighting different enemies with similar weapons. Trends Plant Sci. 2020;25:80–91. doi: 10.1016/j.tplants.2019.09.008. [DOI] [PubMed] [Google Scholar]
- Meyers B.C., Kaushik S., Nandety R.S. Evolving disease resistance genes. Curr. Opin. Plant Biol. 2005;8:129–134. doi: 10.1016/j.pbi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Meyers B.C., Kozik A., Griego A., Kuang H., Michelmore R.W. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell. 2003;15:809–834. doi: 10.1105/tpc.009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Morinaka T., Fujii K., Kimura T. Inheritance of resistance to rice varieties Kogyoku and Java 14. Annu. Phytopathol. Soc. Jpn. 1978;49:69–72. [Google Scholar]
- Read A.C., Moscou M.J., Zimin A.V., Pertea G., Meyer R.S., Purugganan M.D., Leach J.E., Triplett L.R., Salzberg S.L., Bogdanove A.J. Genome assembly and characterization of a complex zfBED-NLR gene-containing disease resistance locus in Carolina Gold Select rice with Nanopore sequencing. Plos Genet. 2020;16:e1008571. doi: 10.1371/journal.pgen.1008571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read A.C., Rinaldi F.C., Hutin M., He Y.Q., Triplett L.R., Bogdanove A.J. Suppression of Xo1-mediated disease resistance in rice by a truncated, non-DNA-binding TAL effector of Xanthomonas oryzae. Front. Plant Sci. 2016;7:1516. doi: 10.3389/fpls.2016.01516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer P., Recht S., Lahaye T. A single plant resistance gene promoter engineered to recognize multiple TAL effectors from disparate pathogens. Proc. Natl. Acad. Sci. U S A. 2009;106:20526–20531. doi: 10.1073/pnas.0908812106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Liu J., Xie K., Xing F., Xiong F., Xiao J., Li X., Xiong L. Translational repression by a miniature inverted-repeat transposable element in the 3′ untranslated region. Nat. Commun. 2017;8:14651. doi: 10.1038/ncomms14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W.Y., Wang G.L., Chen L.L., Kim H.S., Pi L.Y., Holsten T., Gardner J., Wang B., Zhai W.X., Zhu L.H. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science. 1995;270:1804–1806. doi: 10.1126/science.270.5243.1804. [DOI] [PubMed] [Google Scholar]
- Sun X., Cao Y., Yang Z., Xu C., Li X., Wang S., Zhang Q. Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J. 2004;37:517–527. doi: 10.1046/j.1365-313x.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D., Wang J., Zeng X., Gu K., Qiu C., Yang X., Zhou Z., Goh M., Luo Y., Murata-Hori M. The rice TAL effector-dependent resistance protein XA10 triggers cell death and calcium depletion in the endoplasmic reticulum. Plant Cell. 2014;26:497–515. doi: 10.1105/tpc.113.119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett L.R., Cohen S.P., Heffelfinger C., Schmidt C.L., Huerta A.I., Tekete C., Verdier V., Bogdanove A.J., Leach J.E. A resistance locus in the American heirloom rice variety Carolina Gold Select is triggered by TAL effectors with diverse predicted targets and is effective against African strains of Xanthomonas oryzae pv. oryzicola. Plant J. 2016;87:472–483. doi: 10.1111/tpj.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wersch S., Tian L., Hoy R., Li X. Plant NLRs: the whistleblowers of plant immunity. Plant Commun. 2020;1:100016. doi: 10.1016/j.xplc.2019.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R., Schmidt L.K., Lohse M., Hashimoto K., Bock R., Kudla J. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J. 2008;56:505–516. doi: 10.1111/j.1365-313X.2008.03612.x. [DOI] [PubMed] [Google Scholar]
- Wang C., Zhang X., Fan Y., Gao Y., Zhu Q., Zheng C., Qin T., Li Y., Che J., Zhang M. XA23 is an executor R protein and confers broad-spectrum disease resistance in rice. Mol. Plant. 2015;8:290–302. doi: 10.1016/j.molp.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Wang C.T., Wen G.S., Lin X.H., Liu X.Q., Zhang D.P. Identification and fine mapping of the new bacterial blight resistance gene, Xa31(t), in rice. Eur. J. Plant Pathol. 2009;123:235–240. [Google Scholar]
- Wang J., Hu M., Qi J., Han Z., Wang G., Qi Y., Wang H.W., Zhou J.M., Chai J. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science. 2019;364:eaav5870. doi: 10.1126/science.aav5870. [DOI] [PubMed] [Google Scholar]
- Xie W., Wang G., Yuan M., Yao W., Lyu K., Zhao H., Yang M., Li P., Zhang X., Yuan J. Breeding signatures of rice improvement revealed by a genomic variation map from a large germplasm collection. Proc. Natl. Acad. Sci. U S A. 2015;112:E5411–E5419. doi: 10.1073/pnas.1515919112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Lv Q., Shang J., Pang Z., Zhou Z., Wang J., Jiang G., Tao Y., Xu Q., Li X. Excavation of Pid3 orthologs with differential resistance spectra to Magnaporthe oryzae in rice resource. PLoS One. 2014;9:e93275. doi: 10.1371/journal.pone.0093275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Sugio A., White F.F. Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proc. Natl. Acad. Sci. U S A. 2006;103:10503–10508. doi: 10.1073/pnas.0604088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S., Yamanouchi U., Katayose Y., Toki S., Wang Z.X., Kono I., Kurata N., Yano M., Iwata N., Sasaki T. Expression of Xa1, a bacterial blight-resistance gene in rice, is induced by bacterial inoculation. Proc. Natl. Acad. Sci. U S A. 1998;95:1663–1668. doi: 10.1073/pnas.95.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Ke Y., Huang R., Ma L., Yang Z., Chu Z., Xiao J., Li X., Wang S. A host basal transcription factor is a key component for infection of rice by TALE-carrying bacteria. Elife. 2016;5:e19605. doi: 10.7554/eLife.19605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T., Li X., Xiao J., Wang S. Characterization of Xanthomonas oryzae-responsive cis-acting element in the promoter of rice race-specific susceptibility gene Xa13. Mol. Plant. 2011;4:300–309. doi: 10.1093/mp/ssq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Wang S. Rice versus Xanthomonas oryzae pv. oryzae: a unique pathosystem. Curr. Opin. Plant Biol. 2013;16:188–195. doi: 10.1016/j.pbi.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang S. Progress in functional genomic studies of rice disease resistance. Chin. Bull. Life Sci. 2016;28:1189–1199. [Google Scholar]
- Zhao Y., Huang J., Wang Z., Jing S., Wang Y., Ouyang Y., Cai B., Xin X.F., Liu X., Zhang C. Allelic diversity in an NLR gene BPH9 enables rice to combat planthopper variation. Proc. Natl. Acad. Sci. U S A. 2016;113:12850–12855. doi: 10.1073/pnas.1614862113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Peng Z., Long J., Sosso D., Liu B., Eom J.S., Huang S., Liu S., Vera Cruz C., Frommer W.B. Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J. 2015;82:632–643. doi: 10.1111/tpj.12838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.