Abstract
Xanthomonas oryzae pathovar oryzae (Xoo) uses transcription activator-like effectors (TALEs) to cause bacterial blight (BB) in rice. In turn, rice has evolved several mechanisms to resist BB by targeting TALEs. One mechanism involves the nucleotide-binding leucine-rich repeat (NLR) resistance gene Xa1 and TALEs. Reciprocally, Xoo has evolved TALE variants, C-terminally truncated versions (interfering TALEs or iTALEs), to overcome Xa1 resistance. However, it remains unknown to what extent the two co-adaptive mechanisms mediate Xoo–rice interactions. In this study, we cloned and characterized five additional Xa1 allelic R genes, Xa2, Xa31(t), Xa14, CGS-Xo111, and Xa45(t) from a collection of rice accessions. Sequence analysis revealed that Xa2 and Xa31(t) from different rice cultivars are identical. These genes and their predicted proteins were found to be highly conserved, forming a group of Xa1 alleles. The XA1 alleles could be distinguished by the number of C-terminal tandem repeats consisting of 93 amino acid residues and ranged from four in XA14 to seven in XA45(t). Xa1 allelic genes were identified in the 3000 rice genomes surveyed. On the other hand, iTALEs could suppress the resistance mediated by Xa1 allelic R genes, and iTALE genes were prevalent (∼95%) in Asian, but not in African Xoo strains. Our findings demonstrate the prominence of a defense mechanism in which rice depends on Xa1 alleles and a counteracting mechanism in which Xoo relies on iTALEs for BB.
Keywords: Xa2, Xa14, Xa45(t), TAL effector, iTAL effector, bacterial blight
Xa1 confers TAL effector-dependent resistance to Xanthomonas oryzae pathovar oryzae (Xoo) in rice, and resistance is suppressed by interfering TAL effectors (iTALEs). This study reports the characterization of five additional R genes, namely Xa2, Xa14, CGS-Xo111, Xa31(t), and Xa45(t), all allelic to Xa1 that mediate similar host-susceptible or -resistant phenotypes to the infection of Xoo strains carrying or lacking iTALEs.
Introduction
Major food crops suffer significant yield losses to an average of 20% due to damage caused by pathogens and pests (Savary et al., 2019). To ward off infections caused by microbial pathogens, host plants depend on defense responses that are induced upon the recognition of pathogen-derived molecules by plant immune receptors. The receptors are either intracellular nucleotide-binding leucine-rich repeat receptor proteins (NLRs) or plasma membrane-bound receptor proteins. NLRs recognize effectors inside plant cells that are delivered by pathogens, whereas membrane immune receptors recognize a wide range of extracellular pathogen-derived signals at the plant–pathogen interface (Boller and Felix, 2009; Cook et al., 2015; Monteiro and Nishimura, 2018). The extracellular signals include general pathogen-associated molecular patterns or compounds released from plant cells during infection, and the recognition by membrane immune receptors activates basic and broad immunity to plants. To establish successful infections, pathogens have evolved a diverse repertoire of effectors that are delivered into plant cells to interfere withimmunity by acting as virulence factors. However, these effectors can be recognized by NLRs and the recognition triggers effector-specific immunity, including the hypersensitive response (HR) at the infection site (Boller and Felix, 2009; Cook et al., 2015). Pathogens can also use virulence mechanisms to prepare the host for pathogen infection by evading or suppressing the effector triggered immunity (Jackson et al., 1999; Feng and Zhou, 2012).
Bacterial blight (BB), caused by Xanthomonas oryzae pathovar oryzae (Xoo), is one of the most important diseases in rice. BB also provides a well-established model for studying host–microbe interactions (Niño-Liu et al., 2006). Xoo causes blight disease by colonizing and spreading along the vascular tissue of rice leaves, causing severe yield loss and damaging grain quality (Mew et al., 1993; Niño-Liu et al., 2006). Host resistance bestowed by genetically inheritable R genes is one of the most economically and environmentally sustainable approaches to control diseases, other than the use of chemicals and antibiotics (White and Yang, 2009). Forty-four R genes for BB have been identified, and several have been cloned and characterized (He et al., 2012; Triplett et al., 2016; Dilla-Ermita et al., 2017; Kim, 2018). The cloned R genes for BB can be classified into five groups based on their structural characteristics and mechanisms of action. They are receptor-like kinase genes (e.g., Xa21), NLR genes (e.g., Xa1), executor genes (e.g., Xa27), recessive R genes derived from the sugar transporter SWEET genes (e.g., xa13), and a variant of the transcription factor gamma subunit gene (xa5) (Song et al., 1995; Yoshimura et al., 1998; Iyer and McCouch, 2004; Gu et al., 2005; Chu et al., 2006). Unfortunately, an increasing number of R genes, such as Xa10, Xa4, and Xa21, when used as single major BB-resistant genes in rice breeding, were defeated in fields due to the rapidly emerging virulent strains of Xoo (Adhikari et al., 1999; Lee et al., 1999; Vera Cruz et al., 2000). Altering R gene recognizable avirulence (avr) genes and recruiting new virulence factors or resistance suppressors are two common mechanisms used by Xoo to evade and counteract rice immunity (Jiang et al., 2020).
Transcription activator-like effectors (TALEs), from Xanthomonas and Ralstonia bacteria, comprise the largest family of type III effector proteins in bacteria. TALEs are highly conserved and differ from each other by the number of central 34-amino acid (aa) repeats and the composition of two amino acids at positions 12 and 13 of each repeat, two unique features that determine the specificity of DNA binding to the promoters of host target genes (Boch et al., 2009; Moscou and Bogdanove, 2009). In addition, the C termini of TALEs contain nuclear localization signals and transcription activation domains, characteristic of eukaryotic transcription activators. A group of atypical TALE variants lacking C-terminal transcription activation domains, the so-called iTALEs (interfering TALEs [Ji et al., 2016]) or truncTALEs (truncated TALEs [Read et al., 2016]) have been identified. iTALEs exist in two forms; type A iTALEs are characterized by the C-terminal truncation of 103 aa due to a premature stop codon introduced by a C-to-T change in the coding sequence of the genes, whereas type B iTALEs are characterized by a loss of 229 aa and an addition of 10 aa due to a large deletion and frameshift of the coding sequence at the 3′ end of the genes (Salzberg et al., 2008; Ji et al., 2016; Read et al., 2016).
Members of the NLR superfamily, the critical components of the innate immune system in plants and animals, can detect a variety of microbial pathogens and protect hosts from the threat of disease (Jones et al., 2016; Bentham et al., 2017). Xa1 is the only cloned NLR gene against BB from rice, and it can confer race-specific resistance to certain Xoo strains (Yoshimura et al., 1998). Xa1 resistance is triggered by TALEs but suppressed by iTALEs (Ji et al., 2016), resulting in race specificity that is determined by the presence or absence of both TALEs and iTALEs, particularly in the Xoo strain (Ji et al., 2016). Xa1 plants, including transgenic rice plants expressing Xa1, were resistant to Xoo strains lacking iTALE genes (e.g., ΔTal3, a mutant of PXO99A with its iTALE genes inactivated), and resistance was suppressed in the presence of iTal3a or iTal3b (Ji et al., 2016). Resistance responses include the HR (brown coloring at the inoculation site). Similar to Xa1, Xo1, which has not yet been cloned, in the Carolina Gold Select (CGS) rice variety was shown to confer resistance against several African Xoc (Xanthomonas oryzae pathovar oryzicola) strains in a TALE-dependent manner, and resistance was suppressed by truncated TALEs (Read et al., 2016; Triplett et al., 2016). It has been reported that Xo1 in CGS is located within a region harboring 14 NLR R genes, and hypothesized that one of them (CGS-Xo111, allelic to Xa1) might be Xo1 (Read et al., 2020).
The main feature of the predicted protein encoded by Xa1 includes six nearly identical leucine-rich repeats at the C terminus, and the leucine-rich repeat (LRR) region is hypothesized to function in pathogenic molecule recognition and protein–protein interactions. It has been reported that all TALEs are the avirulent triggers for Xa1 resistance, and they are widely present in Xanthomonas strains (Ji et al., 2016). iTALEs, probably the evolutionary derivatives of TALEs, share unique and conserved functional protein structures, namely two internal deletions at the N terminus and the lack of transcription activation domains at the C terminus. However, further studies are needed to determine how XA1 recognizes TALEs to initiate resistance and how iTALEs suppress Xa1 resistance to Xoo in rice (Zuluaga et al., 2017).
We previously reported that Xa1 is a broad-spectrum R gene that recognizes TALEs independent of the TALE central repeat regions. However, the function of this presumably excellent R gene was masked by iTALEs (Ji et al., 2016). In this study, we report the cloning of Xa1 allelic genes from different rice varieties and show that they have high variations at their C termini, mainly in the LRR regions. Surprisingly, iTALEs have different suppression profiles compared with Xa1 allelic R genes. Although the resistance of Xa1 R alleles can be defeated by new virulence factors, an investigation into the structural and resistance features of Xa1 R alleles can help us to understand the resistance mechanism and suppression basis of iTALEs, and potentially help us to engineer new R genes for an expanded spectrum of BB resistance.
Results
Four Rice Varieties, Each with an R Gene, Display a Resistance Spectrum Similar to Xa1
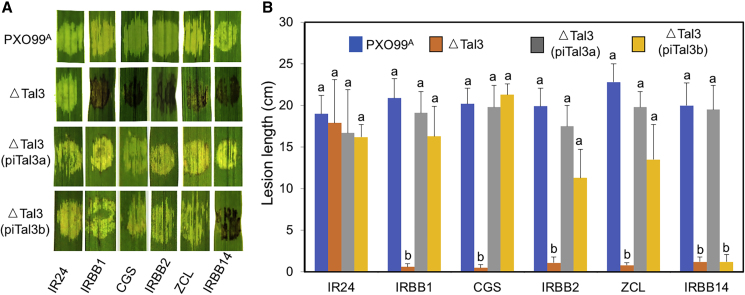
Previous studies have mapped Xa2 in IRBB2 (He et al., 2006), Xa14 in IRBB14 (Bao et al., 2010), Xa31(t) in Zhachanglong (ZCL) (Wang et al., 2009), and Xo1 in CGS (Triplett et al., 2016) within the regions corresponding to the Xa1 locus in IRBB1. We asked whether these loci, in which all four respective R genes have not yet been cloned, could confer disease responses similar or different from that of Xa1, i.e., susceptible to PXO99A and resistant to its mutant strain ΔTal3. As shown in Figure 1 and Table 1, rice varieties (IRBB2, CGS, ZCL, and IRBB14), such as IRBB1, were resistant to ΔTal3 and susceptible to PXO99A in terms of the HR and lesion length. Except for IRBB14, their resistance to ΔTal3 could be suppressed by the introduction of iTal3a or iTal3b. Their resistance spectra were also examined with an additional 24 Xoo isolates that contained different iTALE genes as confirmed by PCR with iTALE gene-specific primers and the analysis of their available genome sequences. Five Xoo strains (KXO85, JW89011, T7174, Aust-2013, and Aust-R3) were avirulent to the five rice varieties (IRBB1, IRBB2, CGS, ZCL, and IRBB14), whereas a PXO86 mutant containing only a type B iTALE gene and PXO112 were incompatible with IRBB14. The African strain AXO1947, which contains no known iTALE gene, was avirulent to IRBB1 and CGS, but virulent to three rice varieties (IRBB2, ZCL, and IRBB14). The remaining Xoo strains were virulent to the five varieties, which exhibited a susceptible phenotype, such as IR24, a rice variety that contained no known dominant R gene and served as a control (Table 1). These findings indicate that the four rice varieties are similar to IRBB1 (Xa1) in response to TALE- and iTALE-containing Xoo strains, except for IRBB14 (Xa14).
Figure 1.
Several Rice Varieties Show Disease (Resistant or Susceptible) Responses to Xoo Strains Lacking or Carrying iTALE Genes.
(A) Hypersensitive responses (dark brown inoculation spots) or water soaking symptoms (clear inoculation spots) in different rice varieties. CGS, Carolina Gold Select; ZCL, Zhachanglong.
(B) Lesion length measurements of different rice varieties infected with Xoo strains as indicated. Error bars indicate standard deviations. Identical lower-case letters indicate no significant difference (p < 0.01). The experiments were repeated three times with similar results.
Table 1.
Disease Spectrum of Cultivars and Transgenic Plants Containing the Genomic Clones of Xa1 Allelic R Genes.
| Xoo strain | iTALE type A | iTALE type B | IR24 | IRBB1 | CGS | IRBB2 | ZCL | IRBB14 | Kit | Kit-Xa1 | Kit-CGS-Xo111 | Kit-Xa2 | Kit-Xa31(t) | Kit-Xa14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PXO99A | + | + | S | S | S | S | S | S | S | S | S | S | S | S |
| ΔTal3 | − | − | S | R | R | R | R | R | S | R | R | R | R | R |
| ΔTal3/iTAL3a | + | − | S | S | S | S | S | S | S | S | S | S | S | S |
| ΔTal3/iTAL3b | − | + | S | S | S | S | S | R | S | S | S | S | S | R |
| PXO86 | + | + | S | S | S | S | S | S | S | S | S | S | S | S |
| PXO83ΔTal3 | − | + | S | S | S | S | S | R | S | S | S | S | S | R |
| PXO83ΔTal6 | + | − | S | S | S | S | S | S | S | S | S | S | S | S |
| PXO61 | + | + | S | S | S | S | S | S | S | S | S | S | S | S |
| PXO79 | + | + | S | S | S | S | S | S | S | S | S | S | S | S |
| PXO112 | − | + | S | S | S | S | S | R | S | S | S | S | S | S |
| KXO85 | − | − | S | R | R | R | R | R | S | R | R | R | R | R |
| JW89011 | − | + | S | R | R | R | R | R | S | R | R | R | R | R |
| K202 | + | + | S | S | S | S | S | S | S | S | S | S | S | S |
| T7174 | − | + | S | R | R | R | R | R | S | R | R | R | R | R |
| H75373 | + | + | S | S | S | S | S | S | S | S | S | S | S | S |
| Xoo2 | + | − | S | S | S | S | S | S | S | S | S | S | S | S |
| A3842 | + | + | S | S | S | S | S | S | S | S | S | S | S | S |
| PbXo7 | + | − | S | S | S | S | S | S | S | S | S | S | S | S |
| IXO56 | + | + | S | S | S | S | S | S | S | S | S | S | S | S |
| NXO260 | + | + | S | S | S | S | S | S | S | S | S | S | S | S |
| CIAT1185 | + | + | S | S | S | S | S | S | S | S | S | S | S | S |
| ZHE173 | + | − | S | S | S | S | S | S | S | S | S | S | S | S |
| C1 | − | + | S | S | S | S | S | S | S | S | S | S | S | S |
| GD1358 | + | + | S | S | S | S | S | S | S | S | S | S | S | S |
| HB21 | − | + | S | S | S | S | S | S | S | S | S | S | S | S |
| Aust-2013 | − | + | S | R | R | R | R | R | S | R | R | R | R | R |
| Aust-R3 | − | + | S | R | R | R | R | R | S | R | R | R | R | R |
| AXO1947 | − | − | S | R | R | S | S | S | S | R | R | S | S | S |
Note: CGS, Carolina Gold Select; ZCL, Zachanglong; Kit, Kitaake. The disease scores were determined by lesion length, followed by leaf tip clipping. R represents the disease reaction with a lesion length shorter than 5 cm, whereas S indicates the reaction with a lesion length longer than 5 cm (usually longer than 10 cm).
Xa2, Xa31(t), CGS-Xo111, and Xa14 Confer Xa1-like Resistance
We hypothesized that the R genes in the four tested rice varieties that conferred resistance to ΔTal3 and susceptibility to PXO99A were allelic to Xa1. To test this hypothesis, we PCR-amplified the respective genomic fragments from IRBB2, IRBB14, and ZCL using primers 53120-F4b and 53120-R4b, and from CGS, we used 53120-F6b as the forward primer, which was specific to both Xa1 in IRBB1 and xa1 in Nipponbare, and 53120-R4b as the reverse primer (see sequence information in Supplemental Table 1). The individual genomic fragments were sequenced and used to transform Kitaake, a japonica variety susceptible to most Xoo strains (Oliva et al., 2019). The transgenic T0 plants, as long as they were positive for transgenes as verified by PCR, were resistant to ΔTal3, with the lesion length ranging from 0.1 to 0.7 cm, whereas the wild-type control and transgene-negative lines were susceptible to ΔTal3, with the lesion length ranging from 11.3 to 14.7 cm (Supplemental Figure 1). Similar results were obtained by the segregation analysis of transgenes and resistance phenotypes in selected T1 and T2 families derived from more than two independent resistant T0 plants. The transgenic plants also showed resistance or susceptibility to the 26 Xoo strains, similar to their donor rice (Table 1). These results demonstrate that the genes from these four rice varieties are allelic to Xa1 and function similar to Xa1, in that they are resistant to Xoo strains lacking iTALE genes.
Xa1, Xa2, Xa31(t), CGS-Xo111, and Xa14 Form a Group of Xa1 Allelic R Genes
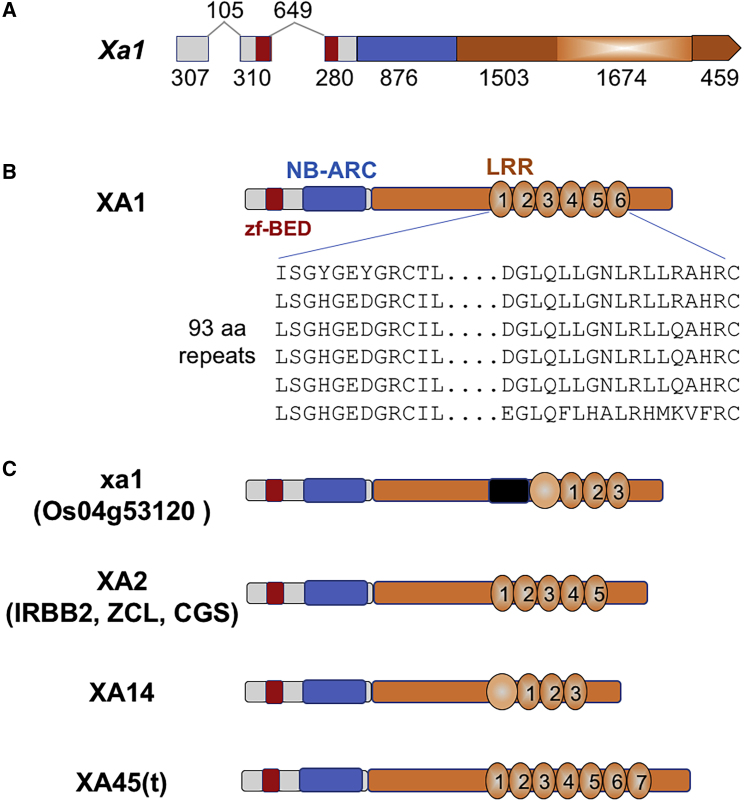
We then analyzed the sequences derived from the genomic clones that complemented Kitaake with resistance to ΔTal3. The clone from IRBB2 contained 8997 nucleotides with a predicted gene structure similar to that of Xa1, consisting of two introns and three exons. The predicted amino acid sequences of XA2 consisted of an N-terminal zinc finger BED (zfBED) domain, an NB-ARC domain, and strikingly, the C-terminal, a highly conserved tandem repeat of 93-aa residues within the LRR region (Supplemental Figure 2). XA2 had five 93-aa repeats, whereas XA1 contained six 93-aa repeats (Figure 2). In addition to the repeat regions, the remaining 1244 aa were identical between XA2 and XA1 (Supplemental Figure 2). By comparison, Xa31(t) encoded a protein almost identical to XA2 (with only a 1-aa difference in the entire 1709-aa predicted protein), including the five 93-aa tandem repeats. In addition, the CGS clone contained a gene encoding five 93-aa repeats. It was 99.6% identical to Xa2 and 99.2% identical to XA2, and very similar to CGS-Xo111 as reported by Read et al. (2020). Our CGS-Xo111 differed from that of Read et al., 2020 in 17 nucleotides, which corresponded to 7 aa all occurring within the repeat region, probably due to sequencing errors. For example, the CGS-Xo111 of Read et al., 2020 had a frameshift “A” deletion at position 78 in the fourth repeat. Due to the high similarity, we refer to the gene in our genomic clone as CGS-Xo111 (Supplemental Figure 2). It is obvious that Xa2 and Xa31(t) are identical, whereas CGS-Xo111 is not. Furthermore, Xa14 encodes a protein that is slightly different from XA1 in terms of structure (Figure 2). XA14 contains an imperfect first repeat of 135 aa, followed by three 93-aa repeats. Beyond their repeat regions, XA14 and XA1 are 96% (1199/1245 aa) identical (Supplemental Figure 2).
Figure 2.
Gene and Protein Domain Structures of XA1 Allelic Members.
(A)Xa1 consists of three exons (bars) and two introns (lines). Sequences encoding zf-BED, NB-ARC, and LRR are denoted as red, blue, and orange bars, respectively. Nucleotide numbers are shown below the bars or above the lines.
(B) Domains of XA1 are presented as zf-BED, NB-ARC, and LRR. Partial sequences of 99 amino acids are denoted below the numbered circles.
(C) Domains of XA1 alleles similar to XA1 are presented. xa1 from Nipponbare contains a sequence (black bar) upstream of the first imperfect repeat.
Prevalence of Xa1 Allelic R Genes in Rice
Based on the conserved sequences between xa1 (susceptible) and Xa1 (resistant) alleles in the database, as well as our newly cloned Xa1 allelic R genes, the significant difference between the resistant and susceptible alleles was an insertion of 1305 bp preceding the first 279-bp (for 93-aa) repeat in xa1 (Figure 2C). We therefore chose a 36-bp sequence (5′-tctctgccaccttccgca/atcagtggttatggagaa-3′) that spanned the junction of the first 279-bp repeat and its preceding region as the feature sequence for the functional Xa1 allelic R genes. The 36-bp feature sequence was used to scan 3000 rice genome sequences to identify rice accessions that contained the feature sequence. A total of 463 accessions were fount to contain at least ten reads with perfect matches to the feature sequence, suggesting that approximately 15.4% of the accessions contained functional Xa1 allelic R genes. There were 994 (33.1%) accessions that contained Xa1 alleles, that is, if the reads of the matches were extended to include at least one hit (Supplemental Table 2).
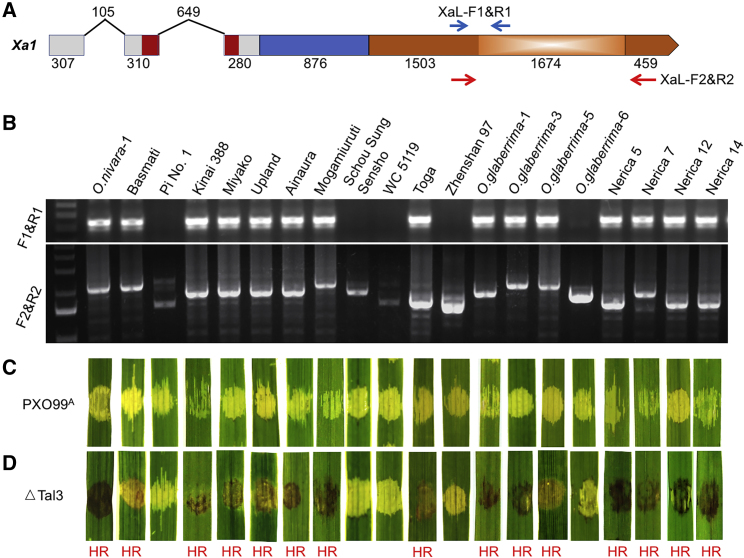
We next screened our collection of rice accessions using a PCR approach with two pairs of primers that specifically detect the presence of the Xa1 feature sequence (XaL-F1 and XaL-R1). We also determined the number of repeat sequences (XaL-F2 and XaL-R2) (Figure 3A). As shown in Supplemental Table 3, 16 out of 87 accessions were positive for the Xa1 signature sequence. Among them, the repeats ranged from four to seven, with the majority of accessions having five or six repeats (Figure 3B). To determine whether alleles confer BB resistance similar to Xa1, 22 accessions, including several accessions lacking the Xa1 signature sequence, were inoculated with PXO99A, ΔTal3, and the complemented strains of ΔTal3 with either iTal3a or iTal3b. Indeed, accessions that contained Xa1 allelic genes exhibited the HR phenotype in response to ΔTal3, and the disease phenotype (water soaking) after the inoculation of both PXO99A and ΔTal3 with either iTal3a or iTal3b (Figure 3C and 3D and Supplemental Table 3), indicating that the resistance by Xa1 allelic R genes was specifically suppressed by iTALEs.
Figure 3.
Prevalence of Xa1 Allelic Genes in Representative Rice Accessions.
(A) Gene structure of Xa1 in which two pairs of primers were used to detect the signature sequences of Xa1 alleles in 20 rice varieties.
(B) Gel images of PCR amplicons of the junction of the first repeat of 279 nucleotides and its upstream region derived from primers XaL-F1 and XaL-R1 (upper panel), as well as the whole repeat region derived from primers XaL-F2 and XaL-R2 (lower panel).
(C) Water soaking (susceptible) phenotypes of 20 rice varieties in response to PXO99A inoculation through the syringe infiltration of leaves.
(D) Hypersensitive reaction (HR) and water soaking phenotypes of 20 rice varieties in response to ΔTal3 inoculation through the syringe infiltration of leaves.
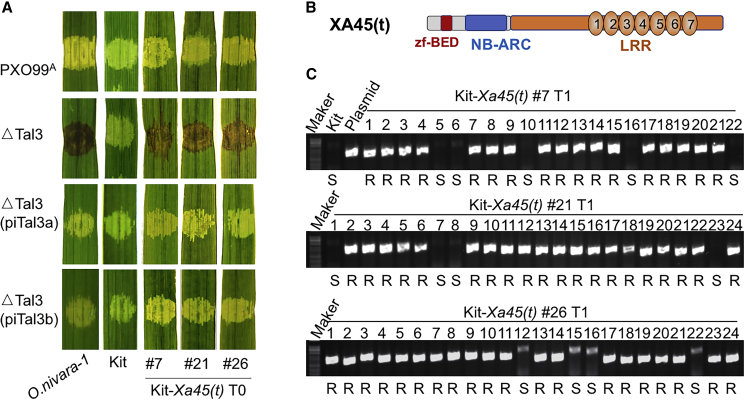
To further confirm whether the gene predicted to encode the seven 93-aa LRR repeats was indeed functional, the genomic fragment was PCR-amplified with 53120-F4b and 53120-R4b from one accession (Oryza nivara-1, IRGC 102463), similar to the approach used to clone the other Xa1 allelic genes. The genomic clone in pCAMBIA1300 was introduced into Kitaake and the primary transgenic plants were assessed for resistance. As expected, the transgenic plants (T0 and T1 generations) that were positive for the transgene were resistant to ΔTal3 but susceptible to PXO99A (Figure 4). The genomic clone was Sanger sequenced and found to contain a gene allelic to Xa1 (Supplemental Figure 2). We named the new gene Xa45(t).
Figure 4.
Functional Complementation of Xa45(t).
(A) Three transgenic Kitaake (Kit) lines of the first generation (T0) in response to the inoculation of different Xoo strains as indicated on the left side of each panel. The hypersensitive response (dark brown inoculation spots) or water soaking (clear inoculation spots) symptoms were scored 3 days after inoculation.
(B) Predicted domains of XA45(t) as indicated by zf-BED, NB-ARC, and LRR with seven 93-aa repeats.
(C) Association of genotypes and phenotypes of Xa45(t) transgenic Kitaake plants in the T1 generation. Gel images of PCR amplicons specific for Xa45(t) of individual progeny plants from three lines. Disease reactions are characterized as “S” for susceptibility to bacterial infection when lesion lengths were >10 cm and “R” for resistance when lesion lengths were <5 cm 12 days after leaf tip clipping inoculation with ΔTal3.
Prevalence of iTALE Genes in Xanthomonas oryzae Pathovars
The wide spread of Xa1 allelic R genes in nature might create significant selection pressure for the adaptation of Xanthomonas oryzae (Xo) pathogens, particularly Xoo populations in rice fields. It is believed that cognate iTALE genes exist in the two Xo pathovars. To survey the prevalence of iTALE genes among Xo isolates that represent the geographic and genetic relatedness of the pathogens (Oliva et al., 2019), we surveyed the database for the presence of iTALE genes among the Xo genome sequences deposited in NCBI (https://www.ncbi.nlm.nih.gov, Supplemental Table 5 for a list of strains with a prevalence of iTALE genes). Out of 42 Xoo strains from Asia and Australia, 40 (∼95%) strains contained type A, type B, or both types of iTALE genes, whereas none of the 33 African Xoo genomes contained iTALE genes (Supplemental Table 5). On the other hand, ten (100%) Xoc genomes from Asia contained either type B or both A and B iTALE genes, whereas two out of three African Xoc strains contained type B iTALE genes (Supplemental Table 5). These findings reveal the prevalence of both Xa1 allelic R genes in Asian rice and iTALE genes in Xanthomonas oryzae populations in Asia, as well as the co-adaptation of pathogens and host plants in BB and leaf streak diseases in Asia.
Discussion
In this study, we identified several rice varieties that were resistant to the Xoo strain (ΔTal3) lacking iTALE genes, and the resistance was suppressed by Xoo strains carrying iTALE genes. The resistance was mediated by several previously identified but as-yet uncloned R genes allelic to Xa1. These genes are highly conserved on both nucleotide and predicted amino acid levels, and the most distinguishable feature is the variable repeat number of the C-terminal 93-aa residues, with the repeats ranging from four in XA14 to seven in XA45(t). Based on the signature sequence of Xa1 allelic members, a search of 3000 rice genome sequences revealed that approximately 15% of the accessions contained the Xa1 allelic R genes. Reciprocally, most Asian Xoo and Xoc strains (>95%) contained iTALE genes, probably due to co-adaptation or arms races between the host plant and its pathogens.
The cloning of a gene usually involves mapping of the gene and functional complementation of the lines otherwise lacking the gene or the functional allele. For Xa2, Xa31(t), and Xa14, all three R genes were mapped in their respective donors within the regions, wherein Xa1 in IRBB1 was cloned (He et al., 2006; Wang et al., 2009; Bao et al., 2010). The transgenic Kitaake plants containing putative Xa2, Xa31(t), and Xa14 genomic clones were resistant to Xoo strains lacking iTALEs and were susceptible to Xoo strains carrying iTALE genes. The phenotypes of resistance and susceptibility of the transgenic plants to the tested Xoo strains were identical to the donors, IRBB2, Zhachanglong, and IRBB14, respectively. These data evidence the functional identities of the three R genes. As Xa2 and Xa31(t) are identical, we refer to them as Xa2. However, it remains to be determined whether CGS-Xo111 is Xo1, which confers resistance to Xoc. We currently do not have the avirulent Xoc strains used by Triplett et al. (2016) to determine the Xoc resistance spectrum of the Kitaake CGS-Xo111 transgenic plants. However, CGS-Xo111 is at least an NLR gene resistant to Xoo, which is supported by the notion that the Kitaake transgenic plants with CGS-Xo111 had the same resistance spectrum against the tested Xoo strains as the donor CGS.
Xa1 alleles represent a unique R gene group of the NLR superfamily that encodes proteins with a set of typical NLR domains, such as the NB-ARC, CC (coiled coil), and LRR domains, as well as the characteristic features of the zfBED domain flanked by two nuclear localization signals and variable 93-aa tandem repeats in the LRR region. The XA1 allelic members are highly conserved and can be distinguished by the number of 93-aa repeats. It remains to be determined whether repeat number plays a role in the functionality and specificity of the members. It is also unknown whether the 93-aa repeats play a role in the recognition of TALEs for the initiation of resistance to BB. As TALEs and iTALEs contain variable numbers of 34-aa repeats, it is reasonable to speculate that there are physical interactions between XA1 allelic members and TALEs for resistance and the interference of the interaction by iTALEs as a decoy for susceptibility.
The prevalence of both Xa1 allelic R genes in rice and TALE genes in Xoo creates an extraordinary challenge for Xoo to proliferate in rice, which is the only known host. Therefore, it is important to further investigate the prevalence of iTALE genes in Xoo, particularly in Asia where conditions are more conducive to BB and where epidemics of blight disease frequently occur (Niño-Liu et al., 2006). To understand how iTALEs interfere with the resistance activated by the recognition of TALEs by Xa1 or its allelic members will provide the basis for the production of broad-spectrum resistance against iTALE-containing Xo pathogens and mitigate the yield loss caused by the pathogens in rice.
Methods
Plant Materials, Bacterial Strains, Medium, and Growth Conditions
The seeds of several rice varieties (Supplemental Table 3) used in this research were kindly provided by the International Rice Research Institute and the U.S. National Small Grains Collection.
All plants were grown in a greenhouse and growth chamber at 30°C with a 12-h light period and at 28°C with a 12-h dark period and relative humidity of 60%–75%. Escherichia coli strains were grown in Luria-Bertani medium supplemented with appropriate antibiotics at 37°C. Agrobacterium tumefaciens strains were grown at 30°C in the dark. All Xoo strains were grown at 28°C in TSA medium (10 g/l tryptone, 10 g/l sucrose, 1 g/l glutamic acid). Antibiotics were used at the following concentrations if required: 100 μg/ml ampicillin, 10 μg/ml cephalexin, 25 μg/ml chloramphenicol, 25 μg/ml kanamycin, 100 μg/ml spectinomycin, and 10 μg/ml tetracycline. Bacterial strains and DNA plasmids used in this study are listed in Supplemental Table 4.
Rice Transformation with the Genomic Fragments of R Genes
For the functional complementation of R genes, with Xa2 as an example, an 8997-bp genomic sequence containing a 1596-bp promoter sequence, the entire coding region (5885 bp) of Xa2, and a 1516-bp downstream sequence, were PCR-amplified using primers 53120-F4b and 53120-R4b (Supplemental Table 1), cloned into the binary pCAMBIA1300 vector, and linearized with EcoRI and HindIII using the Gibson Assembly Master Mix (New England BioLabs, Ipswich, MA, USA). After Sanger sequencing, the pCAMBIA1300-Xa2 plasmid was electroporated into the Agrobacterium tumefaciens EHA105 strain and introduced into calli that were induced from the immature embryos of the japonica variety, Kitaake. The rice transformation method was used as described previously (Hiei et al., 1997). Transgenic plants were genotyped with primers BB2-F1 and M13F (Supplemental Table 1). The genomic fragments of the other R genes (Xa31(t), CGS-Xo111, Xa14, and Xa45(t)) were PCR-amplified and introduced into Kitaake by Agrobacterium-mediated transformation as indicated.
iTal3 and iTal6 Deletion in PXO86
Based on the PXO86 genomic sequence (NCBI accession, CP007166.1), two pairs of primers, 86TalKDF1/86TalKDR1 and 86TalKDF2/86TalKDR2 (Supplemental Table 1), were used to amplify the upstream and downstream regions flanking iTal3 (a type A iTALE gene) and iTal6 (a type B iTALE gene) using PXO86 genomic DNA as the template. A pair of primers, KD13-F and KD13-R, was used to amplify the whole expression cassette of the kanamycin resistance gene from plasmid pKD13 (NCBI accession, AY048744.1). The three fragments were fused by overlapping PCR, and the resulting fragment was inserted into the pBluscript SK vector at the EcoRV site. The plasmid was introduced into the competent cells of PXO86 by electroporation, and the transformants were grown on TSA plates supplemented with kanamycin. Single colonies with deletions were identified by PCR using two pairs of primers, 86TAL3-F2/R2 and 86TAL6-F2/R2.
Disease Assays
The assay for the HR through leaf infiltration using a needleless syringe and the assay for the lesion length through leaf tip clipping were performed as described previously (Yang and Bogdanove, 2013). In brief, Xoo stocks, preserved in a −80°C freezer, were streaked on TSA plates supplemented with appropriate antibiotics and incubated at 28°C for 2–4 days. Cells were harvested from the plates, suspended in sterilized water and washed twice, re-suspended in water, and adjusted to an optical density of 0.5 at 600 nm. Bacterial cells in suspension were infiltrated into the leaves of 3-week-old rice plants through a blunt (needleless) syringe pressed against the underside of the leaf. The inoculation spot turned brown, and bacterial spread was restricted in resistant plants 2–3 days after infiltration, whereas the symptoms in susceptible plants were much stronger, with water soaking extending in both directions and bacterial exudate appearing at the inoculation spot. Fully expanded leaves of rice plants (6–8 weeks old) were inoculated using the leaf tip clipping method. Scissor blades were immersed in Xoo suspension and used to clip approximately 1–2 cm from the leaf tip. The lesion length was measured 12–14 days after inoculation. Three replications with approximately ten leaves from two to five plants per replicate were inoculated per strain. One-way analysis of variance (ANOVA) was conducted on all measurements. The Tukey honestly significant difference test was used for post-ANOVA pairwise tests for significance, which was set at 5% (p < 0.05).
Xa1 Feature Sequence searching in 3000 Rice Genomes
The raw read data of 3000 rice genomes were downloaded from the Sequence Read Archive (Wang et al., 2018). Reads were subjected to adaptor clipping and quality trimming with Trimmomatic software (v0.36), keeping trimmed reads longer than 50 bp (Bolger et al., 2014). The resistant Xa1 allele-associated sequence 5′-tctctgccaccttccgca/atcagtggttatggagaa-3′ (36 bp) was used to identify rice accessions potentially harboring resistant alleles. Reads carrying perfectly matched sequences were counted and normalized to the number of total reads. The sequence was absent in the reference genome (IRGSP-1.0) that carried the susceptible allele. Reads were also directly aligned to the 279-bp repeat sequence that encoded the first 93-aa LRR repeat of XA1. Alignments were conducted with the mem module of bwa (0.7.12-r1039) (Li and Durbin, 2009). Read alignments required matches of at least 50 bp with identities of 95%. Aligned reads were counted and normalized to the number of total reads.
Sequence Analysis of iTALE Genes in Sequenced Xo Genomes
The complete or partial genome sequences of Xoo and Xoc strains were obtained from the NCBI (Supplemental Table 5) via BLASTn using a 690-bp sequence at the 5′ coding region of iTal3a from PXO99A. Distinctive C-terminal sequences of iTALE genes (iTal3a and iTal3b) of PXO99A were used to categorize the iTALE genes as type A and type B. All annotations were performed through NCBI Blast and further confirmed using SnapGene software.
Funding
This work was supported by the National Natural Science Foundation of China (31830072 to G.C.), the United States Department of Agriculture National Institute of Agriculture and Food (2017-67 013-26 521 to B.Y.), the Young Elite Scientist Sponsorship of the China Association for Science and Technology (2017QNRC001 to Z.J.), and the Chinese National Transgenic Major Program (2016ZX08001-002 to G.C.).
Author Contributions
C.J., S.L., G.C., and B.Y. conceived the experiments. C.J., Z.J., H.C., B.L., and H.L. performed the experiments. C.J., Z.J., and B.Y. wrote the manuscript with input from all other co-authors.
Acknowledgments
We are grateful to the U.S. National Small Grains Collection (NSGC), Dr. Zhikang Li (Institute of Crop Sciences, Chinese Academy of Agricultural Sciences), and Dr. Bin Han (Institute of Plant Physiology and Ecology, Chinese Academy of Sciences) for providing rice accessions. No conflict of interest is declared.
Published: June 20, 2020
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and IPPE, CAS.
Supplemental Information is available at Plant Communications Online.
Contributor Information
Bing Yang, Email: yangbi@missouri.edu.
Gongyou Chen, Email: gyouchen@sjtu.edu.cn.
Accession Numbers
The NCBI GenBank accession numbers for Xa2, Xa31(t), CGS-Xo111, Xa14, and Xa45(t) are as follows: MT395899, MT395900, MT395901, MT395902, and MT395903, respectively.
Supplemental Information
References
- Adhikari T.B., Basnyat R.C., Mew T.W. Virulence of Xanthomonas oryzae pv. oryzae on rice lines containing single resistance genes and gene combinations. Plant Dis. 1999;83(1):46–50. doi: 10.1094/PDIS.1999.83.1.46. [DOI] [PubMed] [Google Scholar]
- Bao S., Tan M., Lin X. Genetic mapping of a bacterial blight resistance gene Xa14 in rice. Acta Agronomica Sin. 2010;36:6. [Google Scholar]
- Bentham A., Burdett H., Anderson P.A., Williams S.J., Kobe B. Animal NLRs provide structural insights into plant NLR function. Ann. Bot. 2017;119 doi: 10.1093/aob/mcw171. 827–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch J., Scholze H., Schornack S., Landgraf A., Hahn S., Kay S., Lahaye T., Nickstadt A., Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- Chu Z., Yuan M., Yao J., Ge X., Yuan B., Xu C., Li X., Fu B., Li Z., Bennetzen J.L. Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev. 2006;20:1250–1255. doi: 10.1101/gad.1416306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D.E., Mesarich C.H., Thomma B.P. Understanding plant immunity as a surveillance system to detect invasion. Annu. Rev. Phytopathol. 2015;53:541–563. doi: 10.1146/annurev-phyto-080614-120114. [DOI] [PubMed] [Google Scholar]
- Dilla-Ermita C.J., Tandayu E., Juanillas V.M., Detras J., Lozada D.N., Dwiyanti M.S., Vera Cruz C., Mbanjo E.G.N., Ardales E., Diaz M.G. Genome-wide association analysis tracks bacterial leaf blight resistance loci in rice diverse germplasm. Rice (N Y) 2017;10:8. doi: 10.1186/s12284-017-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng F., Zhou J.M. Plant-bacterial pathogen interactions mediated by type III effectors. Curr. Opin. Plant Biol. 2012;15:469–476. doi: 10.1016/j.pbi.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Gu K., Yang B., Tian D., Wu L., Wang D., Sreekala C., Yang F., Chu Z., Wang G.L., White F.F. R gene expression induced by a type-III effector triggers disease resistance in rice. Nature. 2005;435:1122–1125. doi: 10.1038/nature03630. [DOI] [PubMed] [Google Scholar]
- He Q., Li D., Zhu Y., Tan M., Zhang D., Lin X. Fine mapping of Xa2, a bacterial blight resistance gene in rice. Mol. Breed. 2006;17:6. [Google Scholar]
- He W., Huang D., Li R. Identification of a resistance gene bls1 to bacterial leaf streak in wild rice Oryza rufipogon Griff. J. Integr. Agric. 2012;11:8. [Google Scholar]
- Hiei Y., Komari T., Kubo T. Transformation of rice mediated by Agrobacterium tumefaciens. Plant Mol. Biol. 1997;35:205–218. [PubMed] [Google Scholar]
- Iyer A.S., McCouch S.R. The rice bacterial blight resistance gene xa5 encodes a novel form of disease resistance. Mol. Plant Microbe Interact. 2004;17:1348–1354. doi: 10.1094/MPMI.2004.17.12.1348. [DOI] [PubMed] [Google Scholar]
- Jackson R.W., Athanassopoulos E., Tsiamis G., Mansfield J.W., Sesma A., Arnold D.L., Gibbon M.J., Murillo J., Taylor J.D., Vivian A. Identification of a pathogenicity island, which contains genes for virulence and avirulence, on a large native plasmid in the bean pathogen Pseudomonas syringae pathovar phaseolicola. Proc. Natl. Acad. Sci. U S A. 1999;96:10875–10880. doi: 10.1073/pnas.96.19.10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z., Ji C., Liu B., Zou L., Chen G., Yang B. Interfering TAL effectors of Xanthomonas oryzae neutralize R-gene-mediated plant disease resistance. Nat. Commun. 2016;7:13435. doi: 10.1038/ncomms13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N., Yan J., Liang Y., Shi Y., He Z., Wu Y., Zeng Q., Liu X., Peng J. Resistance genes and their interactions with bacterial blight/leaf streak pathogens (Xanthomonas oryzae) in rice (Oryza sativa L.)—an updated review. Rice (N Y) 2020;13:3. doi: 10.1186/s12284-019-0358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.D., Vance R.E., Dangl J.L. Intracellular innate immune surveillance devices in plants and animals. Science. 2016;354:aaf6395. doi: 10.1126/science.aaf6395. [DOI] [PubMed] [Google Scholar]
- Kim S.M. Identification of novel recessive gene xa44(t) conferring resistance to bacterial blight races in rice by QTL linkage analysis using an SNP chip. Theor. Appl. Genet. 2018;131:2733–2743. doi: 10.1007/s00122-018-3187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.W., Choi S.H., Han S.S., Lee D.G., Lee B.Y. Distribution of Xanthomonas oryzae pv. oryzae strains virulent to Xa21 in Korea. Phytopathology. 1999;89:928–933. doi: 10.1094/PHYTO.1999.89.10.928. [DOI] [PubMed] [Google Scholar]
- Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mew T.W., Alvarez A.M., Leach J.E., Swing J. Focus on bacterial blight of rice. Plant Dis. 1993;77:8. [Google Scholar]
- Monteiro F., Nishimura M.T. Structural, functional, and genomic diversity of plant NLR proteins: an evolved resource for rational engineering of plant immunity. Annu. Rev. Phytopathol. 2018;56:243–267. doi: 10.1146/annurev-phyto-080417-045817. [DOI] [PubMed] [Google Scholar]
- Moscou M.J., Bogdanove A.J. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- Niño-Liu D.O., Ronald P.C., Bogdanove A.J. Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol. Plant Pathol. 2006;7:303–324. doi: 10.1111/j.1364-3703.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- Oliva R., Ji C., Atienza-Grande G., Huguet-Tapia J.C., Perez-Quintero A., Li T., Eom J.S., Li C., Nguyen H., Liu B. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 2019;37:1344–1350. doi: 10.1038/s41587-019-0267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read A.C., Moscou M.J., Zimin A.V., Pertea G., Meyer R.S., Purugganan M.D., Leach J.E., Triplett L.R., Salzberg S.L., Bogdanove A.J. Genome assembly and characterization of a complex zfBED-NLR gene-containing disease resistance locus in Carolina Gold Select rice with Nanopore sequencing. Plos Genet. 2020;16:e1008571. doi: 10.1371/journal.pgen.1008571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read A.C., Rinaldi F.C., Hutin M., He Y.Q., Triplett L.R., Bogdanove A.J. Suppression of Xo1-mediated disease resistance in rice by a truncated, non-DNA-binding TAL effector of Xanthomonas oryzae. Front. Plant Sci. 2016;7:1516. doi: 10.3389/fpls.2016.01516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg S.L., Sommer D.D., Schatz M.C., Phillippy A.M., Rabinowicz P.D., Tsuge S., Furutani A., Ochiai H., Delcher A.L., Kelley D. Genome sequence and rapid evolution of the rice pathogen Xanthomonas oryzae pv. oryzae PXO99A. BMC Genomics. 2008;9:204. doi: 10.1186/1471-2164-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savary S., Willocquet L., Pethybridge S.J., Esker P., McRoberts N., Nelson A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019;3:430–439. doi: 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- Song W.Y., Wang G.L., Chen L.L., Kim H.S., Pi L.Y., Holsten T., Gardner J., Wang B., Zhai W.X., Zhu L.H. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science. 1995;270:1804–1806. doi: 10.1126/science.270.5243.1804. [DOI] [PubMed] [Google Scholar]
- Triplett L.R., Cohen S.P., Heffelfinger C., Schmidt C.L., Huerta A.I., Tekete C., Verdier V., Bogdanove A.J., Leach J.E. A resistance locus in the American heirloom rice variety Carolina Gold Select is triggered by TAL effectors with diverse predicted targets and is effective against African strains of Xanthomonas oryzae pv. oryzicola. Plant J. 2016;87:472–483. doi: 10.1111/tpj.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera Cruz C.M., Bai J., Ona I., Leung H., Nelson R.J., Mew T.W., Leach J.E. Predicting durability of a disease resistance gene based on an assessment of the fitness loss and epidemiological consequences of avirulence gene mutation. Proc. Natl. Acad. Sci. U S A. 2000;97:13500–13505. doi: 10.1073/pnas.250271997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Wen G., Lin X., Liu X., Zhang D. Identification and fine mapping of the new bacterial blight resistance gene, Xa31(t), in rice. Eur. J. Plant Pathol. 2009;123:6. [Google Scholar]
- Wang W., Mauleon R., Hu Z., Chebotarov D., Tai S., Wu Z., Li M., Zheng T., Fuentes R.R., Zhang F. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature. 2018;557:43–49. doi: 10.1038/s41586-018-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F.F., Yang B. Host and pathogen factors controlling the rice-Xanthomonas oryzae interaction. Plant Physiol. 2009;150:1677–1686. doi: 10.1104/pp.109.139360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Bogdanove A. Inoculation and virulence assay for bacterial blight and bacterial leaf streak of rice. Methods Mol. Biol. 2013;956:249–255. doi: 10.1007/978-1-62703-194-3_18. [DOI] [PubMed] [Google Scholar]
- Yoshimura S., Yamanouchi U., Katayose Y. Expression of Xa1, a bacterial blight-resistance gene in rice, is induced by bacterial inoculation. Proc. Natl. Acad. Sci. U S A. 1998;95:1663–1668. doi: 10.1073/pnas.95.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuluaga P., Szurek B., Koebnik R., Kroj T., Morel J.B. Effector mimics and integrated decoys, the never-ending arms race between rice and Xanthomonas oryzae. Front. Plant Sci. 2017;8:431. doi: 10.3389/fpls.2017.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.