Abstract
Green plants on the earth have evolved intricate mechanisms to acclimatize to and utilize sunlight. In Arabidopsis, light signals are perceived by photoreceptors and transmitted through divergent but overlapping signaling networks to modulate plant photomorphogenic development. COP1 (CONSTITUTIVE PHOTOMORPHOGENIC 1) was first cloned as a central repressor of photomorphogenesis in higher plants and has been extensively studied for over 30 years. It acts as a RING E3 ubiquitin ligase downstream of multiple photoreceptors to target key light-signaling regulators for degradation, primarily as part of large protein complexes. The mammalian counterpart of COP1 is a pluripotent regulator of tumorigenesis and metabolism. A great deal of information on COP1 has been derived from whole-genome sequencing and functional studies in lower green plants, which enables us to illustrate its evolutionary history. Here, we review the current understanding about COP1, with a focus on the conservation and functional diversification of COP1 and its signaling partners in different taxonomic clades.
Key words: COP1, E3 ubiquitin ligase, photomorphogenesis, gravitropism, light protection, evolution
This review summarizes and discusses the progress of the studies on COP1, a central repressor in photomorphogenesis, over the last 30 years, and highlights the functional conservation and divergence of COP1 during plant evolution.
Introduction
Light is one of the most important environmental factors for photosynthetic plants, not only as an energy source but also as an informational signal to modulate various developmental processes (McNellis and Deng, 1995, Wei and Deng, 1996). In Arabidopsis, light modulates plant growth and development throughout the whole life cycle, which is most dramatically illustrated by seedling morphogenesis (Kendrick and Kronenberg, 1994, von Arnim and Deng, 1996, Jiao et al., 2007). The developmental pattern in light conditions, termed photomorphogenesis, allows seedling morphology to be optimally developed for photosynthesis (von Arnim and Deng, 1996). A class of Arabidopsis mutants, cop/det/fus (constitutive photomorphogenic/de-etiolated/fusca), was initially identified in genetic screens as displaying constitutively photomorphogenic phenotypes in darkness (Schwechheimer and Deng, 2000, Lau and Deng, 2012). Among a total of nine COP1/DET/FUS loci characterized later (Chory et al., 1989, Deng et al., 1991, Wei and Deng, 1992, Miséra et al., 1994, Wei et al., 1994, Kwok et al., 1996), COP1 is the most extensively studied repressor of plant photomorphogenesis (Ma et al., 2002, Yi and Deng, 2005, Lau and Deng, 2012). Loss-of-function mutations of COP1 result in photomorphogenic phenotypes in darkness, and null cop1 alleles also cause lethality after the seedling phase (McNellis et al., 1994, Stoop-Myer et al., 1999).
Accumulated evidence has indicated that COP1 functions at the heart of light-signaling networks; it is capable of integrating signals from various photoreceptors and regulating a batch of downstream factors to mediate light responses (Lau and Deng, 2012, Podolec and Ulm, 2018). COP1 is regarded as a central switch of global light-responsive gene expression in Arabidopsis (Ma et al., 2002), exerting its effect through destabilizing HY5 (ELONGATED HYCOTYL 5) and other transcriptional regulators critical for photomorphogenesis in darkness (Osterlund et al., 2000a, Wang et al., 2016).
COP1 is well conserved in plants and animals, and its orthologs share high similarities in their cellular properties, biochemical activities, and predicted molecular structures (Deng et al., 1992, Chamovitz and Deng, 1995, Osterlund et al., 2000a, Yi et al., 2002, Marine, 2012, Sanchez-Barcelo et al., 2016). Besides the studies on Arabidopsis photomorphogenesis, recent reports have revealed the crucial roles of COP1 in different plant systems and developmental processes, such as photoprotection in algae (Schierenbeck et al., 2015, Tilbrook et al., 2016, Gabilly et al., 2019, Tokutsu et al., 2019), and gravitropism in mosses (Artz et al., 2019). In addition to plant development, COP1 also plays a role in mammalian metabolism (Sanchez-Barcelo et al., 2016, Ren et al., 2019a), tumorigenesis (Dornan et al., 2004, Wertz et al., 2004, Yi and Deng, 2005, Marine, 2012, Choi and Lee, 2015), and neuron development (Newton et al., 2018). Although it functions in distinct biological contexts in plants and animals, COP1 is proposed to be functionally conserved according to the energy supply transition hypothesis: COP1 takes part in regulating the dark-to-light transition in plants and the feeding-to-fasting transition in mammals (Sanchez-Barcelo et al., 2016). The functional conservation and divergence implies an intriguing evolutionary history of COP1 in plants and animals.
In this review, we summarize the studies on COP1 during the past 30 years and highlight the latest discoveries. Accumulating evidence from parallel comparison of COP1 roles in Arabidopsis, early-originating plants, and mammals, will help extend our understanding of the evolutionary conservation and functional diversification of the COP1-containing regulatory apparatus.
COP1 Is a Conserved E3 Ubiquitin Ligase in Eukaryotes
E3 ubiquitin ligases containing the RING domain represent the largest E3 family and interact with both protein substrates and E2 ubiquitin-conjugating enzymes (Zheng et al., 2000). COP1 protein contains three functional domains required for its activity: an N-terminal RING-finger domain, a middle coiled-coil domain, and a C-terminal WD40 domain (Figure 1A; Deng and Quail, 1992, Deng et al., 1992, Stoop-Myer et al., 1999, Holm et al., 2001). COP1 was first identified as an E3 ligase of the downstream photomorphogenesis-promoting transcription factor HY5; COP1 was found to directly interact with HY5 via its WD40 domain and degrade HY5 in darkness through the ubiquitin–proteasome system (Ang and Deng, 1994, Ang et al., 1998, Osterlund et al., 2000a, Osterlund et al., 2000b). Later a batch of light-signaling factors were identified as COP1's targets, including HYH (HY5-HOMOLOG) (Holm et al., 2002), LAF1 (LONG AFTER FAR-RED LIGHT 1) (Seo et al., 2003), HFR1 (LONG HYPOCOTYL IN FAR-RED 1) (Kim et al., 2002,Jang et al., 2005, Yang et al., 2005), PHYA (PHYTOCHROME A) (Seo et al., 2004), PHYB (PHYTOCHROME B) (Jang et al., 2010), and a series of BBX (B-BOX) proteins (Xu et al., 2016, Lin et al., 2018). The ubiquitination activity of COP1 can be detected toward both its target proteins and itself in vitro (Saijo et al., 2003, Seo et al., 2003). Light conditions and COP1-interacting proteins affect its E3 ligase activity in vivo (Saijo et al., 2003, Yi and Deng, 2005, Lau and Deng, 2012). Notably, the activity of COP1 was reported to be regulated by several post-translational mechanisms. In darkness, SUMO (small ubiquitin-like modifier) modification of COP1 enhances its trans-ubiquitination activity, which causes increased degradation of COP1 targets (Lin et al., 2016). In addition, a Ser/Thr kinase, PID (PINOID), directly interacts with COP1 and phosphorylates it to repress its activity in Arabidopsis (Lin et al., 2017).
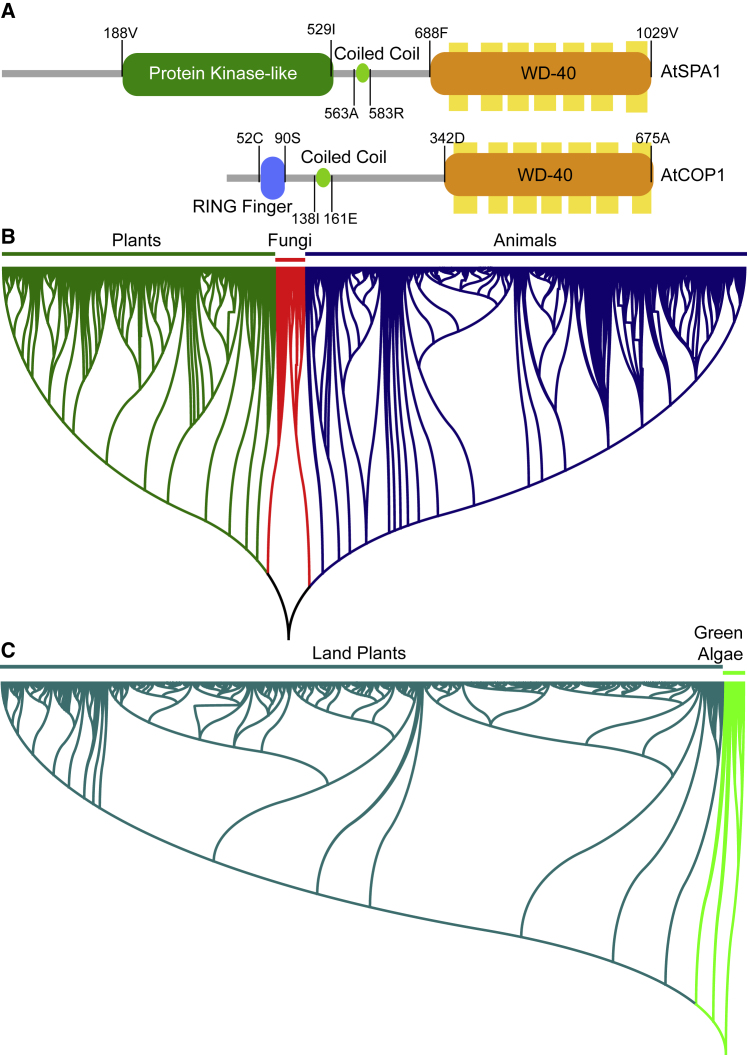
Figure 1.
The Protein Domains and Phylogenetic Analyses of COP1 and SPAs.
(A) Schematic diagrams showing functional domains of Arabidopsis SPA1 and COP1. Conserved domains are shown in the same shapes and colors. Numbers indicate the positions of amino acids.
(B) The phylogenetic relationships of COP1 orthologs. Using AtCOP1 as query, all significantly similar proteins in HMMER reference proteomes containing the COP1 structural domains shown in (A) were identified as COP1 orthologs. The maximum-likelihood tree was constructed following our previous study (Han et al., 2019). COP1 exists widely in eukaryotic clades, and only in eukaryotes.
(C) The phylogenetic relationships of SPA1 orthologs. Candidate SPA1 orthologs were identified as described above. SPA1 orthologs found in HMMER reference proteomes were combined with those previously identified in representative plant genomes (Han et al., 2019). SPA1 orthologs are only present in green plant lineages.
Arabidopsis seedlings carrying cop1-weak alleles exhibit strong photomorphogenic developmental patterns even in complete darkness, with typical short hypocotyls as well as open and expanded cotyledons (Deng and Quail, 1992, McNellis et al., 1994). The nuclear accumulation of COP1 can be drastically reduced by extended light exposure (von Arnim and Deng, 1994), but restored under shade (Pacín et al., 2013). Photoactivated photoreceptors interact with the WD40 domain of COP1 via their VP domains, sequestering COP1 from its interacting substrate proteins, so as to repress COP1 E3 activity and direct photomorphogenic development (Osterlund et al., 2000a, Osterlund et al., 2000b, Lau and Deng, 2012, Lau et al., 2019, Ponnu et al., 2019).
COP1 is also a hotspot of investigation in mammals because of its multi-faceted roles in mammalian development, metabolism, and tumorigenesis (Liu et al., 2008, Marine, 2012, Sanchez-Barcelo et al., 2016). It has E3 ligase activity toward various substrate proteins, including the oncoprotein c-Jun (Bianchi et al., 2003, Yi et al., 2005) and tumor suppressor p53 (Dornan et al., 2004, Koeppen and Dixit, 2004). Although it is ubiquitously expressed and harbors three conserved domains like Arabidopsis COP1, mammalian COP1 failed to rescue the defects of Arabidopsis cop1 mutants (Bianchi et al., 2003, Marine, 2012). However, similarly with Arabidopsis COP1, the localization of mammalian COP1 could be regulated by light when expressed in plant cells (Wang et al., 1999). In addition, human COP1 possesses intrinsic E3 ligase activity in its RING domain together with the coiled-coil domain in vitro (Dornan et al., 2004), and targets tumorigenic factors for degradation in vivo (Bianchi et al., 2003, Migliorini et al., 2011, Vitari et al., 2011). Human COP1 basically localizes in the nuclear envelope, and extracellular cues rapidly induce its nucleoplasm localization (Ouyang et al., 2020). Human COP1 also undergoes nuclear export. This process, correlated with its stability, is controlled by a DNA-damage stimulus and a regulatory chaperone, 14-3-3σ (Dornan et al., 2006, Su et al., 2010).
COP1 orthologs exist extensively in eukaryotes in addition to those studied previously, (Figure 1B). Although fungal COP1 orthologs have not been reported to function in physiological and developmental regulation, they may potentially play key roles relying on the three conserved functional domains. No candidate COP1 ortholog was found in the HMMER reference proteomes of eubacteria, archaea, or viruses. Therefore, COP1 likely originated in the common ancestor of all eukaryotic organisms.
COP1 Is a Core Subunit of Several Protein Complexes
COP1/SPA Complexes
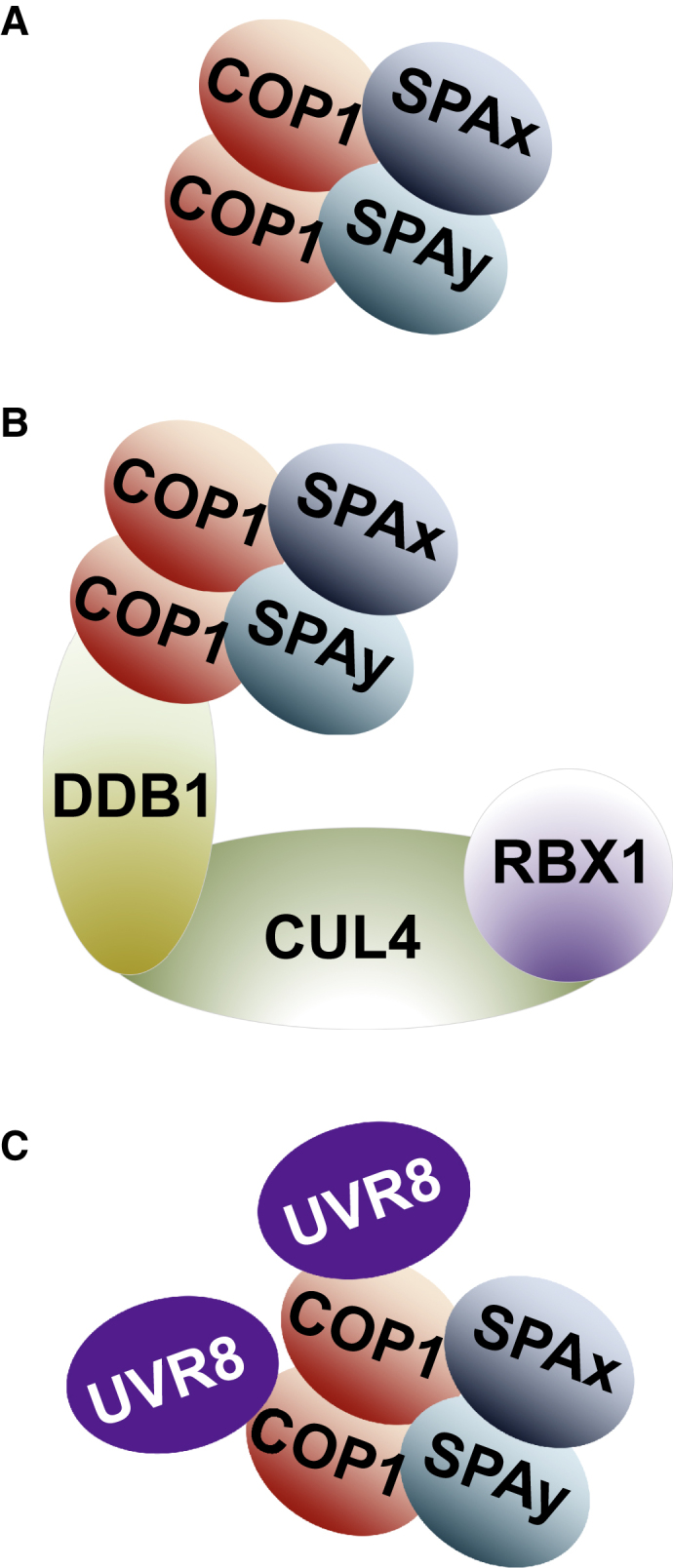
Size-fractionation analyses in higher plants have indicated that COP1 is a part of a ∼700-kDa multimeric protein complex in dark-grown Arabidopsis seedlings (Saijo et al., 2003). SPA1 (SUPPRESSOR OF PHYA-105 1) was the first biochemically identified new component of the COP1 complex (Saijo et al., 2003, Saijo et al., 2008, Zhu et al., 2008), and contains an N-terminal kinase-like domain, a middle coiled-coil domain, and C-terminal WD40 repeats (Figure 1A), with the latter two domains being highly similar to those of COP1 (Hoecker et al., 1999, Laubinger et al., 2004). SPA1 is a nucleus-localized repressor of phyA signaling (Hoecker et al., 1999) and physically interacts with COP1 and HY5 to mediate HY5 degradation (Saijo et al., 2003). SPA1 belongs to an Arabidopsis gene family of consisting of four members that function redundantly in photomorphogenesis (Laubinger et al., 2004, Fittinghoff et al., 2006). These members associate tightly with COP1 to form hetero-tetrameric complexes (Figure 2A) (Saijo et al., 2008, Zhu et al., 2008), and probably act as regulatory subunits of large COP1/SPA complexes in multiple light-signaling events (Lau and Deng, 2012, Hoecker, 2017, Podolec and Ulm, 2018). Besides the dark-induced degradation of HY5, SPAs are also necessary for the light-induced phosphorylation, ubiquitination, and degradation of PIF1 (Saijo et al., 2003, Zhu et al., 2008, Zhu et al., 2015, Paik et al., 2019). To modulate the E3 ligase activity of COP1, SPAs not only interact with COP1-interacting photoreceptors (Lian et al., 2011, Liu et al., 2011, Zuo et al., 2011, Lu et al., 2015, Sheerin et al., 2015) but also regulate the subcellular localization of COP1 (Balcerowicz et al., 2017). The stability of SPA2 itself is rapidly downregulated by light exposure in a phytochrome-dependent fashion1 (Balcerowicz et al., 2011; Chen et al., 2015).
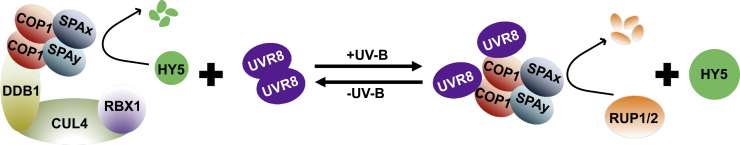
Figure 2.
Three COP1/SPA-Containing Complexes Are Involved in Light Regulation of Plant Development.
(A) COP1/SPA core tetramer complex.
(B) CUL4–DDB1–COP1/SPA ubiquitin ligase complex.
(C) UVR8–COP1/SPA complex.
Unlike COP/DET/FUS proteins, SPA proteins are only found in green algae and land plants (Han et al., 2019, Figure 1C). Therefore, COP1/SPA protein complexes are specific to the green plant lineage. As mammalian COP1 also exists in a large protein complex of ∼700 kDa (Yi et al., 2002), the molecular mechanism for multimerization is possibly conserved and dependent on COP1-interacting, SPA-like, but mammalian-lineage-specific proteins. Since COP1 and the SPAs have conserved coiled-coil and WD40 domains (Figure 1A), formation of a core COP1 tetramer in mammals is also a possibility.
CUL4-Based COP1 Complexes
CUL4 (CULLIN4) is a well-conserved scaffold protein in multimeric CULLIN-based E3 ligases (Jackson and Xiong, 2009). Through an adaptor protein, DDB1 (DNA DAMAGE BINDING PROTEIN 1), CUL4 recruits DWD (DDB1 BINGING WD40) proteins, which serve as substrate receptors, to form multimeric E3 ligases regulating diverse biological processes in plants and animals (Lee and Zhou, 2007). In Arabidopsis, CUL4 negatively regulates photomorphogenesis and flowering by biochemically linking three COP/DET/FUS complexes: COP1/SPA complexes, CSN (COP9 signalosome), and the CDD (COP10, DDB1, and DET1) complex (Yanagawa et al., 2004, Chen et al., 2006, Chen et al., 2010, Zhu et al., 2008). Distinct from human COP1, which associates with CUL4–DDB1 by interacting with DET1 (Wertz et al., 2004), Arabidopsis COP1 and SPA proteins are all DWD proteins that directly associate with CUL4–DDB1 independently of DET1 (Figure 2B; Chen et al., 2010). Consequently, CUL4–DDB1–COP1/SPA works in concert with CUL4–CDD to destabilize HY5 and repress photomorphogenesis in darkness (Figure 2B; Yanagawa et al., 2004, Chen et al., 2006, Chen et al., 2010, Huang et al., 2014). One way to attenuate CUL4–DDB1–COP1/SPA E3 ligase activity is to degrade its cofactor PIF1 in response to light (Zhu et al., 2015).
UVR8–COP1/SPA Complexes
Unlike its repressive role under visible light, COP1 positively regulates UV-B light signaling that is initiated by the UV-B photoreceptor UVR8 (UV RESISTANCE LOCUS 8) (Figure 3). In a rapid response to UV-B light, UVR8 is photoactivated and monomerized, allowing COP1 to recruit it into COP1/SPA complexes via direct interaction Wu et al., 2012 (Favory et al., 2009, Heijde and Ulm, 2013, Huang et al., 2013). It is worth noting that the interaction between COP1 and UVR8 relies on the flexible C-terminal WD40 domain of COP1 and the VP motif of UVR8 (Figure 2C; Rizzini et al., 2011, Cloix et al., 2012, Yin et al., 2015, Lau et al., 2019). Such an interaction mode is generally employed by COP1-targeted photoreceptors and transcription factors in order to compete for the binding domain and modulate the E3 ligase activity of COP1 (Lau et al., 2019, Ponnu et al., 2019). In addition, the assembly of the UV-B-specific UVR8–COP1/SPA complexes leads to a reduced amount of CUL4–DDB1–COP1/SPA complexes in vivo (Huang et al., 2013) and a switch of the substrate receptor for HY5 degradation from COP1/SPA to RUP1/2 (REPRESSOR OF UV-B PHOTOMORPHOGENESIS 1/2), which is the substrate-recognizing module in another CUL4–DDB1 E3 ligase (Ren et al., 2019b). Furthermore, the positive role of COP1 in UVR8 signaling is closely tied to its role in the polyubiquitination and degradation of RUP1/2 (Ren et al., 2019b). Therefore, the disruption of these two E3 ligases (CUL4–DDB1–COP1/SPA and CUL4–DDB1–RUP1/2) that target HY5 protects HY5 from degradation and allows it to accumulate and mediate downstream UV-B-responsive gene expression (Ren et al., 2019b, Jin and Zhu, 2019). Besides RUP1/2, UVR8 and COP1 also destabilize PIF4/5, which leads to UV-B-induced inhibition of hypocotyl elongation (Sharma et al., 2019).
Figure 3.
A Model Decipicting the Conversion between Two Distinct COP1/SPA-Containing Complexes in the Presence or Absence of UV-B Light.
Without UV-B irradiation, the CUL4–DDB1–COP1/SPA complex is the primary functional COP1/SPA core tetramer, and targets HY5 and other promoters of light signaling for degradation to repress photomorphogenic or photoprotective gene expression. RUP1 and RUP2 function redundantly to mediate UVR8 redimerization and halt UVR8 signaling (Heijde and Ulm, 2013). Upon UV-B irradiation, UVR8 monomerizes and associates with the COP1/SPA core tetramer complex to form a new complex. The E3 ligase responsible for HY5 degradation switches from CUL4–DDB1–COP1/SPA to CUL4–DDB1–RUP1/2. COP1, possibly in the UVR8–COP1/SPA complex, targets RUP1/2 for proteolysis and thus stabilizes HY5.
COP1 Plays Evolutionarily Conserved and Divergent Roles in Plants
COP1: A Central Repressor of Photomorphogenesis in Arabidopsis
In the past 30 years, tremendous progress has been achieved in the characterization of hierarchical regulatory networks in Arabidopsis photomorphogenesis (Figure 4). Readers are encouraged to consult these specific reviews for detailed information (Yi and Deng, 2005, Jiao et al., 2007, Lau and Deng, 2012, Huang et al., 2014, Pan and Shi, 2017). Photoreceptors responsible for light perception are categorized into three groups according to the light wavelengths they detect. Far-red and red light is perceived by phytochromes (PHYs) (Bae and Choi, 2008). Blue and UV-A light is perceived by cryptochromes (CRYs), phototropins (PHOTs), and Zeitlupes (ZTLs) (Lin and Shalitin, 2003, Christie, 2007, Suetsugu and Wada, 2013). UV-B light is perceived by UVR8 (Rizzini et al., 2011). Light modulates the protein conformation, subcellular localization, and molecular activity of photoreceptors. Downstream of activated photoreceptors a series of regulatory factors, which usually function in the form of multimeric protein complexes, mediate light signal transduction (Chen et al., 2010, Huang et al., 2013, Paik et al., 2019, Ren et al., 2019b). COP/DET/FUS proteins have been widely recognized as the central repressors of photomorphogenesis networks, functioning in the form of E3 ligase complexes, such as COP1/SPA complexes and their regulators (Lau and Deng, 2012). The key transcription factors that are targeted by central repressor complexes directly control the transcription of light-responsive genes.
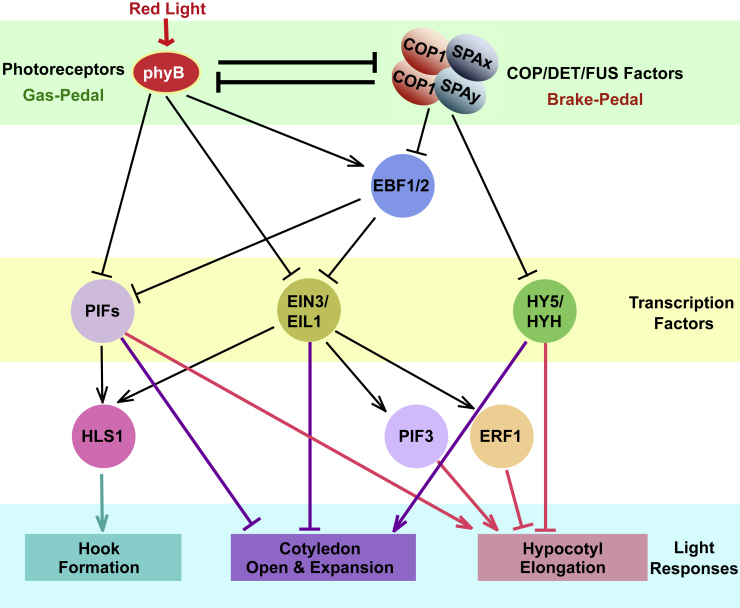
Figure 4.
A Simplified Network Antagonistically Regulated by phyB and the COP1/SPA Complex during Photomorphogenesis in Arabidopsis.
Photoreceptors perceive environmental light signals to promote light responses, while COP/DET/FUS proteins function as central repressors to inhibit light responses. Under red light conditions, activated phyB inhibits the activity of the photomorphogenesis-repressing transcription factors PIFs (Huq and Quail, 2002, Khanna et al., 2004, Leivar et al., 2008), and EIN3/EIL (Shi et al., 2016). On the contrary, the COP1/SPA complex stabilizes these transcription factors by targeting their E3 ligase EBF1/2 for degradation (Dong et al., 2017, Shi et al., 2016), and meanwhile destabilizes photomorphogenesis-promoting transcription factor HY5/HYH (Ang et al., 1998, Holm et al., 2002, Oyama et al., 1997, Saijo et al., 2003, Yanagawa et al., 2004) to maintain skotomorphogenesis in darkness. PIFs and EIN3/EIL1 act in parallel to activate HLS1 expression to promote the formation of the apical hook (Lyu et al., 2019, Zhang et al., 2018). These two groups of transcription factors cooperatively repress cotyledon development but promote hypocotyl elongation (Shi et al., 2018, Zhang et al., 2018, Zhong et al., 2012). In contrast, HY5/HYH promote cotyledon expansion but inhibit hypocotyl elongation by regulating the expression of light-responsive genes.
In darkness, photoreceptors are inactive (Heijde and Ulm, 2012, Ahmad, 2016, Pham et al., 2018a), and COP1 is largely present in the nucleus as part of COP1/SPA E3 ligase complexes (von Arnim and Deng, 1994, Zhu et al., 2008). COP1/SPA complexes are most likely to form larger E3 complexes with the CUL4 scaffold via the DDB1 adaptor (Chen et al., 2010). These COP1-containing complexes, in concert with other E3 complexes (Saijo et al., 2008, Zhu et al., 2008, Chen et al., 2010), target photomorphogenesis-promoting transcription factors such as HY5/HYH (Holm et al., 2002, Saijo et al., 2003) and LAF1 (Seo et al., 2003) for ubiquitination and degradation through the 26S-proteasome pathway (Lau and Deng, 2012). On the other hand, COP1 positively regulates photomorphogenesis-repressing transcription factors, PIFs (PHYTOCHROME-INTERACTING FACTORS) (Bauer et al., 2004, Ling et al., 2017, Pham et al., 2018b), and EIN3/EIL (ETHYLENE-INSENSITIVE3/EIN3-LIKE 1) (Shi et al., 2016) to maintain skotomorphogenesis (Pan and Shi, 2017). COP1 stabilizes PIFs and EIN3/EIL by targeting CUL1–EBF1/2, the E3 ligases for PIFs and EIN3/EIL, for ubiquitination and degradation (Shi et al., 2016, Dong et al., 2017). PHYs and CRYs are the primary receptors sensing visible light. Photoactivation of these photoreceptors impairs the assembly and activity of CUL4–COP1/SPA complexes (Hoecker, 2017, Podolec and Ulm, 2018). Release of its substrates from COP1 alters the expression levels of light-responsive genes (Lee et al., 2007, Paik et al., 2019). In negative feedback regulation, CUL4–DDB1–COP1/SPA complexes target photoreceptors including PHYs and CRYs for 26S-proteosome-mediated degradation (Seo et al., 2004, Weidler et al., 2012, Debrieux et al., 2013). Therefore, COP/DET/FUS proteins, which act like a brake, antagonize the photoreceptors in the photomorphogenesis regulatory network, which act like the gas pedal (accelerator). The COP/DET/FUS proteins and photoreceptors thus coordinate to precisely control downstream signaling regulators and events (Figure 4).
Notably, although initiated by specific photoreceptors, multiple light-signaling pathways converge at COP1 and directly modulate COP1 activity on specific targets (Lau and Deng, 2012, Podolec and Ulm, 2018). COP1 interacts with both photoreceptors and its target transcription factors via its WD40 repeats. The components of such photoreceptor–COP1–target modules seem to have co-evolved to preserve their tripartite interaction pattern during plant terrestrialization (Lau et al., 2019, Wang and Lin, 2019).
Role of COP1 in Seedling Emergence from Soil in Arabidopsis
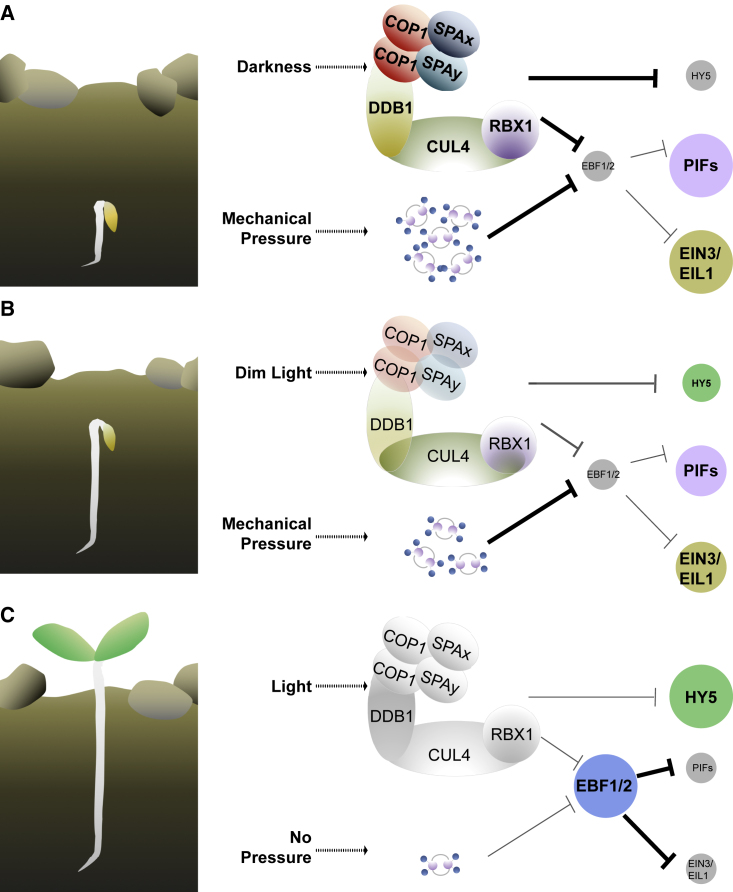
A COP1-containing apparatus is employed in Arabidopsis while seedlings effectively penetrate upward through soil. In nature, a germinating seed buried under the soil has to deal with two issues before it reaches the light: the distance from the soil surface and mechanical pressure by the covering soil. The mechanical pressure leads to the production of ethylene while the reduction of distance to soil surface is measured by a slight increase of dim light, which downregulates COP1 activity (Figure 5). Recent studies have revealed that COP1 directly targets EBF1/2 (EIN3-BINDING F-BOX protein 1/2) for ubiquitination and degradation through its E3 activity, while EBF1/2 degrades the central transcription factors EIN3/EIL1 (EIN3-LIKE 1) and PIFs in darkness (Shi et al., 2016, Dong et al., 2017). It is therefore thought that the light-signaling repressor COP1 is also involved in measuring the distance from a germinating seedling to soil surface and coordinates with the mechanical pressure-triggered ethylene signaling cascade to optimally allow seedlings to emerge from soil (Figure 5). So far, three families of transcription factors, HY5, EIN3/EIL, and PIFs, have been reported to play keys roles in seedling emergence, all of which are regulated by COP1 (Shi et al., 2018). Upon seed germination in soil (Figure 5A), Under mechanical pressure, which triggers an ethylene response, and darkness, HY5 is degraded by the nuclear COP1 apparatus, whereas PIFs and EIN3/EIL1, the negative regulators of photomorphogenesis, are relatively stable and promote skotomorphogenesis. During the course of hypocotyl elongation, the increasing amount of dim light that penetrates through soil pores negatively modulates COP1 activity and indirectly modulates EIN3/EIL activities (Figure 5B). When seedlings finally break out of the soil, COP1 is inactivated by light, and EIN3/EIL and PIFs are targeted by accumulated EBF1/2 for degradation, which switches the developmental pattern from skotomorphogenesis to photomorphogenesis (Figure 5C).
Figure 5.
The Seedling Emergence Signaling Networks Regulated by Light and Mechanical Pressure.
(A) Upon germination deep in the soil, the seedlings covered by soil face two stress stimuli: darkness and mechanical pressure. The active COP1/SPA complexes promote skotomorphogenesis by regulating three transcription factors (HY5, PIFs, and EIN3). In addition, mechanical impedance boosts endogenous ethylene accumulation, which induces crosstalk between ethylene and light signals.
(B) During the course of hypocotyl elongation under soil, dim light penetrates into soil and reduces the activity of COP1/SPA complexes. However, ethylene responses remain highly variable depending on soil conditions.
(C) After breaking out of the soil, seedlings free from mechanical pressure and under sunlight switch to photomorphogenic development. The activity of COP1/SPA complexes is completely repressed.
COP1 Regulates Gravitropism in Physcomitrella
Nine COP1 orthologs and two SPA orthologs have been found in the moss Physcomitrella patens. PpCOP1 partially rescued the defects of the Arabidopsis cop1 null mutant, indicating a conserved function of COP1 in land plants (Ranjan et al., 2014). However, PpSPAb failed to rescue the Arabidopsis spa triple mutant, implying the functional divergence of SPA genes during the evolution of land plants (Ranjan et al., 2014). Dark-cultivated moss grows in an opposite direction to gravity, presumably to break through the soil and get back under light as soon as possible (Sack et al., 2001). This reversed gravitropic response in moss is regarded as a physiological response comparable with skotomorphogenesis in Arabidopsis. PpCOP1 is required for this process and physically interacts with PpHY5 and PpSPA (Yamawaki et al., 2011, Artz et al., 2019, Kreiss et al., 2019). PpspaAB double mutants exhibited reduced gametophore gravitropism but normal etiolation in darkness (Ranjan et al., 2014, Artz et al., 2019). Light-responsive gene expression in these mutants is mostly not constitutive, further supporting functional divergence of moss and Arabidopsis SPA genes (Artz et al., 2019). Therefore, it is reasonable to speculate that COP1 was more functionally conserved than SPA during the evolution of plants.
COP1 Is Involved in Photoprotective Responses in Chlamydomonas
To minimize the damage from high light irradiation, photosynthetic organisms have evolved a protection mechanism termed non-photochemical quenching (Niyogi and Truong, 2013). In the green alga Chlamydomonas reinhardtii grown under high light conditions, LHCSR (LIGHT-HARVESTING COMPLEX STRESS RELATED PROTEIN) genes are activated to mediate energy-dependent quenching (Peers et al., 2009). The newly evolved Chlamydomonas photoreceptor PHOT is responsible for the initiation of photoprotective signal transduction and light-dependent LHCSR expression under high light intensity (Li and Mathews, 2016, Petroutsos et al., 2016).
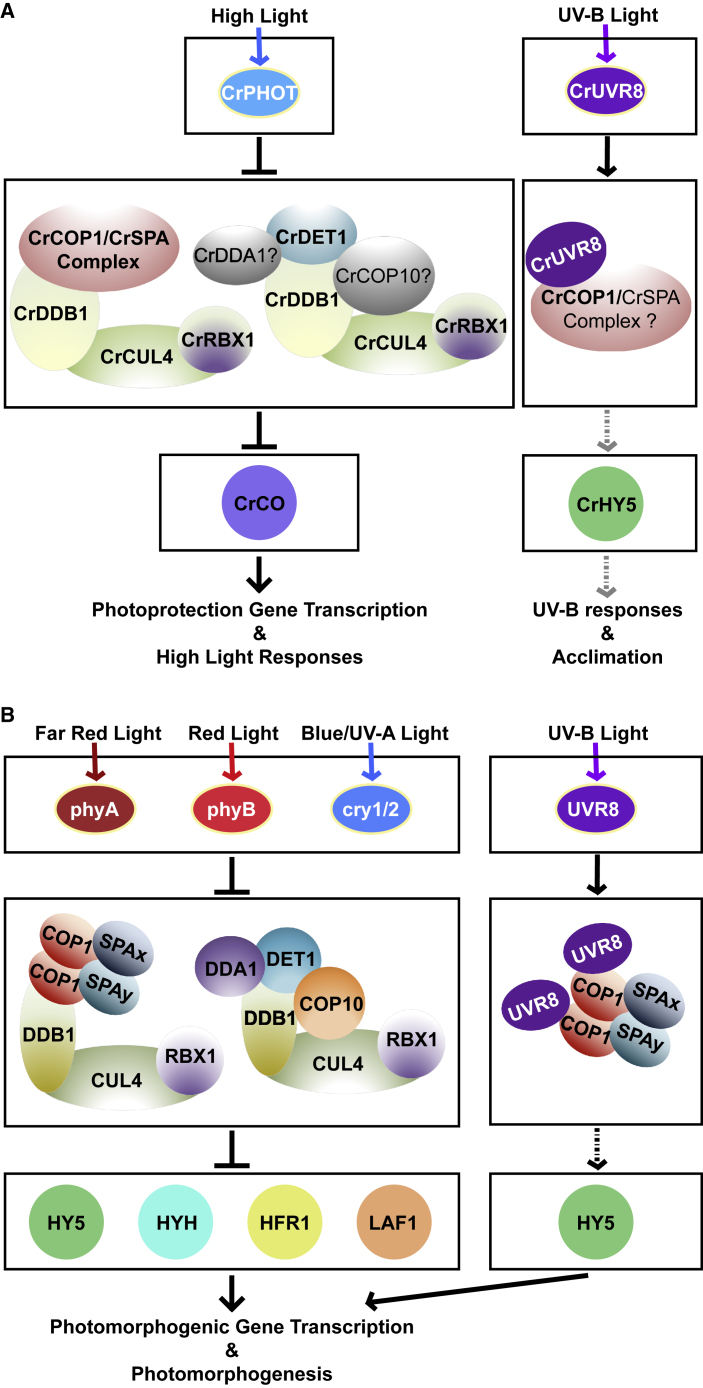
In 2015, two Chlamydomonas mutants showing tolerance to high light were identified to harbor different mutations within the COP1 locus. This study uncovered a novel role of COP1 in high light adaptation in unicellular green algae (Schierenbeck et al., 2015). A subsequent suppressor screen for phot mutants identified DET1 and DDB1, which both encode the components of the CUL4-based E3 ligase (Aihara et al., 2019). Another two genetic screens based on deficient LHCSR expression in Chlamydomonas also identified a series of core regulators: CO (CONSTANS), CUL4, and SPA (Gabilly et al., 2019, Tokutsu et al., 2019). It has been suggested that CrCOP1 and CrSPA form complexes and that they associate with the CUL4–DDB1 scaffold under high light conditions (Figure 6A; Gabilly et al., 2019). Similarly to their coordinated roles in Arabidopsis photomorphogenesis, both CUL4–DDB1–COP1/SPA and CUL4–DDB1–DET1 function as putative E3 ligases in the photoprotective response to high light in Chlamydomonas (Figure 6A; Aihara et al., 2019, Gabilly et al., 2019). Remarkably, CrCO is a target of the COP1/SPA E3 complex in Chlamydomonas under high light conditions (Figure 6A; Gabilly et al., 2019, Tokutsu et al., 2019). While CO controls flowering time in Arabidopsis, CrCO is functionally more similar to Arabidopsis HY5 in activating high light-responsive gene expression. A regulatory module consisting of COP/DET/FUS E3 ligases and CO seems to have been established in an ancestral green photosynthetic organism when both SPA and PHOT evolutionarily appeared (Han et al., 2019, Tokutsu et al., 2019).
Figure 6.
Conserved COP/DET/FUS Proteins Are Involved in Distinct Light-Regulatory Pathways in Chlamydomonas and Arabidopsis.
(A) In Chlamydomonas, CrPHOT senses high light and induces photoprotection responses. In low light, both the CDD complex and COP1/SPA complex repress high light responses. In high light the activity of these E3 ubiquitin ligases is inhibited, allowing CrCO accumulation and the expression of high light-responsive genes. Under UV-B light, activated CrUVR8 monomers interact with CrCOP1 and induce the accumulation of CrHY5, and thus promote UV-B responses and acclimation.
(B) In Arabidopsis, light-activated photoreceptors inactivate COP/DET/FUS protein complexes, thus allowing the accumulation of photomorphogenesis-promoting regulators such as HY5 to globally activate light-responsive genes and photomorphogenic development. Similarly to Chlamydomonas CrUVR8, Arabidopsis UVR8 senses UV-B light and forms a new complex with the COP1/SPA core apparatus, which indirectly promotes the accumulation of HY5 and photomorphogenic development.
In Chlamydomonas, the UV-B photoreceptor UVR8 is responsible for UV-B acclimation and responses (Allorent et al., 2016). The cop1 mutation resulted in the loss of UV-B response and acclimation, suggesting a critical role of COP1 (Tilbrook et al., 2016). Although the UV-B photoreceptor UVR8 only originated in Chlorophytes including Chlamydomonas, the CrUVR8–CrCOP1 regulatory module seems to function in the same manner as in Arabidopsis (Tilbrook et al., 2016). Additionally, the interaction among CrUVR8, CrCOP1, and CrSPA implies the formation of a UVR8–COP1/SPAs complex under UV-B light (Tilbrook et al., 2016, Tokutsu et al., 2019), in a manner conserved with that in Arabidopsis (Figure 6A). Although the transcript level of CrHY5 is upregulated in response to UV-B light in a UVR8-dependent manner (Tilbrook et al., 2016), whether CrHY5 is the core regulator of UV-B photomorphogenesis in green algae is still unresolved.
It is worth noting that PHOT, UVR8, and SPAs are green plant lineage specific, whereas COP1/DET/FUS proteins are ubiquitously present in all eukaryotic organisms. COP1/SPA complexes are involved in both PHOT-mediated high light protection and UVR8-mediated UV-B response in Chlamydomonas, although SPA, PHOT, and UVR8 only originated in Chlorophytes. Thus, COP1/SPA complexes in higher plants have evolved a role in seedling photomorphogenesis, and have maintained their conserved roles in UV-B response. Taken together, the roles of COP1/SPA complexes in the light regulation of plant development differ in specific green plant lineages, from photoprotection, to gravitropism, to photomorphogenesis.
Concluding Remarks and Future Perspectives
COP1 is a highly conserved E3 ligase that possibly originated in the common ancestor of all eukaryotes. COP1 is involved in diverse biological processes in plants and animals, including development and metabolism, and responses to abiotic and biotic stimuli (Lau and Deng, 2012, Marine, 2012, Artz et al., 2019, Sharma et al., 2019, Tokutsu et al., 2019, Wang and Lin, 2019). The pleiotropic roles of COP1 are at least partly determined by its functional domains, which are responsible for flexible protein–protein interactions (Uljon et al., 2016). COP1 selectively targets interacting proteins to function in specific contexts. From an evolutionary point of view, COP1 and its interacting partners display an intricate co-evolutionary pattern and their regulatory activity has been optimized during plant terrestrialization (Han et al., 2019, Lau et al., 2019). However, it is certainly of great interest to study how exactly COP1 acts at the biochemical and cellular levels in each biological process in future investigations.
There are recent reports that in a number of cases, COP1/SPA-interacting proteins somehow become activated/stabilized rather than degraded, such as PIF3 in darkness (Ling et al., 2017), PIF5 under shady environments (Sharma et al., 2019), and HY5 under UV-B light (Huang et al., 2013). Thus, non-canonical roles, e.g., certain COP1 activities not resulting from its proteolysis mechanism, may be involved. While COP1 directly interacts with BIN2 and modulates its kinase activity (Ling et al., 2017), the initially reported stabilization of HY5 by COP1/SPA under UV-B light is likely due to the UV-B-induced formation of the new UVR8–COP1/SPA complex, which targets RUP1/2 instead of HY5 for degradation (Ren et al., 2019b). It is likely that more possible non-canonical roles of COP1/SPA will be uncovered in future studies.
As tightly related partners of COP1, SPA proteins are absent in all other organisms except green lineage plants. Therefore, COP1 functions without assistance from SPA “stabilizers” in ancient algae systems, such as red alga, or in animals. In the case of algae systems without SPAs, what is the configuration of the core COP1 complex? Does COP1 act via similar mechanisms in early-originating algae and in animal systems? These open questions, together with the exact structural and functional significance of SPAs in COP1 complexes in green plants, await further investigation.
Funding
This work was supported by grants from National Key R&D Program of China (2017YFA0503800), National Natural Science Foundation of China (31330048, 31621001), Peking-Tsinghua Center for Life Sciences, Peking University, Southern University of Science and Technology, and Xiamen University.
Author Contributions
X. Han, X. Huang, and X.W.D. wrote the manuscript.
Acknowledgments
We thank our colleagues for their excellent work on photomorphogenesis. No conflict of interest declared.
Published: April 12, 2020
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and IPPE, CAS.
References
- Ahmad M. Photocycle and signaling mechanisms of plant cryptochromes. Curr. Opin. Plant Biol. 2016;33:108–115. doi: 10.1016/j.pbi.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Aihara Y., Fujimura-Kamada K., Yamasaki T., Minagawa J. Algal photoprotection is regulated by the E3 ligase CUL4-DDB1DET1. Nat. Plants. 2019;5:34–40. doi: 10.1038/s41477-018-0332-5. [DOI] [PubMed] [Google Scholar]
- Allorent G., Lefebvre-Legendre L., Chappuis R., Kuntz M., Truong T.B., Niyogi K.K., Ulm R., Goldschmidt-Clermont M. UV-B photoreceptor-mediated protection of the photosynthetic machinery in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. U S A. 2016;113:14864–14869. doi: 10.1073/pnas.1607695114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang L.H., Deng X.-W. Regulatory hierarchy of photomorphogenic loci: allele-specific and light-dependent interaction between the HY5 and COP1 loci. Plant Cell. 1994;6:613–628. doi: 10.1105/tpc.6.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang L.-H., Chattopadhyay S., Wei N., Oyama T., Okada K., Batschauer A., Deng X.-W. Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol. Cell. 1998;1:213–222. doi: 10.1016/s1097-2765(00)80022-2. [DOI] [PubMed] [Google Scholar]
- von Arnim A.G., Deng X.-W. Light inactivation of Arabidopsis photomorphogenic repressor COP1 involves a cell-specific regulation of its nucleocytoplasmic partitioning. Cell. 1994;79:1035–1045. doi: 10.1016/0092-8674(94)90034-5. [DOI] [PubMed] [Google Scholar]
- von Arnim A.G., Deng X.-W. Light control OF seedling development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:215–243. doi: 10.1146/annurev.arplant.47.1.215. [DOI] [PubMed] [Google Scholar]
- Artz O., Dickopf S., Ranjan A., Kreiss M., Abraham E.T., Boll V., Rensing S.A., Hoecker U. Characterization of spa mutants in the moss Physcomitrella provides evidence for functional divergence of SPA genes during the evolution of land plants. New Phytol. 2019;224:1412–14134. doi: 10.1111/nph.16004. [DOI] [PubMed] [Google Scholar]
- Bae G., Choi G. Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 2008;59:281–311. doi: 10.1146/annurev.arplant.59.032607.092859. [DOI] [PubMed] [Google Scholar]
- Balcerowicz M., Fittinghoff K., Wirthmueller L., Maier A., Fackendahl P., Fiene G., Koncz C., Hoecker U. Light exposure of Arabidopsis seedlings causes rapid de-stabilization as well as selective post-translational inactivation of the repressor of photomorphogenesis SPA2: functional divergence between SPA1 and SPA2. Plant J. 2011;65:712–723. doi: 10.1111/j.1365-313X.2010.04456.x. [DOI] [PubMed] [Google Scholar]
- Balcerowicz M., Kerner K., Schenkel C., Hoecker U. SPA proteins affect the subcellular localization of COP1 in the COP1/SPA ubiquitin ligase complex during photomorphogenesis. Plant Physiol. 2017;174:1314–1321. doi: 10.1104/pp.17.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D., Viczián A., Kircher S., Nobis T., Nitschke R., Kunkel T., Panigrahi K.C.S., Ádám É., Fejes E., Schäfer E. Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell. 2004;16:1433–1445. doi: 10.1105/tpc.021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E., Denti S., Catena R., Rossetti G., Polo S., Gasparian S., Putignano S., Rogge L., Pardi R. Characterization of human constitutive photomorphogenesis protein 1, a RING finger ubiquitin ligase that interacts with jun transcription factors and modulates their transcriptional activity. J. Biol. Chem. 2003;278:19682–19690. doi: 10.1074/jbc.M212681200. [DOI] [PubMed] [Google Scholar]
- Chamovitz D.A., Deng X.W. The novel components of the Arabidopsis light signaling pathway may define a group of general developmental regulators shared by both animal and plant kingdoms. Cell. 1995;82:353–354. doi: 10.1016/0092-8674(95)90423-9. [DOI] [PubMed] [Google Scholar]
- Chen H., Shen Y., Tang X., Yu L., Wang J., Guo L., Zhang Y., Zhang H., Feng S., Strickland E. Arabidopsis CULLIN4 forms an E3 ubiquitin ligase with RBX1 and the CDD complex in mediating light control of development. Plant Cell. 2006;18:1991–2004. doi: 10.1105/tpc.106.043224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Huang X., Gusmaroli G., Terzaghi W., Lau O.S., Yanagawa Y., Zhang Y., Li J., Lee J.-H., Zhu D. Arabidopsis CULLIN4-damaged DNA binding protein 1 interacts with CONSTITUTIVELY PHOTOMORPHOGENIC1-SUPPRESSOR OF PHYA complexes to regulate photomorphogenesis and flowering time. Plant Cell. 2010;22:108–123. doi: 10.1105/tpc.109.065490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.H., Lee M.-H. CSN6-COP1 axis in cancer. Aging. 2015;7:461–462. doi: 10.18632/aging.100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J., Peto C., Feinbaum R., Pratt L., Ausubel F. Arabidopsis thaliana mutant that develops as a light-grown plant in the absence of light. Cell. 1989;58:991–999. doi: 10.1016/0092-8674(89)90950-1. [DOI] [PubMed] [Google Scholar]
- Christie J.M. Phototropin blue-light receptors. Annu. Rev. Plant Biol. 2007;58:21–45. doi: 10.1146/annurev.arplant.58.032806.103951. [DOI] [PubMed] [Google Scholar]
- Cloix C., Kaiserli E., Heilmann M., Baxter K.J., Brown B.A., O’Hara A., Smith B.O., Christie J.M., Jenkins G.I. C-terminal region of the UV-B photoreceptor UVR8 initiates signaling through interaction with the COP1 protein. Proc. Natl. Acad. Sci. U S A. 2012;109:16366–16370. doi: 10.1073/pnas.1210898109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrieux D., Trevisan M., Fankhauser C. Conditional involvement of CONSTITUTIVE PHOTOMORPHOGENIC1 in the degradation of phytochrome A. Plant Physiol. 2013;161:2136–2145. doi: 10.1104/pp.112.213280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X.-W., Quail P.H. Genetic and phenotypic characterization of cop1 mutants of Arabidopsis thaliana. Plant J. 1992;2:83–95. [Google Scholar]
- Deng X.-W., Caspar T., Quail P.H. cop1: a regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev. 1991;5:1171–1182. doi: 10.1101/gad.5.7.1172. [DOI] [PubMed] [Google Scholar]
- Deng X.W., Matsui M., Wei N., Wagner D., Chu A.M., Feldmann K.A., Quail P.H. COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a Gβ homologous domain. Cell. 1992;71:791–801. doi: 10.1016/0092-8674(92)90555-q. [DOI] [PubMed] [Google Scholar]
- Dong J., Ni W., Yu R., Deng X.W., Chen H., Wei N. Light-dependent degradation of PIF3 by SCFEBF1/2 promotes a photomorphogenic response in Arabidopsis. Curr. Biol. 2017;27:2420–2430.e6. doi: 10.1016/j.cub.2017.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornan D., Wertz I., Shimizu H., Arnott D., Frantz G.D., Dowd P., O’ Rourke K., Koeppen H., Dixit V.M. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature. 2004;429:86–92. doi: 10.1038/nature02514. [DOI] [PubMed] [Google Scholar]
- Dornan D., Shimizu H., Mah A., Dudhela T., Eby M., O’Rourke K., Seshagiri S., Dixit V.M. ATM engages autodegradation of the E3 ubiquitin ligase COP1 after DNA damage. Science. 2006;313:1122–1126. doi: 10.1126/science.1127335. [DOI] [PubMed] [Google Scholar]
- Favory J.-J., Stec A., Gruber H., Rizzini L., Oravecz A., Funk M., Albert A., Cloix C., Jenkins G.I., Oakeley E.J. Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J. 2009;28:591–601. doi: 10.1038/emboj.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittinghoff K., Laubinger S., Nixdorf M., Fackendahl P., Baumgardt R.-L., Batschauer A., Hoecker U. Functional and expression analysis of Arabidopsis SPA genes during seedling photomorphogenesis and adult growth. Plant J. 2006;47:577–590. doi: 10.1111/j.1365-313X.2006.02812.x. [DOI] [PubMed] [Google Scholar]
- Gabilly S.T., Baker C.R., Wakao S., Crisanto T., Guan K., Bi K., Guiet E., Guadagno C.R., Niyogi K.K. Regulation of photoprotection gene expression in Chlamydomonas by a putative E3 ubiquitin ligase complex and a homolog of CONSTANS. Proc. Natl. Acad. Sci. U S A. 2019;116:17556–17562. doi: 10.1073/pnas.1821689116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Chang X., Zhang Z., Chen H., He H., Zhong B., Deng X.W. Origin and evolution of core components responsible for monitoring light environment changes during plant terrestrialization. Mol. Plant. 2019;12:847–862. doi: 10.1016/j.molp.2019.04.006. [DOI] [PubMed] [Google Scholar]
- Heijde M., Ulm R. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 2012;17:230–237. doi: 10.1016/j.tplants.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Heijde M., Ulm R. Reversion of the Arabidopsis UV-B photoreceptor UVR8 to the homodimeric ground state. Proc. Natl. Acad. Sci. U S A. 2013;110:1113–1118. doi: 10.1073/pnas.1214237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecker U. The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr. Opin. Plant Biol. 2017;37:63–69. doi: 10.1016/j.pbi.2017.03.015. [DOI] [PubMed] [Google Scholar]
- Hoecker U., Tepperman J.M., Quail P.H. SPA1, a WD-repeat protein specific to phytochrome A signal transduction. Science. 1999;284:496–499. doi: 10.1126/science.284.5413.496. [DOI] [PubMed] [Google Scholar]
- Holm M., Hardtke C.S., Caudet R., Deng X.-W. Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1. EMBO J. 2001;20:118–127. doi: 10.1093/emboj/20.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm M., Ma L.-G., Qu L.-J., Deng X.-W. Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev. 2002;16:1247–1259. doi: 10.1101/gad.969702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Ouyang X., Yang P., Lau O.S., Chen L., Wei N., Deng X.W. Conversion from CUL4-based COP1-SPA E3 apparatus to UVR8-COP1-SPA complexes underlies a distinct biochemical function of COP1 under UV-B. Proc. Natl. Acad. Sci. U S A. 2013;110:16669–16674. doi: 10.1073/pnas.1316622110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Ouyang X., Deng X.W. Beyond repression of photomorphogenesis: role switching of COP/DET/FUS in light signaling. Curr. Opin. Plant Biol. 2014;21:96–103. doi: 10.1016/j.pbi.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Huq E., Quail P.H. PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J. 2002;21:2441–2450. doi: 10.1093/emboj/21.10.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S., Xiong Y. CRL4s: the CUL4-RING E3 ubiquitin ligases. Trends Biochem. Sci. 2009;34:562–570. doi: 10.1016/j.tibs.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I.-C., Yang J.-Y., Seo H.S., Chua N.-H. HFR1 is targeted by COP1 E3 ligase for post-translational proteolysis during phytochrome A signaling. Genes Dev. 2005;19:593–602. doi: 10.1101/gad.1247205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I.-C., Henriques R., Seo H.S., Nagatani A., Chua N.-H. Arabidopsis PHYTOCHROME INTERACTING FACTOR proteins promote phytochrome B polyubiquitination by COP1 E3 ligase in the nucleus. Plant Cell. 2010;22:2370–2383. doi: 10.1105/tpc.109.072520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Lau O.S., Deng X.W. Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 2007;8:217–230. doi: 10.1038/nrg2049. [DOI] [PubMed] [Google Scholar]
- Jin H., Zhu Z. Dis-RUP for COP1 role-switch under UV-B light. Trends Plant Sci. 2019;24:7. doi: 10.1016/j.tplants.2019.04.006. [DOI] [PubMed] [Google Scholar]
- Kendrick R.E., Kronenberg G.H.M. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1994. Photomorphogenesis in Plants. [Google Scholar]
- Khanna R., Huq E., Kikis E.A., Al-Sady B., Lanzatella C., Quail P.H. A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell. 2004;16:3033–3044. doi: 10.1105/tpc.104.025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-M., Woo J.-C., Song P.-S., Soh M.-S. HFR1, a phytochrome A-signalling component, acts in a separate pathway from HY5, downstream of COP1 in Arabidopsis thaliana. Plant J. 2002;30:711–719. doi: 10.1046/j.1365-313x.2002.01326.x. [DOI] [PubMed] [Google Scholar]
- Koeppen H., Dixit V.M. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature. 2004;429:86–92. doi: 10.1038/nature02514. [DOI] [PubMed] [Google Scholar]
- Kreiss, M., Artz, O., and Hoecker, U. (2019). Evolutionary analysis of the COP1/SPA complex in the moss Physcomitrella patens. Poster presented at: World Congress on Light and Life (17th Congress of the International Union of Photobiology and 18th Congress of the European Society for Photobiology); August 25–30, Barcelona, Spain.
- Kwok S.F., Piekos B., Miséra S., Deng X.-W. A complement of ten essential and pleiotropic Arabidopsis COP/DET/FUS genes is necessary for repression of photomorphogenesis in darkness. Plant Physiol. 1996;110:731–742. doi: 10.1104/pp.110.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O.S., Deng X.W. The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci. 2012;17:584–593. doi: 10.1016/j.tplants.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Lau K., Podolec R., Chappuis R., Ulm R., Hothorn M. Plant photoreceptors and their signaling components compete for COP 1 binding via VP peptide motifs. EMBO J. 2019;38:e102140. doi: 10.15252/embj.2019102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubinger S., Fittinghoff K., Hoecker U. The SPA quartet: a family of WD-repeat proteins with a central role in suppression of photomorphogenesis in Arabidopsis. Plant Cell. 2004;16:2293–2306. doi: 10.1105/tpc.104.024216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Zhou P. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol. Cell. 2007;26:775–780. doi: 10.1016/j.molcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Lee J., He K., Stolc V., Lee H., Figueroa P., Gao Y., Tongprasit W., Zhao H., Lee I., Deng X.W. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell. 2007;19:731–749. doi: 10.1105/tpc.106.047688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Monte E., Al-Sady B., Carle C., Storer A., Alonso J.M., Ecker J.R., Quail P.H. The Arabidopsis phytochrome-interacting factor PIF7, together with PIF3 and PIF4, regulates responses to prolonged red light by modulating phyB levels. Plant Cell. 2008;20:337–352. doi: 10.1105/tpc.107.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F.-W., Mathews S. Evolutionary aspects of plant photoreceptors. J. Plant Res. 2016;129:115–122. doi: 10.1007/s10265-016-0785-4. [DOI] [PubMed] [Google Scholar]
- Lian H.-L., He S.-B., Zhang Y.-C., Zhu D.-M., Zhang J.-Y., Jia K.-P., Sun S.-X., Li L., Yang H.-Q. Blue-light-dependent interaction of cryptochrome 1 with SPA1 defines a dynamic signaling mechanism. Genes Dev. 2011;25:1023–1028. doi: 10.1101/gad.2025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Shalitin D. Cryptochrome structure and signal transduction. Annu. Rev. Plant Biol. 2003;54:469–496. doi: 10.1146/annurev.arplant.54.110901.160901. [DOI] [PubMed] [Google Scholar]
- Lin X.-L., Niu D., Hu Z.-L., Kim D.H., Jin Y.H., Cai B., Liu P., Miura K., Yun D.-J., Kim W.-Y. An Arabidopsis SUMO E3 ligase, SIZ1, negatively regulates photomorphogenesis by promoting COP1 activity. PLoS Genet. 2016;12:e1006016. doi: 10.1371/journal.pgen.1006016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Xu D., Jiang Y., Chen H., Fan L., Holm M., Deng X.W. Phosphorylation and negative regulation of CONSTITUTIVELY PHOTOMORPHOGENIC 1 by PINOID in Arabidopsis. Proc. Natl. Acad. Sci. U S A. 2017;114:6617–6622. doi: 10.1073/pnas.1702984114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Jiang Y., Li J., Yan T., Fan L., Liang J., Chen Z.J., Xu D., Deng X.W. B-BOX DOMAIN PROTEIN28 negatively regulates photomorphogenesis by repressing the activity of transcription factor HY5 and undergoes COP1-mediated degradation. Plant Cell. 2018;30:2006–2019. doi: 10.1105/tpc.18.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J.-J., Li J., Zhu D., Deng X.W. Noncanonical role of Arabidopsis COP1/SPA complex in repressing BIN2-mediated PIF3 phosphorylation and degradation in darkness. Proc. Natl. Acad. Sci. U S A. 2017;114:3539–3544. doi: 10.1073/pnas.1700850114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Dentin R., Chen D., Hedrick S., Ravnskjaer K., Schenk S., Milne J., Meyers D.J., Cole P., Iii J.Y. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456:269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Zuo Z., Liu H., Liu X., Lin C. Arabidopsis cryptochrome 1 interacts with SPA1 to suppress COP1 activity in response to blue light. Genes Dev. 2011;25:1029–1034. doi: 10.1101/gad.2025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X.-D., Zhou C.-M., Xu P.-B., Luo Q., Lian H.-L., Yang H.-Q. Red-light-dependent interaction of phyB with SPA1 promotes COP1-SPA1 dissociation and photomorphogenic development in Arabidopsis. Mol. Plant. 2015;8:467–478. doi: 10.1016/j.molp.2014.11.025. [DOI] [PubMed] [Google Scholar]
- Lyu M., Shi H., Li Y., Kuang K., Yang Z., Li J., Chen D., Li Y., Kou X., Zhong S. Oligomerization and photo-deoligomerization of HOOKLESS1 controls plant differential cell growth. Dev. Cell. 2019;51:78–88.e3. doi: 10.1016/j.devcel.2019.08.007. [DOI] [PubMed] [Google Scholar]
- Ma L., Gao Y., Qu L., Chen Z., Li J., Zhao H., Deng X.W. Genomic evidence for COP1 as a repressor of light-regulated gene expression and development in Arabidopsis. Plant Cell. 2002;14:2383–2398. doi: 10.1105/tpc.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marine J.-C. Spotlight on the role of COP1 in tumorigenesis. Nat. Rev. Cancer. 2012;12:455–464. doi: 10.1038/nrc3271. [DOI] [PubMed] [Google Scholar]
- McNellis T.W., Deng X.W. Light control of seedling morphogenetic pattern. Plant Cell. 1995;7:1749–1761. doi: 10.1105/tpc.7.11.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNellis T.W., von Arnim A.G., Araki T., Komeda Y., Miséra S., Deng X.-W. Genetic and molecular analysis of an allelic series of cop1 mutants suggests functional roles for the multiple protein domains. Plant Cell. 1994;6:487–500. doi: 10.1105/tpc.6.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliorini D., Bogaerts S., Defever D., Vyas R., Denecker G., Radaelli E., Zwolinska A., Depaepe V., Hochepied T., Skarnes W.C. Cop1 constitutively regulates c-Jun protein stability and functions as a tumor suppressor in mice. J. Clin. Invest. 2011;121:1329–1343. doi: 10.1172/JCI45784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miséra S., Müller A.J., Weiland-Heidecker U., Jürgens G. The FUSCA genes of Arabidopsis: negative regulators of light responses. Mol. Genet. Genomics. 1994;244:242–252. doi: 10.1007/BF00285451. [DOI] [PubMed] [Google Scholar]
- Newton K., Dugger D.L., Sengupta-Ghosh A., Ferrando R.E., Chu F., Tao J., Lam W., Haller S., Chan S., Sa S. Ubiquitin ligase COP1 coordinates transcriptional programs that control cell type specification in the developing mouse brain. Proc. Natl. Acad. Sci. U S A. 2018;115:11244–11249. doi: 10.1073/pnas.1805033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi K.K., Truong T.B. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr. Opin. Plant Biol. 2013;16:307–314. doi: 10.1016/j.pbi.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Osterlund M.T., Hardtke C.S., Wei N., Deng X.W. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature. 2000;405:462–466. doi: 10.1038/35013076. [DOI] [PubMed] [Google Scholar]
- Osterlund M.T., Wei N., Deng X.W. The roles of photoreceptor systems and the COP1-targeted destabilization of HY5 in light control of Arabidopsis seedling development. Plant Physiol. 2000;124:1520–1524. doi: 10.1104/pp.124.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W., Guo P., Takeda K., Fu Q., Fang H., Frucht D.M. Erk1/2 inactivation promotes a rapid redistribution of COP1 and degradation of COP1 substrates. Proc. Natl. Acad. Sci. U S A. 2020 doi: 10.1073/pnas.1913698117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T., Shimura Y., Okada K. The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 1997;11:2983–2995. doi: 10.1101/gad.11.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacín M., Legris M., Casal J.J. COP1 re-accumulates in the nucleus under shade. Plant J. 2013;75:631–641. doi: 10.1111/tpj.12226. [DOI] [PubMed] [Google Scholar]
- Paik I., Chen F., Ngoc Pham V., Zhu L., Kim J.-I., Huq E. A phyB-PIF1-SPA1 kinase regulatory complex promotes photomorphogenesis in Arabidopsis. Nat. Commun. 2019;10:4216. doi: 10.1038/s41467-019-12110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Shi H. Stabilizing the transcription factors by E3 ligase COP1. Trends Plant Sci. 2017;22:999–1001. doi: 10.1016/j.tplants.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Peers G., Truong T.B., Ostendorf E., Busch A., Elrad D., Grossman A.R., Hippler M., Niyogi K.K. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature. 2009;462:518–521. doi: 10.1038/nature08587. [DOI] [PubMed] [Google Scholar]
- Petroutsos D., Tokutsu R., Maruyama S., Flori S., Greiner A., Magneschi L., Cusant L., Kottke T., Mittag M., Hegemann P. A blue-light photoreceptor mediates the feedback regulation of photosynthesis. Nature. 2016;537:563–566. doi: 10.1038/nature19358. [DOI] [PubMed] [Google Scholar]
- Pham V.N., Kathare P.K., Huq E. Phytochromes and phytochrome interacting factors. Plant Physiol. 2018;176:1025–1038. doi: 10.1104/pp.17.01384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham V.N., Kathare P.K., Huq E. Dynamic regulation of PIF5 by COP1-SPA complex to optimize photomorphogenesis in Arabidopsis. Plant J. 2018;96:260–273. doi: 10.1111/tpj.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolec R., Ulm R. Photoreceptor-mediated regulation of the COP1/SPA E3 ubiquitin ligase. Curr. Opin. Plant Biol. 2018;45:18–25. doi: 10.1016/j.pbi.2018.04.018. [DOI] [PubMed] [Google Scholar]
- Ponnu J., Riedel T., Penner E., Schrader A., Hoecker U. Cryptochrome 2 competes with COP1 substrates to repress COP1 ubiquitin ligase activity during Arabidopsis photomorphogenesis. Proc. Natl. Acad. Sci. U S A. 2019;116:27133–27141. doi: 10.1073/pnas.1909181116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan A., Dickopf S., Ullrich K.K., Rensing S.A., Hoecker U. Functional analysis of COP1 and SPA orthologs from Physcomitrella and rice during photomorphogenesis of transgenic Arabidopsis reveals distinct evolutionary conservation. BMC Plant Biol. 2014;14:178. doi: 10.1186/1471-2229-14-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Chen N., Chen Y., Liu W., Hu Y. TRB3 stimulates SIRT1 degradation and induces insulin resistance by lipotoxicity via COP1. Exp. Cell Res. 2019;382:111428. doi: 10.1016/j.yexcr.2019.05.009. [DOI] [PubMed] [Google Scholar]
- Ren H., Han J., Yang P., Mao W., Liu X., Qiu L., Qian C., Liu Y., Chen Z., Ouyang X. Two E3 ligases antagonistically regulate the UV-B response in Arabidopsis. Proc. Natl. Acad. Sci. U S A. 2019;116:4722–4731. doi: 10.1073/pnas.1816268116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzini L., Favory J.-J., Cloix C., Faggionato D., O’Hara A., Kaiserli E., Baumeister R., Schäfer E., Nagy F., Jenkins G.I. Perception of UV-B by the Arabidopsis UVR8 protein. Science. 2011;332:103–106. doi: 10.1126/science.1200660. [DOI] [PubMed] [Google Scholar]
- Sack F.D., Schwuchow J.M., Wagner T., Kern V. Gravity sensing in moss protonemata. Adv. Space Res. 2001;27:871–876. doi: 10.1016/s0273-1177(01)00151-x. [DOI] [PubMed] [Google Scholar]
- Saijo Y., Sullivan J.A., Wang H., Yang J., Shen Y., Rubio V., Ma L., Hoecker U., Deng X.W. The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev. 2003;17:2642–2647. doi: 10.1101/gad.1122903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo Y., Zhu D., Li J., Rubio V., Zhou Z., Shen Y., Hoecker U., Wang H., Deng X.W. Arabidopsis COP1/SPA1 complex and FHY1/FHY3 associate with distinct phosphorylated forms of phytochrome A in balancing light signaling. Mol. Cell. 2008;31:607–613. doi: 10.1016/j.molcel.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Barcelo E.J., Mediavilla M.D., Vriend J., Reiter R.J. Constitutive photomorphogenesis protein 1 (COP1) and COP9 signalosome, evolutionarily conserved photomorphogenic proteins as possible targets of melatonin. J. Pineal Res. 2016;61:41–51. doi: 10.1111/jpi.12340. [DOI] [PubMed] [Google Scholar]
- Schierenbeck L., Ries D., Rogge K., Grewe S., Weisshaar B., Kruse O. Fast forward genetics to identify mutations causing a high light tolerant phenotype in Chlamydomonas reinhardtii by whole-genome-sequencing. BMC Genomics. 2015;16:57. doi: 10.1186/s12864-015-1232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C., Deng X.-W. The COP/DET/FUS proteins—regulators of eukaryotic growth and development. Semin. Cell Dev. Biol. 2000;11:495–503. doi: 10.1006/scdb.2000.0203. [DOI] [PubMed] [Google Scholar]
- Seo H.S., Yang J.-Y., Ishikawa M., Bolle C., Ballesteros M.L., Chua N.-H. LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature. 2003;423:995–999. doi: 10.1038/nature01696. [DOI] [PubMed] [Google Scholar]
- Seo H.S., Watanabe E., Tokutomi S., Nagatani A., Chua N.-H. Photoreceptor ubiquitination by COP1 E3 ligase desensitizes phytochrome A signaling. Genes Dev. 2004;18:617–622. doi: 10.1101/gad.1187804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Sharma B., Hayes S., Kerner K., Hoecker U., Jenkins G.I., Franklin K.A. UVR8 disrupts stabilisation of PIF5 by COP1 to inhibit plant stem elongation in sunlight. Nat. Commun. 2019;10:4417. doi: 10.1038/s41467-019-12369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheerin D.J., Menon C., zur Oven-Krockhaus S., Enderle B., Zhu L., Johnen P., Schleifenbaum F., Stierhof Y.-D., Huq E., Hiltbrunner A. Light-activated phytochrome A and B interact with members of the SPA family to promote photomorphogenesis in Arabidopsis by reorganizing the COP1/SPA complex. Plant Cell. 2015;27:189–201. doi: 10.1105/tpc.114.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Liu R., Xue C., Shen X., Wei N., Deng X.W., Zhong S. Seedlings transduce the depth and mechanical pressure of covering soil using COP1 and ethylene to regulate EBF1/EBF2 for soil emergence. Curr. Biol. 2016;26:139–149. doi: 10.1016/j.cub.2015.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Lyu M., Luo Y., Liu S., Li Y., He H., Wei N., Deng X.W., Zhong S. Genome-wide regulation of light-controlled seedling morphogenesis by three families of transcription factors. Proc. Natl. Acad. Sci. U S A. 2018;115:6482–6487. doi: 10.1073/pnas.1803861115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop-Myer C., Torii K.U., McNellis T.W., Coleman J.E., Deng X.-W. Short communication: the N-terminal fragment of Arabidopsis photomorphogenic repressor COP1 maintains partial function and acts in a concentration-dependent manner. Plant J. 1999;20:713–717. doi: 10.1046/j.1365-313x.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- Su C.-H., Zhao R., Velazquez-Torres G., Chen J., Gully C., Yeung S.-C.J., Lee M.-H. Nuclear export regulation of COP1 by 14-3-3s in response to DNA damage. Mol. Cancer. 2010;9:243. doi: 10.1186/1476-4598-9-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N., Wada M. Evolution of three LOV blue light receptor families in green plants and photosynthetic stramenopiles: phototropin, ZTL/FKF1/LKP2 and aureochrome. Plant Cell Physiol. 2013;54:8–23. doi: 10.1093/pcp/pcs165. [DOI] [PubMed] [Google Scholar]
- Tilbrook K., Dubois M., Crocco C.D., Yin R., Chappuis R., Allorent G., Schmid-Siegert E., Goldschmidt-Clermont M., Ulm R. UV-B perception and acclimation in Chlamydomonas reinhardtii. Plant Cell. 2016;28:966–983. doi: 10.1105/tpc.15.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokutsu R., Fujimura-Kamada K., Matsuo T., Yamasaki T., Minagawa J. The CONSTANS flowering complex controls the protective response of photosynthesis in the green alga Chlamydomonas. Nat. Commun. 2019;10:4099. doi: 10.1038/s41467-019-11989-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uljon S., Xu X., Durzynska I., Stein S., Adelmant G., Marto J.A., Pear W.S., Blacklow S.C. Structural basis for substrate selectivity of the E3 ligase COP1. Structure. 2016;24:687–696. doi: 10.1016/j.str.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitari A.C., Leong K.G., Newton K., Yee C., O’Rourke K., Liu J., Phu L., Vij R., Ferrando R., Couto S.S. COP1 is a tumour suppressor that causes degradation of ETS transcription factors. Nature. 2011;474:403–406. doi: 10.1038/nature10005. [DOI] [PubMed] [Google Scholar]
- Wang Q., Lin C. Photoreceptor signaling: when COP1 meets VPs. EMBO J. 2019;38:e102962. doi: 10.15252/embj.2019102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Kang D., Deng X.-W., Wei N. Evidence for functional conservation of a mammalian homologue of the light-responsive plant protein COP1. Curr. Biol. 1999;9:711–S2. doi: 10.1016/s0960-9822(99)80314-5. [DOI] [PubMed] [Google Scholar]
- Wang W., Lian H., Zhang L., Mao Z., Li X., Xu F., Li L., Yang H. Transcriptome analyses reveal the involvement of both C and N termini of cryptochrome 1 in its regulation of phytohormone-responsive gene expression in Arabidopsis. Front. Plant Sci. 2016;7:294. doi: 10.3389/fpls.2016.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei N., Deng X.W. COP9: a new genetic locus involved in light-regulated development and gene expression in Arabidopsis. Plant Cell. 1992;4:1507–1518. doi: 10.1105/tpc.4.12.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei N., Deng X.W. The role of the COP/DET/FUS genes in light control of Arabidopsis seedling development. Plant Physiol. 1996;112:871–878. doi: 10.1104/pp.112.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei N., Kwok S.F., von Arnim A.G., Lee A., McNellis T.W., Piekos B., Deng X.W. Arabidopsis COP8, COP10, and COP11 genes are involved in repression of photomorphogenic development in darkness. Plant Cell. 1994;6:629–643. doi: 10.1105/tpc.6.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidler G., zur Oven-Krockhaus S., Heunemann M., Orth C., Schleifenbaum F., Harter K., Hoecker U., Batschauer A. Degradation of Arabidopsis CRY2 is regulated by SPA proteins and phytochrome A. Plant Cell. 2012;24:2610–2623. doi: 10.1105/tpc.112.098210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz I.E., O’Rourke K.M., Zhang Z., Dornan D., Arnott D., Deshaies R.J., Dixit V.M. Human de-etiolated-1 regulates c-Jun by assembling a CUL4A ubiquitin ligase. Science. 2004;303:1371–1374. doi: 10.1126/science.1093549. [DOI] [PubMed] [Google Scholar]
- Wu Di, Hu Q., Yan Z., Chen W., Yan C., Huang X., Zhang J., Yang P., Deng H., Wang J. Structural basis of ultraviolet-B perception by UVR8. Nature. 2012;484:214–219. doi: 10.1038/nature10931. [DOI] [PubMed] [Google Scholar]
- Xu D., Jiang Y., Li J., Lin F., Holm M., Deng X.W. BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc. Natl. Acad. Sci. U S A. 2016;113:7655–7660. doi: 10.1073/pnas.1607687113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki S., Yamashino T., Nakanishi H., Mizuno T. Functional characterization of HY5 homolog genes involved in early light-signaling in Physcomitrella patens. Biosci. Biotechnol. Biochem. 2011;75:1533–1539. doi: 10.1271/bbb.110219. [DOI] [PubMed] [Google Scholar]
- Yanagawa Y., Sullivan J.A., Komatsu S., Gusmaroli G., Suzuki G., Yin J., Ishibashi T., Saijo Y., Rubio V., Kimura S. Arabidopsis COP10 forms a complex with DDB1 and DET1 in vivo and enhances the activity of ubiquitin conjugating enzymes. Genes Dev. 2004;18:2172–2181. doi: 10.1101/gad.1229504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Lin R., Sullivan J., Hoecker U., Liu B., Xu L., Deng X.W., Wang H. Light regulates COP1-mediated degradation of HFR1, a transcription factor essential for light signaling in Arabidopsis. Plant Cell. 2005;17:804–821. doi: 10.1105/tpc.104.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C., Deng X.W. COP1—from plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 2005;15:618–625. doi: 10.1016/j.tcb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Yi C., Wang H., Wei N., Deng X.W. An initial biochemical and cell biological characterization of the mammalian homologue of a central plant developmental switch, COP1. BMC Cell Biol. 2002;3:30. doi: 10.1186/1471-2121-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C., Li S., Chen X., Wiemer E.A.C., Wang J., Wei N., Deng X.W. Major vault protein, in concert with constitutively photomorphogenic 1, negatively regulates c-Jun-mediated activator protein 1 transcription in mammalian cells. Cancer Res. 2005;65:5835–5840. doi: 10.1158/0008-5472.CAN-05-0423. [DOI] [PubMed] [Google Scholar]
- Yin R., Arongaus A.B., Binkert M., Ulm R. Two distinct domains of the UVR8 photoreceptor interact with COP1 to initiate UV-B signaling in Arabidopsis. Plant Cell. 2015;27:202–213. doi: 10.1105/tpc.114.133868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Ji Y., Xue C., Ma H., Xi Y., Huang P., Wang H., An F., Li B., Wang Y. Integrated regulation of apical hook development by transcriptional coupling of EIN3/EIL1 and PIFs in Arabidopsis. Plant Cell. 2018;30:1971–1988. doi: 10.1105/tpc.18.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N., Wang P., Jeffrey P.D., Pavletich N.P. Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell. 2000;102:533–539. doi: 10.1016/s0092-8674(00)00057-x. [DOI] [PubMed] [Google Scholar]
- Zhong S., Shi H., Xue C., Wang L., Xi Y., Li J., Quail P.H., Deng X.W., Guo H. A molecular framework of light-controlled phytohormone action in Arabidopsis. Curr. Biol. 2012;22:1530–1535. doi: 10.1016/j.cub.2012.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Maier A., Lee J.-H., Laubinger S., Saijo Y., Wang H., Qu L.-J., Hoecker U., Deng X.W. Biochemical characterization of Arabidopsis complexes containing CONSTITUTIVELY PHOTOMORPHOGENIC1 and SUPPRESSOR OF PHYA proteins in light control of plant development. Plant Cell. 2008;20:2307–2323. doi: 10.1105/tpc.107.056580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Bu Q., Xu X., Paik I., Huang X., Hoecker U., Deng X.W., Huq E. CUL4 forms an E3 ligase with COP1 and SPA to promote light-induced degradation of PIF1. Nat. Commun. 2015;6:7245. doi: 10.1038/ncomms8245. [DOI] [PubMed] [Google Scholar]
- Zuo Z., Liu H., Liu B., Liu X., Lin C. Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. Curr. Biol. 2011;21:841–847. doi: 10.1016/j.cub.2011.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Lory N., Stauber J., Hoecker U. Photoreceptor specificity in the light-Induced and COP1-mediated rapid degradation of the repressor of photomorphogenesis SPA2 in Arabidopsis. PLOS Genet. 2015;11:e1005516. doi: 10.1371/journal.pgen.1005516. [DOI] [PMC free article] [PubMed] [Google Scholar]