Abstract
This study was performed to investigate pneumothorax characteristics and association with clinical outcomes in patients with osteosarcoma treated with apatinib. We retrospectively reviewed the medical records of osteosarcoma patients treated with apatinib between January 2016 and April 2020 at three institutions. We evaluated the prevalence, healing time, recurrence, severity, clinical management, and prognosis of pneumothorax in these patients. A total of 54 osteosarcoma patients who received apatinib treatment were enrolled in this study. Among them, 14 patients had pneumothorax. There were significant differences between the patients with and without pneumothorax with regard to the cavitating rate of lung metastases (92.86 vs. 32.50%, respectively, P < 0.001), objective response rate (42.86 vs. 10.00%, P = 0.013), disease control rate (85.71 vs. 42.50%, P = 0.006), 4-month progression-free survival (PFS) rate (57.10 vs. 20.00%, P < 0.001), and median PFS (5.65 vs. 2.90 months, P = 0.011). Compared with pneumothorax patients treated with chest tube drainage only [non-staphylococcal enterotoxin C (SEC) group], those treated with chest tube drainage and SEC thoracic perfusion in parallel (SEC group) had a shorter pneumothorax healing time (12.00 ± 4.50 days vs. 24.00 ± 14.63 days for SEC group and non-SEC group, respectively, P = 0.103), a lower recurrence rate of pneumothorax (25.00% vs. 66.67%, P = 0.277), and a longer median PFS (5.9 months vs. 4.75 months, P = 0.964). however, these numerical differences for the SEC/non-SEC data did not reach statistical significance. Pneumothorax and cavitation in lung metastases may be effective prognostic markers for patients with osteosarcoma treated with apatinib. SEC may be effective for treatment of such pneumothorax patients, warranting further study.
Keywords: apatinib, cavitation, osteosarcoma, pneumothorax, staphylococcal enterotoxin C, tyrosine kinase inhibitors
Introduction
Osteosarcoma is a mesenchymal malignancy with a worldwide incidence of 3.4 per million people per year [1]. Despite the low incidence, there are still more than 4000 new cases diagnosed in China each year. The malignancy shows a predilection for the limbs in adolescent patients. Growth of osteosarcoma at the primary site causes impaired limb function, and metastasis often leads to death; 95% of metastases occur in the lung [2]. Current treatment of osteosarcoma includes surgical resection of all gross disease in conjunction with systemic chemotherapy to control micro-metastatic disease. This treatment yields a 5-year event-free survival rate of approximately 70% for patients with localized osteosarcoma, whereas patients with metastatic or recurrent disease have a poorer prognosis, with a 4-month progression-free survival (PFS) rate of only 12% and overall survival rates lower than 20% [3].
In recent years, with the great success of receptor tyrosine kinase inhibitors (TKIs) in the treatment of malignant tumors, the treatment of osteosarcoma has entered a new era. Sorafenib, apatinib, and regorafenib are TKIs that have been shown to be effective in the treatment of osteosarcoma in clinical trials [4–7]. These TKIs inhibit a variety of tyrosine kinases, and although their multitarget nature has led to effective treatment of osteosarcoma, it has also caused various adverse events. A growing number of studies have shown that pneumothorax is one of adverse events associated with treatment of sarcomas using multitarget TKIs [5,8,9].
As a multitarget TKI marketed for the treatment of advanced or metastatic gastric cancer in China, apatinib has been shown to be effective for the treatment of osteosarcoma. In our previous work, we found that the incidence of pneumothorax in patients with osteosarcoma treated with apatinib was 18.18% [10], which was similar to the results of other studies [5,11]. To further investigate this issue, we retrospectively analyzed data for patients with osteosarcoma treated with apatinib at three institutions, focusing on clinical characteristics related to pneumothorax, with the aim of providing more clinical data to support the treatment of osteosarcoma using TKIs.
Material and methods
Study design and eligibility criteria
This study was approved by the Institutional Review Board of the Affiliated Cancer Hospital of Zhengzhou University and performed according to the principles and guidelines of the Declaration of Helsinki. All patients provided written informed consent for data collection and research purposes. This was a multicenter retrospective study of osteosarcoma patients treated at three hospitals: Affiliated Cancer Hospital of Zhengzhou University, First Affiliated Hospital of Zhengzhou University, and Affiliated People’s Hospital of Zhengzhou University. We retrospectively reviewed the medical records of osteosarcoma patients treated with apatinib between January 2016 and April 2020.
Inclusion criteria were as follows: (1) histologically proven osteosarcoma; (2) presence of metastatic lung lesions; (3) treatment with apatinib; (4) no history of treatment with other targeted drugs before apatinib treatment; (5) measurable lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; and (6) complete clinical data that could be statistically analyzed.
Treatment
Patients received a once-daily oral dose of 500 mg apatinib. This apatinib dose was reduced to 250 mg per day for patients with intolerable adverse events. Apatinib was administered continuously until intolerable adverse events or progressive disease (PD) occurred. Adverse events were assessed using the US National Center Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0. If a severe adverse event occurred, apatinib administration was delayed for a maximum of 14 days to enable recovery.
If pneumothorax occurred, patients were treated by chest tube drainage. In the later stage, the patients were treated with staphylococcal enterotoxin C injection (SEC, Xiehe Biology Group, Shenyang, China) thoracic perfusion in parallel. SEC thoracic perfusion was performed as follows: 10 mL SEC, 5 mL 1% lidocaine solution, and 30 mL 0.9% sodium chloride solution were mixed and then injected into the chest cavity via the chest tube. The body position of the patients was changed after injection to make SEC mixture to be evenly distributed throughout the chest. The perfusion was repeated every 3 days for up to five cycles. Removal of the chest tubes was performed based on a strict algorithm and required complete cessation of any air leakage and fluid output <250 mL (clear fluid) per 24 h.
Evaluation
We reviewed the baseline characteristics of all the osteosarcoma patients enrolled in this study. Specifically, we determined the time interval between the first pneumothorax and apatinib treatment and assessed the prevalence, healing time, recurrence, severity, and clinical management of pneumothorax in these patients. We also evaluated the impact of pneumothorax by comparing the characteristics of the patients with and without pneumothorax. The effectiveness of SEC was evaluated by comparing the characteristics of the patients with pneumothorax treated with and without SEC thoracic perfusion.
Statistical analysis
The objective response rate (ORR), disease control rate (DCR), 4-month PFS rate, and cavitating rate of lung metastases during apatinib treatment were evaluated and compared. PFS was estimated by the Kaplan–Meier method and compared using the log-rank test. Group-wise comparison was conducted using Fisher’s exact test and the Wilcoxon rank-sum test with continuity correction. Quantitative variables are presented as medians (range) or number of patients (percentage). In all analyses, the P values were two-sided, and P < 0.05 was considered significant.
The prevalence of pneumothorax was calculated as the percentage of patients suffering from pneumothorax. The healing time for pneumothorax was calculated as the time from chest tube drainage treatment to removal of the chest tubes. The severity of pneumothorax events was graded based on NCI-CTCAE (version 4.0). The objective response and PD were defined based on RECIST (version 1.1). PFS was calculated from the date of initiation of apatinib therapy until radiological progression of disease.
Results
Patient characteristics
A total of 54 osteosarcoma patients who received apatinib treatment were enrolled in this study. Among them, 14 patients had pneumothorax. The characteristics of the patients are shown in Table 1. Comparison of various characteristics revealed no statistically significant difference between the patients with and without pneumothorax (Table 1).
Table 1.
Basic characteristics of the two osteosarcoma groups
| Characteristics | Patients with pneumothorax (n = 14) |
Patients without pneumothorax (n = 40) |
P value |
|---|---|---|---|
| Gender | 1 | ||
| Male | 8 (57.14%) | 21 (52.50%) | |
| Female | 6 (42.86%) | 19 (47.50%) | |
| Age (years) | 22.00 ± 11.70 | 20.00 ± 9.80 | 0.564 |
| ECOG PS | 0.546 | ||
| 0 | 7 (50.00%) | 24 (60.00%) | |
| 1 | 7 (50.00%) | 16 (40.00%) | |
| Primary site | 0.973 | ||
| Femur | 5 (35.71%) | 13 (32.50%) | |
| Tibia | 3 (21.43%) | 11 (27.50%) | |
| Humerus | 3 (21.43%) | 6 (15.00%) | |
| Other | 1 (7.14%) | 4 (10.00%) | |
| Axial skeleton | 1 (7.14%) | 3 (7.50%) | |
| Radial | 1 (7.14%) | 1 (2.50%) | |
| Fibula | 0 (0.00%) | 2 (5.00%) | |
| Excision of primary lesion | 1 | ||
| No | 2 (14.29%) | 5 (12.50%) | |
| Yes | 12 (85.71%) | 35 (87.50%) | |
| Metastatic site | 0.681 | ||
| Only lung | 11 (78.57%) | 34 (85.00%) | |
| Both bone and lung | 3 (21.43%) | 6 (15.00%) | |
| Previous MAP/I chemotherapy | 1 | ||
| No | 1 (7.14%) | 5 (12.50%) | |
| Yes | 13 (92.86%) | 35 (87.50%) | |
| Time interval (months) | 4.36 ± 2.68 | 4.30 ± 2.41 | 0.504 |
| Apatinib dosage per administration (mg) | 435.46 ± 31.72 | 428.95 ± 33.87 | 1 |
Data are presented as numbers (percentages) or means ± SD.
ECOG PS, Eastern Cooperative Oncology Group performance status; MAP/I, high-dose methotrexate, doxorubicin, cisplatin, and/or ifosfamide; Time interval, time interval between the end of chemotherapy and oral apatinib administration.
Clinical outcomes
As shown in Table 2, there were significant differences between the patients with and without pneumothorax with regard to the cavitating rate of lung metastases (92.86 vs. 32.50%, respectively, P < 0.001), ORR (42.86 vs. 10.00%, P = 0.013), DCR (85.71 vs. 42.50%, P = 0.006), 4-month PFS rate (57.10 vs. 20.00%, P < 0.001; Fig. 1), and median PFS (5.65 vs. 2.90 months, P = 0.011; Fig. 1).
Table 2.
Clinical outcomes of the two osteosarcoma groups
| Characteristics | Patients with pneumothorax (n = 14) |
Patients without pneumothorax (n = 40) |
P value |
|---|---|---|---|
| Cavitation in lung metastases | <0.001 | ||
| Yes | 13 (92.86%) | 13 (32.50%) | |
| No | 1 (7.14%) | 27 (67.50%) | |
| ORR (%) | 6 (42.86%) | 4 (10.00%) | 0.013 |
| DCR (%) | 12 (85.71%) | 17 (42.50%) | 0.006 |
| Median PFS (months) | 5.65 (3–8) | 2.90 (2–3) | 0.011 |
| 4-month PFS rate | 57.10% (0.284–0.780) | 20.00% (0.094–0.335) | <0.001 |
Data are presented as numbers (percentages), medians (95% confidence interval), or rates (deviations).
DCR, disease control rate; ORR, objective response rate; PFS, progression-free survival.
Fig. 1.
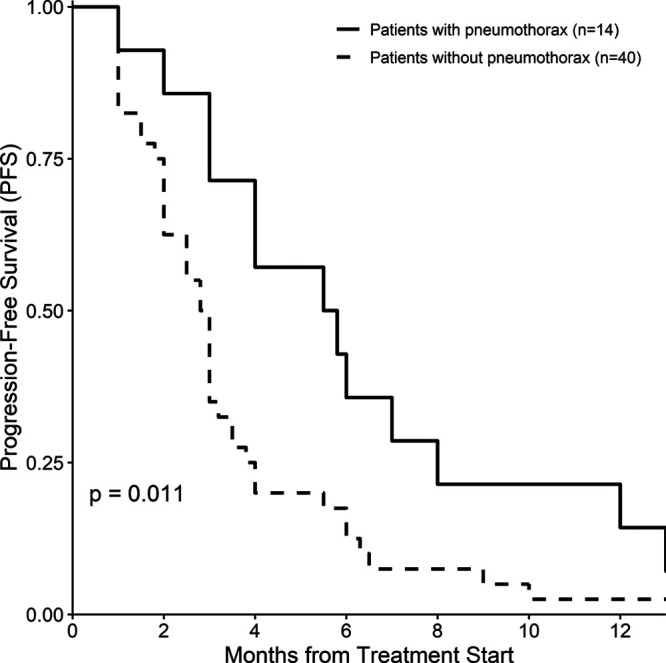
Kaplan–Meier estimates of progression-free survival among osteosarcoma patients with or without pneumothorax.
Characteristics of pneumothorax
The prevalence of pneumothorax was 25.93%. The time interval between the first pneumothorax and apatinib treatment was 3.3 ± 2.8 months. Among the 14 patients with pneumothorax, 6 patients were treated with chest tube drainage (non-SEC group), and 8 patients were treated with chest tube drainage and SEC thoracic perfusion in parallel (SEC group). As shown in Table 3, compared with the non-SEC group, the SEC group had a shorter pneumothorax healing time (12.00 ± 4.50 days vs. 24.00 ± 14.63 days for SEC group and non-SEC group, respectively), a lower recurrence rate of pneumothorax (25.00 vs. 66.67%), and a longer median PFS (5.9 vs. 4.75 months). Nevertheless, there was no statistically significant difference in these numerical differences and other characteristics between the two groups (Table 3).
Table 3.
Basic characteristics of the two pneumothorax groups
| Characteristics | SEC group (n = 8) |
Non-SEC group (n = 6) |
P value |
|---|---|---|---|
| Gender | 1 | ||
| Male | 5 (62.50%) | 21 (52.50%) | |
| Female | 3 (37.50%) | 19 (47.50%) | |
| Age | 23.62 ± 15.32 | 20.00 ± 9.80 | 0.557 |
| ECOG PS | 1 | ||
| 0 | 4 (50.00%) | 24 (60.00%) | |
| 1 | 4 (50.00%) | 16 (40.00%) | |
| Primary site | 1 | ||
| Femur | 2 (25.00%) | 3 (50.00%) | |
| Humerus | 2 (25.00%) | 1 (16.67%) | |
| Tibia | 1 (12.50%) | 2 (33.33%) | |
| Other | 1 (12.50%) | 0 (0.00%) | |
| Axial skeleton | 1 (7.14%) | 0 (0.00%) | |
| Radial | 1 (12.50%) | 0 (0.00%) | |
| Excision of primary lesion | 1 | ||
| No | 1 (12.50%) | 1 (16.67%) | |
| Yes | 7 (87.50%) | 5 (83.33%) | |
| Metastatic site | 0.538 | ||
| Only lung | 7 (87.50%) | 4 (66.67%) | |
| Both bone and lung | 1 (12.50%) | 2 (33.33%) | |
| Previous MAP/I chemotherapy | 1 | ||
| No | 1 (12.50%) | 0 (0.00%) | |
| Yes | 7 (87.50%) | 6 (100.00%) | |
| Pneumothorax grade >2 | 3 (37.50%) | 3 (50.00%) | 1 |
| Pneumothorax healing time (days) | 12.00 ± 4.50 | 24.00 ± 14.63 | 0.103 |
| Recurrence of pneumothorax | 0.277 | ||
| Yes | 2 (25.00%) | 4 (66.67%) | |
| No | 6 (75.00%) | 2 (33.33%) | |
| Median PFS (months) | 5.9 (2-12) | 4.75 (1-NA) | 0.964 |
Data are presented as numbers (percentages), means ± standard or medians (95% CI range) deviations.
ECOG PS, Eastern Cooperative Oncology Group performance status; MAP/I, high-dose methotrexate, doxorubicin, cisplatin, and/or ifosfamide; PFS, progression-free survival; SEC, staphylococcal enterotoxin C.
Discussion
To our knowledge, this study is the first to focus on pneumothorax in osteosarcoma patients treated with apatinib. The results of this study showed that the incidence of pneumothorax in osteosarcoma patients treated with apatinib was 25.93%, which was similar to previous reports [5,11]. However, the incidence of pneumothorax in osteosarcoma patients treated with sorafenib and regorafenib, the other two multitarget TKIs that have been shown to be effective in osteosarcoma treatment, was 3 and 0%, respectively [4,6,7]. We speculate that these differences may be attributable to the different targets of these TKIs (Table 4) [12–15]. Nonetheless, the exact reasons for the discrepancy are unknown. Pazopanib is another multitarget TKI that has a high probability of causing pneumothorax in patients with sarcomas [8,16,17]; the targets of pazopanib are also different from those of apatinib (Table 4) [18]. Different targets result in different effectiveness of the two TKIs for osteosarcoma treatment. Based on current evidence, apatinib is more effective than pazopanib for osteosarcoma treatment [5,19]. Although the targets and therapeutic effects of the two drugs are different, the pathological process of pneumothorax caused by the two drugs appears to be the same; this process involves cavitation in lung metastases and finally pneumothorax formation (as shown in Fig. 2) [17,20]. This suggests that both drugs inhibit key targets involved in pneumothorax formation. As shown in Table 4, the shared targets of apatinib and pazopanib include vascular endothelial growth factor receptors (VEGFRs) and stem cell factor receptor (KIT). We performed a literature search and found that bevacizumab and ramucirumab, single-target inhibitors of the VEGFR signaling pathway, can cause pneumothorax [21–23]. However, imatinib, which targets KIT but not VEGFRs (Table 4) [24], has not been reported to cause pneumothorax. This evidence suggests that VEGFRs are involved in pneumothorax development during treatment of sarcomas with TKIs. However, the detailed mechanisms remain unclear and warrant further study.
Table 4.
Targets of apatinib, regorafenib, sorafenib, pazopanib, and imatinib
| TKI | Targets (RTKs) and IC50 (nM, mean) | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| VEGFR1 | VEGFR2 | VEGFR3 | KIT | RET | PDGFRα | PDGFRβ | FGFR1 | ||
| Apatinib | 70 | 1 | – | 429 | 13 | >1000 | – | >10 000 | 12 |
| Regorafenib | 13 | 4.2 | 46 | 7 | 1.5 | – | 22 | 202 | 15 |
| Sorafenib | – | 4 | 20 | 68 | 0.4 | – | 57 | 580 | 13 |
| Pazopanib | 10 | 30 | 47 | 74 | – | 71 | 84 | 140 | 18 |
| Imatinib | 19 500 | 10 700 | 5700 | 97 | – | 72 | – | 31 200 | 24 |
IC50, half maximal inhibitory concentration; nM, nmol/L; KIT, stem cell factor receptor; RTKs, receptor tyrosine kinases; TKIs, receptor tyrosine kinase inhibitors; VEGFR, vascular endothelial growth factor receptor.
Fig. 2.
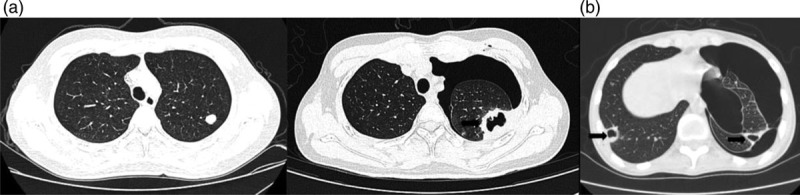
Typical pathological process of pneumothorax after apatinib treatment in two osteosarcoma patients. Computed tomography scans were obtained at (a1) treatment initiation in case 1, (a2) 6 months after treatment in case 1, and (b) 4 months after treatment in case 2. Arrows indicate cavitation in lung metastases.
In this study, patients with pneumothorax had a higher cavitating rate in lung metastases than patients without pneumothorax (Table 2, Fig. 2), which was consistent with other reports [8,9]. We speculate that pneumothorax and cavitation in lung metastasis are the result of tumor necrosis and wound healing disorders caused by apatinib. In other words, pneumothorax and cavitation in lung metastasis are manifestations of the effectiveness of the treatment. This is demonstrated by the prolongation of median PFS in patients with pneumothorax (Table 2, Fig. 1). Interestingly, another study also identified cavitation in lung metastasis as a common effect of apatinib therapy and as a potential prognostic marker for the treatment of gastric and non-small-cell lung cancer patients [25]. And most recently, a study suggests that pneumothorax might be a marker for the favorable clinical outcome following apatinib-treated refractory osteosarcoma [26].
Some of the pneumothorax patients in this study were treated with SEC thoracic perfusion. SEC is the most frequent superantigenic toxin produced by Staphylococcus aureus, which was isolated from bovine mastitis [27,28]. SEC can be instilled via an indwelling pleural catheter to induce pleurodesis. SEC is used in pneumothorax treatment because it can cause an inflammatory reaction and adhesion of pleura, leading to resolution of the pneumothorax. SEC is commonly used in the treatment of spontaneous pneumothorax in China [29,30]. To our knowledge, there is no report of SEC for treatment of secondary pneumothorax caused by TKIs. In this study, we found that the healing time was shortened and recurrence rate was reduced in pneumothorax patients treated with SEC. This suggests that SEC may also be effective for treatment of secondary pneumothorax caused by TKIs. For treatment of spontaneous pneumothorax, pleurodesis and thoracoscopic surgery have been widely studied [31–33]; however, we cannot determine whether pleurodesis, including the use of SEC, is superior to thoracoscopic surgery for the treatment of secondary pneumothorax caused by TKIs. The effectiveness of these treatments requires further study.
This study preliminarily evaluated secondary pneumothorax caused by apatinib treatment in osteosarcoma patients with lung metastasis. However, this study had some limitations, including its retrospective design, small sample size, and the absence of a control group. To further investigate secondary pneumothorax caused by TKIs, prospective clinical studies must be performed. The mechanisms underlying this form of pneumothorax also require further study. More importantly, the treatment of secondary pneumothorax, which may involve pleurodesis or thoracoscopic surgery, requires further investigation.
In conclusion, pneumothorax and cavitation in lung metastasis are common adverse events associated with apatinib therapy and may be effective prognostic markers in osteosarcoma patients undergoing apatinib treatment. In addition, SEC may be effective for treatment of pneumothorax in these cases, warranting further study.
Acknowledgements
We are grateful to all the patients and investigators in this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009; 125:229–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattasali O, Vo AT, Roth M, Geller D, Randall RL, Gorlick R, et al. Variability in the reported management of pulmonary metastases in osteosarcoma. Cancer Med. 2015; 4:523–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. 2018; 18:39–50 [DOI] [PubMed] [Google Scholar]
- 4.Grignani G, Palmerini E, Ferraresi V, D’Ambrosio L, Bertulli R, Asaftei SD, et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol. 2015; 16:98–107 [DOI] [PubMed] [Google Scholar]
- 5.Xie L, Xu J, Sun X, Tang X, Yan T, Yang R, et al. Apatinib for Advanced Osteosarcoma after failure of standard multimodal therapy: an open label phase ii clinical trial. Oncologist. 2018; 24:e542–e550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffaud F, Mir O, Boudou-Rouquette P, Piperno-Neumann S, Penel N, Bompas E, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019; 20:120–133 [DOI] [PubMed] [Google Scholar]
- 7.Davis LE, Bolejack V, Ryan CW, Ganjoo KN, Loggers ET, Chawla S, et al. Randomized double-blind phase II study of regorafenib in patients with metastatic osteosarcoma. J Clin Oncol. 2019; 37:1424–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabath B, Muhammad HA, Balagani A, Ost DE, Vakil E, Ahmed T, et al. Secondary spontaneous pneumothorax in patients with sarcoma treated with pazopanib, a case control study. BMC Cancer. 2018; 18:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Interiano RB, McCarville MB, Wu J, Davidoff AM, Sandoval J, Navid F. Pneumothorax as a complication of combination antiangiogenic therapy in children and young adults with refractory/recurrent solid tumors. J Pediatr Surg. 2015; 50:1484–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian Z, Gu Z, Wang X, Liu Z, Yao W, Wang J, et al. Efficacy and safety of apatinib in treatment of osteosarcoma after failed standard multimodal therapy: an observational study. Medicine (Baltimore). 2019; 98:e15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie L, Guo W, Wang Y, Yan T, Ji T, Xu J. Apatinib for advanced sarcoma: results from multiple institutions’ off-label use in China. BMC Cancer. 2018; 18:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011; 102:1374–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004; 64:7099–7109 [DOI] [PubMed] [Google Scholar]
- 14.Mao WF, Shao MH, Gao PT, Ma J, Li HJ, Li GL, et al. The important roles of RET, VEGFR2 and the RAF/MEK/ERK pathway in cancer treatment with sorafenib. Acta Pharmacol Sin. 2012; 33:1311–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011; 129:245–255 [DOI] [PubMed] [Google Scholar]
- 16.Nakano K, Motoi N, Tomomatsu J, Gokita T, Ae K, Tanizawa T, et al. Risk factors for pneumothorax in advanced and/or metastatic soft tissue sarcoma patients during pazopanib treatment: a single-institute analysis. BMC Cancer. 2016; 16:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verschoor AJ, Gelderblom H. Pneumothorax as adverse event in patients with lung metastases of soft tissue sarcoma treated with pazopanib: a single reference centre case series. Clin Sarcoma Res. 2014; 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyamoto S, Kakutani S, Sato Y, Hanashi A, Kinoshita Y, Ishikawa A. Drug review: pazopanib. Jpn J Clin Oncol. 2018; 48:503–513 [DOI] [PubMed] [Google Scholar]
- 19.Longhi A, Paioli A, Palmerini E, Cesari M, Abate ME, Setola E, et al. Pazopanib in relapsed osteosarcoma patients: report on 15 cases. Acta Oncol. 2019; 58:124–128 [DOI] [PubMed] [Google Scholar]
- 20.Nakano K, Inagaki L, Tomomatsu J, Motoi N, Gokita T, Ae K, et al. Incidence of pneumothorax in advanced and/or metastatic soft tissue sarcoma patients during pazopanib treatment. Clin Oncol (R Coll Radiol). 2014; 26:357. [DOI] [PubMed] [Google Scholar]
- 21.Alrifai T, Saba R, Rifai D, Pandit S, Kozma KE. Pneumothorax following combination chemotherapy with bevacizumab: a case report and review of the literature. Mol Clin Oncol. 2019; 11:173–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiorean EG, Hurwitz HI, Cohen RB, Schwartz JD, Dalal RP, Fox FE, et al. Phase I study of every 2- or 3-week dosing of ramucirumab, a human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2 in patients with advanced solid tumors. Ann Oncol. 2015; 26:1230–1237 [DOI] [PubMed] [Google Scholar]
- 23.Fuchs CS, Shitara K, Di Bartolomeo M, Lonardi S, Al-Batran SE, Van Cutsem E, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019; 20:420–435 [DOI] [PubMed] [Google Scholar]
- 24.Waller CF. Imatinib mesylate. Recent Results Cancer Res. 2018; 212:1–27 [DOI] [PubMed] [Google Scholar]
- 25.Jiang M, Zhang C, Liu D, Wang Y, Wang H, Li T, et al. Influence and mechanism of lung cavitation development on antiangiogenic therapy. Transl Lung Cancer Res. 2019; 8:500–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie L, Xu J, Sun X, Tang X, Yan T, Yang R, et al. Anorexia, hypertension, pneumothorax, and hypothyroidism: potential signs of improved clinical outcome following apatinib in advanced osteosarcoma. Cancer Manag Res. 2020; 12:91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang R, Cui J, Cui T, Guo H, Ono HK, Park CH, et al. Staphylococcal enterotoxin C is an important virulence factor for mastitis. Toxins. 2019; 11:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuroishi T, Komine K, Asai K, Kobayashi J, Watanabe K, Yamaguchi T, et al. Inflammatory responses of bovine polymorphonuclear neutrophils induced by staphylococcal enterotoxin C via stimulation of mononuclear cells. Clin Diagn Lab Immunol. 2003; 10:1011–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng C, Chen X, Zhang X. Efficacy of staphylococcal enterotoxin C combined with hypertonic glucose intrathoracic injection in the treatment of refractory pneumothorax. Chin J Postgrad Med. 2014; 37:54–56 [Google Scholar]
- 30.Bai X, Shi G, Yu C. Clinical observation on the treatment of spontaneous pneumothorax with microtubule drainage and intrathoracic injection of staphylococcal enterotoxin C. Hebei Med J. 2019; 31:3210–3211 [Google Scholar]
- 31.Mercer RM, Hassan M, Rahman NM. The role of pleurodesis in respiratory diseases. Expert Rev Respir Med. 2018; 12:323–334 [DOI] [PubMed] [Google Scholar]
- 32.Goto T. Is surgery the choice for treatment for first presentation of pneumothorax. J Thorac Dis. 2019; 11:S1398–S1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KH, Kim BT, Kim HK, Han KN, Choi YH. Comparison of additional minocycline versus iodopovidone pleurodesis during video-assisted thoracoscopic bleb resection for primary spontaneous pneumothorax: a propensity score-matched analysis. J Thorac Dis. 2018; 10:5443–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]


