Supplemental digital content is available in the text.
Key Words: HPV, HPV genotyping, systematic review, cervical cancer screening, triage
Abstract
Objective
The aim of the study was to examine whether high-grade cervical intraepithelial neoplasia (CIN) was more closely associated with human papillomavirus (HPV) same-genotype persistence (SGTP) versus clearance of prior infection with a subsequent infection by a new genotype (genotype switch [GS]), clearance of HPV infection, or acquisition of a new HPV infection after a negative infection status, during a follow-up testing subsequent to abnormal screening results.
Materials and Methods
MEDLINE, Cochrane Library, Health Technology Assessment, and clinicaltrials.gov were searched from January 2000 to July 2019 for prospective controlled trials and observational studies of women and retrospective studies using HPV assays with extended- or full-genotype reporting. The primary outcome was high-grade CIN after at least 2 rounds of testing. Overall quality of evidence for the risk estimate outcomes was assessed. Of the 830 identified abstracts, 66 full-text articles were reviewed, and 7 studies were included in the synthesis. The study protocol was registered with the PROSPERO International Prospective Register of Systematic Reviews (CRD42018091093).
Results
Continued HPV-positive women falls in 2 equally large groups: SGTP and GS. Sensitivity, positive predictive value, and positive likelihood ratio of SGTP were significantly higher than for GS. Human papillomavirus genotypes may be ranked into 3 tiers (immediate colposcopy, follow-up testing, return to routine screening), according to associated risk of persistence for high-grade CIN and to prevailing clinical action thresholds.
Conclusions
There is moderately high-quality evidence to support the clinical utility of SGTP to improve risk discrimination for high-grade CIN compared with qualitative HPV testing without genotype-specific information.
Guidelines for human papillomavirus (HPV) genotyping in management of cervical cancer screening are gaining momentum internationally. The continued technologic advances in HPV screening methods increase the number of validated HPV assays allowing for reporting of genotype-specific test outcomes. The clinical implications of specific genotypes, i.e., HPV 16 and 18, is reflected in differential management compared with the other carcinogenic HPV genotypes with lower risk for high-grade cervical intraepithelial neoplasia (CIN) or cancer. Several countries already use limited genotyping for HPV 16 and 18 as an integral element in algorithms to stratify women for a specific follow-up reflecting the increased risk profiles of infection with these genotypes. However, for an HPV infection to cause high-grade CIN and cancer, it must be more than a transient infection; it must be persistent.1,2 To date, guidelines only factor in persistence as a qualitatively measure of finding HPV in several samples spaced in time. However, today's HPV technology for routine screening with individual reporting of multiple HPV genotypes offers a more refined approach allowing assessment of same-genotype persistence (SGTP) over time; clinical information can then be included in the risk-based clinical action threshold calculations. This systematic review summarizes the findings from published studies evaluating the risk of high-grade CIN 2 or 3 after persistent HPV infection.
Today, it is well documented that individual oncogenic HPV genotypes have differential carcinogenic potential, with HPV 16, 18, 31, and 33 having the highest absolute risks of CIN 2 or higher or CIN 3 or higher.1,3–5 A recent systematic review has summarized the current evidence on oncogenic HPV genotypes with respect to risk stratification in cervical screening.6 Along this line, countries such as Denmark and Norway are actively looking to extend the number of HPV genotypes, which are individually reported with specific management recommendations in screening algorithms aimed at increasing the specificity of molecular screening algorithms. The International Agency for Research on Cancer classifies 12 HPV genotypes as carcinogenic (group 1: HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59), whereas HPV 68 is probably carcinogenic (group 2A) and HPV 66 is possibly carcinogenic (group 2B).7–9
Same-genotype persistence is strongly associated with a high risk of progression to high-grade disease and development of cervical cancer,1,8,10–22 and practically all cervical cancers are caused by SGTP, but conversely, not all SGTP results in cervical cancer. The key evidence in this respect is that women with SGTP have an increased risk of CIN 2 or higher (hazard ratio [HR] = 75, 95% CI = 32 to 179) compared with women who are HPV negative at 2 subsequent visits. Furthermore, if a baseline HPV infection(s) was cleared at the subsequent visits (HPV clearance), the woman's risk of cervical cancer risk was not significant compared with a noninfected woman.1
From a management standpoint, the challenge is the ability to compare a concurrent screening result with previous results. This requires detailed testing, reporting, and registration, not only of qualitative (“positive” versus “negative”) HPV test results but also of genotype of the individual findings. With current (pooled qualitative) technology, the reported data resolution does not allow the clinician to determine whether a given HPV infection is most likely new (transient) or most likely a repeat finding (persistence). The relative carcinogenic potential over time of individual HPV genotypes, on the other hand, is well established in literature. For risk-based algorithms to work optimally, knowledge on present and former test outcomes with detailed reporting of HPV genotype finding(s) is required to develop the optimal follow-up recommendation. In this systematic review, we will distinguish between qualitative-pooled HPV persistence (QPHP) test results (any HPV genotype infection at both the first time point and the subsequent time point) and results with reporting of findings with respect to individual HPV genotypes detected.
Despite the established evidence involving persistence and risk for CIN 2/3 and cancer, most clinical practice guidelines as of 2019 do not include SGTP as a risk parameter outside referring HPV women for a retest within a defined timeframe; typically 12–18 months.23
Today, from a clinical management perspective, women with HPV-positive test results can be triaged into high-grade cytology, low-grade cytology, or cytology triage negative subgroups. If triage include high-grade squamous intraepithelial lesion, atypical squamous cells – high-grade squamous intraepithelial lesion cannot be excluded (ASC-H), or atypical glandular cells, the woman would be referred to colposcopy according to most guidelines. If the subsequent colposcopy result is CIN 1 or negative, the management decision is typically surveillance with retesting, usually at 12 months. With qualitative HPV testing, continued surveillance is an option only for HPV-negative women. On the other hand, evidence of clearance of a prior genotype and new genotype infection could instead result in a management decision to retest after an interval and avoidance of colposcopy.24–26
Human papillomavirus–positive women with negative for intraepithelial lesions or malignancies (NILM) or low-grade cytology triage outcomes represent the predominant subgroup in the screening population. These women may be managed by retesting after 12 months or by referral to colposcopy, depending on the guideline. The most often-seen recommendation in this respect when using limited genotyping is women positive for HPV 16 or HPV 18 who are referred to colposcopy, regardless of cytology results.25,27–30 If the surveillance test result is HPV negative, the woman may be recommended to return in 3 years for retesting.28,30 If the absolute risk of a new infection after a prior abnormal screening result is less than the colposcopy threshold, then a woman could undergo continued surveillance rather than colposcopy. For these cases, genotyping would greatly enhance the ability to clinically distinguish between women with the highest-risk genotypes like HPV 16, 18, 31, and 33, compared with those with an infection by lower-risk oncogenic HPV genotypes.6
Recently, the US American Society for Colposcopy and Cervical Pathology 2019 guideline panel adapted a risk-based guideline for revision of cervical cancer screening and management of abnormal results.26,30–33 Risk factors included prior results, age, vaccination history, and concurrent screening results. Here, the clinical utility of genotyping results for persistence tracking would be determined by several factors. First, the sensitivity of SGTP can never be greater than the sensitivity of high-risk QPHP result (no genotyping), as all positives are covered by the pooled results. The specificity of SGTP, however, can be significantly improved compared with pooled outcomes as SGTP may be used to identify women with a genotype switch (GS; i.e., GS new infection places the woman at a lower risk for ≥CIN 2) rather than a persistent infection with the same genotype. Several studies have shown that 40%–93% of repeatedly HPV-positive women at 6–12 months after a baseline positive HPV result had SGTP, whereas 7%–60% had a GS.34–38 This implies that a significant proportion (median of 40%) of QPHP is the result of a GS. Hence, using qualitative-pooled HPV assays masks this distinction between true persistence and GS.
The objective of this systematic review was to investigate the differential clinical utility of persistence tracking when the QPHP is positive in combination with genotype risk discrimination, compared with clinical action risk thresholds when the triage cytology is NILM, atypical squamous cells–undetermined significance (ASC-US)/low-grade squamous intraepithelial lesion (LSIL), or when colposcopy yielded negative or CIN 1 results. We approached this task by analyzing studies that compared SGTP results with QPHP results for women undergoing cervical cancer screening condensing the included data for evidence of differences in specificity, positive predictive value (PPV), and positive likelihood ratio (PLR), relative to the outcome of CIN 2/3 or higher. If the PPV of SGTP is significantly different from QPHP associated with GS, then genotype results during surveillance could have clinical utility.
The PICO of this systematic review was as follows:
(P) population who underwent surveillance or follow-up after positive HPV screening results with/without colposcopy,
(I) intervention test was extended or full genotyping assay at screening and at follow-up,
(C) comparators were QPHP (due to GS) result and HPV clearance, with/without HPV-negative referent or comparison across genotypes, and
(O) outcomes were CIN 2, CIN 2 or higher, CIN 3, and CIN 3 or higher.
SOURCES
Eligible studies included prospective controlled trials, observational studies, and retrospective studies of residual specimens after HPV-positive screening results with at least 1 subsequent testing at 6-month persistent infection (6MPI) or more. Studies were included that used HPV DNA or RNA assays reporting extended or full genotyping (beyond genotypes 16/18/45).
Medline, Cochrane Database of Systematic Reviews, Health Technology Assessment, and clinicaltrials.gov electronic databases were searched between January 2000 and July 2019. The study protocol was developed, and the review was performed in accordance with the Preferred Reporting Items for Systematic Reviews (PRISMA)39 and the Institutes of Medicine Standards for Systematic Reviews.40 No similar published systematic review or similar study protocol was found. This study protocol was registered with the PROSPERO International Prospective Register of Systematic Reviews in 2018 (PROSPERO: CRD42018091095).41 The search term string was: (HPV OR “human papillomavirus”) AND (genotyp*) AND (persistence) AND (cervical or cervix) OR colposcopy OR SIL OR “squamous intraepithelial lesion” OR CIN OR “cervical intraepithelial neoplasia” in title, abstract, keywords; publication, or published-ahead-of-print date from January 2000 and July 2019.
STUDY SELECTION
Relevance of all retrieved abstracts was assessed through applied inclusion/exclusion criteria. Full-text review was performed for articles passing abstract review to confirm that studies met inclusion/exclusion criteria. Peer-reviewed publications that used HPV DNA or RNA detection in humans were eligible if they either reported or had calculable relative risks (risk ratios, rate ratios, odds ratios, or HRs, hereafter termed “relative risks”) and corresponding 95% CI for the association between HPV persistence and CIN. Figure 1 details the PRISMA flow diagram.
FIGURE 1.
The PRISMA flow diagram detailing the process of search and selection. Article search and selection occurred over 4 stages (identification, screening, eligibility, and included). Selection began with 830 abstracts, and after exclusion of duplicates (n = 58), removal based on exclusion criteria (n = 686), removal based on outcomes missing (n = 21), missing pre/post data (n = 18), missing genotyping (n = 14), testing interval too large (n = 2), and not original research (n = 1) resulted in 10 articles included for this systematic review.
Data extraction tables were prepared in Excel, piloted, and used for study characteristics and for risk estimates or risk ratios with 95% CIs. Genotyping risk estimates were grouped according to histology outcomes. For cervical neoplasia risk, relative risk values and 95% CIs were extracted if they were reported; alternatively, these were calculated for this synthesis. Risk values associated with all outcome measures were compared in women with QPHP; when possible, women with SGTP and women with a GS were compared. Women with SGTP were also compared with those with HPV clearance, a new infection, or no infection at either time point. Two independent reviewers extracted and confirmed data from each article to ensure accuracy.
As there is no definitive international definition for the duration of infection defining HPV persistence, here, we define persistent infections as 6MPI, with true persistence defined as the SGTP lasting equal to or longer than 6MPI.11,42,43 We correlated the duration of persistent infection to risk of CIN 2/3 or higher and determined to extract the number of MPI data reported by the original research.
The definitions of no genotyping (QPHP), limited genotyping (individual reporting of HVP 16 and/or 18, with or without 45, and the other oncogenic genotypes as a group), extended genotyping (6 or more individually reported genotypes including HPV 16 and HPV 18, plus 1 or more grouped result[s]), and full genotyping (individual reporting of all oncogenic types) were ascribed according to the VALidation of HPV GENotyping Tests framework definition.44
Risk of bias (RoB, individual study quality) was evaluated with Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) that included domains: selection, index test, reference standard, flow, and timing.45,46 Summary assessment of RoB for individual studies, combining all authors' evaluations, was assessed as high, low, or unclear.
Each author assessed the overall quality of evidence for the risk estimate outcomes (all included studies by outcome) using a modified Grading of Recommendations, Assessment, Development and Evaluation (GRADE) QUADAS for observational diagnostic studies and included the summary assessment of RoB for the individual studies, indirectness, imprecision, inconsistency, publication bias, magnitude of effect, and whether all plausible confounders or other biases increased the confidence in the estimated effect. Summary levels of certainty, combining all authors' evaluations, were assessed as high, moderate, or low.
RESULTS
The systematic review identified 830 unique abstracts, of which, 66 were assessed for full-text review: 56 studies were excluded (see PRISMA flow diagram in Figure 1). Ten studies that reported genotyping results and the risk of CIN after testing were included in the synthesis.2,42,43,47–53 The 10 studies had a combined entry population size of 115,840; 12,364 had high-risk HPV-positive results at baseline (see Table 1, study characteristics).
TABLE 1.
Characteristics of Study Design, HPV Status, and Persistence Definition for Studies Included in the Current Review
| Study characteristics | Jaisamrarn et al.42 (2013) | Skinner et al.43 (2016) | Schiffman et al.2 (2005) and Castle et al.47 (2009) | Gage et al.49 (2010) | Elfgren et al.48 (2017) | Kjaer et al.52 (2010) | Kitchener et al.51 (2014) and Gilham et al.50 (2019) | Sand et al.53 (2019) |
|---|---|---|---|---|---|---|---|---|
| Design | PRO; RT | PRO; RT | PRO; cohort | PRO; post hoc; cohort | PRO; post hoc; cohort | PRO; cohort | PRO; RT | PRO; cohort |
| Months of persistent infection | 6 | 6 and 12 | 12 (range = 9–21) | 12 | 12 (median = 19) | 24 | 36 | 36 |
| Age, y | ||||||||
| Median | — | nr; >25 | 37 | 23 | Range = 32–38 | 28 | 39.4 | 31 |
| Mean | 19.7 | — | — | — | — | — | ||
| Recruitment | 9,337 | 2,870 | 10,049 | 5,060 | 12,527 | 11,088 | 24,510 | 40,399 |
| Analysis | 4,825 | 2,838 | 7,278;a 2,282b | 671 | 6,257 | 7,482 | 24,510 | 5,528 |
| HPV(+) at baseline | 3,363 | 507 | 542 | 584 | 433; 341 | 1,222 | 3,813 | 2,875 |
| Specimen collection dates | 2004–2009 | 2006–2011 | 1993–1994 | 2000–2001 | 1997–2012 | 1991–1995 | 2001–2009 | 2002–2005 |
| Year follow-up ended | 2009 | 2014 | nr | 2002 | 2012 | 2007 | 2010–2015 | 2015 |
| Median follow-up | 4 y | 2 y | 5 y | 2 y | 13 y | 12.9 y | 6 y; 13 y | 8 y |
| Country | Multinational | Multinational | Costa Rica | United States | Sweden | Denmark | England, United Kingdom | Denmark |
| HPV assay | DEIA/LiPA25 | DEIA/LiPA25 | MY09/11 PCR | HC2; Line Blot | GP5+/6+ PCR | HC2; INNO-LiPA | LBA; LA; Pap√ of HC2 | HC2; INNO-LiPAv2 |
aTreatment based on colposcopic impression.
bIncluded use of AmpliTaq Gold polymerase.
LA, liner array; OBS, observational study; PCR, polymerase chain reaction; PRO, Prospective design; RETRO, retrospective design; RT, randomized clinical trial.
Risk of Bias
The QUADAS-2 quality assessment is reported in Table 2. The overall quality of evidence for the reported outcomes was assessed using a modified GRADE-QUADAS methodology for observational diagnostic studies and judged to be moderate for the report of proportional split between SGTP and GS. The quality of evidence for the performance statistics comparing SGTP to GS for high-grade CIN outcomes was judged high for sensitivity, specificity, PPV, and PLR. The quality of evidence for genotype risk discrimination, with SGTP, by high-grade CIN outcomes was judged moderate (see Table 3).
TABLE 2.
| Domainsa | ||||
|---|---|---|---|---|
| Article | Selection | Index test | Reference standard | Flow and timing |
| Jaisamrarn et al.42 (2013) | High | Uncertain | Low | Low |
| Skinner et al.43 (2016) | Uncertain | Uncertain | Low | Low |
| Schiffman et al.2 (2005) | Low | Uncertain | Low | Low |
| Castle et al.47 (2009) | Low | Uncertain | Low | Low |
| Gage et al.49 (2010) | Uncertain | Low | Low | Uncertain |
| Elfgren et al.48 (2017) | Uncertain | Low | Low | Uncertain |
| Kjaer et al.52 (2010) | Low | Low | Low | Low |
| Kitchener et al.51 (2014) | Low | Low | Low | Low |
| Gilham et al.50 (2019) | Low | Low | Low | Low |
| Sand et al.53 (2019) | Low | Uncertain | Low | Low |
aIn all cases, answers are “low,” “high,” or “uncertain.”
TABLE 3.
Overall Quality of Evidence for Outcomes (Modified GRADE)54
| Outcome | Summary RoB assessmenta | Indirectness | Imprecision | Inconsistency | Publication bias | Magnitude of effect | Confounder effectb | Overall |
|---|---|---|---|---|---|---|---|---|
| SGTP vs GT switch43,47,49,51 | Unclear | Direct | Precise | Consistent | No | Large | No | Moderate |
| High-grade CIN; SGTP vs GT switch47,49–51 | Low | Direct | Precise | Consistent | No | Large | No | High |
| Genotype risk discrimination; high-grade CIN; SGTP2,42,43,48,50–53 | Unclear | Direct | Precise | Consistent | No | Moderate | No | Moderate |
aSee RoB table (see Table 2).
bCofounder effect characterizes the degree to which all plausible confounders would tend to increase confidence in the estimated effect.
Analysis of SGTP Versus GS Proportions of QPHP
Four studies contributed to the percentages of SGTP versus GS in HPV-positive women with QPHP.43,47,49,51 Same-genotype persistence averaged 53% of QPHP results (864/1,624), with a range of 42%–80% across the studies; GS accounted for 47% of QPHP results.
Analysis of SGTP Versus GS and High-Grade CIN Outcomes for Performance (Sensitivity, Specificity, PPV, PLR)
Four studies provided sensitivity, specificity, PPV, and negative predictive value data for SGTP versus GS in women with QPHP.47,49–51 The 4 reports could not be combined in a meta-analysis because 2 studies used a 12MPI definition of persistence47,49 and 2 used 36MPI.50,51 Furthermore, 1 study was limited to ASC-US and LSIL cytology and postcolposcopy if results were CIN 1 or lower,49 whereas 3 analyzed a screening population.47,50,51
An analysis of a subcohort of 2,282 women from a population-based screening study (Guanacaste cohort, Costa Rica) evaluated the cumulative incidence of CIN 2 or higher and CIN 3 or higher after HPV persistence.47 Women with QPHP, who tested positive for HPV at and after approximately 1 year (9–21 months), had a 3-year CIN 2 or higher cumulative incidence risk (CIR) of 17.0% (95% CI = 12.5% to 22.0%). Women with SGTP had a 3-year CIR for CIN 2 or higher of 21.3% (95% CI = 15.2% to 27.3%). Of the 51 women with GS, the 3-year CIR for CIN 2 or higher was zero. In comparison, those with HPV clearance had a 3-year CIN 2 or higher CIR of 1.2% (95% CI = −0.2% to 2.5%) and those repeatedly HPV negative displayed a significantly lower risk (3-year CIR = 0.5%, 95% CI = 0.1% to 0.9%). Same-genotype persistence PPV exceeded the US clinical action threshold for colposcopy, whereas GS provided no risk detection for CIN 2 or higher (see Table S1, http://links.lww.com/LGT/A182). Among those who tested repeatedly positive for carcinogenic HPV, all incident CIN 2 or higher and CIN 3 or higher end points during follow-up were linked to SGTP.47
Similarly, a post hoc analysis of all women who underwent colposcopy in the ALTS trial (Atypical squamous cells of undetermined significance and Low-grade squamous intraepithelial Triage Study)55 identified 671 women with CIN 1 or lower.49 Of these, 329 had QPHP 12 months after colposcopy. The 1-year risk for CIN 3 or higher was 9.6% in the women with SGTP and 8.0% in the women with a GS; however, no hierarchical assignment for mixed infections was undertaken. Comparing CIN 3 or higher risk by genotype, only HPV 16 was associated with a significantly higher sensitivity and PPV, if persistent, versus all results. The median age of the women was 23 years, and the follow-up interval (12–24 months) was short. Moreover, in the ALTS trial, the number of women with the clinical end point of CIN 3 or higher was relatively small (32).49
In the British ARTISTIC study cohort undergoing cervical screening during primary care in Greater Manchester, United Kingdom, women were recalled for 3 rounds of screening and surveillance after lesser abnormal cytology results.50,51 Here, 24,510 women underwent liquid-based cytology and HPV genotyping, with colposcopy-directed biopsy taken after abnormal results at the third round. The ARTISTIC trial includes the verification bias that genotyping was only performed for Hybrid Capture 2 (HC2)–positive women. The 3,813 women with NILM-HPV–positive results in the first and second rounds were analyzed. In total, 66 CIN 2 or higher outcomes and 31 CIN 3 or higher outcomes were recorded as the cumulative outcomes 2.5 years after the second round.51 The HPV status of individual women over 3 rounds allowed estimates of the impact of SGTP or GS. The median time for defining persistence was 3 years; the long interval suggests that a new infection, which developed after the baseline visit, could have been present for more than 2 years and therefore CIN 2 or higher risk differences between SGTP and GS would be less than expected in comparison with a situation in which persistence had been defined as 12MPI. Another publication from the ARTISTIC trial in 2014 provided 3-year risk values51 and a recent 2019 publication provided 5- and 10-year risk values.50 The 5-year CIN 3 or higher risk estimation for SGTP (11.3% in association with NILM cytology, 23.7% in association with low-grade cytology) exceeds the clinical action thresholds for colposcopy, whereas The 5-year CIN 3 or higher risk values for GS (0% in association with NILM cytology, 5.1% in association with low-grade cytology) correspond to the recommendation of repeat testing at 12 months (see Table 4). In conclusion, SGTP was associated with significantly higher sensitivity for detection of disease, PPV, and PLR compared with GS (see Tables S2, S3, http://links.lww.com/LGT/A182).50,51
TABLE 4.
Cervical Intraepithelial Neoplasia 2 or Higher and CIN 3 or Higher 3, 5, 10-Year Risks Comparing SGTP With GS
| Kitchener et al.51 (2014) and Gilham et al.50 (2019) | Pooled HPV persistent | SGTP | GS | HPV(+), then HPV(−) as referent | HPV(−), then HPV(−) as referent |
|---|---|---|---|---|---|
| ≥CIN 2 three-year risk51 | 12.9% | 21.0% | 6.2 | 0.59% | 0.02% |
| ≥CIN 3 three-year risk51 | 7.0% | 13.5% | 1.7% | 0.17% | 0% |
| ≥CIN 3 five-year CIR; NILM50 | 7.0 (3.9–12.2) | 11.3 (6.5–19.5) | 0 | 0.13 (0.02–0.90) | 0.05 (0.02–0.11) |
| ≥CIN 3 ten-year CIR; NILM50 | 8.9 (5.4–14.5) | 13.4 (8.0–22.0) | 1.7 (0.2–11.3) | 0.26 (0.06–1.03) | 0.09 (0.05–0.17) |
| ≥CIN 3 five-year CIR; low-grade cytologya50 | 14.5 (7.8–26.0) | 23.7 (14.8–36.8) | 5.1 (2.5–10.4) | 1.6 (0.2–10.7) | 0 |
| ≥CIN 3 ten-year CIR; low-grade cytology50 | 16.2 (9.0–28.0) | 23.7 (14.8–36.8) | 5.9 (3.0–11.4) | 1.6 (0.2–10.7) | 0 |
aLow-grade cytology = ASC-US and LSIL combined.
Analysis of SGTP by Genotype
Eight study articles contributed to genotype-specific risk.2,42,43,48,50–53 However, they could not be combined into a meta-analysis because definitions of persistence ranged from 6 months (2 studies),42,43 to 12 months (3 studies),2,48,49 to 24 months (1 study),52 to 36 months (3 studies).50,51,53 The median ages of the women differed considerably from 19.5 years42 to 39.4 years.51 Two of the subcohorts were selected after colposcopy,48,49 5 were created from a general screening population,2,50–53 and 2 were selected from the control arms of randomized controlled trials.42,43 Two studies assigned the same risk to all genotypes in mixed infections,43,48 2 studies excluded mixed infections and used single genotype infections to assign risk,52,53 and 3 studies used a statistical method to assign risk by hierarchical assignment to genotypes; each study used a different methodology.2,42,51 The studies were assessed to determine if there was significant risk differentiation by genotype when there was SGTP and to determine if there were specific genotype risk values that were below the clinical action threshold for colposcopy, even when the risk was associated with SGTP (see Figure 2).
FIGURE 2.
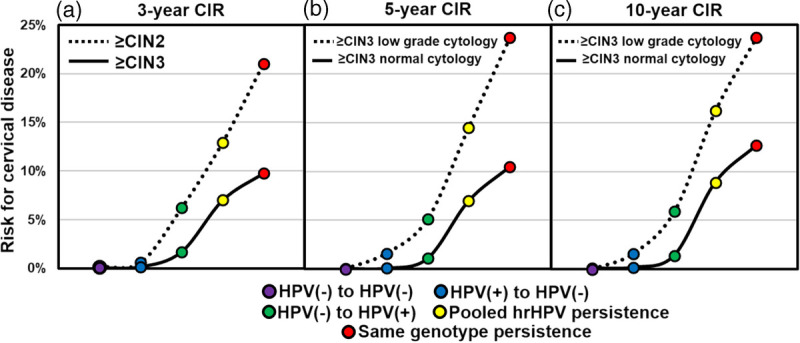
Cumulative incident risk for high-grade cervical disease according to HPV status at the first and subsequent test. Five HPV status classifications are shown, including HPV negative (purple; negative at first and subsequent testing), HPV clearance (blue; HPV positive at first test and HPV negative at second test), new HPV infection (green; HPV negative at first test and HPV positive at the subsequent test), pooled (any genotype) HPV persistence (yellow; any HPV genotype at first test and any HPV genotype at the subsequent test), and genotype-specific HPV persistence (red; positive for the same genotype at the first and subsequent test). Risk estimation, plotted along the y-axis and based on HPV status, is shown in (a) for CIN 2 or higher and CIN 3 or higher over 3 years, in (b) for CIN 3 or higher among individuals with normal and low-grade cytology across 5-years, and in (c) for CIN 3 or higher among individuals with normal and low-grade cytology across 10 years. The disease outcome, 5-, and 10-year time points.
The first population-based study reporting the long-term absolute risk of high-grade CIN associated with SGTP involved a screening cohort nested within a parent study (N = 40,399) involving residual samples from women participating in the organized screening program in Greater Copenhagen (baseline period from 2002 to 2005). The screening cohort (n = 7,482) involved only those with NILM cytology at baseline. From these, 1,281 were HC2 positive, then retested after 2 years, and followed up passively via a registry over 13.4 years.52 The definition of persistence was 24MPI. As in the ARTISTIC study, genotyping was performed on HC2-positive samples and therefore represents a verification bias. The genotype analysis excluded mixed infections. The rate of SGTP (detected 2 years after baseline) varied across genotypes from 7% (HPV 66) to 29.4% (HPV 16). Human papillomavirus 16 was the HPV genotype with the greatest carcinogenic potential. Women with NILM cytology who were positive for non–HPV 16 genotypes, including HPV 18, 31, 33, and 58, also had high risks for developing high-grade cervical lesions, but those risks were much lower than that associated with HPV 16 positivity. Here, the authors reported the absolute risk of high-grade CIN after a single infection with a specific HPV genotype to isolate the effect of the individual HPV genotypes. Women who had a single infection with HPV 16 (26%) or HPV 18 (15.4%) had the highest absolute risk for CIN 3 or higher, followed by women who had a single infection with HPV 33 (12.8%), HPV 31 (9.8%), HPV 35 (9.1%), HPV 58 (8.3%), or HPV 45 (6.4%). Single infections with 5 high-risk HPV types (HPV 39, 59, 68, 53, 66) did not result in any CIN 3 or higher during the 13.4-year follow-up time (see Table S4, http://links.lww.com/LGT/A182).52
In a follow-up study involving the same Danish population as hereinabove, an analysis by genotype excluded mixed infections. Here, 5,528 women had baseline NILM cytology/HPV positive; of these, 2,875 had repeat testing during the 2005–2008 period after intervals between 1 and 4.5 years. Complete follow-up records were retrieved through the national Danish Pathology Data Bank using CIN 3 or higher as the clinical end point. Women were defined as having SGTP infection if they were positive for the same HPV type in the 2 separate cervical samples. In total, 874 (30.4%) of 2,875 had QPHP and 761 of the 874 women had SGTP (see Table S4, http://links.lww.com/LGT/A182). In agreement with Kjaer et al.52 (2010), this study reported the highest CIN 3 or higher risk for SGTP with HPV 16, 18, 33, and 31.53 Importantly, this study adjusted for age and time interval (3 years in this study) in-between HPV testing regarding the definition of persistence. In addition, the authors adjusted for possible effects of HPV 16 clearance or acquisition during the 3-year interim to more accurately assess the CIN 3 or higher risk associated with persistent non–HPV 16 infections.
A population-based prospective study of HPV infections and CIN 3/cancer in Guanacaste, Costa Rica, included 10,000 women.2 The definition of persistence was 12MPI, with a range of 9–21 months. Mixed infections were assigned by hierarchical method based on rank of single-type infections. Uniquely, HPV 16 was likely both to persist and to cause neoplastic progression when it persisted; 19.9% of HPV 16–infected women were diagnosed with CIN 3/cancer at enrollment or during the 5-year follow-up. Other carcinogenic types were not particularly persistent but could cause neoplastic progression (see Table S4, http://links.lww.com/LGT/A182).2
Among 12,527 women from the SWEDESCREEN double-blind randomized controlled trial, a longitudinal analysis of women from the colposcopy arm reported on 100 women who had NILM cytology, an HPV-positive result at the index screen, and persistent HPV positive at least 12 months later (median = 19 months). A random selection of 95 women from the control arm was also included.48 Women in the HPV testing arm were followed up with repeat HPV test and genotyping, cytology, and colposcopy if they had QPHP without CIN 2 or higher. A similar number of random colposcopies and tests were carried out in the control arm. Women were followed up over 13 years for the main outcome measures of cumulative incidence of CIN 2 or higher/CIN 3 or higher. There were 57 CIN 2 or higher outcomes, and 33 CIN 3 or higher outcomes. Among women who continued to attend and had continuous HPV genotype persistence, all developed CIN 2 or higher within 7 years (n = 40, 100%; 95% CI = 91% to 100%). No CIN 2 or higher cases were observed among women who cleared their HPV persistence within 13 years (0 of 35; 0%: 95% CI = 0% to 10%, p < .001). NILM with concurrent HPV 16 persistence conferred the highest CIN 2 or higher risk (68%), followed by HPV 18 (64%), 31 (58%), 33 (50%), and 45 (44%). NILM and HPV positive with the group of the other 9 types conferred a CIN 2 or higher risk of 30%. The same risk was assigned to all genotypes in mixed infection; this tends to decrease the absolute risk of the higher oncogenic genotype and increase the absolute risk of the less oncogenic genotypes.48
A post hoc analysis of young women (mean = 19.7 years) in the control arm of the Cervarix randomized, controlled HPV vaccination trial (PATRICIA) reported persistence and clearance of same-genotype positivity, after 6 months and after 12 months,42 with a definition of persistence of 6 months. A multivariate analysis was used to assign a mixed infection to the genotype of higher risk. A total of 4,825 women had at least 1 HPV-positive result during the 4 years. Of these, 3,363 had at least 1 record of 6-month persistence of the same genotype (69.7%); 2,283 had at least 1 record of 12 months persistence of the same genotype (67.9% of the group with 6-month persistence). Overall, 53% of HPV infections were cleared at 12 months. There were 217 CIN 2 outcomes, 114 CIN 3 outcomes, and 10 cancer outcomes. The HR for CIN 3 or higher if SGTP for 6 months was 5.3 (95% CI = 3.3 to 8.4) compared with a transient infection. Human papillomavirus 16 and HPV 31 had the least chance of being cleared and the highest risk of progression to high-grade CIN, followed by HPV 33, HPV 18, and HPV 45. The HRs reported demonstrate substantially higher risk for HPV 16 and 33, followed by HPV 31, 45, and 18 (see Table S5, http://links.lww.com/LGT/A182).42
The control arm of the phase 3 VIVIANE study in women, older than 25 years, was analyzed for the risk of progression from cervical HPV infection to detectable CIN.43 Infections were categorized depending on persistence as 6MPI or infection of any duration. There were 40 CIN 2 outcomes and 18 CIN 3 or higher outcomes. The same risk was assigned to all genotypes in mixed infection, which tends to decrease the absolute risk of the higher oncogenic genotype and increase the absolute risk of the less oncogenic genotypes. For 6MPI, the highest risk was associated with HPV 33 (HR = 31.9 [8.3–122.2, p < .0001]). The second highest risk was HPV 16 (21.1 [6.3–70.0], p < .0001). Similar findings were seen for infections of any duration. Significant risk was also observed for HPV 18, HPV 31, and HPV 45 (see Table S5, http://links.lww.com/LGT/A182).43
Lastly, the ARTISTIC study cohort was recalled for 3 rounds of screening and surveillance of lesser abnormal results.50,51 Here, SGTP was confirmed post hoc by extended genotyping. Mixed infections were assigned by hierarchical method based on rank of single-type infections and then analyzed in strata. Same-genotype persistence conferred a 3-fold greater likelihood of having CIN 2 or higher in round 2 than the likelihood for women with divergent HPV types in the 2 rounds (21.0% compared with 6.2%). In round 3, this was 2-fold, 14.7% versus 7.9%. The comparison of PPV by genotype across different genotypes and duration of follow-up demonstrated that the PPV of persistent HPV 16 is more than twice that of HPV 18. The PPV values of persistent HPV 31/33/52/58/45 were less than the PPV value for HPV 16, but comparable with that of HPV 18. The group of persistent HPV 51/35/39/68/56/59/66 had the lowest PPV values (see Table 5).50,51
TABLE 5.
| SGTP and all cytology | SGTP and NILM | SGTP and low-grade cytology | SGTP and all cytology | ||||
|---|---|---|---|---|---|---|---|
| HPV genotype result | 3-y ≥ CIN 351 | 5-y ≥ CIN 350 | 10-y ≥ CIN 350 | 5-y ≥ CIN 350 | 10-y ≥ CIN 350 | 5-y ≥ CIN 350 | 10-y ≥ CIN 350 |
| 16 | nr | 19.5 (10.3–35.3) | 22.0 (12.1–38.1) | 53.9 (30.4–80.8) | 53.9 (30.4–80.8) | 31.8 (22.1–44.5) | 33.4 (23.4–46.1) |
| 18 | nr | 7.7 (1.1–43.4) | 7.7 (1.1–43.4) | 25.0 (6.9–28.5) | 25.0 (6.9–28.5) | 13.0 (4.4–35.2) | 13.0 (4.4–35.2) |
| 16/18a | 18.5 (14.4–23.5) | 16.7 (10.4–25.6) | 18.5 (11.2–29.1) | 42.9 (26.0–61.6) | 42.9 (26.0–61.6) | 27.0 (20.2–35.0) | 28.1 (21.1–36.3) |
| 31, 33, 52, 58, 45 | 11.1 (7.4–16.4) | 9.3 (4.0–20.8) | 13.0 (6.4–25.3) | 13.6 (4.6–36.6) | 13.6 (4.6–36.6) | 15.7 (9.4–25.4) | 18.1 (11.3–28.2) |
| 51, 35, 39, 68, 56, 59, 66 | 5.0 (1.5–15.7) | 0 | 0 | 12.5 (3.3–41.4) | 12.5 (3.3–41.4) | 9.1 (3.5–22.4) | 9.1 (3.5–22.4) |
| Any SGTP | 11.9 (9.6–14.6) | 10.5 (6.3–17.0) | 12.7 (8.1–19.7) | 23.7 (14.8–36.8) | 23.7 (14.8–36.8) | 19.0 (14.4–24.9) | 20.4 (15.6–26.4) |
| HPV-positive at round 1 and round 2 | 6.6 (5.5–7.7) | 5.1 (3.2–8.1) | 6.4 (4.2–9.6) | 12.1 (7.7–18.8) | 12.9 (8.3–19.7) | 9.6 (7.4–12.5) | 10.6 (8.2–13.6) |
| Clear new | 1.7 (0.7–3.8) | 1.1 (0.4–3.0) | 1.4 (0.6–3.4) | 5.1 (2.5–10.4) | 5.9 (3.0–11.4) | 3.0 (1.8–4.9) | 3.4 (2.1–5.4) |
| Clearance (HPV negative at round 2) | 0.4 (0.1–1.5) | 0.09 (0.01–0.62) | 0.18 (0.04–0.71) | 1.6 (0.2–10.7) | 1.6 (0.2–10.7) | 0.16 (0.04–0.65) | 0.25 (0.08–0.76) |
aThe values for HPV 16/18 were imputed from data reported in Gilham et al.50 (2019).
nr, not reported.
DISCUSSION
Main Findings
Persistent infection with carcinogenic HPV is the cause of high-grade CIN and cancer1,2 However, current guidelines only includes, if any, HPV 16 or HVP 18 same-genotype persistence as an integral part of the algorithms.
Using qualitative HPV tests, persistence is a composite of true persistence, SGTP (44% and GS (56%) where the ability to distinguish is limited by the data resolution of the HPV test used.48 The clinical value of the distinction is well founded in current evidence from long-term follow-up studies that convincingly show that women who experience GS or outright clearance of infection rarely develop cancer and almost all cancers are associated with SGTP. Qualitative HPV tests cannot unlock the diagnostic information that may clear many women from having to attend surveillance testing and/or colposcopy.
In follow-up after abnormal screening test results, SGTP was reported to pose a much greater risk of high-grade CIN than GS. Women with GS and either NILM or low-grade cytology had immediate CIN 3 or higher risks consistently below the US clinical action threshold for colposcopy. Consequently, if genotyping information on screening samples were used, these women could safely undergo repeat testing rather than colposcopy, thus lowering the burden of screening. Of the women followed-up for prior abnormal results of lesser severity and those followed-up after colposcopy, up to one half with repeat positive QPHP could avoid colposcopy if the genotyping had been reported and recorded.
Different genotypes posed different risks of high-grade CIN when persistent, supporting a tiered-risk stratification approach to HPV genotype-specific risks associated with SGTP.2,42,43,48,50–53 Human papillomavirus 16 is clearly a much stronger viral carcinogen and is more likely to persist than any other HPV genotype.2,15,52,56 Moreover, HPV 16 persists longer than most other HPV types, which contributes to higher prevalence.2 Human papillomavirus 16 persistence was associated with high absolute risks for progression to high-grade CIN, at a level 2 1/2 times higher than the next highest-risk genotype, HPV 18, which is included in the top-tier genotypes HPV 33, 31, 18, and 45 all with a high HR for progression to high-grade CIN when persistent.2,37,42,43,48,50–53 Genotype-specific risk discrimination with persistence was reported in 3 risk tiers: HPV 16; HPV 18/31/33/52/58/45; and HPV 51/39/68/56/59/66. The long-term follow-up in the ARTISTIC study did not identify any cases of CIN 3 or higher if there was SGTP and NILM cytology. The failure rate for colposcopy to detect CIN 3 is higher in cases of QPHP with NILM cytology compared with QPHP and abnormal cytology.57 These findings require further research before guideline panels could consider an alternative to colposcopy for women with SGTP and NILM cytology and genotypes of lowest risk.
Strengths and Limitations
The inclusion of studies from various countries spanning more than a decade, with different population demographics, and HPV tests used is a strength of the analysis, given the similar conclusions reached across studies. That risk estimates are not entirely uniform across the studies can be explained from the population characteristics and differences in screening efforts. Most notably, some studies provided genotyping only for samples positive for HC2 representing an obvious verification bias. Other studies used genotyping assays without a clinical or analytically defined cutoff, which means that the assays used vary substantially in the ability to detect different HPV genotypes. Another limitation of this analysis is that across the published literature, researchers have developed different methods for assigning genotype results in the case of mixed infections. For useful genotype risk assessment, genotypes must be included in order from most to least discriminatory. In this analysis, a variety of hierarchical methods and restrictions to single-genotype infection and reporting of mixed infections by assigning the same risks to all the genotypes in a mixed infection were used. Restriction of analysis to single-genotype infection introduces bias. The simple proportional method of according equal risk to each genotype found in mixed infection results in totals exceeding 100% and overestimation of risk for genotypes of lesser rank order. In addition, it was difficult to determine in some studies whether HPV-positive women included in analyses were QPHP or SGTP, because of incomplete reporting at screening or because of no screening. This confounder might have artificially inflated risk values for high-grade cervical disease associated with QPHP. Moreover, an underlying assumption for all the hierarchical models is that mixed infections do not involve synergism that leads to risk greater than the risk associated with either individual genotype, although patterns of cervical HPV coinfection with different genotypes are not independent of one another.58 However, our knowledge of HPV biology is limited and so is our understanding of the interplay between risk and multiple infections.
The period over which risk was estimated differed in the studies from 2 to 13 years. Rare cases of premalignant and invasive cervical lesions can be related to non–high-risk HPV genotypes, although it is unknown whether these cases are the result of a previous multiple infection. Although these cases were not a focus of this systematic review, they are no doubt a confounding factor in some of the studies included in this synthesis.
Comparison With Other Reviews
Recently, our author group reported a systematic review supporting clinical utility of HPV genotyping in risk discrimination in cervical cancer screening.6 A systematic review by Koshiol et al11 reported that HPV persistence was consistently and strongly associated with high-grade CIN and called for precise definition and standardization of HPV testing, sampling procedure, and test interval for reliable clinical testing. Rositch et al59 reported from a meta-analysis that type-specific HPV testing would improve clinical specificity for high-grade CIN over qualitative-pooled HPV tests reporting “positive” and “negative” only. Mittal et al60 reported that the cumulative incidence of CIN 2 or higher in women after a colposcopy with results of CIN 1 or lower was significantly higher in women with true persistence compared with those with clearance of oncogenic HPV. Mariani et al61 stated that persistent positivity of HPV-DNA testing was considered a prognostic index of recurrent disease in patients treated for CIN 2 or higher and that HPV genotyping methods, as a biological indicator of persistent disease, were more suitable for a predictive role and risk stratification than pooled HPV-based testing. Bottari et al34 supported improved clinical utility of HPV genotyping compared with qualitative-pooled HPV positivity in follow-up after treatment of high-grade CIN. The overwhelming conclusion is that improvement to screening sensitivity and specificity can be achieved if HPV screening includes genotype reporting, at both screening and follow-up, to identify women with SGTP.
Clinical Implication of the Findings
If genotyping results are available from the index screening test and from subsequent visits, then the risk associated with SGTP is much higher than that observed with QPHP associated with a GS. Guideline panels could decide to differentiate management of women with SGTP by short-interval retests or colposcopy compared with those with GS where the risk of disease is substantially less. Ultimately, systematic reporting of genotype in positive samples could allow for differentiated follow-up among SGTP dependent on the persistent genotype, at the same time as allowing for less intensive follow-up of women with new infection(s) and clearance of the prior genotype by extending the follow-up intervals from the current 1 year. Today, guidelines rely on “cotest” (US) or “HPV test” (EU/AUS) after a selected interval (often 1 year), yet near-future guidelines might benefit from the implicit information available when genotyping is integrated into screening in an organized fashion and medical data are stored in electronic health records. A GS infers a low risk (compared with SGTP) of CIN 2 or higher at the time testing and a newly detected genotype infection could be followed by retesting after intervals, similar to the NILM cytology interval.
As one of the first countries, Japan's Joint Medical Society Guidelines in 2012 recommended that HPV genotyping should be used for women with histopathologic confirmed CIN 1/2 to characterize their risk of disease progression more precisely. Women who tested positive for HPV 16, 18, 31, 33, 35, 45, 52, and 58 were considered to be at increased risk of disease progression and to be managed separately from women who were negative for those 8 genotypes.62 Similarly, Denmark has included HPV genotyping and cytology as a combined triage of HPV screening positive women in the 2018 guidelines.
For women with SGTP, there is broad risk discrimination dependent on the specific genotype. However, from a practical screening program administration perspective, risk-based clinical action thresholds and the recommendations for follow-up could vary according to specific genotype or genotype risk tier. The duration of the SGTP infection could be factored in during subsequent assessments. In conclusion, HPV genotyping to identify true persistence is readily at hand from a technological perspective.
Supplementary Material
Footnotes
D.S.G. and J.C.A. were full-time employees of Becton, Dickinson and Company. M.T.S. has in the past served as a paid advisor to Roche and received honoraria from Roche and BD. J.B. is the principal investigator of studies supported with reagents and limited co-funding to his institution by BD Diagnostics, Agena Bioscience, Genomica SAU, LifeRiver Biotech, and QIAGEN. He has received honoraria for lectures from BD Diagnostics and Hologic. J.B. is appointed member of the National Danish Cervical Screening Committee by the Danish Health Authority and a member of the regional cervical screening steering committee of the Capital Region of Denmark. The other authors have declared they have no conflicts of interest.
J.A. was responsible for the study concept, study design, search, and screening of titles and abstracts. J.C.A. and D.S.G. were responsible for data extraction. J.H.B., D.M.E., F.Bot., A.D.I., C.E.C., F.Bog., M.T.S., K.S.K., D.S.G., and J.C.A. were responsible for data interpretation, risk of bias assessment, overall quality of evidence assessment, and preparation of the manuscript. K.S.K. is a distinguished investigator at University of Granada funded by the Beatriz Galindo (senior modality) program of the Spanish Ministry of Education. All authors have attended meetings with manufacturers of human papillomavirus assays discussed in this work.
This study was presented at the oral presentation at EUROGIN—Monaco, December 6, 2019.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jlgtd.com).
Contributor Information
Fabio Bottari, Email: Fabio.bottari@ieo.it.
Anna D. Iacobone, Email: AnnaDaniela.Iacobone@ieo.it.
Clementina E. Cocuzza, Email: Clementina.cocuzza@unimib.it.
Maria-Teresa Sandri, Email: Maria.sandri@humanitas.it.
Fabrizio Bogliatto, Email: qadosh2011@gmail.com.
Khalid S. Khan, Email: profkkhan@gmail.com.
Ditte M. Ejegod, Email: Ditte.Ejegod@regionh.dk.
Devin S. Gary, Email: devin.gary@bd.com.
Jeffrey C. Andrews, Email: Jeffrey.Andrews@bd.com.
REFERENCES
- 1.Chen HC Schiffman M Lin CY, et al. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011;103:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiffman M Herrero R Desalle R, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology 2005;337:76–84. [DOI] [PubMed] [Google Scholar]
- 3.Marks MA Castle PE Schiffman M, et al. Evaluation of any or type-specific persistence of high-risk human papillomavirus for detecting cervical precancer. J Clin Microbiol 2012;50:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto K Oki A Furuta R, et al. Predicting the progression of cervical precursor lesions by human papillomavirus genotyping: a prospective cohort study. Int J Cancer 2011;128:2898–910. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura Y Matsumoto K Satoh T, et al. HPV genotyping for triage of women with abnormal cervical cancer screening results: a multicenter prospective study. Int J Clin Oncol 2015;20:974–81. [DOI] [PubMed] [Google Scholar]
- 6.Bonde JH Sandri MT Gary DS, et al. Clinical utility of human papillomavirus genotyping in cervical cancer screening: a systematic review. J Low Genit Tract Dis 2020;24:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum 2012;100B:255–314. [Google Scholar]
- 8.Schiffman M Castle PE Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet 2007;370:890–907. [DOI] [PubMed] [Google Scholar]
- 9.Schiffman M, Clifford G, Buonaguro FM. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer 2009;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castle PE. Invited commentary: is monitoring of human papillomavirus infection for viral persistence ready for use in cervical cancer screening? Am J Epidemiol 2008;168:138–44; discussion 45-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koshiol J, Lindsay L, Pimenta JM. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am J Epidemiol 2008;168:123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacs K Varnai AD Bollmann M, et al. A 7.5-year prospective study of longer than 18 months type-specific human papillomavirus persistence in a routine cytology-based cervical screening population of about 31,000 women in West Germany. Eur J Cancer Prev 2009;18:307–15. [DOI] [PubMed] [Google Scholar]
- 13.Munoz N Castellsague X de Gonzalez AB, et al. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006;24(suppl 3):S3/1–10. [DOI] [PubMed] [Google Scholar]
- 14.Munoz N Hernandez-Suarez G Mendez F, et al. Persistence of HPV infection and risk of high-grade cervical intraepithelial neoplasia in a cohort of Colombian women. Br J Cancer 2009;100:1184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez AC Schiffman M Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010;102:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walboomers JM Jacobs MV Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12–9. [DOI] [PubMed] [Google Scholar]
- 17.Cuschieri KS Cubie HA Whitley MW, et al. Persistent high risk HPV infection associated with development of cervical neoplasia in a prospective population study. J Clin Pathol 2005;58:946–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kjaer SK van den Brule AJ Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ 2002;325:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radley D, Saah A, Stanley M. Persistent infection with human papillomavirus 16 or 18 is strongly linked with high-grade cervical disease. Hum Vaccin Immunother 2016;12:768–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rebolj M Brentnall AR Mathews C, et al. , Group HPVPS . 16/18 genotyping in triage of persistent human papillomavirus infections with negative cytology in the English cervical screening pilot. Br J Cancer 2019;121:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlecht NF Kulaga S Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001;286:3106–14. [DOI] [PubMed] [Google Scholar]
- 22.Schmeink CE Melchers WJ Siebers AG, et al. Human papillomavirus persistence in young unscreened women, a prospective cohort study. PloS one 2011;6:e27937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundstrom K Eloranta S Sparen P, et al. Prospective study of human papillomavirus (HPV) types, HPV persistence, and risk of squamous cell carcinoma of the cervix. Cancer Epidemiol Biomarkers Prev 2010;19:2469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massad LS Einstein MH Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2013;17:S1–s27. [DOI] [PubMed] [Google Scholar]
- 25.Demarco M Egemen D Raine-Bennett TR, et al. A study of partial human papillomavirus genotyping in support of the 2019 ASCCP Risk-Based Management Consensus Guidelines. J Low Genit Tract Dis 2020;24:144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egemen D Cheung LC Chen X, et al. Risk estimates supporting the 2019 ASCCP Risk-Based Management Consensus Guidelines. J Low Genit Tract Dis 2020;24:132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katki HA Gage JC Schiffman M, et al. Follow-up testing after colposcopy: five-year risk of CIN 2+ after a colposcopic diagnosis of CIN 1 or less. J Low Genit Tract Dis 2013;17:S69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katki HA Schiffman M Castle PE, et al. Five-year risks of CIN 3+ and cervical cancer among women who test Pap-negative but are HPV-positive. J Low Genit Tract Dis 2013;17:S56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke MA Darragh TM Nelson E, et al. Reporting and assessing the quality of diagnostic accuracy studies for cervical cancer screening and management. J Low Genit Tract Dis 2020;24:157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perkins RB Guido RS Castle PE, et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis 2020;24:102–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiffman M Wentzensen N Perkins RB, et al. An introduction to the 2019 ASCCP Risk-Based Management Consensus Guidelines. J Low Genit Tract Dis 2020;24:87–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiffman M Wentzensen N Khan MJ, et al. Preparing for the next round of ASCCP-sponsored cervical screening and management guidelines. J Low Genit Tract Dis 2017;21:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stockdale CK. ASCCP president 2019-2020 vision. J Low Genit Tract Dis 2019;23:187. [DOI] [PubMed] [Google Scholar]
- 34.Bottari F Iacobone AD Passerini R, et al. Human papillomavirus genotyping compared with a qualitative high-risk human papillomavirus test after treatment of high-grade cervical intraepithelial neoplasia: a systematic review. Obstet Gynecol 2019;134:452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu T-CJ Carter JJ Hughes JP, et al. Re-detection vs. new acquisition of high-risk human papillomavirus in mid-adult women. Int J Cancer 2016;139:2201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gravitt PE Rositch AF Silver MI, et al. A cohort effect of the sexual revolution may be masking an increase in human papillomavirus detection at menopause in the United States. J Infect Dis 2013;207:272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahlgren H Elfstrom KM Lamin H, et al. Colposcopic and histopathologic evaluation of women with HPV persistence exiting an organized screening program. Am J Obstet Gynecol 2020;222:253.e1–8. [DOI] [PubMed] [Google Scholar]
- 38.Rebolj M Bonde J Preisler S, et al. Human papillomavirus assays and cytology in primary cervical screening of women aged 30 years and above. PLoS One 2016;11:e0147326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moher D Liberati A Tetzlaff J, et al. , PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Institute of Medicine (US) Committee on Standards for Systematic Reviews of Comparative Effectiveness Research, Eden J Levit L Berg A, et al. , eds. In: Finding What Works in Health Care: Standards for Systematic Reviews. Washington, DC: National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 41.Booth A Clarke M Ghersi D, et al. An international registry of systematic-review protocols. Lancet 2011;377:108–9. [DOI] [PubMed] [Google Scholar]
- 42.Jaisamrarn U Castellsague X Garland SM, et al. Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study. PLoS One 2013;8:e79260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skinner SR Wheeler CM Romanowski B, et al. Progression of HPV infection to detectable cervical lesions or clearance in adult women: analysis of the control arm of the VIVIANE study. Int J Cancer 2016;138:2428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arbyn M Depuydt C Benoy I, et al. VALGENT: a protocol for clinical validation of human papillomavirus assays. J Clin Virol 2016;76(suppl 1):S14–21. [DOI] [PubMed] [Google Scholar]
- 45.Naaktgeboren CA de Groot JA van Smeden M, et al. Evaluating diagnostic accuracy in the face of multiple reference standards. Ann Intern Med 2013;159:195–202. [DOI] [PubMed] [Google Scholar]
- 46.Whiting PF Rutjes AW Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- 47.Castle PE Rodriguez AC Burk RD, et al. , Proyecto Epidemiológico Guanacaste (PEG) Group . Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ 2009;339:b2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elfgren K Elfstrom KM Naucler P, et al. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol 2017;216:264.e1–7. [DOI] [PubMed] [Google Scholar]
- 49.Gage JC Schiffman M Solomon D, et al. Comparison of measurements of human papillomavirus persistence for postcolposcopic surveillance for cervical precancerous lesions. Cancer Epidemiol Biomarkers Prev 2010;19:1668–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gilham C Sargent A Kitchener HC, et al. HPV testing compared with routine cytology in cervical screening: long-term follow-up of ARTISTIC RCT. Health Technol Assess 2019;23:1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitchener HC Canfell K Gilham C, et al. The clinical effectiveness and cost-effectiveness of primary human papillomavirus cervical screening in England: extended follow-up of the ARTISTIC randomised trial cohort through three screening rounds. Health Technol Assess 2014;18:1–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kjaer SK Frederiksen K Munk C, et al. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010;102:1478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sand FL Munk C Frederiksen K, et al. Risk of CIN3 or worse with persistence of 13 individual oncogenic HPV types. Int J Cancer 2019;144:1975–82. [DOI] [PubMed] [Google Scholar]
- 54.Schünemann HJ Oxman AD Brozek J, et al. GRADE: assessing the quality of evidence for diagnostic recommendations. Evid Based Med 2008;13:162–3. [DOI] [PubMed] [Google Scholar]
- 55.Walker JL Wang SS Schiffman M, et al. Predicting absolute risk of CIN3 during post-colposcopic follow-up: results from the ASCUS-LSIL Triage Study (ALTS). Am J Obstet Gynecol 2006;195:341–8. [DOI] [PubMed] [Google Scholar]
- 56.Khan MJ Castle PE Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005;97:1072–9. [DOI] [PubMed] [Google Scholar]
- 57.Horn J Denecke A Luyten A, et al. Reduction of cervical cancer incidence within a primary HPV screening pilot project (WOLPHSCREEN) in Wolfsburg, Germany. Br J Cancer 2019;120:1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldman B Rebolj M Rygaard C, et al. Patterns of cervical coinfection with multiple human papilloma virus types in a screening population in Denmark. Vaccine 2013;31:1604–9. [DOI] [PubMed] [Google Scholar]
- 59.Rositch AF Koshiol J Hudgens MG, et al. Patterns of persistent genital human papillomavirus infection among women worldwide: a literature review and meta-analysis. Int J Cancer 2013;133:1271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mittal S Basu P Muwonge R, et al. Risk of high-grade precancerous lesions and invasive cancers in high-risk HPV-positive women with normal cervix or CIN 1 at baseline-a population-based cohort study. Int J Cancer 2017;140:1850–9. [DOI] [PubMed] [Google Scholar]
- 61.Mariani L Sandri MT Preti M, et al. HPV-testing in follow-up of patients treated for CIN2+ lesions. J Cancer 2016;7:107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawaguchi R Matsumoto K Akira S, et al. Guidelines for office gynecology in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2017 edition. J Obstet Gynaecol Res 2019;45:766–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


