Supplemental Digital Content is available in the text.
Keywords: aortic stenosis; arteries; elastin; genes; hypertension; muscle, smooth
Background:
Elastin insufficiency causes recurrent vascular stenoses. Hemizygous deletion of the elastin gene (ELN) causes Williams-Beuren syndrome (WBS), while single nucleotide variants in ELN cause nonsyndromic supravalvar aortic stenosis (SVAS). Our objective was to compare cardiovascular disease outcomes in patients with WBS and nonsyndromic SVAS.
Methods:
Patients (81 WBS, 42 nonsyndromic SVAS) with cardiovascular disease were included in this retrospective single center study. Freedom from surgical and catheter interventions and reinterventions was compared. Vascular tissue from 8 patients and 6 controls was analyzed for arterial wall architecture.
Results:
Patients with nonsyndromic SVAS presented at a younger age (median 0.3 [0.4–0.7] years) compared with patients with WBS (1.3 [0.2–3.0] years) and had lower freedom from surgical/catheter interventions compared with patients with WBS, with median event-free survival 1.1 (0.3–5.9) versus 4.7 (2.4–13.3) years, respectively (hazard ratio, 1.62 [95% CI, 1.02–2.56]; P=0.04). Patients with nonsyndromic SVAS also had a lower freedom from reinterventions (P=0.054 by log-rank test). This was related in part to a higher frequency of primary and reinterventions for concomitant valvar aortic stenosis. Histology revealed abnormal intimal and medial thickening, disorganized and fragmented elastic fibers, reduced smooth muscle calponin expression, and increased macrophage marker, CD68, expression in the arterial walls in patients with WBS and nonsyndromic SVAS compared with controls.
Conclusions:
Patients with nonsyndromic SVAS require early and more frequent vascular and valvular interventions and reinterventions, in particular for concomitant valvar aortic stenosis compared with patients with WBS. This provides important prognostic information to guide counseling of affected families with cardiovascular disease and may guide primary intervention strategies based on predicted risk of restenosis.
Williams-Beuren syndrome (WBS) is a rare autosomal dominant disorder affecting 1 in 10 000 live births.1,2 It is caused by microdeletion of chromosome 7q11.23 typically involving 26 to 28 genes resulting in clinical manifestations that affect multiple organ systems and include short stature, cognitive impairment, cardiovascular involvement, and hypercalcemia. The cardiovascular phenotype is predominantly due to elastin haplo-insufficiency caused by the deletion of one copy of the elastin (ELN) gene within this region.3
Elastin in the vasculature is produced primarily from smooth muscle cells as elastin polymers which form elastic fibers that arrange as concentric rings of elastic lamellae in the arterial wall alternating with a ring of smooth muscle cells.4 This organization is critical for the arterial response to hemodynamic stress during systole and for maintaining blood pressure during diastole.5 Elastin insufficiency results in impaired elastin assembly and lack of elasticity of the arterial tree causing increased arterial stiffness.6 Also, without elastin, smooth muscle cells migrate and proliferate in the sub-endothelial region to cause medial hypertrophy and lumen occlusion.7–9 Important consequences of decreased functioning elastin include reduced integrity of elastic fibers in the skin, lungs and large blood vessels, and proliferation of smooth muscle cells, which ultimately leads to a thickened media and arterial narrowing. This results in supravalvar aortic stenosis (SVAS) in 70% of patients but can also involve other large arteries such as the pulmonary, aortic, renal and coronary arteries.10–12 In addition to discrete stenoses, the phenotype may include a generalized arteriopathy characterized by stiff arteries and systemic hypertension.13 Risk of progression of stenosis in WBS is variable and associated with severity of presentation.14 Thirty percent of patients require surgery to relieve stenoses with a mortality risk of 3% to 7%.15 Common alternatives to surgery for the repair of aortic stenosis are balloon angioplasty and stent insertion, but they have a higher risk of restenosis and vessel rupture.11 In addition, patients are susceptible to sudden death risk due to coronary spasm and ischemia in the context of coronary stenosis.16 Patients, therefore, require lifelong follow-up even after surgical or catheter interventions.10
Nonsyndromic SVAS differs from WBS in that patients only have a cardiovascular phenotype without the extra-cardiac phenotype seen in WBS. Isolated nonsyndromic SVAS, in a majority of cases, is autosomal dominant, caused by pathogenic variants in the ELN gene.10 Almost 90% are heterozygous loss-of-function variants and include frameshift, splice site, and 3′UTR variants that affect initiation of translation, and only a small proportion are missense variants. The severity of SVAS and other arterial stenoses varies among individuals. The objective of our study was to compare the cardiovascular phenotype and outcomes in patients with WBS and those with nonsyndromic SVAS. This knowledge would be important in risk prognostication and tailored management of patients presenting with SVAS as well as in appropriate counseling of affected families.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The study was approved by the institutional Research Ethics Board, and waiver of consent was obtained for retrospective analysis of clinical data. In the subset of patients that were enrolled in the Heart Centre Biobank Registry with collection of DNA and tissue samples for research, DNA was used to confirm genetic diagnosis on a research basis. Written informed consent for biobanking was obtained from the patient, parent, or legal guardian, and the protocol was approved by the institutional Research Ethics Board. In addition, arterial samples acquired at the time of surgical repair from consented patients were retrieved from the biobank or from leftover tissue in Surgical Pathology and evaluated histologically for structural abnormalities and elastin expression in the arterial wall.
The full methods are available in the Data Supplement.
Results
Clinical Characteristics
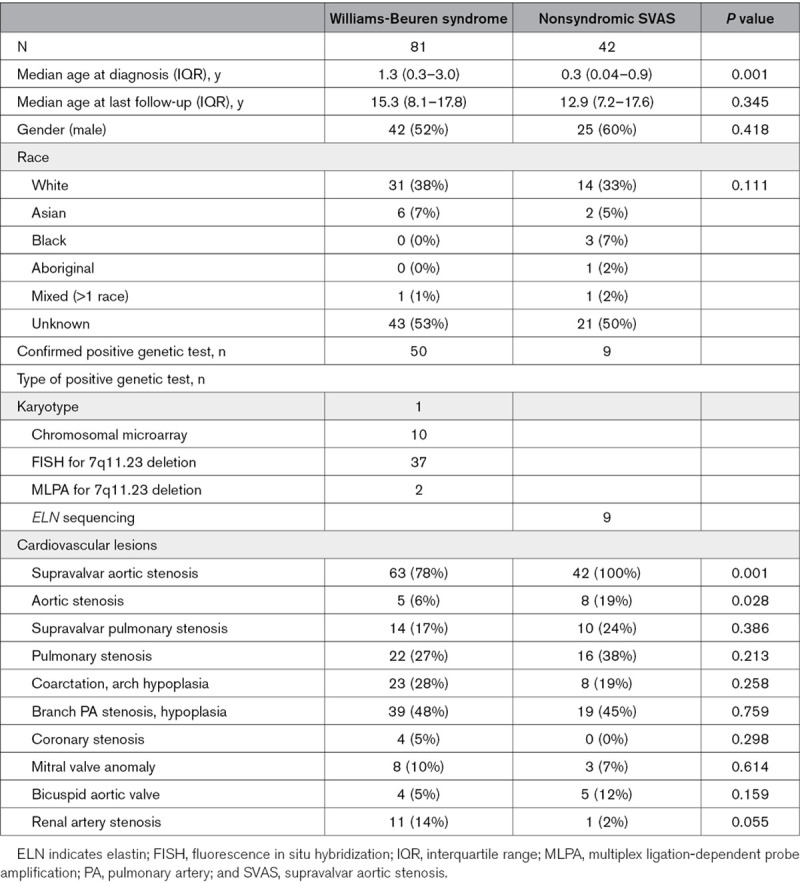
Patients <18 years old diagnosed with (1) WBS based on clinical evaluation and cytogenetic confirmation of 7q11.23 deletion or (2) nonsyndromic SVAS with/without those with ELN variants were screened in a single center retrospective cohort study. Only patients with a confirmed cardiovascular lesion were included. The presence or absence of syndromic features was determined through clinical evaluation by a clinical geneticist and a cardiologist. A total of 135 patients (86 WBS, 49 nonsyndromic SVAS) seen at our institution between 1995 and 2017 were screened; 123 patients with cardiovascular disease were included in the analysis—81 with WBS and 42 with nonsyndromic SVAS. There were no significant differences between the WBS and nonsyndromic SVAS group in baseline characteristics except for younger age at diagnosis in nonsyndromic SVAS compared with patients with WBS (P=0.001; Table 1). The most common cardiovascular lesion was SVAS affecting 78% of WBS and 100% of patients with nonsyndromic SVAS (P=0.001). The frequency of valvar aortic stenosis was higher in nonsyndromic SVAS compared with patients with WBS (P=0.028). The frequency of other cardiovascular lesions was not different between the 2 groups. Although frequency of confirmed coronary artery stenosis and of renal artery stenosis was higher in WBS compared with nonsyndromic SVAS, the differences were not statistically significant (Table 1).
Table 1.
Patient Characteristics (N=123)
Systemic hypertension, requiring antihypertensive medications at any time during follow-up, was diagnosed in 39 (32%) patients in the overall cohort. Median age at diagnosis of hypertension was 2.7 (0.8–9.9) years. Thirty-six percent of patients with WBS and 24% of patients with nonsyndromic SVAS had a history of hypertension with no difference in age at diagnosis (P=0.44). One-, 5-, and 10-year freedom from antihypertensive medications was 90%, 78%, and 68%, respectively, in patients with WBS and 90%, 83%, and 81% in patients with nonsyndromic SVAS (P=0.25 by log-rank test; Figure 1). Of patients receiving antihypertensive medications, 10% were on angiotensin-converting enzyme inhibitors, 19% on β-blockers, 14% on calcium channel blockers, 10% on diuretics, and 47% on combination therapy. Since fewer than 50% of patients in the overall cohort were on antihypertensive medications, median hypertension-free survival could not be calculated.
Figure 1.
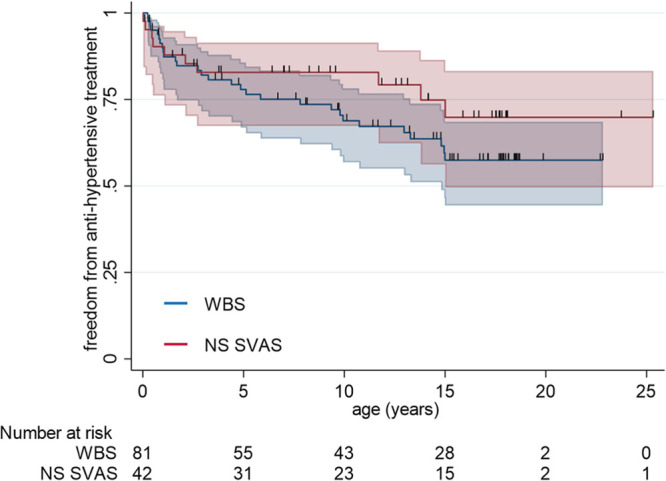
Kaplan-Meier survival curve with 95% CIs for freedom from antihypertensive treatment in Williams-Beuren syndrome (WBS; blue) and patients with nonsyndromic supravalvar aortic stenosis (NS-SVAS; red; P=0.253 by log-rank test).
Genetic Characteristics
In the WBS group, 50 patients had confirmed evidence of a 1.5 MB deletion or larger in 7q11.23 on clinical testing available in the medical records or through research testing. Fourteen of 42 patients with nonsyndromic SVAS had ELN sequencing done, of which 8 patients had confirmed pathogenic variants in ELN (including 2 patients with the same variant). All variants were heterozygous, and 4 variants were novel variants, that is, not previously reported. The details of ELN variants along with associated patient phenotype and outcomes at last follow-up are described in Table 2 and their genomic locations are shown in Figure 2. Results of evaluation by a clinical geneticist were available in 73 (59%) patients (56 WBS and 17 nonsyndromic SVAS). There was no difference in the clinical characteristics of patients who had genetic testing versus those who did not (data not shown).
Table 2.
Genetic Characteristics of Nonsyndromic SVAS

Figure 2.

Map of the elastin (ELN) gene illustrating positions of variants (red) from 9 patients with nonsyndromic supravalvar aortic stenosis (NS-SVAS). (1) 1_28del; (2) c.862_863insG; (3) c.890-9 T>A; (4) Q442X variant; (5) c.1234_1235ins; (6) c.1785T>A; (7) c.1909delG; and (8) c.1918+1G>A. Chr indicates chromosome.
Cardiovascular Outcomes
During a median follow-up of 14 years (interquartile range, 8–17 years), there were 3 deaths. One patient with nonsyndromic SVAS died preoperatively at age 26 days, one postoperatively at age 3.8 years, and 1 patient with WBS died postoperatively at age 6 months. Forty-eight patients with WBS (59%) and 30 (71%) nonsyndromic SVAS required at least one surgical or catheter intervention for cardiovascular lesions. The median (95% CI) intervention-free survival was 1.1 (0.3–5.9) years in the patients with nonsyndromic SVAS compared with 4.7 (2.4–13.3) years in the patients with WBS (Figure 3A). The risk of primary surgical or catheter intervention was higher in nonsyndromic SVAS compared with WBS after adjusting for sex (hazard ratio, 1.62 [95% CI, 1.02–2.56]; P=0.04). The types of primary surgical and catheter procedures are shown in Figure 3B. Patients with nonsyndromic SVAS had a significantly higher proportion of aortic valve procedures than patients with WBS (19% versus 2%, respectively; P=0.003). To adjust for years of follow-up, we compared the intervention incidence rates per 100 patient years of follow-up and found that the aortic valve intervention rates remained higher in the patients with nonsyndromic SVAS compared with patients with WBS (P=0.001; Table 3). Of the 19 patients with aortic valve lesions, 9 had bicuspid aortic valves. The proportion of patients with bicuspid aortic valve was not different between the 2 groups suggesting that valve morphology was likely not the basis of the differences in need for valve intervention (Table 1).
Figure 3.

Surgical or catheter interventions (n=123).A, Kaplan Meier survival curve of freedom from any surgical or catheter interventions. Patients with nonsyndromic supravalvar aortic stenosis (NS-SVAS; red, n=42) had lower freedom from interventions compared with patients with Williams-Beuren syndrome (WBS; blue, n=81; hazard ratio, 1.62 [95% CI, 1.02] 2.56; P=0.04). B, Types of primary surgical and/or catheter procedures in WBS (blue) and nonsyndromic SVAS (red). Other category=septal myectomy, mitral valve replacement, tetralogy of Fallot repair, ventricular septal defect repair, inter-atrial communication repair, LV outflow tract fibromyectomy, and pacemaker insertion. **P<0.01 between WBS and NS-SVAS. PA indicates pulmonary artery; and SVPS, supravalvar pulmonary stenosis.
Table 3.
Surgical or Catheter Intervention Rates per 100 Patient Years (95% CI) in WBS and NS-SVAS

Reinterventions
Of the 78 patients with a primary intervention, follow-up data was available in 72 patients. Of these, 46 (64%) patients (24 WBS and 22 nonsyndromic SVAS) required surgical or catheter re-interventions for recurrence of vascular stenoses. One-, 5-, and 10-year reintervention free survival was 60%, 49%, and 39%, respectively, in patients with WBS and 46%, 33%, and 19% in the patients with nonsyndromic SVAS (P=0.054 by log-rank test; Figure 4A). The types of reintervention procedures are shown in Figure 4B. When adjusted for follow-up duration, rates of reintervention per 100 years of patient follow-up were significantly higher in patients with nonsyndromic SVAS compared with patients with WBS for SVAS (P=0.006), aortic valve (P=0.002), aorta (P=0.03), and other lesions (P=0.013; Table 3).
Figure 4.

Surgical or catheter reinterventions (n=72).A, Kaplan-Meier survival curve of freedom from surgical or catheter reinterventions. Patients with nonsyndromic supravalvar aortic stenosis (NS-SVAS; red, n=29) had lower freedom from reinterventions compared with patients with Williams-Beuren syndrome (WBS; blue, n=43; P=0.054 by log-rank test). B, Types of reintervention procedures in WBS (blue) and NS-SVAS (red; n=72). Other category=mitral valve replacement, interatrial communication repair and pacemaker replacement. *P<0.05 between WBS and NS-SVAS.
Arterial Wall Architecture in Patients With WBS and NS-SVAS
Histological studies were performed on arterial tissue obtained at the time of cardiac surgery. This included 6 control patients with transposition of the great arteries (TGA) undergoing arterial switch procedure in whom aortic tissue was obtained, 7 patients with WBS (6 aorta, 1 pulmonary artery), and 1 patient (patient 5) with nonsyndromic SVAS harboring the heterozygous Q442X variant (pulmonary artery). Representative findings from arterial samples are shown in Figure 5. Compared with TGA, patients with WBS had thicker aortic walls due to more intimal and medial thickening (P=5.2×10−05; Figure 5A and 5B), as well as more disorganized and fragmented elastic fibers (Figure 5C, 5E, and 5F). Abnormal elastic fiber assembly was also prominent in the one patient with nonsyndromic SVAS (Figure 5D). Compared with TGA, staining for calponin, a marker of smooth muscle cell differentiation, was lower in the arterial wall of WBS compared with patients with nonsyndromic SVAS (P=0.03 WBS versus TGA; Figure 5C through 5E and 5G), and staining for CD68, a macrophage marker, was higher in patients with nonsyndromic SVAS and WBS (P=0.01 WBS versus TGA; Figure 5C through 5E and 5H).
Figure 5.

Arterial wall structure in transposition of the great arteries (TGA; n=6, aortic), Williams-Beuren syndrome (WBS; n=7, 6 aortic and 1 pulmonary artery), and nonsyndromic supravalvar aortic stenosis (NS-SVAS; n=1, pulmonary artery from patient number 5 with Q442X ELN variant). WBS and NS-SVAS arterial tissue showed disorganized elastic lamellae and increased macrophage expression compared with TGA controls. A, Representative images of Movat pentachrome stained sections from TGA and patients with WBS showing elastic fibers (black), collagen (yellow), and muscle cells (red). B, The transmural aortic wall thickness was higher in patients with WBS than TGA. C–E, Elastic lamellae showed parallel alignment in TGA but showed disorganized alignment in WBS and NS-SVAS arterial walls. C–E and F, Elastin fragmentation was higher in WBS compared with TGA (*P<0.05). C–E and G, The expression of smooth muscle differentiation marker, calponin, was lower on immunostaining in NS-SVAS and WBS compared with TGA (*P<0.05, WBS vs TGA). C–E and H, The expression of macrophage marker, CD68, on immunostaining was higher in WBS compared with TGA (*P<0.05).
Discussion
Elastin arteriopathy is a serious genetic condition caused by elastin insufficiency that leads to severe and recurrent vascular stenoses.17 Our study found that patients with nonsyndromic SVAS had a more severe cardiovascular phenotype requiring earlier and more frequent interventions for vascular stenoses as well as earlier and more frequent reinterventions for recurrence of stenoses compared with patients with WBS. This appeared to be related to both a higher frequency of concomitant valvar aortic stenosis and a higher requirement for primary and reinterventions for aortic valve lesions in the patients with nonsyndromic SVAS which is consistent with the findings in a previous study by Wu et al.18 The reintervention rates for SVAS and aortic lesions were also significantly higher in patients with nonsyndromic SVAS compared with patients with WBS in our study. Wu et al18 who did not find a difference in reinterventions between patients with WBS and non-WBS likely related to the shorter median duration of follow-up of 3.7 years for patients in their study.
Analysis of the genetic findings revealed that the most commonly deleted locus in the 7q11.23 region in our patients was from D7S489B to D7S1870 with an estimated deletion size of 1.5-2.5mbp. The deletion of one copy of ELN results in reduced but functionally normal elastin.19–21 Loss-of-function variants in ELN, on the other hand, can either cause a haplo-insufficiency or a dominant-negative effect. Our in silico predictions suggested that all the observed variants would lead to a premature stop codon that would result in nonsense-mediated decay of ELN mRNA.22 Nonsense-mediated decay leads to destruction of the prematurely terminated mRNA and loss of elastin production from the mutated allele, resulting in a single functioning allele and an elastin haplo-insufficiency phenotype.23 However, if truncated elastin escapes nonsense mediated decay, it could result in truncated functionally abnormal protein being generated that could affect the assembly of even normal elastin protein that is, a dominant-negative effect.24 While we were not powered for a genotype-phenotype analysis in the patients with nonsyndromic SVAS and did not have tissue to measure elastin protein levels in all our patients, we did note that patients with variants in distal exons (20 or higher) required more interventions than those with variants in proximal exons 1 to 17. Others have hypothesized that a less severe phenotype in some WBS could be related to a deletion involving NCF1 (neutrophil cytosolic Factor 1). NCF1 encodes a cytosolic subunit of neutrophil NADPH oxidase that produces superoxide anion on activation resulting in oxidative stress. Previous studies have reported that patients with WBS in whom the deletion involves the loss of a functioning copy of NCF1 gene, are protected against hypertension and vascular stiffness.25,26 This protective effect would not be observed in patients with point variants in ELN.
Our histology analyses confirmed the abnormal vascular structure with intimal and medial thickening and disorganized elastic fibers in all patients with ELN insufficiency, including the patient with nonsyndromic SVAS with a Q442X nonsense variant involving exon 21. We also assessed the expression of the macrophage marker, CD68, since smooth muscle cells within atherosclerotic plaques have been shown to undergo phenotype switching attracting immune cells, for example, macrophages, T cells, and B cells in the adventitia.27 Lineage tracing studies in human atheromas have reported that vascular smooth muscle cell phenotypic switching can also result in macrophage-like cells, expressing the CD68 marker.28 The finding of increased CD68 expression in the intimal and medial layers of arteries from patients with WBS and nonsyndromic SVAS raises the intriguing possibility that elastin insufficiency can predispose to a proinflammatory process which can potentially contribute to the high incidence of vascular restenoses after initial surgical or catheter intervention.
Limitations
The study had limitations inherent to a retrospective study including small cohort size and missing data. Since all patients did not have a confirmed genotype, we were unable to do genotype-phenotype comparisons within the WBS and nonsyndromic SVAS groups. Also, we were unable to analyze if elastin levels varied by genotype in the patients with nonsyndromic SVAS as a way to explain differences in phenotype. Coronary artery stenoses may be under-estimated since routine echocardiography may miss distal coronary stenoses, and renal artery stenoses may be underestimated since routine surveillance is not performed.
In summary, our findings indicate that patients with nonsyndromic SVAS are more likely to require early and more frequent vascular and valvular interventions and reinterventions, in particular for concomitant valvar aortic stenosis compared with patients with WBS with cardiovascular disease, although subgroup analyses of different intervention types was limited by sample size. Our findings have important clinical implications for patients with cardiovascular disease that is confirmed or suspected to be caused by elastin insufficiency. They highlight the importance of increasing physician awareness regarding the need for ELN sequencing in patients with nonsyndromic SVAS and in patients with SVAS who are negative for 7q11.23 deletion. The identification of a genetic cause of elastin insufficiency in patients with cardiovascular disease can help in family and reproductive counseling regarding recurrence risk in future pregnancies as an autosomal dominant condition. Importantly, our findings that patients with nonsyndromic SVAS have more severe disease with higher reintervention rates, provides important prognostic information for surgeons, interventionalists, and families although a larger study with more genetic and tissue information is needed to validate genotype-phenotype associations. Future efforts should be directed towards understanding the genetic basis of inter-individual variability in disease phenotype and severity.
Sources of Funding
The work was funded by the Canadian Institutes of Health Research grant number 126146 (S. Mital), the Ted Rogers Centre for Heart Research (S. Mital), and the Heart and Stroke Foundation/Robert M Freedom Chair in Cardiovascular Science (S. Mital).
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- SVAS
- supravalvar aortic stenosis
- TGA
- transposition of the great arteries
- WBS
- Williams-Beuren syndrome
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCGEN.120.002971.
For Sources of Funding and Disclosures, see page 695.
Contributor Information
Sandar Min, Email: sandar.min@sickkids.ca.
Caroline Kinnear, Email: Caroline.kinnear@sickkids.ca.
Jade Bouwmeester, Email: jade.bouwmeester@sickkids.ca.
Roderick Yao, Email: roderick.yao@sickkids.ca.
David Chiasson, Email: david.chiasson@sickkids.ca.
Fred Keeley, Email: fwk@sickkids.ca.
References
- 1.Strømme P, Bjørnstad PG, Ramstad K. Prevalence estimation of Williams syndrome. J Child Neurol. 2002;17:269–271. doi: 10.1177/088307380201700406 [DOI] [PubMed] [Google Scholar]
- 2.Pober BR. Williams-Beuren syndrome. N Engl J Med. 2010;362:239–252. doi: 10.1056/NEJMra0903074 [DOI] [PubMed] [Google Scholar]
- 3.Delio M, Pope K, Wang T, Samanich J, Haldeman-Englert CR, Kaplan P, Shaikh TH, Cai J, Marion RW, Morrow BE, et al. Spectrum of elastin sequence variants and cardiovascular phenotypes in 49 patients with williams–beuren syndrome. Am. J. Med. Genet A. 2013;161:527–533. doi: 10.1002/ajmg.a.35784 [DOI] [PubMed] [Google Scholar]
- 4.Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20:99–111. doi: 10.1161/01.res.20.1.99 [DOI] [PubMed] [Google Scholar]
- 5.Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest. 1998;102:1783–1787. doi: 10.1172/JCI4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salaymeh KJ, Banerjee A. Evaluation of arterial stiffness in children with Williams syndrome: does it play a role in evolving hypertension? Am Heart J. 2001;142:549–555. doi: 10.1067/mhj.2001.116763 [DOI] [PubMed] [Google Scholar]
- 7.Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, et al. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest. 2003;112:1419–1428. doi: 10.1172/JCI19028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223 [DOI] [PubMed] [Google Scholar]
- 9.Rein AJ, Preminger TJ, Perry SB, Lock JE, Sanders SP. Generalized arteriopathy in Williams syndrome: an intravascular ultrasound study. J Am Coll Cardiol. 1993;21:1727–1730. doi: 10.1016/0735-1097(93)90394-g [DOI] [PubMed] [Google Scholar]
- 10.Merla G, Brunetti-Pierri N, Piccolo P, Micale L, Loviglio MN. Supravalvular aortic stenosis: elastin arteriopathy. Circ Cardiovasc Genet. 2012;5:692–696. doi: 10.1161/CIRCGENETICS.112.962860 [DOI] [PubMed] [Google Scholar]
- 11.Pieles GE, Ofoe V, Morgan GJ. Severe left main coronary artery stenosis with abnormal branching pattern in a patient with mild supravalvar aortic stenosis and Williams-Beuren syndrome. Congenit Heart Dis. 2014;9:E85–E89. doi: 10.1111/chd.12087 [DOI] [PubMed] [Google Scholar]
- 12.Pober BR, Johnson M, Urban Z. Mechanisms and treatment of cardiovascular disease in Williams-Beuren syndrome. J Clin Invest. 2008;118:1606–1615. doi: 10.1172/JCI35309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins RT., 2nd.Cardiovascular disease in Williams syndrome. Circulation. 2013;127:2125–2134. doi: 10.1161/CIRCULATIONAHA.112.000064 [DOI] [PubMed] [Google Scholar]
- 14.Collins RT, 2nd, Kaplan P, Somes GW, Rome JJ. Long-term outcomes of patients with cardiovascular abnormalities and williams syndrome. Am J Cardiol. 2010;105:874–878. doi: 10.1016/j.amjcard.2009.10.069 [DOI] [PubMed] [Google Scholar]
- 15.Hickey EJ, Jung G, Williams WG, Manlhiot C, Van Arsdell GS, Caldarone CA, Coles J, McCrindle BW. Congenital supravalvular aortic stenosis: defining surgical and nonsurgical outcomes. Ann Thorac Surg. 2008;86:1919–1927. discussion 1927. doi: 10.1016/j.athoracsur.2008.08.031 [DOI] [PubMed] [Google Scholar]
- 16.Bird LM, Billman GF, Lacro RV, Spicer RL, Jariwala LK, Hoyme HE, Zamora-Salinas R, Morris C, Viskochil D, Frikke MJ, et al. Sudden death in Williams syndrome: report of ten cases. J Pediatr. 1996;129:926–931. doi: 10.1016/s0022-3476(96)70042-2 [DOI] [PubMed] [Google Scholar]
- 17.Kececioglu D, Kotthoff S, Vogt J. Williams-Beuren syndrome: a 30-year follow-up of natural and postoperative course. Eur Heart J. 1993;14:1458–1464. doi: 10.1093/eurheartj/14.11.1458 [DOI] [PubMed] [Google Scholar]
- 18.Wu FY, Mondal A, Del Nido PJ, Gauvreau K, Emani SM, Baird CW, Kaza AK. Long-term surgical prognosis of primary supravalvular aortic stenosis repair. Ann Thorac Surg. 2019;108:1202–1209. doi: 10.1016/j.athoracsur.2019.04.094 [DOI] [PubMed] [Google Scholar]
- 19.Urbán Z, Michels VV, Thibodeau SN, Donis-Keller H, Csiszár K, Boyd CD. Supravalvular aortic stenosis: a splice site mutation within the elastin gene results in reduced expression of two aberrantly spliced transcripts. Hum Genet. 1999;104:135–142. doi: 10.1007/s004390050926 [DOI] [PubMed] [Google Scholar]
- 20.Tassabehji M, Metcalfe K, Donnai D, Hurst J, Reardon W, Burch M, Read AP. Elastin: genomic structure and point mutations in patients with supravalvular aortic stenosis. Hum Mol Genet. 1997;6:1029–1036. doi: 10.1093/hmg/6.7.1029 [DOI] [PubMed] [Google Scholar]
- 21.Pérez Jurado LA, Peoples R, Kaplan P, Hamel BC, Francke U. Molecular definition of the chromosome 7 deletion in Williams syndrome and parent-of-origin effects on growth. Am J Hum Genet. 1996;59:781–792 [PMC free article] [PubMed] [Google Scholar]
- 22.Baker KE, Parker R. Nonsense-mediated mRNA decay: terminating erroneous gene expression. Curr Opin Cell Biol. 2004;16:293–299. doi: 10.1016/j.ceb.2004.03.003 [DOI] [PubMed] [Google Scholar]
- 23.Urbán Z, Michels VV, Thibodeau SN, Davis EC, Bonnefont JP, Munnich A, Eyskens B, Gewillig M, Devriendt K, Boyd CD. Isolated supravalvular aortic stenosis: functional haploinsufficiency of the elastin gene as a result of nonsense-mediated decay. Hum Genet. 2000;106:577–588. doi: 10.1007/s004390000285 [DOI] [PubMed] [Google Scholar]
- 24.Reichheld SE, Muiznieks LD, Lu R, Sharpe S, Keeley FW. Sequence variants of human tropoelastin affecting assembly, structural characteristics and functional properties of polymeric elastin in health and disease. Matrix Biol. 2019;84:68–80. doi: 10.1016/j.matbio.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 25.Del Campo M, Antonell A, Magano LF, Muñoz FJ, Flores R, Bayés M, Pérez Jurado LA. Hemizygosity at the NCF1 gene in patients with Williams-Beuren syndrome decreases their risk of hypertension. Am J Hum Genet. 2006;78:533–542. doi: 10.1086/501073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozel BA, Danback JR, Waxler JL, Knutsen RH, de Las Fuentes L, Reusz GS, Kis E, Bhatt AB, Pober BR. Williams syndrome predisposes to vascular stiffness modified by antihypertensive use and copy number changes in NCF1. Hypertension. 2014;63:74–79. doi: 10.1161/HYPERTENSIONAHA.113.02087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu D, Yin C, Luo S, Habenicht AJR, Mohanta SK. Vascular smooth muscle cells contribute to atherosclerosis immunity. Front Immunol. 2019;10:1101. doi: 10.3389/fimmu.2019.01101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. doi: 10.1038/nm.3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


