Abstract
Immunotherapy has been shown to prolong survival in recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN) in front-line use; however, subsequent systemic therapy has not been optimized. This study aimed to evaluate the safety and efficacy of cetuximab-containing chemotherapy after immunotherapy. We retrospectively analyzed patients with recurrent or metastatic SCCHN who underwent cetuximab-containing regimens after progression on immunotherapy. Of the 22 patients who met the inclusion criteria, 21 received paclitaxel and cetuximab, and 1 carboplatin and fluorouracil and cetuximab after immunotherapy. Nine patients achieved a partial response, 10 patients had stable disease as their best response on cetuximab-containing chemotherapy, yielding an overall response rate and disease control rate of 40.9 and 86.4%, respectively. The median progression-free survival was 5.2 months, and the median overall survival was 14.5 months. Ten patients developed grade 3–4 adverse events, including neutropenia (31.8%), acneiform rash (9.1%), anemia (4.5%), hypertransaminasemia (4.5%) and stomatitis (4.5%). The most frequent cetuximab-related toxicities across all grades were skin reactions (77.3%), hypomagnesemia (40.9%), stomatitis (27.3%), paronychia (13.6%) and keratitis (4.5%). There was no treatment-related death. Taken together, cetuximab-containing chemotherapy was effective and feasible even after immunotherapy.
Keywords: anti-epidermal growth factor receptor antibody, cetuximab, immune checkpoint inhibitor, salvage chemotherapy, squamous cell carcinoma of the head and neck
Introduction
Squamous cell carcinoma of the head and neck (SCCHN) is one of the most common malignancies worldwide [1]. A combination of surgery, radiation therapy and chemotherapy play a key role in improving life expectancy with this condition [2,3]. However, despite recent advances in multimodality treatment, the prognosis of metastatic or recurrent disease remains poor. The addition of cetuximab, an anti-epidermal growth factor receptor (EGFR) antibody, to platinum/5-fluorouracil (5-FU) chemotherapy prolongs survival and has been the standard front-line chemotherapy for recurrent or metastatic SCCHN (R/M-SCCHN) [4]. Nonetheless, cetuximab plus platinum-based chemotherapy (EXTREME regimen) yielded a median overall survival (OS) of only 10.1 months, indicating that further improvements are necessary.
The emergence of immune checkpoint inhibitors (ICIs) targeting programmed cell death-1 (PD-1) or PD-ligand 1 (PD-L1) has dramatically changed the treatment paradigm in various types of solid tumors. For example, the global phase III CheckMate 141 trial compared nivolumab, an anti-PD-1 antibody, versus standard therapies (methotrexate, docetaxel or cetuximab monotherapy) for R/M SCCHN refractory to platinum agents [5]. In this study, the nivolumab group exhibited a significantly longer OS, which resulted in the approval of nivolumab. Furthermore, the results of the use of another anti-PD-1 antibody, pembrolizumab, in first-line settings, were recently reported [6]. The KEYNOTE-048 trial was a phase III randomized trial that randomly assigned a total of 825 patients with R/M SCCHN into three cohorts: pembrolizumab alone, pembrolizumab + platinum/5-FU and the EXTREME regimen. Pembrolizumab alone is effective in patients with PD-L1-positive tumors and pembrolizumab plus chemotherapy showed survival benefits compared with the EXTREME regimen, thus establishing pembrolizumab as first-line therapy for R/M SCCHN. However, since cetuximab remains a key chemotherapy drug for R/M SCCHN, it is expected that cetuximab is increasingly used as a second- or later-line setting after exposure to immunotherapy. Several studies have demonstrated potential improvements in the treatment outcomes of cytotoxic chemotherapy after exposure to immunotherapy in various types of malignancies, including malignant melanoma, non-small cell lung cancer and stomach cancer [7–9]. In R/M SCCHN, two retrospective studies assessed patients who progressed on ICIs and subsequently underwent cytotoxic chemotherapy; these studies reported a relatively higher overall response rate (ORR) [10,11]. Meanwhile, previous studies suggested that the administration of EGFR tyrosine kinase inhibitors (TKIs) increased the risk of adverse events such as interstitial lung disease after treatment with anti-PD-1 antibodies [12,13].
Currently, following the KEYNOTE-048 trial, many prospective trials are ongoing in an attempt to evaluate the front-line use of ICIs [14]. Therefore, considering the treatment outcomes of subsequent cetuximab therapies after immunotherapy is essential. In the above-mentioned two retrospective studies of R/M SCCHN, subsequent therapies included various types of regimens and did not focus on cetuximab-containing regimens [10,11]. In particular, the safety of cetuximab use has not been adequately evaluated. Therefore, the present study aimed to evaluate the effectiveness and safety of the combined use of cetuximab and cytotoxic chemotherapy after exposure to ICIs.
Methods
Patients
We retrospectively reviewed the medical records of all patients with R/M SCCHN who underwent cetuximab-containing regimens after progressing while receiving ICI treatment at Kindai University Hospital between September 2017 and March 2020. The main eligibility criteria were as follows: an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, adequate organ function, and a receipt of at least one cycle of immunotherapy. Patients who had a previous cetuximab-containing treatment history before immunotherapy were also eligible. Cetuximab was administered at an initial dose of 400 mg/m2, followed by subsequent weekly doses of 250 mg/m2. This study was approved by the institutional review boards.
Data collection
The following information was acquired from medical records: age, sex, smoking status, site of the primary tumor and ECOG performance status at the start of cetuximab-containing chemotherapy after immunotherapy. We also retrieved tumor response and adverse events to each treatment line for R/M SCCHN patients. Tumor response was assessed by the investigator according to the Response Evaluation Criteria in Solid Tumors version 1.1 [15]. We defined the ORR as the proportion of patients with a best overall response of complete response or partial response. We defined the disease control rate (DCR) as the proportion of patients with a best overall response of complete response, partial response or stable disease. Progression-free survival (PFS) was measured from the time of treatment initiation to disease progression or death from any cause. OS was measured from the time of treatment initiation to death from any cause. Patients without documented clinical or radiographic disease progression or who were still alive were censored at the last follow-up. We evaluated adverse events according to the Common Terminology Criteria for Adverse Events, version 5.0.
Statistical analysis
Survival curves were estimated using the Kaplan–Meier method and hazard ratios with 95% confidence intervals (CIs) were determined with the use of a Cox proportional-hazards model. The ORRs were compared using Fisher’s exact test. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [16]. A P value <0.05 was considered statistically significant.
Results
Patient characteristics
A total of 22 patients met the eligibility criteria. Their clinical characteristics are summarized in Table 1. Sixteen patients developed recurrence after curative treatments, and six developed de novo metastatic SCCHN. Fifteen patients had a history of regional radiation therapy. Among seven patients with oropharyngeal cancer, four patients had a human papillomavirus-positive tumor. All patients received cetuximab after immunotherapy as combination therapy with chemotherapeutic agents; the most commonly administered regimen was paclitaxel plus cetuximab (21 patients, 95.5%) while one patient (4.5%) received carboplatin and fluorouracil plus cetuximab. Two (9.1%) patients had received three prior regimens for R/M SCCHN, 13 (59.1%) had received two prior regimens and 7 (31.8%) had received one prior regimen. For immunotherapy, 17 (77.3%) patients received nivolumab monotherapy and one patient received pembrolizumab plus cisplatin and fluorouracil. The remaining four patients received investigational drugs, including anti-PD-(L)1 antibodies.
Table 1.
Patient characteristics
| Characteristic | No. of patients | % |
|---|---|---|
| Median age (range), years | 65 (39–75) | |
| Sex | ||
| Male | 13 | 59.1 |
| Female | 9 | 40.9 |
| ECOG performance status | ||
| 0 | 5 | 22.7 |
| 1 | 16 | 72.7 |
| 2 | 1 | 4.5 |
| Primary tumor site | ||
| Nasopharynx | 3 | 13.6 |
| Oropharynx | 7 | 31.8 |
| Hypopharynx | 3 | 13.6 |
| Larynx | 3 | 13.6 |
| Oral cavity | 4 | 18.2 |
| Other | 2 | 9.1 |
| Type of relapse | ||
| Locoregional only | 7 | 31.8 |
| Distant metastases with or without locoregional recurrences | 15 | 68.2 |
| No. of previous regimens for R/M SCCHN | ||
| 1 | 7 | 31.8 |
| 2 | 13 | 59.1 |
| 3 | 2 | 9.1 |
| Prior immunotherapy regimens | ||
| Nivolumab monotherapy | 17 | 77.3 |
| Investigational drugs including anti-PD-(L)1 antibodies | 4 | 18.2 |
| Pembrolizumab plus cisplatin and fluorouracil | 1 | 4.5 |
| Prior cetuximab treatment | ||
| Yes | 13 | 59.1 |
| No | 9 | 40.9 |
Anti-PD-(L)1 antibodies, anti-programmed cell death- (ligand) 1 antibodies; ECOG performance status, Eastern Cooperative Oncology Group performance status; R/M SCCHN, recurrent/metastatic squamous cell carcinoma of head and neck.
Thirteen (59.1%) patients had a cetuximab-containing treatment history for R/M SCCHN before immunotherapy. The most frequent adverse events related to cetuximab among the patients receiving their first cetuximab-containing treatment were skin reactions (100%), paronychia (53.8%), hypomagnesemia (38.5%) and stomatitis (15.4%). Only one patient experienced a grade 4 adverse event, hypomagnesemia, which was manageable with intravenous magnesium supplementation. All other adverse events were grades 1–2. No patients discontinued the first cetuximab due to adverse events.
Immunotherapy
The patients underwent a median of seven immunotherapy cycles (range, 1–13). Three (13.6%) patients achieved a partial response, 10 (45.5%) achieved stable disease, and 9 (40.9%) achieved progressive disease as their best overall response on immunotherapy. Three patients developed immune-related adverse events (irAEs). Two patients developed irAEs of grade 3 or worse, including Stevens-Johnson syndrome (SJS) (grade 4) and nephritis (grade 3). The patient who developed an SJS discontinued immunotherapy. On the other hand, the patient who developed nephritis restarted immunotherapy after a serum creatinine level was resolved as grade 1.
The efficacy of cetuximab-containing chemotherapy after immunotherapy
The median time to cetuximab-containing chemotherapy from the last dose of immunotherapy was 21 days (range; 11–308). Fifteen patients (68.2%) were first administered cetuximab within 30 days after the last dose of immunotherapy. Of the total population, nine (40.9%) patients achieved a partial response, ten (45.5%) achieved stable disease and three (13.6%) achieved progressive disease as their best response on cetuximab-containing chemotherapy after immunotherapy, yielding an ORR and DCR of 40.9% (9 of 22 patients, 95% CI, 20.7–63.6%) and 86.4% (19 of 22 patients, 95% CI, 65.1–97.1%), respectively. The patients received a median of 12 cetuximab infusions (range, 2–51). With a median follow-up of 13.6 months, the median PFS and OS were 5.2 (95% CI, 3.6–7.2) and 14.5 (95% CI, 9.8–27.1) months, respectively (Fig. 1).
Fig. 1.
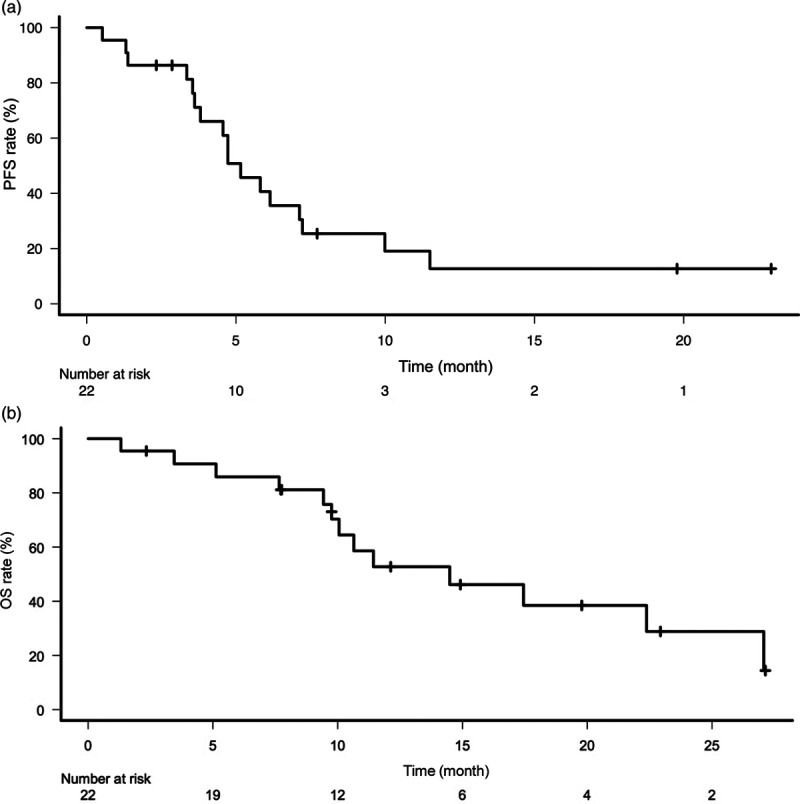
Kaplan–Meier curve of PFS (a) and OS (b) for patients with cetuximab-containing chemotherapy after immunotherapy. PFS, progression-free survival; OS, overall survival.
When comparing efficacy between patients with or without a history of prior cetuximab before immunotherapy, the former had a significantly longer PFS (median PFS = 7.1 vs. 3.8 months; hazard ratio: 0.34; 95% CI, 0.13–0.92; P = 0.03) (Fig. 2a), a numerically longer OS (median OS = 22.4 vs. 10.6 months; hazard ratio: 0.63; 95% CI, 0.20–1.97; P = 0.43) (Fig. 2b) and a numerically higher ORR (46.2 vs. 33.3%; P = 0.67).
Fig. 2.
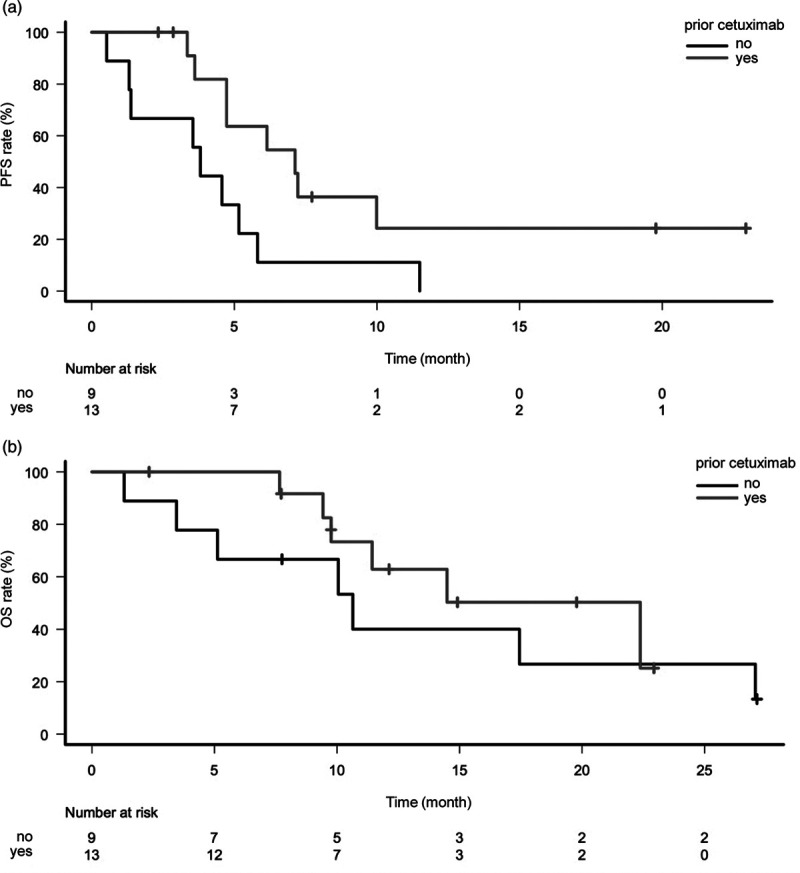
Kaplan–Meier curve of PFS (a) and OS (b) for patients with cetuximab-containing chemotherapy after immunotherapy according to with or without a history of prior cetuximab before immunotherapy. PFS, progression-free survival; OS, overall survival.
The safety of cetuximab-containing chemotherapy after immunotherapy
Adverse events related to cetuximab that were observed during cetuximab-containing chemotherapy after immunotherapy are listed in Table 2. The most common were skin reactions (77.3%), hypomagnesemia (40.9%), stomatitis (27.3%), paronychia (13.6%) and keratitis (4.5%). Grades 3–4 adverse events related to cetuximab were observed in two patients. One developed both a grade 3 acneiform rash and grade 3 stomatitis; the other patient developed a grade 3 acneiform rash. Grades 3–4 adverse events not related to cetuximab were observed in seven patients during cetuximab-containing chemotherapy after immunotherapy (Table 3), including grades 3–4 neutropenia (27.3%), grade 3 anemia (4.5%) and grade 3 hypertransaminasemia (4.5%). No unexpected adverse events were observed. Furthermore, there were no treatment-related deaths.
Table 2.
Adverse events related to cetuximab
| Adverse events | Patients who received cetuximab-containing chemotherapy after immunotherapy (n = 22) |
||
|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | |
| Hypomagnesemia | 8 | 1 | 0 |
| Skin reactionsa | 10 | 5 | 2 |
| Paronychia | 1 | 2 | 0 |
| Stomatitis | 3 | 2 | 1 |
| Keratitis | 0 | 1 | 0 |
aSkin reactions include acneiform rash, dry skin, eczema and pruritus.
Table 3.
Grades 3–4 adverse events not related to cetuximab
| Adverse events | Patients who received cetuximab-containing chemotherapy after immunotherapy (n = 22) |
|
|---|---|---|
| Grade 3 | Grade 4 | |
| Neutropenia | 5 | 1 |
| Anemia | 1 | 0 |
| Hypertransaminasemia | 1 | 0 |
Discussion
To the best of our knowledge, this is the first study to investigate subsequent chemotherapy after immunotherapy in R/M SCCHN, focusing on cetuximab-containing therapies. Given that pembrolizumab alone and pembrolizumab in combination with chemotherapy have been established as the standard treatments in the first-line setting for R/M SCCHN and that several phase III studies are ongoing to evaluate the efficacy of ICIs in the first-line setting, in line with other malignancies [17], there is increasing concern regarding cetuximab use after immunotherapy. Therefore, determining the efficacy and safety of the combined use of cetuximab and cytotoxic chemotherapy after immunotherapy in R/M SCCHN is an important issue. In a later-line setting, combination therapies of cetuximab with taxanes were also proven to be effective and tolerable [18,19]. However, patients who had received prior immunotherapy were not included in these studies. Previous retrospective studies assessing chemotherapy after immunotherapy in R/M SCCHN included various types of regimens and did not report detailed safety profiles [10,11]. It remains unclear whether the combined use of cetuximab and cytotoxic chemotherapy is valid after failure with ICIs treatment. The present study indicated that cetuximab-containing chemotherapy had a meaningful therapeutic value and a manageable toxicity profile for patients with R/M SCCHN who were pretreated with ICIs.
When it comes to efficacy, we observed an ORR of 40.9% (9 of 22 patients, 95% CI, 20.7–63.6%) and a median PFS of 5.2 (95% CI, 3.6–7.2) months for combination therapy with cetuximab after immunotherapy. In a previous study, Saleh et al. analyzed 82 R/M SCCHN patients who underwent salvage chemotherapy after treatment with ICIs and reported an ORR of 30% and a median PFS of 3.6 months [10]. A report by Pestana et al., including 43 R/M SCCHN patients who underwent systemic therapy after treatment with ICIs, indicated that the ORR was 42% and the median PFS was 4.2 months [11]. Our data were similar to or slightly higher than those of previous studies. Higher chemosensitivity in subsequent therapies after immunotherapy has been supported by the protracted effect of immunotherapy [20,21]. Recent preclinical studies have suggested that EGFR inhibition affects the tumor microenvironment and induces an immune-modulatory effect [22], which leads to our hypothesis that the residual therapeutic efficacy of ICIs may be synergistic with cetuximab. Approximately half of the subjects of this analysis were pretreated with cetuximab and underwent re-administration of cetuximab; these patients tended to have a better outcome compared with patients without prior cetuximab therapy. The CRICKET study suggested that readministering cetuximab provided a clinical benefit in patients with metastatic colorectal cancer displaying disease progression while receiving anti-EGFR antibodies in an earlier treatment line and thereafter, other regimens [23]. These results may be explained by the fact that the clones resistant to anti-EGFR therapies were reduced by subsequent chemotherapy without anti-EGFR antibodies [24]. Likewise, immunotherapy might remove tumor resistance to cetuximab. Although our findings should be interpreted with caution and further studies are necessary, patients with R/M SCCHN may also gain clinical benefits from the re-administration of cetuximab.
With respect to safety, all toxicities that were observed during cetuximab-containing chemotherapy after immunotherapy was well tolerated, and no unexpected adverse events were observed. The safety profile of cetuximab in the present study was consistent with that in previous studies evaluating cetuximab plus taxanes [18,19]. Notably, most of the subjects were administered cetuximab immediately after the last infusion of ICIs. Previous studies reported that combining EGFR-TKI with immunotherapy may increase the incidence of interstitial lung disease and lead to increased alanine aminotransferase/aspartate aminotransferase levels in nonsmall cell lung cancer harboring EGFR mutations [12,13]. These reports proposed certain prudence in using anti-EGFR agents after immunotherapy, which encouraged us to conduct the present study. However, on the basis of our findings, cetuximab could be used safely in such settings, unlike EGFR-TKI.
This study has some limitations. First, it was a retrospective single-institution study evaluating a small number of patients. Second, we lacked a control arm assessing chemotherapy without cetuximab; therefore, we cannot clarify the additional benefits of cetuximab to salvage chemotherapy after immunotherapy. In addition, from this study, the optimal cytotoxic agent to combine with cetuximab could not be identified because most of our subjects received paclitaxel plus cetuximab. Third, the possibility of selection bias cannot be excluded because approximately half of the subjects received prior cetuximab before immunotherapy, which meant that patients who were tolerable to anti-EGFR antibodies in earlier lines of therapy were included. A future prospective study is warranted to confirm our results.
In conclusion, our findings suggest that cetuximab-containing chemotherapy is effective and well tolerated even just after ICIs in R/M SCCHN. Our findings will help physicians select second-line treatment after failure to ICIs as a first-line therapy in clinical practice.
Acknowledgements
Conflicts of interest
S.M. reports research funding from Taiho Pharmaceutical Co.; honoraria from Taiho Pharmaceutical Co., Eli Lilly, Takeda Pharmaceutical Co. and Ono Pharmaceutical Co. K.H. reports grants from AstraZeneca K.K. and MSD K.K.; lecture fees from AstraZeneca K.K., AS ONE Corporation, Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., MSD K.K., Pfizer Japan Inc. and Ono Pharmaceutical Co. Ltd. H.H. reports research funding from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Chugai Pharmaceutical Co. Ltd. and Ono Pharmaceutical Co. Ltd.; honoraria from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., Kyorin pharmaceutical Co. Ltd., Merck Biopharma Co., Ltd., MSD K.K., Novartis pharmaceuticals K.K., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., Shanghai Haihe Biopharm, Taiho Pharmaceutical Co. Ltd. and Takeda Pharmaceutical Co. Ltd. T.Y. reports honoraria from Daiichi-Sankyo Co. Ltd., Hisamitsu Pharmaceutical (China) Co. Ltd., Mundipharma Pharmaceutical Co. Ltd. and Kyowa Pharmaceutical Industry Co. Ltd. K.N. reports research funding from AbbVie Inc., Astellas Pharma Inc., AstraZeneca K.K., A2 Healthcare Corp., Bayer Yakuhin, Ltd., Bristol Myers Squibb Company, Chugai Pharmaceutical Co. Ltd., CMIC Shift Zero K.K., Daiichi Sankyo Co. Ltd., Eisai Co. Ltd., Eli Lilly Japan K.K., EP-CRSU Co. Ltd., EPS Corporation., EPS International Co. Ltd., Gritsone Oncology Inc., ICON Japan K.K., inVentiv Health Japan, Kissei Pharmaceutical Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Linical Co. Ltd., Merck Serono Co. Ltd., Merck Biopharma Co. Ltd., MSD K.K., Nippon Boehringer Ingelheim Co. Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., PAREXEL International Corp., Pfizer Japan Inc., Pfizer R&D Japan G.K., Quintiles Inc./IQVIA Services JAPAN K.K., SymBio Pharmaceuticals Limited., Syneos Health. and Takeda Pharmaceutical Co. Ltd.; honoraria from AbbVie Inc., Astellas Pharma Inc., AstraZeneca K.K., Bayer Yakuhin, Ltd., Bristol Myers Squibb Company, Chugai Pharmaceutical Co. Ltd., CareNet, Inc., Clinical Trial Co. Ltd., Daiichi Sankyo Co. Ltd., Eli Lilly Japan K.K., KYORIN Pharmaceutical Co. Ltd., Medical Mobile Communications Co. Ltd., Medical Review Co. Ltd., MEDICUS SHUPPAN, Publishers Co. Ltd., Merck Biopharma Co. Ltd., MSD K.K., NANZANDO Co. Ltd., Nichi-Iko Pharmaceutical Co. Ltd., Nikkei Business Publications, Inc., Nippon Boehringer Ingelheim Co. Ltd., Nippon Kayaku Co. Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., Reno. Medical K.K., Roche Diagnostics K.K., SymBio Pharmaceuticals Ltd., Taiho Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Thermo Fisher Scientific K.K., YODOSHA CO. LTD., YOMIURI TELECASTING CORPORATION. and 3H Clinical Trial Inc.; consulting or advisor role from Eli Lilly Japan K.K., KYORIN Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc. and Takeda Pharmaceutical Co. Ltd. There are no conflicts of interest for the remaining authors.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424 [DOI] [PubMed] [Google Scholar]
- 2.D’cruz A, Lin T, Anand AK, Atmakusuma D, Calaguas MJ, Chitapanarux I, et al. Consensus recommendations for management of head and neck cancer in Asian countries: a review of international guidelines. Oral Oncol. 2013; 49:872–877 [DOI] [PubMed] [Google Scholar]
- 3.Nibu KI, Hayashi R, Asakage T, Ojiri H, Kimata Y, Kodaira T, et al. Japanese clinical practice guideline for head and neck cancer. Auris Nasus Larynx. 2017; 44:375–380 [DOI] [PubMed] [Google Scholar]
- 4.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008; 359:1116–1127 [DOI] [PubMed] [Google Scholar]
- 5.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016; 375:1856–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Jr, et al. ; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019; 394:1915–1928 [DOI] [PubMed] [Google Scholar]
- 7.Kato R, Hayashi H, Chiba Y, Miyawaki E, Shimizu J, Ozaki T, et al. Propensity score-weighted analysis of chemotherapy after PD-1 inhibitors versus chemotherapy alone in patients with non-small cell lung cancer (WJOG10217L). J Immunother Cancer. 2020; 8:e000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato K, Narita Y, Mitani S, Honda K, Masuishi T, Taniguchi H, et al. Efficacy of cytotoxic agents after progression on anti-PD-(L)1 antibody for pre-treated metastatic gastric cancer. Anticancer Res. 2020; 40:2247–2255 [DOI] [PubMed] [Google Scholar]
- 9.Hadash-Bengad R, Hajaj E, Klein S, Merims S, Frank S, Eisenberg G, et al. Immunotherapy potentiates the effect of chemotherapy in metastatic melanoma-a retrospective study. Front Oncol. 2020; 10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saleh K, Daste A, Martin N, Pons-Tostivint E, Auperin A, Herrera-Gomez RG, et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer. 2019; 121:123–129 [DOI] [PubMed] [Google Scholar]
- 11.Pestana RC, Becnel M, Rubin ML, Torman DK, Crespo J, Phan J, et al. Response rates and survival to systemic therapy after immune checkpoint inhibitor failure in recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 2020; 101:104523. [DOI] [PubMed] [Google Scholar]
- 12.Mamesaya N, Kenmotsu H, Katsumata M, Nakajima T, Endo M, Takahashi T. Osimertinib-induced interstitial lung disease after treatment with anti-PD1 antibody. Invest New Drugs. 2017; 35:105–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang H, Liu X, Wang M. Immunotherapy combined with epidermal growth factor receptor-tyrosine kinase inhibitors in non-small-cell lung cancer treatment. Onco Targets Ther. 2018; 11:6189–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von der Grün J, Rödel F, Brandts C, Fokas E, Guckenberger M, Rödel C, et al. Targeted therapies and immune-checkpoint inhibition in head and neck squamous cell carcinoma: where do we stand today and where to go. Cancers. 2019; 11:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247 [DOI] [PubMed] [Google Scholar]
- 16.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013; 48:452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi H, Nakagawa K. Combination therapy with PD-1 or PD-L1 inhibitors for cancer. Int J Clin Oncol. 2020; 25:818–830 [DOI] [PubMed] [Google Scholar]
- 18.Knoedler M, Gauler TC, Gruenwald V, Matzdorff A, Schroeder M, Dietz A, et al. Phase II study of cetuximab in combination with docetaxel in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck after platinum-containing therapy: a multicenter study of the Arbeitsgemeinschaft Internistische Onkologie. Oncology. 2013; 84:284–289 [DOI] [PubMed] [Google Scholar]
- 19.Sosa AE, Grau JJ, Feliz L, Pereira V, Alcaraz D, Muñoz-García C, Caballero M. Outcome of patients treated with palliative weekly paclitaxel plus cetuximab in recurrent head and neck cancer after failure of platinum-based therapy. Eur Arch Otorhinolaryngol. 2014; 271:373–378 [DOI] [PubMed] [Google Scholar]
- 20.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010; 28:3167–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osa A, Uenami T, Koyama S, Fujimoto K, Okuzaki D, Takimoto T, et al. Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight. 2018; 3:e59125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saba NF, Chen ZG, Haigentz M, Bossi P, Rinaldo A, Rodrigo JP, et al. Targeting the EGFR and immune pathways in Squamous Cell Carcinoma of the Head and Neck (SCCHN): forging a new alliance. Mol Cancer Ther. 2019; 18:1909–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cremolini C, Rossini D, Dell’Aquila E, Lonardi S, Conca E, Del Re M, et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 2019; 5:343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg RM, Montagut C, Wainberg ZA, Ronga P, Audhuy F, Taieb J, et al. Optimising the use of cetuximab in the continuum of care for patients with metastatic colorectal cancer. ESMO Open. 2018; 3:e000353. [DOI] [PMC free article] [PubMed] [Google Scholar]


