Abstract
Objective
The aim of the study was to describe the features required for diagnosis of differentiated vulvar intraepithelial neoplasia (dVIN) and vulvar aberrant maturation (VAM).
Materials and Methods
The International Society of the Study of Vulvovaginal Diseases tasked the difficult pathologic diagnoses committee to develop consensus recommendations for clinicopathologic diagnosis of vulvar lichen planus, lichen sclerosus, and dVIN. The dVIN subgroup reviewed the literature and formulated diagnostic criteria that were reviewed by the committee and then approved by the International Society of the Study of Vulvovaginal Diseases membership.
Results
Differentiated vulvar intraepithelial neoplasia is the immediate precursor of human papillomavirus (HPV)–independent vulvar squamous cell carcinoma and shows a spectrum of clinical and microscopic appearances, some overlapping with HPV-related neoplasia. The histopathologic definition of dVIN is basal atypia combined with negative or nonblock-positive p16 and basal overexpressed, aberrant negative, or wild-type p53. The most common pattern of dVIN is keratinizing with acanthosis, aberrant rete ridge pattern, and premature maturation. The morphologic spectrum of keratinizing dVIN includes hypertrophic, atrophic, acantholytic, and subtle forms. A few dVIN cases are nonkeratinizing, with basaloid cells replacing more than 60% of epithelium. Vulvar aberrant maturation is an umbrella term for lesions with aberrant maturation that arise out of lichenoid dermatitis and lack the basal atypia required for dVIN.
Conclusions
Evaluation of women at risk for dVIN and VAM requires a collaborative approach by clinicians and pathologists experienced in vulvar disorders. Close surveillance of women with lichen sclerosus and use of these recommendations may assist in prevention of HPV-independent squamous cell carcinoma through detection and treatment of dVIN and VAM.
Key Words: vulva, differentiated VIN, vulvar aberrant maturation, HPV-independent, squamous cell carcinoma, lichen sclerosus, lichen planus, high-grade squamous intraepithelial lesion
There are 2 types of vulvar intraepithelial neoplasia (VIN), both immediate precursors to vulvar squamous cell carcinoma (SCC). High-grade squamous intraepithelial lesion (HSIL, usual VIN), is human papillomavirus (HPV)–related, usually shows warty-basaloid morphology, and comprises more than 80% of VIN but less than 50% of SCC.1–6 Differentiated VIN (dVIN) is HPV-independent and usually shows keratinizing morphology on a background of lichen sclerosus (LS).7–11
High-grade squamous intraepithelial lesion commonly displays full-thickness atypical cells with large dark nuclei and scant basophilic cytoplasm, giving the epithelium a blue appearance on hematoxylin and eosin (H&E)–stained slides, obviously different to nonneoplastic skin conditions.10,11 In contrast, dVIN often recapitulates the pink-colored maturation pattern seen in benign conditions such as LS and lichen simplex chronicus (LSC). The atypia of dVIN is subtle compared with HSIL for 3 reasons—it is often confined to basal and parabasal layers, abnormal mitoses are uncommon, and some or all enlarged nuclei are vesicular rather than hyperchromatic. As a result of these difficulties, traditional definitions of dVIN focused on a distinctive pattern of parakeratosis (PK), elongated branched rete ridges, marked intercellular prickles, and keratin pearls.7,12
Over the past 2 decades, multiple publications highlighted deficiencies in this construct of morphology guiding diagnosis. Researchers identified keratinizing HSIL that mimics dVIN, basaloid dVIN that mimics HSIL, and atrophic dVIN that mimics LS.11,13–18 Researchers also described lesions of uncertain malignant potential adjacent to dVIN and SCC; the term “vulvar aberrant maturation” (VAM) captures their unifying histopathologic characteristic.19–21 Immunohistochemistry (IHC) for p16 emerged as a reliable marker for HPV-related neoplasia, and description of distinctive p53 IHC patterns helped distinguish dVIN and VAM from HSIL.15,22–29 These advancements revealed that 10%–25% of vulvar neoplasia is misclassified when diagnosis relies on a traditional combination of clinical risk factors and morphologic categorization.13,22,30–33
Distinguishing between HPV-related and HPV-independent precursors has important implications for treatment and prognosis. Treatment of dVIN and steroid-resistant VAM is excision, whereas options for HSIL include imiquimod, LASER, and excision. Differentiated VIN is more likely than HSIL to progress to cancer and more often associated with a prior, synchronous, or subsequent SCC.1,34,35 Human papillomavirus–independent SCC is less radiosensitive with higher disease-related mortality.1,4,34–38 High-grade squamous intraepithelial lesion surveillance is within the scope of most gynecologists, whereas evaluation for dVIN and VAM requires skill and experience in vulvar dermatoses.1,39 Correct diagnosis is essential to direct clinical care. The aims of this document are to critically appraise the literature and to formulate consensus recommendations for clinicopathologic diagnosis of dVIN and VAM.
METHODS
The International Society for the Study of Vulvovaginal Diseases (ISSVD) tasked the difficult pathologic diagnoses committee with development of consensus documents for diagnosis of lichen planus (LP), LS, and dVIN. The dVIN subgroup performed a literature search in PubMed-Medline from database inception through March 2020 using terms: “vulvar” and “vulval” “intraepithelial neoplasia,” “VIN,” “differentiated,” “simplex,” “histology,” “pathology,” and “histopathology.” Review of selected studies' references identified additional relevant publications. The subgroup appraised and summarized pertinent studies, synthesized them into a critical review, and then generated diagnostic criteria and recommendations. The manuscript was disseminated within the committee and underwent revisions to achieve consensus, and then was approved by ISSVD membership. Signed written consents were obtained for use of clinical photographs.
Incidence and Epidemiology
Mean age at diagnosis of HPV-independent neoplasia, to include invasive and intraepithelial disease, ranges from 67 to 78 years.1,4,7,12,18,20,24,35,36,40,41 Although characterized as a disease of older women, multiple cases have occurred in women aged 17–39 years.18,42–45 The age-standardized incidence of HPV-independent SCC has fallen for the past 30 years from 0.76 to 0.54/100,000.1 Rates fell from 2.53 to 1.62/100,000 in women older than 50 years; meanwhile, the incidence in younger women remained steady since 1991 at 0.14/100,000. Median interval between biopsy-proven dVIN and SCC is reported as 23–44 months (range = 6–102).8,14,35,46
There is strong epidemiologic, histopathologic, and clinical evidence that LS is the major underlying cause of HPV-independent neoplasia. A Finnish registry study found women with LS have a standardized incidence ratio of 40.3 for vulvar SCC, with a total of 160 cancers.47 Retrospective cohorts of keratinizing and/or HPV-negative SCC identify peritumoral LS in 40% to 88%.14,27,36,40,48–51 A review of 43 cases of HPV-independent SCC found that all were associated with LS when results from previous and subsequent vulvar specimens were incorporated.52 Historically, 5% of women with LS develop neoplasia and this risk seems reduced by tailored long-term topical steroids.1,53 Uncertainties around primary prevention include optimal maintenance regimens, frequency and mechanism of follow-up, and circumstances under which steroid cessation is appropriate.18,52–54 Presence of symptoms and architectural change does not predict neoplasia.20,55 There are minimal data on secondary prevention of SCC. A molecular “point of no return” might negate the impact of steroids on a particular clone, but therapy may slow carcinogenesis in other areas.
Association between erosive LP and dVIN/SCC remains uncertain. Cohort studies report SCC in 1%–3% of affected women but fail to exclude HPV-related disease.52,56 Finnish registry data found the standardized incidence ratio of LP for vulvar cancer of any histologic type is 1.99 (total of 18 cancers), low enough to be explained by misdiagnosis and comorbid HPV-related disease.57–59 Reasons for possible misattribution of HPV-independent neoplasia to LP include underrecognition of comorbid LS and LP, histopathologic similarity of nonsclerotic LS and LP, and disappearance of sclerosis under neoplasia.60–64
The rate of dVIN diagnosis before SCC development varies by health care setting and study methodology, but the trajectory suggests improvements in detection.1 A cohort in 2000 found that 95% of dVIN cases were adjacent to SCC.65 Subsequent expert reviews identified in 24%–28% of dVIN cases preceding SCC.7,35 In 2018, Jin and Liang66 found that 34% of dVIN cases lacked associated SCC, matching the rate documented by Day et al in 2020.20
Clinical Assessment of dVIN and VAM
Symptoms
Symptoms reflect the underlying dermatosis, so most women report pruritus or pain.20 Ten percent of women with LS are asymptomatic with neoplasia occurring in this setting.11,20,31 Some women report focally severe itch or pain at the site of dVIN or VAM.
Vulvar Examination
Differentiated VIN and VAM look different to surrounding abnormal skin, but detection may be challenging. Lichen sclerosus produces changes to vulvar architecture, color, and texture, sometimes accompanied by superimposed mycotic, bacterial, or viral infection. Postinflammatory hyperpigmentation, melanosis, and postoperative changes further complicate assessment. Some specialists find a colposcope facilitates detection through light and magnification. Acetic acid application is not recommended for evaluation of HPV-independent neoplasia.67 Differentiated VIN and VAM do not demonstrate acetowhite uptake nor enhancement in margin visualization.68 Moreover, acetic acid is painful and produces false-positive results in areas of dermatitis and physiologic uptake at the mucocutaneous junction.
Differentiated VIN and VAM most often occur on periclitoral structures and labia minora, areas encompassing hairless skin, mucocutaneous junction, and nonkeratinized squamous epithelium.4,20 The next most common site is perineum and perianus, in keeping with LS distribution.20 Characterization of dVIN as unifocal and HSIL as multifocal is an oversimplification. Clinical photographs of HPV-independent neoplasia often show concurrent lesions with diverse morphologies, and 20%–50% of women have 2 or more noncontiguous disease sites.4,12,18,20,28,69
Vulvar aberrant maturation presents as a well-demarcated white papule or plaque, often with an irregular or verruciform surface19 (see Figure 1). Findings consistent with dVIN include a white papule or plaque, “gray-white discoloration with a roughened surface,” pink-red plaque, glazed red patch, and flat pink center with a raised white border12,16–18,28,31,46,50,65 (see Figures 2–4). Clinicians may describe pink-red patches as ulcers or erosions, but these labels do not uniformly concur with histopathologic findings. Pink-red areas may contain white papules or have a smooth surface, mosaic pattern, or gravel-like texture.16,17 Lesions may be less than 1 cm or extend across the vulva.12,46 The unifying description is any treatment-resistant lesion in a field of dermatosis-affected skin.
FIGURE 1.
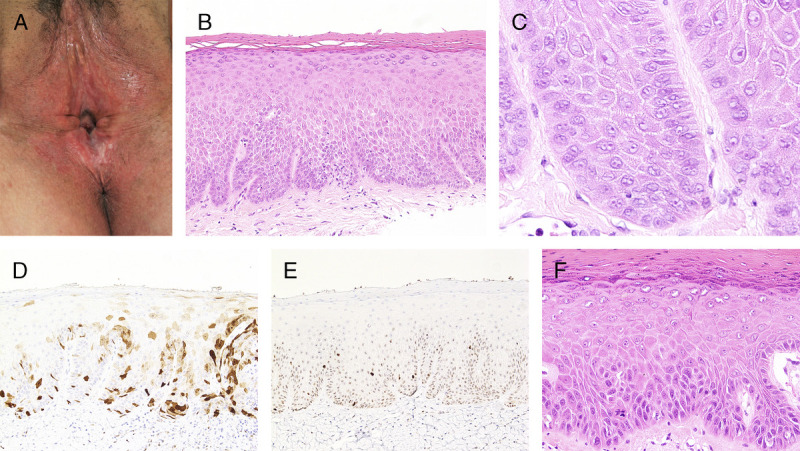
A, Vulvar aberrant maturation—white plaques with a rough surface over posterior fourchette and perineum, background of LS-associated architectural change. B, Parakeratosis, hypergranulosis, acanthosis with clubbed rete ridges, premature maturation, prominent intercellular prickles, and vesicular basal nuclei, H&E ×100. C, Uniform vesicular nuclei with intranuclear vacuoles and occasional prominent nucleoli, H&E ×400. D, p16 is nonblock positive with focal variable cytoplasmic and nuclear staining, ×200. E, p53 is wild type, ×200. F, Two years later, excision of the white plaque shows traditional keratinizing dVIN with PK, anastomosing rete ridges, premature maturation, and basal atypia seen as hyperchromasia, pleomorphism, enlargement, and an abnormal mitosis, H&E ×200.
FIGURE 2.
A, Differentiated vulvar intraepithelial neoplasia—glazed red macule at left clitoral frenulum on a background of LS. B, Basaloid dVIN with erosion, full-thickness atypical nuclei, multiple mitoses, and moderate lymphoplasmacytic infiltrate, H&E ×200. C, p16 is negative, ×200. D, p53 is overexpressed at basal and suprabasal layers, ×200.
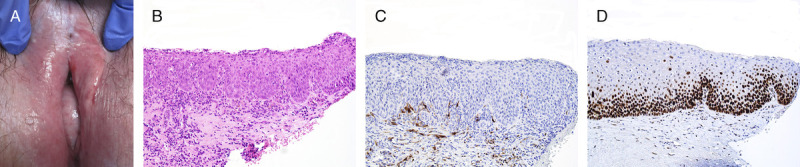
FIGURE 4.
A, Squamous cell carcinoma and dVIN—central tumor surrounded by a white heterogeneous plaque. B, Hypertrophic keratinizing dVIN with thick PK and wide elongated rete ridges, H&E ×100. C, Vesicular nuclei with marked enlargement and multiple nucleoli, H&E ×400. D, p16 is negative ×100. E, p53 is aberrant negative, ×100.
Biopsy—Indications and Recommendations
Lichen sclerosus guidelines recommend biopsy for diagnostic uncertainty and suspicion for neoplasia but provide few additional details.70–72 Survey of 23 expert pathologists identified clinical features thought to support dVIN: visible lesion, history of LS/LP, previous SCC, older than 40 years, and steroid nonresponsiveness; none of these distinguish between leading differential diagnoses nor reliably exclude HSIL.73 Clinicians engaged in LS supervision require (1) familiarity with subtle manifestations of dVIN, (2) equipment and skill to biopsy in the outpatient setting, and (3) a mechanism for prompt operating room access for periclitoral or periurethral disease, multiple lesions, or refusal of office procedures. Adequate tissue sampling and labeling maximizes the chance of correct diagnosis. Accurate, pertinent information on pathology request forms is fundamental to clinicopathologic correlation.
Recommendations for tissue sampling of suspected precursor lesions67,74:
Optimal specimens require a minimum 4-mm width with 5-mm depth for hair bearing skin and 3-mm depth for hairless skin; this may be achieved with punch, suture-assisted snip, or excisional techniques,
Biopsy each morphologically distinct site at the most suspicious part of the lesion(s),
In the case of ulcer or fissure, biopsy where there is intact epithelium, and
In the case of presumed erosion, obtain a biopsy within the red-pink patch.
Recommendations for labeling and communication with pathologists include53,60,62:
Note laterality and location with anatomic terms, not an unoriented “clock-face” position,
Obtain and store clinical photographs with consent,
Write the underlying dermatosis and differential diagnosis on the request form,
Document concern for neoplasia and any previous HSIL, VAM, dVIN, and/or SCC, and
Flatten and pin excisional specimens with labels of surrounding structures.
Mapping biopsies may be required to outline margins and guide excisional procedures. Rates of margin positivity for dVIN range from 45% to 75%.29,35 To preserve capacity for sentinel nodes, generalists should avoid wide local excision unless SCC was recently excluded.
Clinical Differential Diagnosis
The differential diagnosis for a white plaque within abnormal skin includes lichenified LS, LS with mycotic superinfection, the border of erosive LP, VAM, and HSIL.14,53,62 Indicators of mycosis include erythema, labial edema, vaginal discharge, satellite lesions, erosions, fissures, and keratin debris.75 White plaques associated with erosive LP are thin, well demarcated, located beside a glazed red patch, and homogenous in color and texture.59,60 Vulvar aberrant maturation is well demarcated and thick or verrucous. High-grade squamous intraepithelial lesion is variable in size, shape, number of lesions, and thickness but usually presents as a white, gray, red, pink, tan, brown, and/or black plaque, sometimes showing punctuation and mosaicism.6,11 Despite these clues, expert physical examination may not reliably distinguish between diagnoses, so histopathologic assessment is necessary.
Summary of Clinical Findings Consistent With dVIN and VAM
Differentiated VIN presents as treatment-resistant lesions different to surrounding abnormal skin. Color, texture, location, size, and focality are variable. The 3 most common appearances are a thick white plaque, a thin pink-red plaque, and a red glazed patch. Vulvar aberrant maturation manifests as a white nodule or plaque in a field of lichenoid dermatitis. If areas identified histologically as VAM persist despite potent topical and/or intralesional steroids, excision is recommended.
Histopathology of dVIN and VAM
General Principles for Evaluation of Vulvar Squamous Neoplasia
The traditional approach begins with H&E and periodic acid–Schiff (PAS)–stained slides, reviewed first at low power and then sequentially higher power. At each step, interpretation is associated with greater interobserver disagreement. At low power, pathologists identify architectural features such as epithelial thickness, rete ridge length and shape, stromal collagen, and lymphocytic infiltrate (Table 1). At medium power, pathologists assess for surface features, intercellular breakdown, and epithelial maturation (Table 2). Evidence of dyskeratosis emerges, to include intracellular vacuoles, suprabasilar apoptotic bodies, and premature maturation defined as large suprabasilar cells with eosinophilic cytoplasm. At high power, pathologists inspect nuclei for atypical features: abnormal chromatin, pleomorphism, increased and/or abnormal mitoses, and enlargement (Table 3).20 A decision about IHC occurs after review of H&E and PAS.
TABLE 1.
Spectrum of Architecture in dVIN and VAM20
| Diagnosis | Epithelial thickness | Rete ridges—size | Rete ridges—shape | Stroma | Lymphocytic infiltrate |
|---|---|---|---|---|---|
| Nonkeratinizing dVIN | |||||
| Basaloid dVIN | Normal to thick 0.1–0.5 mm |
Reduced | Flat acanthosis Blunted or bulbous |
Normal Fibrosis and/or sclerosis |
Moderate to dense |
| Intermediate dVIN | Normal to thick 0.08–0.6 mm |
Reduced to enlarged | Flat acanthosis Blunted or bulbous |
Normal Fibrosis and/or sclerosis |
Variable |
| Keratinizing dVIN | |||||
| Atrophic | Thin <0.2 mm |
Reduced | N/A | Sclerosis and/or fibrosis Normal |
Variable |
| Subtle | Normal 0.18–0.35 mm |
Reduced to normal | Normal | Sclerosis and/or fibrosis Normal |
Scant to moderate |
| Traditional | Normal to thick 0.1–0.9 mm |
Enlarged | Branched Clubbed |
Sclerosis and/or fibrosis Normal |
Variable |
| Acantholytic | Normal to thick 0.14–1.5 mm |
Enlarged | Branched Clubbed |
Sclerosis and/or fibrosis Normal |
Variable |
| Hypertrophic | Markedly thick 0.6–2.2 |
Enlarged | Branched Complex |
Fibrosis and/or sclerosis | Moderate to dense |
| VAM | Thick 0.35–2.5 |
Reduced to enlarged | Flat acanthosis Branched |
Sclerosis and/or fibrosis Normal |
Scant to moderate |
TABLE 2.
Spectrum of Dyskeratosis in dVIN and VAM20
| Diagnosis | Surface features | Intercellular breakdown | Premature maturation | % of epithelium with cellular maturation |
|---|---|---|---|---|
| Nonkeratinizing dVIN | ||||
| Basaloid dVIN | Thin PK Erosion |
Uncommon | None | <10 |
| Intermediate dVIN | Thin PK Erosion |
Uncommon | None | 10–40 |
| Keratinizing dVIN | ||||
| Atrophic | Thin PK Normal |
Variable | None | 50–80 |
| Subtle | Normal Thin PK |
None | None | 60–90 |
| Traditional | Thin to thick PK/HK | Frequent | Frequent | 40–90 |
| Acantholytic | Thin to thick PK | Marked | Variable | 40–90 |
| Hypertrophic | Thin to thick PK/HK | Frequent | Frequent | 40–90 |
| VAM | Thick PK/HK | Variable | Frequent | >80 |
TABLE 3.
Definition of Basal Nuclear Atypia in Vulvar Squamous Neoplasia
| Nuclear feature | Common feature of atypia | Less common feature of atypia |
|---|---|---|
| Chromatin | Hyperchromatic • Dense chromatin • Nucleoli not well seen |
Vesicular • Open chromatin • Enlarged eosinophilic nucleoli • Double or multiple nucleoli • Occasional binucleation |
| Enlargement | Diffuse marked enlargement • More than triple the size of a lymphocyte nucleus Obviously different to nuclei in nonneoplastic epithelium |
Variable enlargement • Most nuclei double the size of a lymphocyte nucleus Difference between abnormal and normal nuclei highlighted by overexpressed p53 |
| Pleomorphism | Marked cell-to-cell variation Irregularly shaped cells | Cell-to-cell variation present Majority of cells show uniform shape with enlarged vesicular nuclei |
| Mitoses | Increased • >1 per 1-mm basal layer length |
Abnormal mitotic figures • Y-shaped, X-shaped • Pieces of extra chromosomes separate to the main mitotic spindle |
This process provides opportunities for errors that account for dVIN's poor interobserver reproducibility and 25%–61% rate of nondiagnosis.8,29 On low power, dVIN often shows a lichenoid or acanthotic reaction pattern, leading to presumption of LS, LP, or LSC. At high power, the spectrum of atypical nuclear features overlaps with reactive changes, so identification requires a keen eye and comparison with normal. Lack of suspicion for dVIN results in failure to order p16 and p53; clinicians contribute to this through inadequate documentation on request forms. In contrast, diagnosis of warty-basaloid HSIL is straightforward because architectural and maturational abnormalities are evident at low power, and at high power, the atypia and abnormal mitoses are obviously neoplastic.11
Yang and Hart's landmark description of dVIN focused on a morphologic pattern uncommon in nonneoplastic disorders that combines architectural features such as elongated complex rete ridges with maturational abnormalities such as PK, keratin whorls, and pearls and premature maturation. They de-emphasized basal atypia by stating “the range of nuclear atypia in these cells was variable.”12 Multiple authors subsequently endorsed that “pathologists should not be fixated on nuclear atypia in the diagnosis… but should look for supporting features of altered architecture and cell changes,” in an effort to prevent overdiagnosis.7,14,46
However, 20 years of knowledge accumulation yields the conclusion that morphology is an unreliable indicator of diagnosis. This calls into question the framework of low-medium-high power assessment and optional IHC. Basal layer atypia is the single unifying feature of neoplasia, and a panel of p16 and p53 is the best way to distinguish between HSIL and dVIN. Mechanisms to mitigate challenges posed by subversion of long-standing concepts include detailed definitions of terms, standardized descriptions of IHC patterns and their significance, and a categorization system encompassing the spectrum of dVIN and VAM. In practice, clinicopathologic correlation, expert consultation, or multidisciplinary review is often required to arrive at the correct diagnosis.
Pathogenesis of HPV-Independent SCC
Pathogenesis involves overlap of the scar-cancer and itch-scratch-cancer hypotheses; these propose that non-HPV–related SCC arises from traumatized epithelium over scarred stroma. They derive from observations that extragenital LS is not associated with SCC, pruritic conditions such as vulvar psoriasis are not linked to SCC, and hidradenitis suppurativa scars represent an HPV- and LS-independent cancer pathway.10,76,77 The mechanism of LS is T-cell–mediated attack on basal keratinocytes, yielding a cycle of damage and repair associated with oxidative injury, increased cell turnover, and abnormal collagen production.11,78,79 Excoriation may fuel this process. Gradual accumulation of genetic aberrations means that there is no clear histopathologic dividing line between benign and neoplastic.
Molecular underpinning of HPV-independent neoplasia is an area of ongoing investigation. Somatic TP53 mutations occur in 41%–79%, but correlation between histopathology, p53 IHC, mutational analysis, and prognosis remains unclear.5,10,11,29,30,78–80TP53 mutations in SCC and adjacent epithelium are concordant in less than half of cases and noncontiguous dVIN lesions show different genetic aberrations, suggesting tandem development of multiple clones.11,14,78,81 Twenty percent of HPV-independent neoplasia results from pathways related to hypermethylation, chromosome gains, and mutations in NOTCH1 (28%–41%), HRAS (3%–31%), PIK3CA (0%–19%), and CDKN2A (11%–36%).5,10,11,19,82–85 These findings concur with clinical observations of simultaneous VAM, dVIN, verrucous SCC, and conventional SCC in the same woman or specimen.29,53
Previous Work on Histopathologic Diagnosis of dVIN and VAM
Descriptions of dVIN are influenced by methodological approach. Studies of dVIN adjacent to SCC often encounter marked acanthosis, atypia, fibrosis, and lymphocytic infiltrate.26,40,77,78 Research comparing archetypal dVIN cases to selected dermatoses may exclude subtle iterations.12,69,86,87 Cohorts of VIN without SCC overrepresent HPV-related disease.88,89 Publications lacking stringent HPV determination or expert pathologic review may produce misclassification.90–92 Reassessment of pre-SCC biopsies is most likely to identify a morphologic spectrum.8,18,46
Several authors attempted to gain consensus on diagnostic features of dVIN. A Dutch group considered 5 items most predictive: (1) abnormal mitoses in the basal layer, (2) basal cell atypia defined as pleomorphism and enlargement, (3) dyskeratosis, (4) prominent nucleoli, and (5) elongated and anastomosing rete ridges.46 Education on these features improved recognition in gynecologic but not general pathologists. The survey of pathologists found that basal layer atypia and negative p16 were the only essential criteria, whereas premature maturation was the sole nonnuclear feature reaching consensus as “strongly supportive.”73 Dermatopathologists identified acanthosis and branched rete ridges as strongly supportive, but these had uncertain significance for gynecologic pathologists.
A North American group described 4 categories of dVIN occurring in isolation or combination: (1) dVIN resembling LS—thin epidermis, prominent basal atypia, and stromal hyalinization, (2) dVIN resembling LSC—thick epidermis with basal cell expansion and atypia, (3) dVIN with prominent dyskeratosis, and (4) dVIN with marked spongiosis or acantholysis.65,66 The LS-like pattern has been called “atypical LS” and associated with p53 overexpression.14,40 Subsequent documentation of basaloid and hypertrophic patterns of dVIN further supports calls for broader diagnostic criteria.16–18,53,73
Vulvar aberrant maturation is an umbrella term for HPV-independent lesions combining aberrant maturation with minimal nuclear atypia. Multiple names for this have been proposed: differentiated exophytic verruciform intraepithelial lesion, vulvar acanthosis with altered differentiation, atypical epithelial acanthosis, LS with acanthosis or hyperplasia, verruciform LSC, and squamous cell hyperplasia.8,28,40,50,73,93 It is impossible to retroactively describe squamous cell hyperplasia beyond the ISSVD definition of “a hyperplastic process of unknown etiology.”8,10,27,28,78,93,94 The survey of pathologists could not identify a preferred nomenclature and one third of participants wrote in their own unique terms.73 Several proposed names were too narrow to encompass the spectrum of VAM, which extends from lichenified LS and hypertrophic LP at one end to verrucous SCC and dVIN at the other.19–21,53 Where one melds into the next, a clearer understanding of molecular events in the cancerization field should aid in diagnosis and prognosis.10,14,19
Background and Recommended Definitions of Nuclear Atypia, p16, and p53
Nuclear Atypia
In absence of invasion, atypia of squamous keratinocytes has 3 potential etiologies: dVIN, HSIL, and reactive change. Pathologists must itemize features of (1) abnormal chromatin, seen as hyperchromatic or vesicular nuclei, (2) pleomorphism, (3) mitoses, and (4) enlargement.6,20 The most common form of atypia is hyperchromatic and pleomorphic. Often, there are dark narrow nuclei in elongated cells surrounded by edema and intercellular bridges (see Figure 5). These “spindle-shaped” or “angulated” cells occur in more than 30% of keratinizing dVIN but are infrequently seen in benign conditions.20,69 Vesicular nuclei occur in up to 20% of dVIN.12,20,69 These are large and round, contain multiple or bizarre nucleoli, and may have intracellular and intranuclear vacuoles (see Figure 4). Despite epithelial maturation, suprabasilar cells are often atypical with vesicular nuclei.12,69 This may be difficult to detect when combined with premature maturation and poor intercellular cohesion. Increased basal mitoses occur in 40%–80%; thus, absent or rare mitoses do not exclude dVIN.20,69 Abnormal mitoses help exclude an inflammatory process; these are common in HSIL but rare in dVIN.95
FIGURE 5.
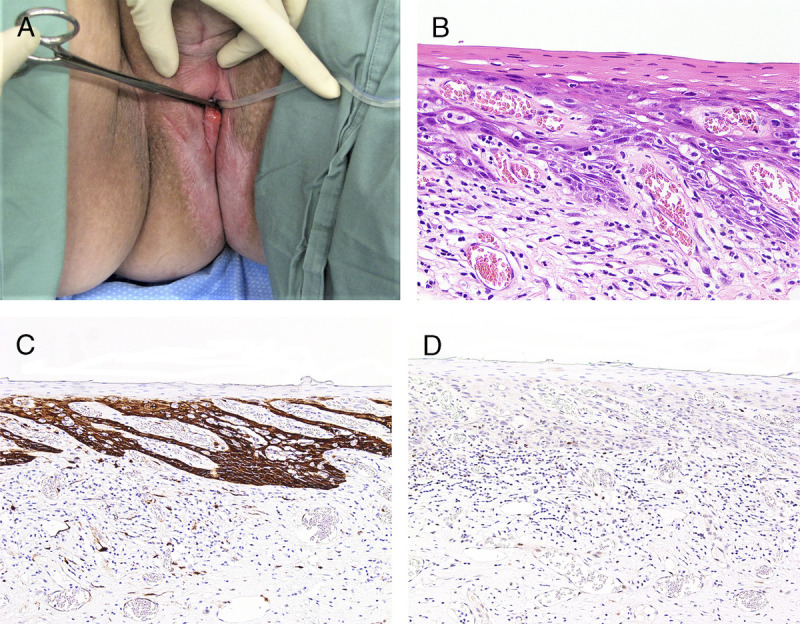
A, Exophytic red HPV-related SCC on a background of uncontrolled LS. B, Excision from the contralateral side with traditional keratinizing dVIN seen as PK, spiky rete ridges, and spindle-shaped hyperchromatic, pleomorphic, enlarged nuclei, H&E ×200. C, p16 is block positive, ×100. D, p53 is aberrant negative suggesting dual carcinogenic etiology, ×100.
Recommended Definition of Nuclear Atypia in Vulvar Squamous Neoplasia
Diagnosis of squamous intraepithelial neoplasia requires a systematic assessment of nuclear atypia. The 4 features are (see Table 3):
-
Abnormal nuclear chromatin,
-
◯
hyperchromatic—dark with inapparent nucleoli
-
◯
vesicular—open chromatin with visible bizarre or multiple eosinophilic nucleoli, binucleation, and/or fluid-filled spaces
-
◯
Pleomorphism,
-
Increased and/or abnormal mitoses,
-
◯
abnormal mitoses show Y-shaped, X-shaped, or bizarre spindle morphology, or contain small pieces of extra chromosomes separate to the main mitotic spindle
-
◯
Enlargement.
Immunohistochemistry for p16 and p53
Block-positive p16 identifies high-risk HPV genomic integration and serves as a reliable biomarker of HPV-related neoplasia. The Lower Anogenital Squamous Terminology project defined block-positive as continuous intense staining across nuclei and cytoplasm.11,96 The CERTAIN trial defined diffuse nuclear and/or cytoplasmic staining as representative of transforming cervical infection and defined focal, noncontiguous, or isolated clusters of stained nuclei as negative.97 Focal or noncontiguous p16 staining, also called nonblock positive, is unrelated to high-risk HPV and corresponds to deletions, point mutations, or promoter hypermethylation.98 The p16 false-negative rate in vulvar and cervical HSIL is less than 5%; manifestations include intense cytoplasmic staining with nuclear sparing and moderate patchy nuclear and cytoplasmic staining.62,99 In rare cases of simultaneous dermatosis-associated neoplasia and HPV integration, p53 may help identify the dominant pathway62 (see Figure 5).
p53 is more complicated to interpret than p16. Basal overexpression is defined as intense nuclear staining in more than 90% of cells in the lower third of epithelium.11 This pattern occurs in 45%–80% of dVIN, usually relating to a missense TP53 mutation.5,14,20,26,27,69,100,101 Staining may extend into mid-epithelium, fading where maturation begins10,20,24,69 (see Figures 2D, 3E). The p53 pattern is wild type in 17%–42%, defined as weak to moderate nuclear staining in less than 50% of cells in the lower third of epithelium.5,20,69 Thirteen to thirty percent of dVIN is aberrant negative for p53, because of a frame-shift, splice site, deletion, or truncating mutation5,20,29,50,69 (see Figures 4–6). The correlation between p53 IHC and TP53 mutation status is imperfect, with a positive predictive value of 67%.5,84
FIGURE 6.
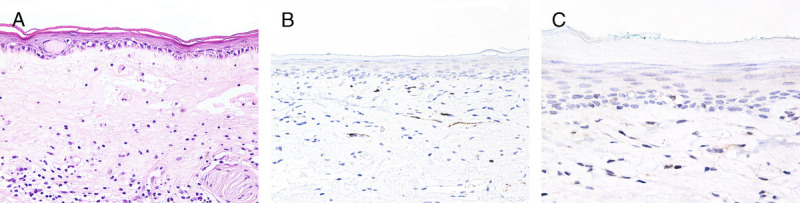
A, Atrophic keratinizing dVIN with LS-like appearance—thin epithelium, absent rete ridges, basal atypia comprising half the epithelium, and band of edematous and hyalinized collagen overlying moderate lymphocytic infiltrate, H&E ×200. B, p16 is negative, ×200. C, p53 is aberrant negative, ×400.
In contrast to dVIN, HSIL shows a suprabasilar dominant p53 pattern of strong nuclear uptake in mid-epithelium, accompanied by absent or weak noncontiguous basal staining.15,23–25 When this pattern is present in combination with nonblock-positive p16, it may reflect HPV-related disease with false-negative p16.62 Rates of p53 basal overexpression in LS and LSC are difficult to determine because of possible misclassification and different grading mechanisms used by researchers. This methodologic and diagnostic variation has produced rates ranging from 0% to 60%.8,26,69,78,101,102 p53 expression in LS is not impacted by topical corticosteroid use.103
Basal overexpression of p53 in dVIN is useful for diagnostic confirmation and margin assessment. At the junction, there is vivid contrast between dVIN's large dark nuclei extending into suprabasilar layers, and the small, regular, lighter-stained basal nuclei of nonneoplastic skin (see Figure 3E). The distinction between null p53 in dVIN and wild-type pattern of benign epithelium is visible but less striking.29 When dVIN has wild-type p53, margin assessment defers to standard histopathology.12,20,28
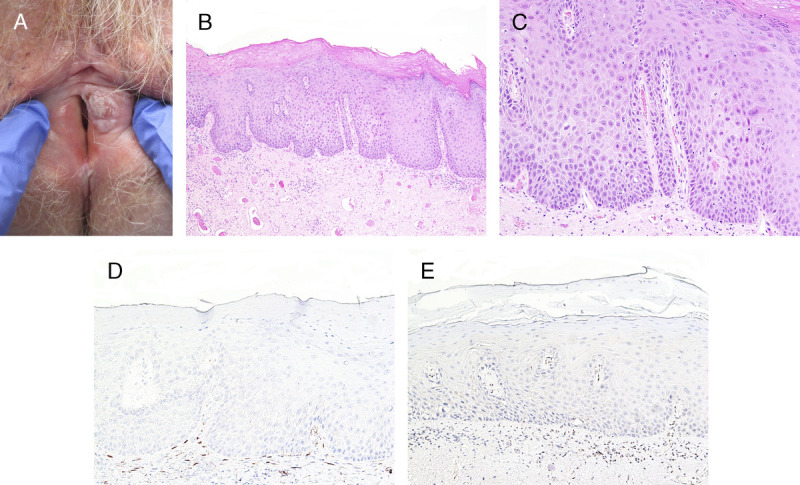
FIGURE 3.
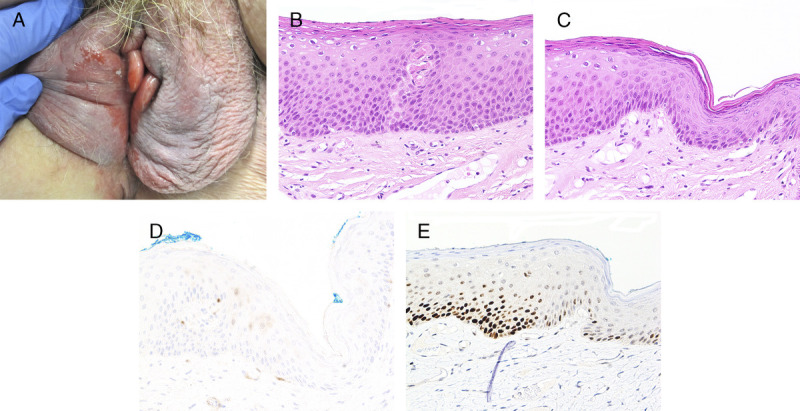
A, Differentiated vulvar intraepithelial neoplasia—large glazed red patch over bilateral labia minora and right interlabial fold. B, Subtle keratinizing dVIN with PK, flat acanthosis, and mildly hyperchromatic enlarged nuclei extending halfway up the epithelium, H&E ×200. C, Junction between dVIN and thinner nonneoplastic epithelium, H&E ×200. D, p16 is negative, ×200. E, Basal and suprabasal overexpressed p53 in dVIN contrasts with wild-type pattern in adjacent epithelium, ×200.
Studies of other stains have not encountered a test for dVIN that excludes HSIL and nonneoplastic disorders.11 Ki-67 is a proliferation marker that stains most dVIN and HSIL more intensely than nonneoplastic epithelium. However, the Ki-67 pattern in dVIN with wild-type p53 resembles normal skin.24,86,104 Ki-67 staining in warty-basaloid HSIL extends to the upper third of epithelium, but the keratinizing HSIL pattern is not well documented.9 Investigators have assessed GATA3, phosphorylated-S6, CK-13 and CK-17, ProEx C, SOX2, and e-cadherin and b-catenin. None of these reliably distinguishes dVIN from diagnoses with similar appearances, so their use remains investigational.11,69,81,86,105–107 No single reliable immunomarker for atypia exists.
Recommended Definitions for p16 Staining Patterns in Vulvar Epithelium
-
Block positive = continuous and intense staining of basal nuclei and cytoplasm
-
◯
supports HSIL
-
◯
rarely, this may occur in dermatosis-associated neoplasia
-
▪
aberrant negative or basal overexpressed p53 supports a dual carcinogenic etiology
-
▪
clinicopathologic correlation is advised
-
▪
-
◯
-
Nonblock positive = focal intense nuclear and/or cytoplasmic staining or diffuse weak to moderate staining of cytoplasm and nuclei
-
◯
supports dVIN
-
◯
rarely, this occurs in HPV-related disease
-
▪
suprabasilar dominant p53 supports an HPV-related etiology
-
▪
HPV genotyping may be useful
-
▪
clinicopathologic correlation is advised
-
▪
-
◯
-
Negative = total absence of staining
-
◯
supports dVIN
-
◯
Recommended Definitions for p53 Staining Patterns in Vulvar Epithelium
-
Basal overexpression = continuous, intense nuclear staining, often extending upwards until the layer at which maturation begins
-
◯
most common pattern in dVIN, but occasionally mimicked in LS and LP
-
◯
-
Wild type = continuous or intermittent weak to moderate nuclear staining, with scant suprabasal extension
-
◯
most common pattern in LS and LP, but does not exclude dVIN
-
◯
-
Suprabasilar dominant = intermittent, variable staining of enlarged suprabasilar nuclei with basal layer sparing
-
◯
supports HSIL and excludes dVIN
-
◯
-
Aberrant negative = total absence of staining
-
◯
supports dVIN
-
◯
Summary of Histopathologic Diagnostic Criteria of dVIN
Diagnosis of dVIN requires basal atypia in combination with negative or nonblock-positive p16 and supportive p53 (see Table 4). Three p53 staining patterns are consistent with dVIN: basal overexpression, wild type, and aberrant negative. Suprabasilar dominant p53 pattern supports HSIL and raises suspicion for false-negative p16. Rarely, lesions may be p16 block-positive with basal overexpressed or aberrant negative p53; this supports dual neoplastic etiology (see Figure 5).
TABLE 4.
Diagnostic Features, p16, and p53 in dVIN and VAM
| Diagnostic features | Supportive features | |||||
|---|---|---|---|---|---|---|
| Basal atypia | p16 | p53 | % of epithelium showing maturation | Architecture | Dyskeratosis | |
| dVIN | Marked abnormalities in 2 or more features | Negative or Nonblock-positive |
Basal overexpression or Wild-type or Aberrant negative |
Keratinizing: >40–90% Nonkeratinizing: <40% |
Variable Keratinizing subtypes • Atrophic • Subtle • Traditional • Acantholytic • Hypertrophic Nonkeratinizing types • Basaloid • Intermediate |
Variable Keratinizing subtypes • Premature maturation • Intercellular breakdown • Intracellular vacuoles Nonkeratinizing types • Minimal |
| VAM | Subtle abnormalities • Vesicular nuclei • No abnormal mitoses • No suprabasilar nuclear atypia |
Negative or Nonblock-positive |
Basal overexpression or Wild-type |
>80%, matures just above basal layer | Acanthosis Variable rete ridge shape |
Premature maturation Thick PK and/or HK |
Summary of Histopathologic Diagnostic Criteria of VAM
The diagnostic features of VAM are (Table 4, Figures 1A–E)20:
Aberrant maturation seen as thick hyperkeratosis (HK) or PK and/or premature maturation
Acanthosis
Minimal basal nuclear atypia identified through qualitative assessment of the 4 features
vesicular nuclei with visible nucleoli
pleomorphism—absent to minimal
mitoses—occasional mitotic figures with normal spindle morphology
enlargement—subtle to moderate
4. p16 is negative or nonblock-positive
5. p53 is wild type or overexpressed with staining confined to the basal layer.
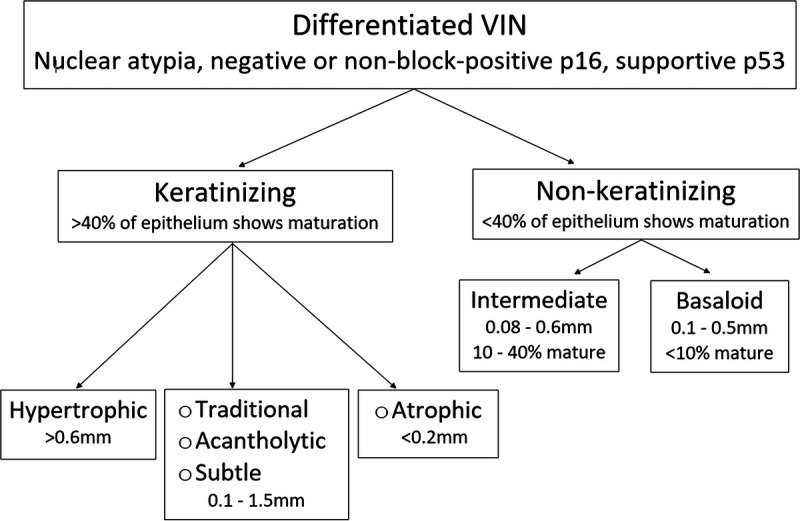
Morphologic Subsets of dVIN
Morphology does not reliably indicate etiology of vulvar neoplasia; 37%–43% of HPV-related cancers show keratinization, whereas 5%–12% of HPV-independent cancers show nonkeratinizing warty/basaloid morphology.22,26,33 However, these 2 categories remain useful as a starting point for the morphologic subsets of dVIN. Keratinizing dVIN divides into types delineated by epithelial thickness and dyskeratosis. Nonkeratinizing dVIN divides into intermediate and basaloid forms based on percentage of epithelium showing cellular maturation (see Figures 2, 7, 8).20
FIGURE 7.
A, Intermediate type of nonkeratinizing dVIN—amphiphilic appearance with PK, uniform acanthosis, and atypical nuclei with cellular maturation occurring at the superficial 30% of epithelium, H&E ×200. B, p16 is negative, ×200. C, p53 is overexpressed at basal and suprabasal layers, ×200.
FIGURE 8.
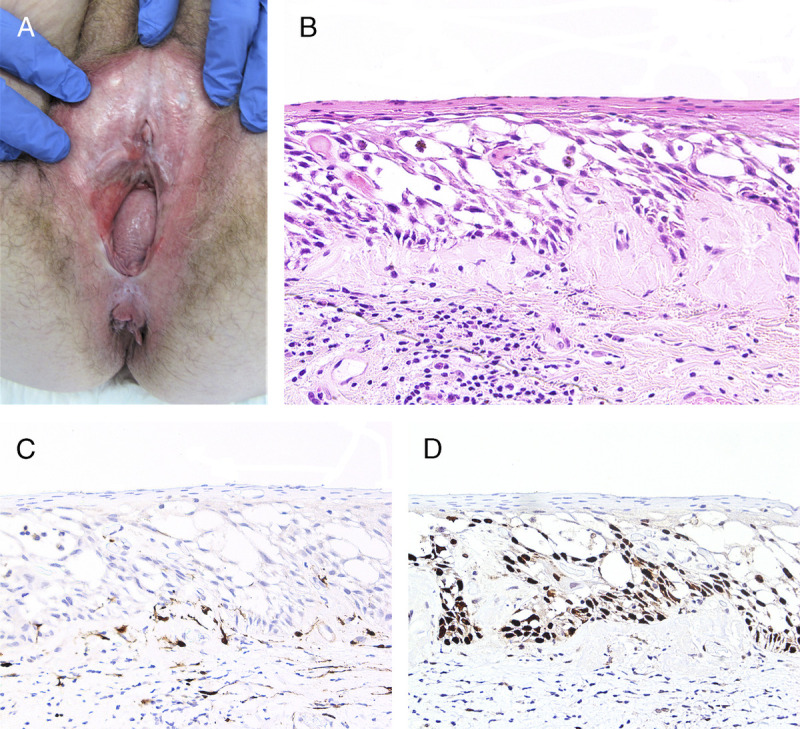
Algorithm for the morphologic subsets of dVIN.
Morphologic Patterns of Keratinizing dVIN—Definitions and Differential Diagnosis
Traditional Keratinizing dVIN
Abell and Gosling108 described this common pattern of dVIN in 1961, refined by Yang and Hart in 2000.12 There is HK and PK, acanthosis, and premature maturation (see Figures 1, 5). Rete ridges are elongated, clubbed, anastomosing, and/or branched.9 Marked intercellular prickles produce a mosaic pattern of keratinocytes with circumferential edema because of loss of cohesion, rather than spongiosis. Suprabasilar findings include enlarged squamous cells with large, sometimes binucleate, vesicular nuclei and abundant eosinophilic cytoplasm, squamous whorls, keratin pearls, mitoses, and apoptotic bodies. The impression of atypia ranges from overt to subtle; usually, all 4 features are seen. Basal overexpressed p53 highlights atypia that appears subtle on H&E. Stroma shows variable infiltrate and often fibrosis or sclerosis; the latter seen in 40%–70%.20,69
Hypertrophic Keratinizing dVIN
The hypertrophic type represents 13% of keratinizing dVIN and shows marked acanthosis with dramatic abnormalities across epithelial layers20,53 (see Figure 4). The surface shows thick HK, PK, and/or scale crust. There may be alternating columns of HK over hypergranulosis and PK over clusters of necrotic keratinocytes within hypogranulosis.109 Rete ridges are deep and irregular with complex branching patterns called “reticular” or “cobblestone.”50,69 Basal layer degeneration at tips or tops of the rete ridges signals a background lichenoid dermatitis.61 Stroma shows moderate to dense infiltrate and fibrosis and/or sclerosis, manifestations of the underlying dermatosis, and itch-scratch cycle.
Atrophic Keratinizing dVIN
Atrophic dVIN occurs in 13% and displays thinned epithelium with flat or reduced rete ridges.20 There are 5 or fewer cell layers, so basal atypia replaces about half of the epithelium. A band of sclerotic collagen overlying scant to moderate lymphocytic infiltrate provokes confusion with LS (see Figure 6). Moderate to dense infiltrate and absent sclerosis produce an appearance resembling lichenoid dermatitis or erosive LP.
Acantholytic Keratinizing dVIN
The acantholytic type occurs in 8% and usually shows PK and acanthosis with complex rete ridges.20 The prominent feature is acantholysis—an extreme form of cellular noncohesion seen as accumulation of intercellular vacuoles, focal disarray of separated cells, and areas replaced by fluid and cellular debris (see Figure 9).69 This limits assessment of premature maturation and suprabasilar atypia.50,69 Intracellular vacuoles are a dyskeratotic feature that enhances the acantholytic appearance.20 Stroma shows variable infiltrate, often with fibrosis and/or sclerosis.
FIGURE 9.
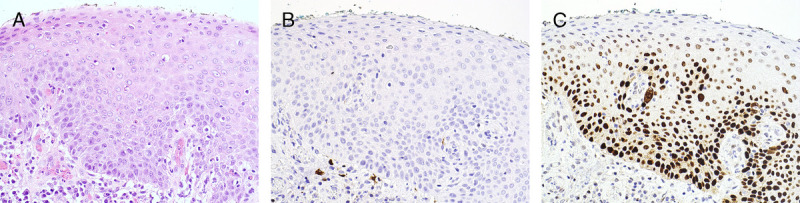
A, Squamous cell carcinoma and dVIN—red plaque at right clitoral frenulum on a background of uncontrolled LS. B, Acantholytic type of keratinizing dVIN—PK, normal thickness, marked intercellular prickles, intercellular and intracellular vacuoles with fluid-filled spaces, and sclerosis, H&E ×200. C, p16 is negative, ×200. D, p53 is overexpressed at basal and suprabasal layers, ×200.
Subtle Keratinizing dVIN
Five percent show normal to mildly increased epithelial thickness, thin PK or stratum corneum, unremarkable rete ridge morphology, minimal dyskeratosis, and nearly normal maturation (see Figure 3).20 In these challenging cases, atypia is the only feature that distinguishes dVIN from benign. This pattern may replace large areas of vulvar epithelium, as if clonal expansion travels rapidly along the basal layer. This may be accompanied by band-like lymphocytic infiltrate and stromal sclerosis/fibrosis, mimicking LS.
Differential Diagnosis of Keratinizing dVIN
Nonneoplastic diagnoses confused for traditional and hypertrophic keratinizing dVIN include LS, LP, LSC, and psoriasis. Assessment for atypia is more challenging when inflammation and excoriation raise the possibility of reactive change; features in keeping with this include dense infiltrate, marked exocytosis, and squamatization.17,20,110 As epithelial thickness increases, diagnoses under consideration are lichenified LS, hypertrophic rather than classic LP, nodular prurigo or severe LSC, and lichenified psoriasis. Superinfection contributes to acanthosis; swabs, scrapings, and PAS facilitate identification of organisms.75 Hypertrophic LP is a morphologic mimic for hypertrophic dVIN, the latter distinguished by atypical nuclei located away from the inflamed dermoepidermal junction.61 Severe LSC and lichenified psoriasis show similar architecture to hypertrophic dVIN but have bland organized basal cells, small nuclei with open chromatin, normal maturation, and minimal vertically oriented papillary dermal fibrosis.28
Vulvar aberrant maturation, verrucous SCC, and keratinizing HSIL are the other mimics for dVIN. Basal nuclear changes in VAM reflect its position on the spectrum between dermatosis and dVIN. Verrucous SCC is a well-differentiated HPV-independent nonmetastasizing neoplasia with minimal atypia and spread through an expansile blunt interface.21,111,112 This downward growth pattern is the salient difference between verrucous SCC versus VAM and dVIN. Cohorts of HPV-related SCC suggest that keratinizing HSIL represents 5% of precursors, but this rate is higher when comorbid with LS/LP.13,26,35,62 When HSIL replaces LS-affected epithelium, 43% of cases retain dermal sclerosis, provoking confusion with dVIN and underscoring the need for p16 and p53.62
The other morphologies of keratinizing dVIN have a limited list of imitators. Atrophic dVIN looks like LS if sclerosis is present and lichenoid dermatitis if sclerosis is absent.8,14 Hailey-Hailey and Darier's disease shows acantholysis and dyskeratotic nuclear enlargement potentially confused with acantholytic keratinizing dVIN. Clinical correlation is helpful because Hailey-Hailey and Darier's affects other intertriginous areas and infrequently occur with LS. Subtle keratinizing dVIN is rare but should be entertained if clinical suspicion is high, and there is no inflammation or excoriation to explain the nuclear changes.
Nonkeratinizing dVIN—Definition and Differential Diagnosis
Intermediate dVIN
Intermediate dVIN reflects the biological continuum between keratinizing and basaloid morphologies and comprises less than 10% of cases.20 It shows crowded basaloid cells replacing more than 60% of epithelium, transitioning to a narrow band of maturation underneath a thin keratin layer (see Figure 7). Epithelial thickness is normal to slightly increased and dyskeratosis is minimal. Rete ridge length is reduced to normal, with clubbed or branched morphology. Two thirds have moderate to dense infiltrate and abnormal collagen.20
Basaloid dVIN
Basaloid dVIN occurs adjacent to 8%–21% of HPV-independent SCC and sometimes is the only precursor lesion present.20,53 It contains full-thickness undifferentiated keratinocytes with scanty cytoplasm and frequent mitotic figures16,17 (see Figure 2). The surface usually shows PK with focal erosion. There is flat acanthosis or reduced clubbed/coalescent rete ridges and minimal intercellular breakdown. There is moderate to dense infiltrate in 93%, and collagen is sclerotic and/or fibrotic in 43%.20
Differential Diagnosis of Nonkeratinizing dVIN
The basaloid morphology of nonkeratinizing dVIN mimics the appearance of regenerative erosive LP and HSIL.17 Erosive LP has thinned epithelium, sometimes with erosion and surface neutrophils. A band-like lymphocytic infiltrate underlies regenerative epithelial changes of maturational disarray, increased mitoses, enlarged nuclei, and nuclear-cytoplasmic ratio reversal. Block-positive p16 identifies HSIL, but basaloid dVIN and erosive LP may be difficult to distinguish. Mild acanthosis, PK, hair bearing site, and prior SCC support dVIN. Aberrant negative p53 confirms dVIN, but basal overexpression and wild-type status are nondiscriminatory. If clinical appearance is consistent with erosive LP, it is reasonable to provide potent topical steroids and rebiopsy if response to therapy is inadequate.
SUMMARY AND RECOMMENDATIONS FOR PRACTICE
Clinicopathologic assessment of dVIN is challenging, and there are serious consequences to erroneous diagnosis. Evaluation of women at risk for dVIN and VAM requires a collaborative approach by clinicians and pathologists experienced in vulvar disorders. Long-term LS surveillance and use of consensus recommendations may decrease vulvar SCC through detection and treatment of dVIN and VAM.1
Aim for universal clinical photography of suspected dVIN and VAM.
Obtain biopsies from morphologically distinct areas, as dVIN and VAM may be multifocal and have a different appearance at each site.
Document presence of LS and/or LP and previous diagnoses of HSIL, VAM, dVIN, or SCC on pathology request forms.
Pathologists may need to solicit additional information from clinicians if clinical notes are insufficient as to history and examination findings.
-
Universal p16 and p53 in cases of suspected squamous neoplasia are advisable because dVIN and HSIL cannot be reliably distinguished by routine microscopy. If this is not possible, p16 and p53 are essential in:
-
◯
biopsies obtained from treatment-resistant lesions within LS
-
◯
suspected dVIN and VAM
-
◯
presumed HSIL in women older than 45 years, with comorbid LS/LP, or nonresponse to LASER or imiquimod.
-
◯
Indicate type of dVIN and presence of LS/LP in pathology reports.
Communication between clinician and pathologist or expert multidisciplinary review is recommended before embarking on cytotoxic, ablative, or extirpative procedures.
Footnotes
Jill Allbritton, Tania Day, Debra S. Heller, Claudia Perrera, Mario Preti, Gianluigi Radici, James Scurry, Maria Angelica Selim, Darion Rowan, Kathryn Welch, Edward J. Wilkinson.
The authors have declared they have no conflicts of interest.
Contributor Information
Debra S. Heller, Email: hellerds@njms.rutgers.edu.
Jill I. Allbritton, Email: jilgardens@gmail.com.
James Scurry, Email: jim.scurry@health.nsw.gov.au.
Gianluigi Radici, Email: gianluigiradici@gmail.com.
Kathryn Welch, Email: kwelch@med.wayne.edu.
Mario Preti, Email: mario.preti@unito.it.
REFERENCES
- 1.Eva LJ Sadler L Fong KL, et al. . Trends in HPV-dependent and HPV-independent vulvar cancers: the changing face of vulvar squamous cell carcinoma. Gynecol Oncol 2020;157:450–5. [DOI] [PubMed] [Google Scholar]
- 2.de Sanjosé S Alemany L Ordi J, et al. , HPV VVAP study group . Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer 2013;49:3450–61. [DOI] [PubMed] [Google Scholar]
- 3.van de Nieuwenhof HP Massuger LF van der Avoort IA, et al. . Vulvar squamous cell carcinoma development after diagnosis of VIN increases with age. Eur J Cancer 2009;45:851–6. [DOI] [PubMed] [Google Scholar]
- 4.Hinten F Molijn A Eckhardt L, et al. . Vulvar cancer: two pathways with different localization and prognosis. Gynecol Oncol 2018;149:310–7. [DOI] [PubMed] [Google Scholar]
- 5.Kashofer K, Regauer S. Analysis of full coding sequence of the TP53 gene in invasive vulvar cancers: implications for therapy. Gynecol Oncol 2017;146:314–8. [DOI] [PubMed] [Google Scholar]
- 6.Preti M Scurry J Marchitelli CE, et al. . Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol 2014;28:1051–62. [DOI] [PubMed] [Google Scholar]
- 7.Scurry J Campion M Scurry B, et al. . Pathologic audit of 164 consecutive cases of vulvar intraepithelial neoplasia. Int J Gynecol Pathol 2006;25:176–81. [DOI] [PubMed] [Google Scholar]
- 8.van de Nieuwenhof HP Bulten J Hollema H, et al. . Differentiated vulvar intraepithelial neoplasia is often found in lesions, previously diagnosed as lichen sclerosus, which have progressed to vulvar squamous cell carcinoma. Mod Pathol 2011;24:297–305. [DOI] [PubMed] [Google Scholar]
- 9.Kokka F Singh N Faruqi A, et al. . Is differentiated vulval intraepithelial neoplasia the precursor lesion of human papillomavirus-negative vulval squamous cell carcinoma? Int J Gynecol Cancer 2011;21:1297–305. [DOI] [PubMed] [Google Scholar]
- 10.Cohen PA Anderson L Eva L, et al. . Clinical and molecular classification of vulvar squamous pre-cancers. Int J Gynecol Cancer 2019;29:821–8. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta S Ewing-Graham PC Swagemakers SMA, et al. . Precursor lesions of vulvar squamous cell carcinoma – histology and biomarkers: a systematic review. Crit Rev Oncol Hematol 2020;147:102866. [DOI] [PubMed] [Google Scholar]
- 12.Yang B, Hart WR. Vulvar intraepithelial neoplasia of the simplex (differentiated) type. Am J Surg Pathol 2000;24:429–41. [DOI] [PubMed] [Google Scholar]
- 13.Rakislova N Alemany L Clavero O, et al. . Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol 2018;42:828–35. [DOI] [PubMed] [Google Scholar]
- 14.Bigby SM Eva LJ Fong KL, et al. . The natural history of vulvar intraepithelial neoplasia, differentiated type: evidence for progression and diagnostic challenges. Int J Gynecol Pathol 2016;35:574–84. [DOI] [PubMed] [Google Scholar]
- 15.Watkins JC Yang E Crum CP, et al. . Classic vulvar intraepithelial neoplasia with superimposed lichen simplex chronicus: a unique variant mimicking differentiated vulvar intraepithelial neoplasia. Int J Gynecol Pathol 2019;38:175–82. [DOI] [PubMed] [Google Scholar]
- 16.Ordi J Alejo M Fuste V, et al. . HPV-negative vulvar intraepithelial neoplasia (VIN) with basaloid histologic pattern. Am J Surg Pathol 2009;33:1659–65. [DOI] [PubMed] [Google Scholar]
- 17.Day T Bowden N Jaaback K, et al. . Distinguishing erosive lichen planus from differentiated vulvar intraepithelial neoplasia. J Low Genit Tract Dis 2016;20:174–9. [DOI] [PubMed] [Google Scholar]
- 18.Regauer S. Residual anogenital lichen sclerosus after cancer surgery has a high risk for recurrence: a clinicopathological study of 75 women. Gynecol Oncol 2011;123:289–94. [DOI] [PubMed] [Google Scholar]
- 19.Watkins JC Howitt BE Horowitz NS, et al. . Differentiated exophytic vulvar intraepithelial lesions are genetically distinct from keratinizing squamous cell carcinomas and contain mutations in PIK3CA. Mod Pathol 2017;30:448–58. [DOI] [PubMed] [Google Scholar]
- 20.Day T Marzol A Pagano R, et al. . Clinicopathologic diagnosis of vulvar intraepithelial neoplasia and vulvar aberrant maturation. J Lower Genit Tract Dis 2020;24:317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nascimento AF Granter SR Cviko A, et al. . Vulvar acanthosis with altered differentiation – a precursor to verrucous carcinoma? Am J Surg Pathol 2004;28:638–43. [DOI] [PubMed] [Google Scholar]
- 22.Rakislova N Clavero O Alemany L, et al. . Histological characteristics of HPV-associated and -independent squamous cell carcinomas of the vulva: a study of 1,594 cases. Int J Cancer 2017;141:2517–27. [DOI] [PubMed] [Google Scholar]
- 23.Jeffreys M Jeffus SK Herfs M, et al. . Accentuated p53 staining in usual type vulvar dysplasia – a potential diagnostic pitfall. Pathol Res Pract 2018;214:76–9. [DOI] [PubMed] [Google Scholar]
- 24.Hoevenaars BM van der Avoort IA de Wilde PC, et al. . A panel of p16INK4a, M1B1, and p53 proteins can distinguish between the 2 pathways leading to vulvar squamous cell carcinoma. Int J Cancer 2008;123:2767–73. [DOI] [PubMed] [Google Scholar]
- 25.Rivero RC Garcia D Hammes LS, et al. . Carcinogenesis of vulvar lesions: morphology and immunohistochemistry evaluation. J Low Genit Tract Dis 2017;21:73–7. [DOI] [PubMed] [Google Scholar]
- 26.Santos M Montagut C Mellado B, et al. . Immunohistochemical staining for p16 and p53 in premalignant and malignant epithelial lesions of the vulva. Int J Gynecol Pathol 2004;23:206–14. [DOI] [PubMed] [Google Scholar]
- 27.Santos M Landolfi S Olivella A, et al. . p16 overexpression identifies HPV-positive vulvar squamous cell carcinomas. Am J Surg Pathol 2006:1347–56. [DOI] [PubMed] [Google Scholar]
- 28.Hart WR. Vulvar intraepithelial neoplasia: historical aspects and current status. Int J Gynecol Pathol 2001;20:16–30. [DOI] [PubMed] [Google Scholar]
- 29.Singh N Leen SL Han G, et al. . Expanding the morphologic spectrum of differentiated VIN (dVIN) through detailed mapping of cases with p53 loss. Am J Surg Pathol 2015;39:52–60. [DOI] [PubMed] [Google Scholar]
- 30.Singh N, Gilks CB. Vulval squamous cell carcinoma and its precursors. Histopathology 2020;76:128–38. [DOI] [PubMed] [Google Scholar]
- 31.Hoang LN Park KJ Soslow RA, et al. . Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology 2016;48:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng AS Karnezis AN Jordan S, et al. . p16 immunostaining allows for accurate subclassification of vulvar squamous cell carcinoma into HPV-associated and HPV-independent cases. Int J Gynecol Pathol 2016;35:385–93. [DOI] [PubMed] [Google Scholar]
- 33.Dong F Kojiro S Borger DR, et al. . Squamous cell carcinoma of the vulva: a subclassification of 97 cases by clinicopathologic, immunohistochemical, and molecular features (p16, p53, and EGFR). Am J Surg Pathol 2015;39:1045–53. [DOI] [PubMed] [Google Scholar]
- 34.Eva LJ Ganesan R Chan KK, et al. . Differentiated-type vulval intraepithelial neoplasia has a high-risk association with vulval squamous cell carcinoma. Int J Gynecol Cancer 2009;19:741–4. [DOI] [PubMed] [Google Scholar]
- 35.McAlpine JN Kim SY Akbari A, et al. . HPV-independent differentiated vulvar intraepithelial neoplasia (dVIN) is associated with an aggressive clinical course. Int J Gynecol Pathol 2017;36:507–16. [DOI] [PubMed] [Google Scholar]
- 36.McAlpine JN Leung SCY Cheng A, et al. . Human papillomavirus (HPV)-independent vulvar squamous cell carcinoma has a worse prognosis than HPV-associated disease: a retrospective cohort study. Histopathology 2017;71:238–46. [DOI] [PubMed] [Google Scholar]
- 37.Horne ZD Dohopolski MJ Pradhan D, et al. . Human papillomavirus infection mediates response and outcome of vulvar squamous cell carcinomas treated with radiation therapy. Gynecol Oncol 2018;151:96–101. [DOI] [PubMed] [Google Scholar]
- 38.Lee LJ Howitt B Catalano P, et al. . Prognostic importance of human papillomavirus (HPV) and p16 positivity in squamous cell carcinoma of the vulva treated with radiotherapy. Gynecol Oncol 2016;142:293–8. [DOI] [PubMed] [Google Scholar]
- 39.MacLean AB Jones RW Scurry J, et al. . Vulvar cancer and the need for awareness of precursor lesions. J Low Genit Tract Dis 2009;13:115–7. [DOI] [PubMed] [Google Scholar]
- 40.Chiesa-Vottero A, Dvoretsky PM, Hart WR. Histopathologic study of thin vulvar squamous cell carcinomas and associated cutaneous lesions: a correlative study of 48 tumors in 44 patients with analysis of adjacent vulvar intraepithelial neoplasia types and lichen sclerosus. Am J Surg Pathol 2006;30:310–8. [DOI] [PubMed] [Google Scholar]
- 41.Zaki I Dalziel KL Solomonsz FA, et al. . The underreporting of skin disease in association with squamous cell carcinoma of the vulva. Clin Exp Dermatol 1996;21:334–7. [PubMed] [Google Scholar]
- 42.Al-Ghamdi A Freedman D Miller D, et al. . Vulvar squamous cell carcinoma in young women: a clinicopathologic study of 21 cases. Gynecol Oncol 2002;84:94–101. [DOI] [PubMed] [Google Scholar]
- 43.Kruse AJ Bottenberg MJ Tosserams J, et al. . The absence of high-risk HPV combined with specific p53 and p16INK4a expression patterns points to the HPV-independent pathway as the causative agent for vulvar squamous cell carcinoma and its precursor simplex VIN in a young patient. Int J Gynecol Pathol 2008;27:591–5. [DOI] [PubMed] [Google Scholar]
- 44.Parva M Miroshnichenko G Holtz DO, et al. . Invasive vulvar cancer in pregnancy: case report and current literature review. J Low Genit Tract Dis 2009;13:264–8. [Google Scholar]
- 45.Roman LD Mitchell MF Burke TW, et al. . Unsuspected invasive squamous cell carcinoma of the vulva in young women. Gynecol Oncol 1991;41:182–5. [DOI] [PubMed] [Google Scholar]
- 46.van den Einden LC de Hullu JA Massuger LF, et al. . Interobserver variability and the effect of education in the histopathological diagnosis of differentiated vulvar intraepithelial neoplasia. Mod Pathol 2013;26:874–80. [DOI] [PubMed] [Google Scholar]
- 47.Halonen P Jakobsson M Heikinheimo O, et al. . Lichen sclerosus and risk of cancer. Int J Cancer 2017;140:1998–2002. [DOI] [PubMed] [Google Scholar]
- 48.Micheletti L Preti M Radici G, et al. . Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis 2016;20:180–3. [DOI] [PubMed] [Google Scholar]
- 49.Carlson JA Ambros R Malfetano J, et al. . Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol 1998;29:932–48. [DOI] [PubMed] [Google Scholar]
- 50.Mulvany NJ, Allen DG. Differentiated intraepithelial neoplasia of the vulva. Int J Gynecol Pathol 2008;27:125–35. [DOI] [PubMed] [Google Scholar]
- 51.Scurry J Flowers LC Wistuba I, et al. . Human papillomavirus, lichen sclerosus, vulvar squamous cell carcinomas. Int J Gynecol Cancer 1998;8:298–306. [Google Scholar]
- 52.Day T Otton G Jaaback K, et al. . Is vulvovaginal lichen planus associated with squamous cell carcinoma? J Low Genit Tract Dis 2018;22:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol 2015;151:1061–7. [DOI] [PubMed] [Google Scholar]
- 54.Corazza M Borghi A Minghetti S, et al. . Clobetasol propionate vs. mometasone furoate in 1-year proactive maintenance therapy of vulvar lichen sclerosus: results from a comparative trial. J Eur Acad Dermatol Venereol 2016;30:956–61. [DOI] [PubMed] [Google Scholar]
- 55.Jones RW Sadler L Grant S, et al. . Clinically identifying women with vulvar lichen sclerosus at increased risk of squamous cell carcinoma: a case-control study. J Reprod Med 2004;49:808–11. [PubMed] [Google Scholar]
- 56.Simpson RC, Murphy R. Is vulval erosive lichen planus a premalignant condition? Arch Dermatol 2012;148:1314–6. [DOI] [PubMed] [Google Scholar]
- 57.Halonen P Jakobsson M Heikinheimo O, et al. . Cancer risk of lichen planus: a cohort study of 13,100 women in Finland. Int J Cancer 2018;142:18–22. [DOI] [PubMed] [Google Scholar]
- 58.Preti M Micheletti L Privitera S, et al. . Vulvar lichen planus: a risk factor for vulvar high-grade squamous intraepithelial lesion recurrence? J Low Genit Tract Dis 2018;22:264–5. [DOI] [PubMed] [Google Scholar]
- 59.Day T Wilkinson E Rowan D, et al. , ISSVD Difficult Pathologic Diagnoses Committee* . Clinicopathologic diagnostic criteria for vulvar lichen planus. J Low Genit Tract Dis 2020;24:317–29. [DOI] [PubMed] [Google Scholar]
- 60.Day T Moore S Bohl TG, et al. . Comorbid vulvar lichen planus and lichen sclerosus. J Low Genit Tract Dis 2017;21:204–8. [DOI] [PubMed] [Google Scholar]
- 61.Day T, Weigner J, Scurry J. Classic and hypertrophic vulvar lichen planus. J Low Genit Tract Dis 2018;22:387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin A Day T Ius Y, et al. . Anogenital high-grade squamous intraepithelial lesion comorbid with vulvar lichen sclerosus and lichen planus. J Low Genit Tract Dis 2020;24:311–6. [DOI] [PubMed] [Google Scholar]
- 63.Lewin MR, Hick RW, Selim MA. Lichenoid dermatitis of the vulva: diagnosis and differential diagnosis for the gynecologic pathologist. Adv Anat Pathol 2017;24:278–93. [DOI] [PubMed] [Google Scholar]
- 64.Roma AA, Hart WR. Progression of simplex (differentiated) vulvar intraepithelial neoplasia to invasive squamous cell carcinoma: a prospective case study confirming its precursor role in the pathogenesis of vulvar cancer. Int J Gynecol Pathol 2007;26:248–53. [DOI] [PubMed] [Google Scholar]
- 65.Medeiros F, Nascimento AF, Crum CP. Early vulvar squamous neoplasia – advances in classification, diagnosis, and differential diagnosis. Adv Anat Pathol 2005;12:20–6. [DOI] [PubMed] [Google Scholar]
- 66.Jin C, Liang S. Differentiated vulvar intraepithelial neoplasia: a brief review of clinicopathologic features. Arch Pathol Lab Med 2019;143:768–71. [DOI] [PubMed] [Google Scholar]
- 67.van den Einden LC, van der Avoort IA, de Hullu JA. Prevention, identification and treatment of vulvar squamous (pre)malignancies: a review focusing on quality of care. Expert Rev Anticancer Ther 2013;13:845–59. [DOI] [PubMed] [Google Scholar]
- 68.Micheletti L, Bogliatto F, Lynch PJ. Vulvoscopy: review of a diagnostic approach requiring clarification. J Reprod Med 2008;53:179–82. [PubMed] [Google Scholar]
- 69.Dasgupta S Ewing-Graham PC van Kemenade FJ, et al. . Differentiated vulvar intraepithelial neoplasia (dVIN): the most helpful histological features and the utility of cytokeratins 13 and 17. Virchows Arch 2018;473:739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lewis FM Tatnall FM Velangi SS, et al. . British Association of Dermatologists guidelines for the management of lichen sclerosus, 2018. Br J Dermatol 2018;178:839–53. [DOI] [PubMed] [Google Scholar]
- 71.Edwards SK Bates CM Lewis F, et al. . 2014 UK national guideline on the management of vulval conditions. Int J STD AIDS 2015;26:611–24. [DOI] [PubMed] [Google Scholar]
- 72.van der Meijden WI Boffa MJ Ter Harmsel WA, et al. . 2016 European guideline for the management of vulval conditions. J Eur Acad Dermatol Venereol 2017;31:925–41. [DOI] [PubMed] [Google Scholar]
- 73.Reutter JC, Walters RA, Selim MA. Differentiated vulvar intraepithelial neoplasia: what criteria do we use in practice? J Low Genit Tract Dis 2016;20:261–6. [DOI] [PubMed] [Google Scholar]
- 74.ACOG Practice Bulletin No. 93: diagnosis and management of vulvar skin disorders. Obstet Gynecol 2008;111:1243–53. [DOI] [PubMed] [Google Scholar]
- 75.Day T Borbolla Foster A Phillips S, et al. . Can routine histopathology distinguish between vulvar cutaneous candidosis and dermatophytosis? J Low Genit Tract Dis 2016;20:267–71. [DOI] [PubMed] [Google Scholar]
- 76.Scurry J. Does lichen sclerosus play a central role in the pathogenesis of human papillomavirus negative vulvar squamous cell carcinoma? The itch-scratch-lichen sclerosus hypothesis. Int J Gynecol Cancer 1999;9:89–97. [DOI] [PubMed] [Google Scholar]
- 77.Scurry J, Vanin K, Ostor A. Comparison of lichen sclerosus with and without adjacent vulvar squamous cell carcinoma. Int J Gynecol Cancer 1997;7:392–9. [Google Scholar]
- 78.Rolfe KJ MacLean AB Crow JC, et al. . TP53 mutations in vulval lichen sclerosus adjacent to squamous cell carcinoma of the vulva. Br J Cancer 2003;89:2249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rolfe KJ Eva LJ Maclean AM, et al. . Cell cycle proteins as molecular markers of malignant change in vulvar lichen sclerosus. Int J Cancer 2001;11:113118. [DOI] [PubMed] [Google Scholar]
- 80.Trietsch MS Nooij LS Gaarenstrom KN, et al. . Genetic and epigenetic changes in vulvar squamous cell carcinoma and its precursors: a review of the current literature. Gynecol Oncol 2015;136:143–57. [DOI] [PubMed] [Google Scholar]
- 81.Pinto AP Lin MC Sheets EE, et al. . Allelic imbalance in lichen sclerosus, hyperplasia, and intraepithelial neoplasia of the vulva. Gynecol Oncol 2000;77:171–6. [DOI] [PubMed] [Google Scholar]
- 82.Aulmann S Schleibaum J Penzel R, et al. . Gains of chromosome region 3q26 in intraepithelial neoplasia and invasive squamous cell carcinoma of the vulva are frequent and independent of HPV status. J Clin Pathol 2008;61:1034–7. [DOI] [PubMed] [Google Scholar]
- 83.Aidé S Lattario FR Almeida G, et al. . Promoter hypermethylation of death-associated protein kinase and p16 genes in vulvar lichen sclerosus. J Low Genit Tract Dis 2012;16:133–9. [DOI] [PubMed] [Google Scholar]
- 84.Choschzick M Hantaredja W Tennstedt P, et al. . Role of TP53 mutations in vulvar carcinomas. Int J Gynecol Pathol 2011;30:497–504. [DOI] [PubMed] [Google Scholar]
- 85.Nooij LS Ter Haar NT Ruano D, et al. . Genomic characterization of vulvar (pre)cancers identifies distinct molecular subtypes with prognostic significance. Clin Cancer Res 2017;23:6781–9. [DOI] [PubMed] [Google Scholar]
- 86.Podoll MB Singh N Gilks CB, et al. . Assessment of CK17 as a marker for the diagnosis of differentiated vulvar intraepithelial neoplasia. Int J Gynecol Pathol 2017;36:273–80. [DOI] [PubMed] [Google Scholar]
- 87.Saglam O Samayoa E Somasekar S, et al. . No viral association found in a set of differentiated VIN cases by human papillomavirus and pan-virus microarray testing. PLoS One 2015;10:e0125292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van der Avoort IA Shirango H Hoevenaars BM, et al. . Vulvar squamous cell carcinoma is a multifactorial disease following two separate and independent pathways. Int J Gynecol Pathol 2006;25:22–9. [DOI] [PubMed] [Google Scholar]
- 89.van Seters M ten Kate FJ van Beurden M, et al. . In the absence of (early) invasive carcinoma, vulvar intraepithelial neoplasia associated with lichen sclerosus is mainly of undifferentiated type: new insights into histology and aetiology. J Clin Pathol 2007;60:504–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davick JJ Samuelson M Krone JT, et al. . The prevalence of lichen sclerosus in patients with vulvar squamous cell carcinoma. Int J Gynecol Pathol 2017;36:305–9. [DOI] [PubMed] [Google Scholar]
- 91.Barbera L Gien LT Sutradhar R, et al. . The added value of pathology review in vulvar cancer: results from a population-based cohort study. Int J Gynecol Pathol 2017;36:107–10. [DOI] [PubMed] [Google Scholar]
- 92.Wakeham K Kavanagh K Cuschieri K, et al. . HPV status and favourable outcome in vulvar squamous cancer. Int J Cancer 2017;140:1134–46. [DOI] [PubMed] [Google Scholar]
- 93.Ridley CM Frankman O Jones ISC, et al. . New nomenclature for vulvar disease from the ISSVD. Hum Pathol 1989;20:495–6. [DOI] [PubMed] [Google Scholar]
- 94.Scurry J, Wilkinson EJ. Review of terminology of precursors of vulvar squamous cell carcinoma. J Low Genit Tract Dis 2006;10:161–9. [DOI] [PubMed] [Google Scholar]
- 95.Scurry J, Baskota SU. HPV-associated atypical mitotic figures in squamous intraepithelial lesions of the lower genital tract. J Low Genit Tract Dis 2016;20:165–8. [DOI] [PubMed] [Google Scholar]
- 96.Darragh TM Colgan TJ Cox JT, et al. . The Lower Anogenital Squamous Terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis 2012;16:205–42. [DOI] [PubMed] [Google Scholar]
- 97.Stoler MH Wright TC Jr. Ferenczy A, et al. . Routine use of adjunctive p16 immunohistochemistry improves diagnostic agreement of cervical biopsy interpretation. Am J Surg Pathol 2018;42:1001–9. [DOI] [PubMed] [Google Scholar]
- 98.Riethdorf S Neffen EF Cviko A, et al. . p16INK4A expression as biomarker for HPV 16-related vulvar neoplasias. Hum Pathol 2004;35:1477–83. [DOI] [PubMed] [Google Scholar]
- 99.Shain AF Wilbur DC Stoler MH, et al. . Test characteristics of specific p16 clones in the detection of high-grade squamous intraepithelial lesions (HSIL). Int J Gynecol Pathol 2018;37:82–7. [DOI] [PubMed] [Google Scholar]
- 100.Del Pino M, Rodriguez-Carunchio L, Ordi J. Pathways of vulvar intraepithelial neoplasia and squamous cell carcinoma. Histopathology 2013;62:161–75. [DOI] [PubMed] [Google Scholar]
- 101.Liegl B, Regauer S. p53 immunostaining in lichen sclerosus is related to ischaemic stress ad is not a marker of differentiated vulvar intraepithelial neoplasia. Histopathol 2006;48:268–74. [DOI] [PubMed] [Google Scholar]
- 102.Hantschmann P Sterzer S Jeschke U, et al. . p53 expression in vulvar carcinoma, vulvar intraepithelial neoplasia, squamous cell hyperplasia and lichen sclerosus. Anticancer Res 2005;25:1739–45. [PubMed] [Google Scholar]
- 103.Rolfe KJ Crow JC Reid WM, et al. . The effect of topical corticosteroids on Ki67 and p53 expression in vulval lichen sclerosus. Br J Dermatol 2002;147:503–8. [DOI] [PubMed] [Google Scholar]
- 104.Scurry J Beshay V Cohen C, et al. . Ki67 expression in lichen sclerosus of vulva in patients with and without associated squamous cell carcinoma. Histopathology 1998;32:399–404. [DOI] [PubMed] [Google Scholar]
- 105.Chen H Gonzalez JL Brennick JB, et al. . Immunohistochemical patterns of ProEx C in vulvar squamous lesions: detection of overexpression of MCM2 and TOP2A. Am J Surg Pathol 2010;34:1250–7. [DOI] [PubMed] [Google Scholar]
- 106.Goyal A, Zhang G, Yang B. Differential expression patterns of GATA3 in usual and differentiated types of vulvar intraepithelial neoplasia: potential diagnostic implications. Mod Pathol 2018;31:1131–40. [DOI] [PubMed] [Google Scholar]
- 107.Li B Zhang Q Ouyang L, et al. . Aberrant staining patterns of E-Cadherin and ß-catenin: a potential diagnostic value for distinguishing vulvar intraepithelial neoplasia from non-neoplastic vulvar lesions. Int J Clin Exp Pathol 2013;6:1362–6. [PMC free article] [PubMed] [Google Scholar]
- 108.Abell MR, Gosling JR. Intraepithelial and infiltrative carcinoma of vulva: Bowen's type. Cancer 1961;14:318–29. [DOI] [PubMed] [Google Scholar]
- 109.Weyers W. Hypertrophic lichen sclerosus sine sclerosis: clues to histopathologic diagnosis when presenting as psoriasiform lichenoid dermatitis. J Cutan Pathol 2015;42:118–29. [DOI] [PubMed] [Google Scholar]
- 110.Reyes MC, Cooper K. An update on vulvar intraepithelial neoplasia: terminology and a practical approach to diagnosis. J Clin Pathol 2014;67:290–4. [DOI] [PubMed] [Google Scholar]
- 111.Liu G Li Q Shang X, et al. . Verrucous carcinoma of the vulva: a 20 year retrospective study and literature review. J Low Genit Tract Dis 2016;20:114–8. [DOI] [PubMed] [Google Scholar]
- 112.Gualco M Bonin S Foglia G, et al. . Morphologic and biologic studies on ten cases of verrucous carcinoma of the vulva supporting the theory of a discrete clinico-pathologic entity. Int J Gynecol Cancer 2003;13:317–24. [DOI] [PubMed] [Google Scholar]


