Abstract
Degeneration of tongue muscles with aging may contribute to swallowing deficits observed in elderly people. However, the capacity for tongue muscle stem cells (SCs) to regenerate and repair the aged tongue and improve tongue strength following tongue exercise (a current clinical treatment) has never been examined. We found that the expression of regenerative, myogenic markers were impaired with age and may be related to increased expression of senescent marker p16INK4a. Tongue strength increased in young adult and old rats following exercise and was related to the expression of Pax7, MyoD, myogenin, and p16INK4a. Our study also suggests that strengthening of tongue muscles via clinical rehabilitation strategies also increased the expression of SC regenerative markers in the tongue throughout the exercise duration.
Keywords: Aging, Swallowing, Dysphagia, Exercise, Muscle stem cells
1. Introduction
Swallowing disorders (dysphagia) affect 15–20% of the elderly population (Roy et al., 2007), including 50–75% of nursing home residents (Nogueira and Reis, 2013). In elderly people, dysphagia impedes the ability to eat a meal, a critical function fundamental to quality of life, and has negative effects on health and rehabilitative potential. Elderly people with swallowing disorders are 44% more likely to develop aspiration pneumonia due to their inability to swallow safely and 46% more likely to die from that aspiration pneumonia (Hu et al., 2014). Despite associated clinical problems, such as malnutrition, depression, impaired airway protection, aspiration pneumonia, and even death (Hu et al., 2014; Roy et al., 2007), current knowledge of underlying mechanisms contributing to the high prevalence of dysphagia in older individuals is sparse.
The degeneration of tongue muscles is a potential pathophysiological mechanism of dysphagia in elderly people. Sarcopenia likely contributes to observed functional changes in the aged swallow, manifested as reductions in lingual forces, increased effort and fatigue, increased variability in swallowing pressures, temporal deficits, and alterations in bolus kinematics and biomechanics (Logemann et al., 2000; Maeda and Akagi, 2015; Robbins et al., 2016). Because of the tongue’s role in swallowing, it is a target for rehabilitative therapies. Tongue muscle strengthening exercises are in current clinical use for the treatment of dysphagia (Rogus-Pulia and Connor, 2016), and have been reported to improve strength, increase pressures generated during the swallow, and improve dietary intake and swallowing function in elderly individuals (Rogus-Pulia and Connor, 2016; Steele, 2012). However, the manner in which tongue exercise modifies molecular and cellular mechanisms of tongue muscle structure is not known. Determination of the biological basis for these changes will provide valuable insight into delivery strategies for exercise-based treatments for people with age-related dysphagia.
Because the use of human subjects is often precluded in the examination of age-related degeneration of the cranial muscles, aging rodent models have been used to study mechanisms of tongue and swallowing dysfunction and to investigate the potential of exercise therapy to reverse/restore tongue muscle function. The human and rat tongue, pharynx, and larynx share many functional, anatomical, and structural similarities despite differences in size and posture (Cenci et al., 2002; Treuting et al., 2018). Degeneration of tongue muscles involved in swallowing have been reported in aged rats, and include alterations in myosin heavy chain (MyHC) isoform and myofiber type composition, atrophy and death of myofibers and myo-nuclei, increased regions of muscle fibrosis, and fragmentation of the neuromuscular junction (Connor et al., 2009; Cullins and Connor, 2017; Johnson and Connor, 2011; Kletzien et al., 2018a; Kletzien et al., 2013; Russell and Connor, 2014; Schaser et al., 2011). These changes may be associated with age-related alterations in muscle contractile properties and contribute to increases in fatigue in the aged tongue (Becker et al., 2015; Kletzien et al., 2018b; Kletzien et al., 2013; Nagai et al., 2008; Ota et al., 2005). Biological and physiological changes that occur in aged tongue musculature are likely contributors to observed alterations in swallowing kinematics and biomechanics of aging rats (Kletzien et al., 2019; Lever et al., 2015; Russell et al., 2013), and probable mechanisms underlying functional swallowing deficits observed in the elderly.
Numerous factors have been identified as potential contributors to age-associated dysfunction in homeostatic skeletal muscle tissue maintenance and to the decline in satellite cell (SCs, adult muscle stem cells) function in aging limb muscle (Sharpless and Depinho, 2007; Sousa-Victor and Munoz-Canoves, 2016). SCs are somatic tissue stem cells that have the ability to self-renew, differentiate, and ultimately regenerate damaged or injured muscle tissue (Fig. S1). Age-related reductions in SC number and function has been reported in limb musculature and shown to be related to changes in the extrinsic environment and dysregulation of cell-intrinsic mechanisms (Almada and Wagers, 2016; Blau et al., 2015; Oh et al., 2014; Sharpless and Depinho, 2007). Deficits in SC function likely contribute to loss in regenerative capacity following injury- or exercise-induced myofiber damage. Compared to limb, the expression of SC regenerative markers in aging tongue muscles has not been investigated. Further, development of therapies that target the age-related failure of SC regenerative processes in tongue muscles may help to ameliorate dysphagia in elderly people by improving tongue strength and function.
Our laboratory has developed an aging rat model of progressive resistance tongue exercise analogous to clinical tongue strengthening therapies implemented by speech-language pathologists for the treatment of dysphagia in elderly patients (Rogus-Pulia and Connor, 2016; Rogus-Pulia et al., 2016). Similar to tongue strengthening therapies where humans are trained to progressively increase voluntary lingual forces over the course of many weeks or months by pressing the tongue against an oral device (Iowa Oral Performance Instrument, Swallow-STRONG) (Lazarus et al., 2003; Rogus-Pulia et al., 2016), our rats are trained to press the tongue against a force incremented disc for a water reward, and forces to attain that reward are progressively increased over a period of 59 days (Fig. 1) (Connor et al., 2009). Comparable gains in tongue strength following progressive resistance tongue exercise therapies have been observed in both humans and rats (Connor et al., 2009; Cullins et al., 2017; Kletzien et al., 2013; Rogus-Pulia et al., 2016; Schaser et al., 2015; Schaser et al., 2012). Previous research from our laboratory, has shown that 59 days of progressive resistance tongue exercise induces considerable musculoplastic changes in the tongues of old rats. Alterations in protrusive tongue muscle contractile properties (increased maximal twitch tension; reduction in fatigue) were detected following the 59-day exercise program (Connor et al., 2009; Kletzien et al., 2013). We also observed exercise-induced biochemical and morphological changes in protrusive muscles of the extrinsic (genioglossus) and intrinsic tongue muscles, such as alterations in MyHC isoform composition and myofiber type, and a trend towards increased myofiber size (Cullins et al., 2017). Clearly, tongue strengthening therapies are capable of inducing physiological, biochemical, and morphological changes in associated regions of the tongue musculature. However, underlying cellular mechanisms of tongue muscle degeneration and regeneration, specifically the contribution of lingual SCs, with age and exercise that accompany MyHC isoform and myofiber type transition, alterations in tongue muscle contractile properties, and increases in voluntary tongue force, have not been elucidated.
Fig. 1.
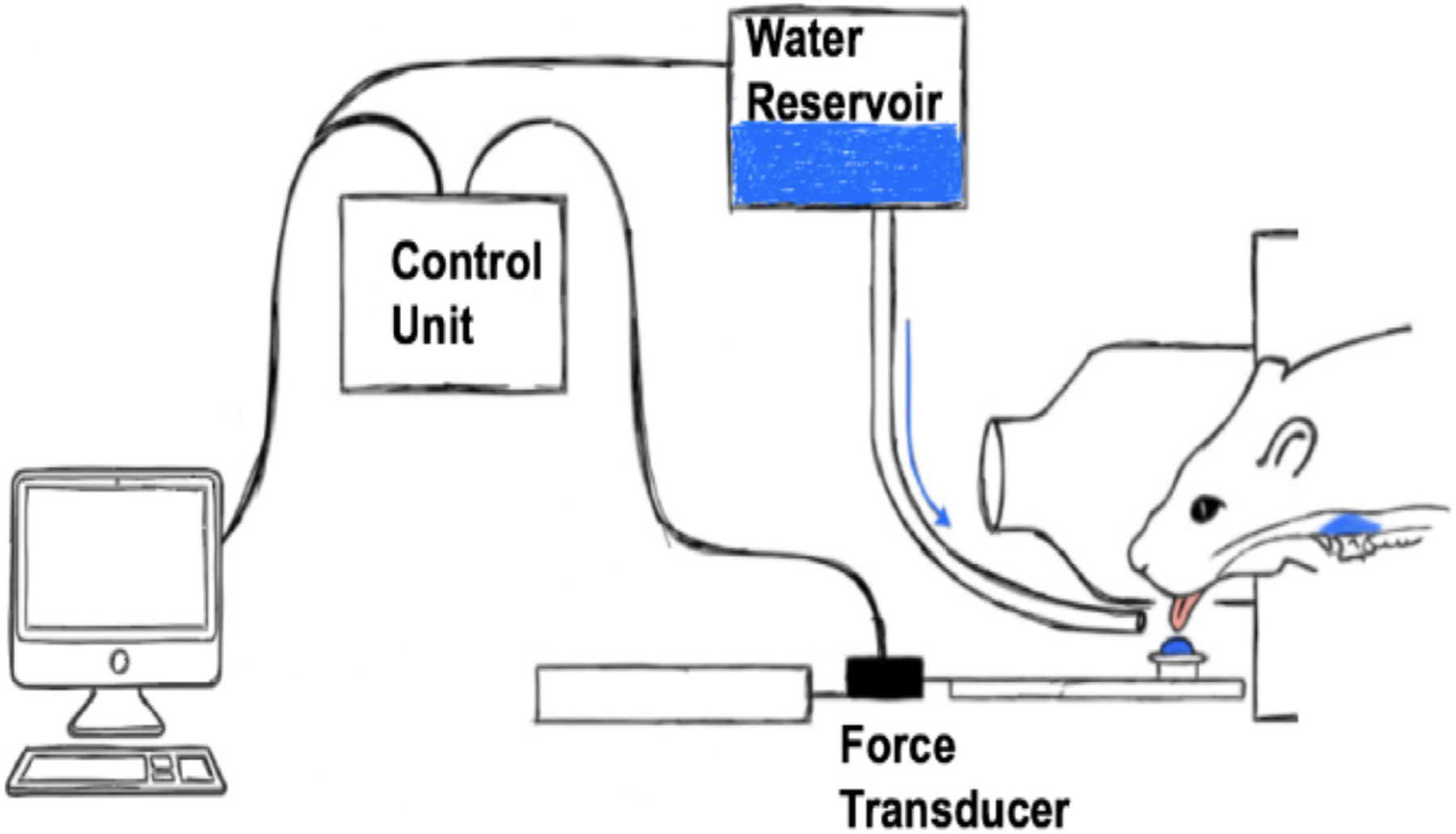
Schematic of tongue exercise operandum.
Here we examine the expression of lingual myogenic regenerative markers in an aging rat model (Fischer 344/Brown Norway), and how a tongue strengthening exercise mitigates age-related signs of tongue muscle degeneration. Our results provide novel insights into the role of lingual SCs for tongue muscle homeostasis in the context of natural aging and provide evidence that SC regenerative turnover is increased in tongue muscles compared to limb. Furthermore, our findings suggest clinical rehabilitation strategies that strengthen the tongue may increase SC regenerative processes.
2. Methods
2.1. Rats
Animal experiments were approved by the University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee, and performed in compliance with the Guide for the Care and Use of Laboratory Animals, 8th Edition (2011). A total of 146 male Fischer 344/ Brown Norway rats were obtained from the National Institute on Aging Aged Rodent Colony (Charles River Laboratory). The Fischer 344/ Brown Norway rat is the most studied and well characterized aging rat strain, and has a median life expectancy of ~36 mo (Turturro et al., 1999). Young adult (7 or 9 mo old; n = 73) and old (30 or 32 mo old; n = 73) rats were randomized into tongue exercise or no exercise, control conditions at 3 timepoints as shown in Fig. 3A to assess: (1) early phases of SC regenerative marker expression following 3 or 17 days of exercise; and (2) later phase of SC regenerative marker expression following 59 days of exercise (n = 10–14 young adult; n = 10–14 old rats per time point by exercise condition) (Bazgir et al., 2017; Bellamy et al., 2014; Cermak et al., 2013; Connor et al., 2009; Cullins et al., 2017; Kadi et al., 2005; Kletzien et al., 2013; Martin and Lewis, 2012; Smith and Merry, 2012).
Fig. 3.
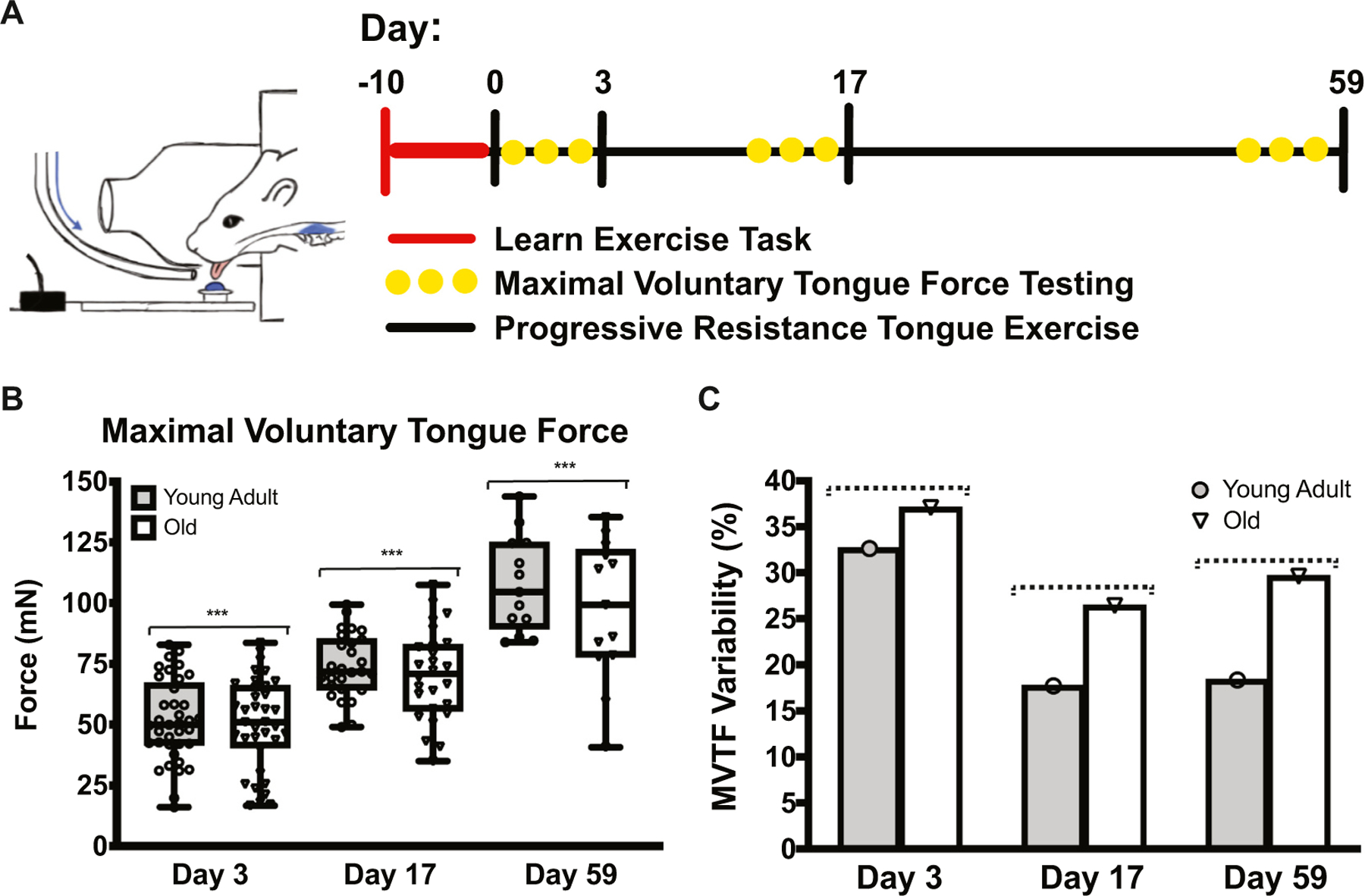
MVTF increased following tongue exercise. A Schematic of exercise timeline. B MVTF increased following tongue exercise at all time points (F1.462,54.81 = 131.9, p < 0.001). C With increasing age, MVTF was more variable and approached significance (F1,2 = 16.80, p = 0.055; dotted line). Over time, MVTF became less variable and approached significance (F2,2 = 16.05, p = 0.059, dotted line). (***HSD p < 0.0001.)
2.1.1. Tongue exercise and no exercise conditions
To improve translation of research from animals and humans, our laboratory developed and validated a rodent model of tongue exercise analogous to that used in clinical care of dysphagia. Immediately upon arrival, rats were randomized, acclimated to the vivarium and the 12:12 h light-dark reverse light cycle, and limited to 3 h of water/day ad libitum.
Over the course of 10 days, rats were trained to press the tongue against a force-incremented disk fitted (Sensotec) to receive water reward (VR5; Fig. 1). Following acclimation, a maximal voluntary tongue force (MVTF; mN), an indication of voluntary tongue strength, was determined at day 3 (average top 10 MVTF values obtained over 3 days). The 17-day exercise group performed tongue exercise at 50% MVTF for 5 days/wk for 10 min/day, and a 17-day MVTF determined. In the 59-day exercise group, force increments were increased every 14 days to mimic a progressive resistance exercise program: 50% MVTF (d 4–17), 60% MVTF (d 18–31), 70% MVTF (d 32–45), and 80% MVTF (d 46–59), and 17-day and 59-day MVTF determined.
No exercise control rats were handled identically to the exercise groups, regulated to 3 h water access/day, and did not perform any tongue exercise for the entire experimental period (3-days, 17-days, or 59-days).
2.1.2. Tissue harvest
Following 3-day, 17-day, or 59-day experimental duration, rats were euthanized, and muscles extracted (Martin and Lewis, 2012). Muscles were either: 1) digested to isolate SC for immunocytochemical (ICC) analysis, or 2) snap frozen and stored at −80° for quantitative real-time reverse transcription polymerase chain reaction (RT-qPCR), western blot, or immunohistochemical (IHC) analysis.
2.2. SC isolation
Following sterile dissection of tongue (GG, SG, HG, IT) and limb (EDL) muscles, connective and non-muscle tissue was removed. Tissue was weighed and finely minced, and enzymatically digested for 1.5 h at 37 °C (Protease from Streptomyces griseus, 1.25 mg mL−1, Sigma-Aldrich). Following incubation, tubes were centrifuged for 4 min at 4000 rpm, and supernatant reserved. Warm DPBS (Dulbecco’s Phosphate-Buffered Saline, Gibco; 5 mL) was added to tubes containing each muscle, vortexed for 20 s, centrifuged for 10 min at 1000 rpm, and supernatant reserved. Warm DPBS (5 mL) was added, vortexed for 20 s, and centrifuged for 8 min at 1000 rpm. Supernatant was reserved, warm DPBS added to the tissue, vortexed for 20 s, and centrifuged for 5 min at 1000 rpm. Supernatant was reserved, and the tissue pellet placed aside. Tubes containing reserved supernatant were centrifuged for 5 min at 3000 rpm to pellet cells.
Supernatant was carefully aspirated, and 5 mL of culture media (DMEM + Glutamax, 10% FBS, 1% penicillin/streptomycin/amphotericin B) added. The cell suspension was incubated for 25 min at 37 °C, passed through a 40-µm, single cell filter (Falcon-Corning), and centrifuged at 3000 rpm for 5 min, and supernatant aspirated. Cells were plated down on glass coverslips coated with PLL (poly-L-lysine) and fibronectin (40% in DMEM + Glutamax), and cultured for 6 h in a humidified incubator at 37 °C in 5% CO2 to maintain a quiescent state (Montarras et al., 2005; Sacco et al., 2008).
2.2.1. ICC
Following the 6-h incubation period, cells were fixed in 4% paraformaldehyde in PBS, blocked in 5% NGS (normal goat serum) and permeabilized in 0.1% Triton X-100 in PBS, and incubated with primary antibodies CDKN2A/p16INK4a (1:100, ab211542, rabbit monoclonal IgG, Abcam), and Pax7 (1:40, mouse monoclonal IgG1; Developmental Studies Hybridoma Bank [DSHB]). Following overnight incubation at 4 °C with primary antibodies, cells were then incubated with secondary antibodies (1:250, A11034 goat anti-rabbit IgG [H + L] highly cross-adsorbed secondary Alexa Fluor 488; 1:500, A21124, goat anti-mouse IgG1 cross-adsorbed secondary antibody, Alexa Fluor 568; Invitrogen) for 1 h. Cells were then cover-slipped with ProLong Gold Antifade Mountant with DAPI (Invitrogen). Negative control coverslips were stained following the identical procedure with omission of the primary antibody. 4–8 random nonoverlapping images at an objective magnification of 20× were acquired using an Olympus BX53 Upright Epifluorescence Microscope with fully automated xyz stage control, and a DP80 Digital Camera. In each field of view the percentage of DAPI, p16INK4a, and Pax7 stained nuclei were determined and averaged using cellSens (Olympus) or Fiji (LOCI). Intensity of p16INK4 expression for Pax7+ cells was also determined. All cell count data are reported as mean ± SEM.
2.3. Whole muscle analyses
2.3.1. RT-qPCR
Tissue snap-frozen in liquid nitrogen was weighed, finely minced, and sonicated on ice in PureZOL RNA reagent (Bio-Rad). Total RNA was extracted with the Aurum Total RNA Fatty and Fibrous Tissue Kit (Bio-Rad) per the manufacturer’s instructions, and measured (ng µL−1; 260/ 280, 260/230) using a Nanodrop system (Thermo Fisher Scientific). DNase-treated RNA (100 n µL−1 per cDNA reaction) was converted into single-stranded cDNA using SuperSricpt III First-Strand Synthesis System (Invitrogen).
NCBI Primer Blast was used to design and confirm primers for control reference genes (Ywaz and GAPDH) and genes of interest (Supplementary Table 1; Pax7, MyoD, Myogenin, p16INK4a) using the rat (Rattus norvegicus) genome. Additionally, Netprimer (PREMIER Biosoft, Palo Alto, CA) was used to examine the secondary primer structure to avoid primer products. Non-template controls were run with each primer pair to check for formation of primer-dimers, as well as non-specific products. Specificity for each primer pair was confirmed using melt curve analysis; all primer runs yielded single-peak melt curves indicating single gene product amplification. To confirm sequence identity, the qPCR reaction product for each gene was sequenced using Sanger sequencing with both forward and reverse primers at the University of Wisconsin Biotechnology Center. Using NCBI BLAST all sequences were confirmed to match intended targets.
Relative gene expression was normalized to GAPDH and Ywaz genes, and determined using RT-qPCR following MIQE (Minimum Information for Publication of Quantitative Real-Time PCR Experiments) guidelines. Samples were prepared in reaction tubes containing the respective sample cDNA, nuclease-free water, characterized forward and reverse primers (5 µM concentration; Integrative DNA Technologies) and SsoFast EvaGreen Supermix (BioRad). On each qPCR plate, five standards were run (1:10 serial dilutions, starting at 500 ng µL−1) with a non-template negative control. Samples and standards were run in triplicate. Plates were read with the Bio-Rad CFX96 Touch Real-Time PCR Detection System (Bio-Rad). Each qPCR run entailed of an initiation step at 95 °C for 30 s, 40 cycles of 95 °C for 5 s, a 30 s annealing phase, a 45 s elongation phase at 72 °C, and a melt curve from 60 to 95 °C, 0.5° for each 5 s step. All plates were read following each elongation and melt curve stages.
Data inclusion criteria consisted of run efficiencies between 90% and 110% as well as an R2 of at least 0.99. The mean Ct value for each cycle was defined as the average cycle number at which each sample triplicate crossed the amplification threshold (set a priori to 200 RFU), and were transformed via the Pfaffl Method.
2.3.2. Western blot
Snap-frozen tissue was weighed, finely minced, and homogenized via sonication on ice in RIPA buffer (Cell Signaling Technology) supplemented with Halt phosphatase and protease inhibitor cocktails, and EDTA (Thermo Fisher Scientific). Following sonication, tissue was placed on ice on an orbital shaker at 4 °C for 1 h and centrifuged at 13,000 rpm for 15 min. Supernatant was reserved, total protein concentration quantified (DC Protein Assay; Bio-Rad) and diluted to a working concentration (8 µg µL−1).
Protein (70 µg) from each tissue sample (GG, combined SG and HG, IT, EDL) was loaded into a precast 4–12% Criterion XT Bis-Tris Protein Gel (Bio-Red), and gel electrophoresis performed. A positive internal control skeletal muscle tissue lysate (70 µg, Abcam, ab29711) and protein standard (Precision Plus Protein Kaleidoscope Prestained Protein Standard, Bio-Rad) were loaded into each gel. Proteins were transferred to a nitrocellulose membrane, and total protein was detected (Pierce Reversible Protein Stain Kit for Nitrocellulose Membranes, Thermo Fisher Scientific) per manufacturer’s instructions. Membrane was then blocked for 1 h at room temperature in filtered 5% nonfat dry milk in TBST (tris buffered saline, 0.15% Tween 20), and incubated with primary antibodies overnight at 4 °C on a revolving tube rotator (Pax7, 1:250, mouse monoclonal IgG1, DSHB; MyoD, MA1–41017, 1:250, mouse monoclonal IgG1, Invitrogen; Myogenin, F5D, 1:250, mouse monoclonal IgG1, DSHB; p16INK4a, PA130670, 1:250, rabbit polyclonal IgG, Invitrogen).
Following primary antibody incubation, membranes were washed, and incubated for 1 h at room temperature in appropriate horseradish peroxidase-conjugated secondary antibodies (hrp linked anti-mouse IgG1 cross-adsorbed, hrp, Invitrogen; hrp linked anti-rabbit IgG, Cell Signaling Technology). Membranes were washed, and developed (SuperSignal West Pico Plus Chemiluminescent Substrate, Thermo Fisher Scientific) using a ChemiDoc-It2 Imaging System (UVP) and imaged, and analyzed using VisionWorks Software (Analytik Jena).
2.3.3. IHC
Tissue embedded in OCT was cut from the muscle mid-belly into serial 10 µm cross-sections using a −16 °C cryostat (Leica CM 1850, Leica Biosystems), mounted on slides, and fixed in cold acetone for 10 min at 4 °C. Slides were washed and blocked (10% NGS, 0.1% Triton X-100, PBS) for 1 h on an orbital platform shaker. Cross-sections were incubated overnight at 4 °C on an orbital platform shaker in primary antibodies: Pax7 (1:10, mouse monoclonal IgG, DSHB), myosin heavy chain (MyHC) Type I (1:50, BA-F8, mouse monoclonal IgG2b, DSHB), MyHC Type IIx (1:90, 6 h1, mouse monoclonal IgM, DSHB), MyHC IIb (1:200, BF-F3, mouse monoclonal IgM, DSHB) and Laminin (1:1000, L9393, rabbit polyclonal, Sigma-Aldrich). MyHC IIa fibers were identified by absence of staining. After primary incubation, slides were washed in PBS, and incubated in secondary antibodies for 1 h on an orbital shaker (1:200, A21140, goat anti-mouse IgG2b cross-adsorbed Alexa Fluor 350; 1:500, A21121, goat anti-mouse IgG1 cross-adsorbed Alexa Fluor 488; 1:500, A21426, goat anti-mouse IgM cross-adsorbed Alexa Fluor 555; 1:500, A21071, goat anti-rabbit IgG (H + L) cross-adsorbed Alexa Fluor 633; Invitrogen). Slides were washed and mounted in ProLong Gold Antifade with DAPI to visualize nuclei.
Cross-sections were imaged (4–6 random nonoverlapping images) using a 20× objective (Olympus BX53 Upright Epifluorescence Microscope with fully automated xyz stage control; DP80 Digital Camera; Olympus). In each field of view the percentage of Pax7+ nuclei by MyHC fiber type was determined and averaged using Fiji (LOCI). Co-localization of Pax7+ nuclei with non-muscle tissue structures (vasculature, nerves, connective tissue bodies) was also determined. The percentage of centralized myofiber nuclei was also quantified. All data are reported as mean ± SEM.
2.4. Statistical analyses
All statistical analyses were conducted with GraphPad Prism version 8.1.2 (GraphPad Software). The critical value for obtaining statistical significance was set at α = 0.05.
2.4.1. MVTF analysis
Mixed-model repeated measures 2-way analysis of variance (ANOVA) was used to examine main effects for age (young adult, old), time point (3-days, 17-days, and 59-days), and their interaction on MVTF, percent change in MVTF, and MVTF variability. Geisser-Greenhouse correction was used, and post-hoc testing completed using Tukey’s honestly significant differences (HSD)..
2.4.2. SC analysis
Two-way ANOVA was used to examine main effects for age, treatment (exercise, no exercise), and interaction effects on Pax7, MyoD, myogenin, and p16INK4a gene expression, the colocalization of Pax7+ nuclei by myofiber type, the percentage of Pax7+ nuclei, and centralized myofiber nuclei. Post-hoc testing was completed using Tukey’s HSD.
To examine differences in protein expression of Pax7, MyoD, myogenin, and p16INK4a among tongue and limb muscles in the no exercise group, 2-way ANOVA was performed to examine main effects for age and muscle (GG, combined SG and HG, IT, EDL). Post-hoc testing was completed using Tukey’s HSD.
Three-way ANOVA was used to examine main effects for age, treatment, time point (3-days, 17-days, and 59-days), and their interactions on Pax7, MyoD, myogenin, and p16INK4a protein expression. Post-hoc testing was completed using Tukey’s HSD.
In isolated SCs, 3-way ANOVA was used to examine main effects for age, treatment, time point, and their interactions on the percentage of Pax7+ SCs, percentage of p16INK4a+/Pax7+ SCs, and intensity of p16INK4a expression in Pax7+ SCs. Post-hoc testing was completed using Tukey’s HSD.
2.4.3. Relationship between muscle regenerative marker expression and tongue strength
Multiple linear regressions were performed to examine the degree to which protein expression of Pax7, MyoD, myogenin, or p16INK4a could predict MVTF at 3-day, 17-day, and 59-day time points.
2.4.4. Missing data
For some statistical comparisons, missing data resulted in a smaller sample size, reflected in smaller degrees of freedom. All other available data were used in all statistical analyses.
3. Results
3.1. Age-associated reduction in Pax7 in genioglossus
We first evaluated expression of SC markers, Pax7, MyoD, and myogenin, in young adult (7 or 9 mo old) and old (30 or 32 mo old) rat genioglossus (GG; primary tongue protrusor), combined styloglossus and hyoglossus (SG & HG, respectively; muscles of tongue retrusion), and intrinsic (IT; tongue shape and position) tongue muscles (Fig. 2A). Gene and protein expression of Pax7, a marker of SC quiescence or activation, was significantly reduced with increasing age in the GG (Fig. 2A, B). However, Pax7 gene and protein expression in the SG and HG, and IT muscles were resistant to aging effects (Fig. 2A, B). No age-related changes in gene and protein expression of MyoD, a marker of activated SCs, or myogenin, a marker of SC differentiation, were detected in any of the tongue muscles studied (Fig. S2). These data indicate the differential expression of myogenic regenerative markers in the tongue is muscle specific in aging tongue musculature. Further, age-related reduction in Pax7 gene and protein expression suggests SC regenerative capacity is impaired with increasing age in the GG.
Fig. 2.
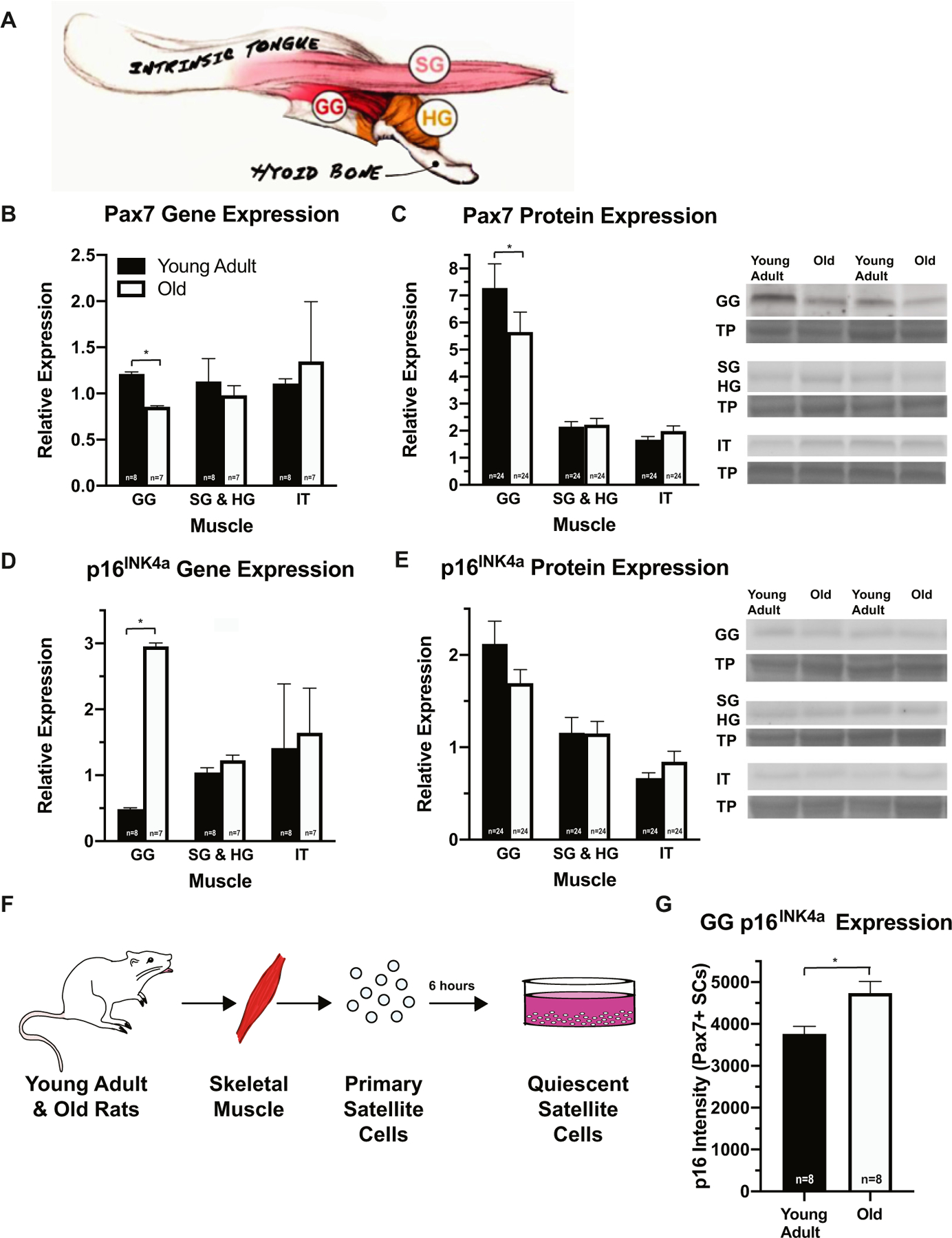
Regenerative marker expression of aged GG is reduced and related to increased p16INK4a expression. A Illustration of the tongue muscles. B, C In GG, Pax7 gene (F1,11 = 5.633, p = 0.037) and protein (F1,36 = 5.154, p = 0.029) expression was reduced with age. D, E p16INK4a gene expression was increased in old GG (F1,11 = 8.704, p = 0.013). F, G In the GG, cell-intrinsic expression of p16INK4a increased in aged SCs (F2,36 = 5.051, p = 0.012). (*p-value < 0.05; sample size indicated in individual bar-plots.)
3.2. p16INK4a may regulate SC function with age in genioglossus
To ascertain whether age-related reductions in Pax7 expression could be related to extrinsic, environmental, or cell-intrinsic mechanisms of senescence, we examined expression of senescent marker p16INK4a in the tongue muscles of young adult and old rats. An age-related increase in p16INK4a gene expression was observed in the GG muscles of the old rat group (Fig. 2D). However, p16INK4a protein expression did not change with increasing age in any of the tongue muscles (Fig. 2E).
Next, we examined p16INK4a expression in isolated Pax7-positive (Pax7+) SCs (Fig. 2F). Primary SC cultures were prepared from young adult and old GG, combined SG and HG, and IT muscles, and cultured for 6 h. In Pax7+ SCs isolated from the GG muscle, p16INK4a expression was elevated with increasing age (Fig. 2G). These data suggest age-related reductions in Pax7 gene and protein expression in the GG may be related to increased extrinsic and cell-intrinsic expression of p16INK4a.
3.3. Maximal voluntary tongue force increases with exercise
Rats in the tongue exercise group completed either 3, 17, or 59 days of progressive resistance tongue exercise to examine gains in tongue strength over short- and long-term exercise treatment periods (Fig. 3A). Maximal voluntary tongue force (MVTF), a measure of behavioral and voluntary maximum tongue strength, significantly increased following 17 and 59 days of progressive resistance tongue exercise (Fig. 3B), and paralleled strength gains observed in elderly patients in clinical studies following completion of tongue strengthening exercises (Rogus-Pulia et al., 2016). There was a trend towards increased MVTF variability at each exercise time point in the old group (Fig. 3C). Although age-related reductions in tongue force were not observed, this finding is consistent with previous studies form our laboratory (Cullins et al., 2017; Kletzien et al., 2013) and with reports from the human population where age-related deficits in voluntary tongue force and swallowing pressures remain inconclusive (Burkhead et al., 2007; Nicosia et al., 2000; Rogus-Pulia and Connor, 2016; Steele, 2013).
3.4. SC differentiation increases with tongue exercise
To determine whether a clinically relevant tongue strengthening exercise program activated tongue SCs, we examined expression profiles of SC markers at three time points following a progressive resistance tongue exercise treatment (3-days, 17-days, 59-days; Fig. 3A). Separate groups of rats were used for each time point to examine early (d 3 & 17) and later stages (d 59) of SC activation and differentiation following exercise. Gene and protein expression of SC markers (Pax7, MyoD, myogenin) were determined in the extrinsic and intrinsic tongue muscles, and Pax7 expression was also examined in isolated SCs.
Myogenin protein expression was significantly elevated following 3, 17, and 59 days of tongue exercise in both young adult and old groups in the SG and HG muscles (Fig. 4A, B). However, no exercise-induced changes were observed in muscle protein expression of Pax7 or MyoD at any time point or in gene expression in the tongue muscles (Fig. S3, Supporting Information). The percentage of Pax7+ SCs isolated from the IT following 17 days of exercise significantly increased in the young adult group and was decreased in the old group (Fig. 4C). With tongue exercise, SC differentiation significantly increased in both the early (d 3 & 17) and late (d 59) stages of the exercise program. The clinical implication of these findings is tongue strengthening exercise may activate SCs in elderly people, representing a putative cellular mechanism for enhancement of muscle regeneration with therapy.
Fig. 4.
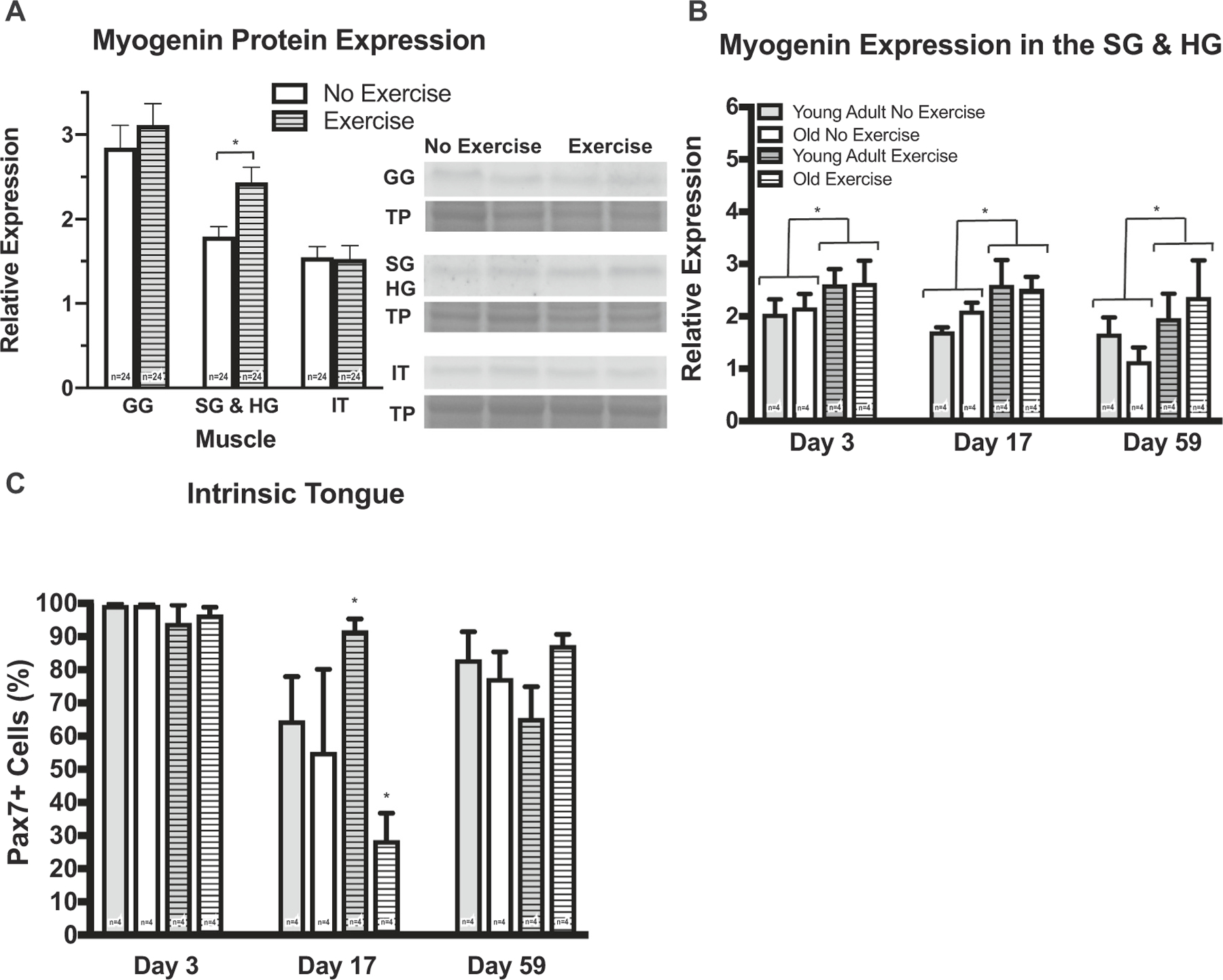
SC differentiation increased following tongue exercise. A, B Myogenin protein expression in SG & HG muscles increased following exercise at all time points (F1,36 = 8.798, p = 0.005; 3-way ANOVA). c Following 17 days of exercise in the IT, the percentage of Pax7+ SCs isolated from the young adult exercise group increased (p = 0.003), and decreased in the old exercise group (p = 0.01; interaction on 3-way ANOVA, F2,36 = 4.441, p = 0.019). (*p-value < 0.05; sample size indicated in individual bar-plots.)
3.5. SC location changes according to myofiber type with exercise
We also examined the percentage of Pax7+ nuclei and Pax7+ myonuclei location by myofiber type in GG and SG muscle mid-belly following 17 days of exercise to determine whether cues from the myofiber influenced SC location (Fig. 5). In the GG, the percentage of Pax7+ nuclei was significantly increased in the young adult exercise group (Fig. 5B), and the exercise group had a significantly lower percentage of Pax7+ myonuclei colocalizing near myosin heavy chain (MyHC) IIa myofibers (Fig. 5E). The percentage of Pax7+ nuclei colocalizing near vessels and/or nerves is significantly increased following tongue exercise in both young adult and old groups (Fig. 5G). Pax7 SC content significantly increased following 17 days of tongue exercise in the SG muscle (Fig. 5H). A significant increase in the percentage of Pax7+ myonuclei colocalizing near MyHC type IIb and IIx myofibers was observed following exercise (Fig. 5I), while a significant reduction of Pax7+ myonuclei colocalizing around MyHC type IIb, IIx, and IIa myofibers was observed (Fig. 5J). The percentage of Pax7+ nuclei colocalizing near vessels and/or nerves in the SG muscle was increased in the old no exercise group, and in the young adult and old tongue exercise groups (Fig. 5M). These findings suggest environmental cues from the myofiber may influence the location of Pax7+ nuclei in tongue muscles with increased age and following 17 days of tongue exercise.
Fig. 5.
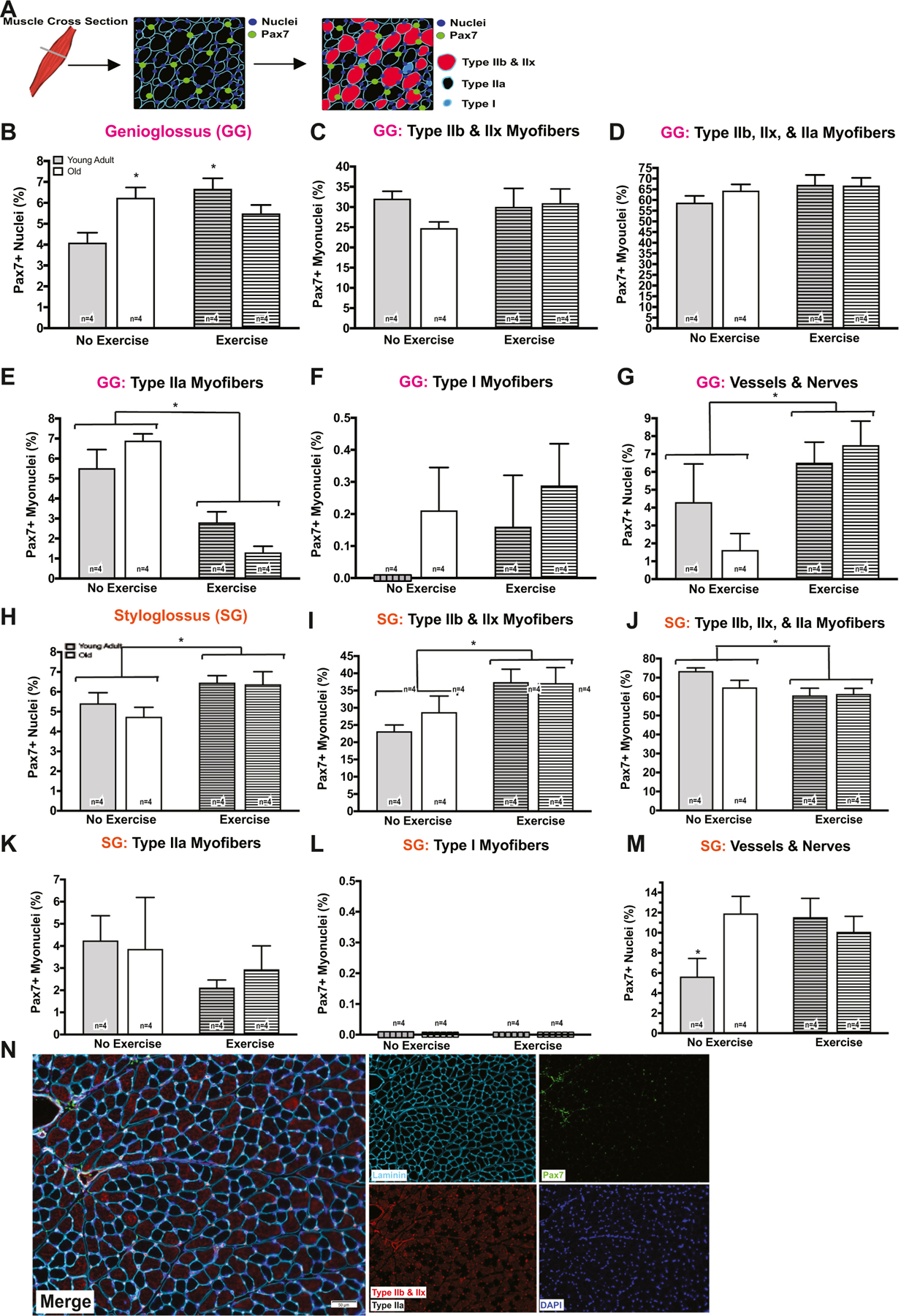
SC location changes according to myofiber type. A, N Schematic of tissue cross section. B SC content increased following 17 days of exercise in the young adult group (p = 0.005) and was greater in the old no exercise group (p = 0.021; 2-way ANOVA, F1,20 = 12.47, p = 0.002). E A significant interaction effect was observed for the percentage of Pax7+ myonuclei colocalizing around MyHC type IIa myofibers (F1,20 = 6.092, p = 0.0227) in the GG. The no exercise, young adult and old groups had a significantly greater percentage Pax7+ SCs near MyHC IIa myofibers in comparison to the young adult exercise (p = 0.018 [young, no exercise]; p < 0.001 [old, no exercise]) and the old exercise (p < 0.001 [young, no exercise]; p < 0.001 [old, no exercise]) groups. The percentage of Pax7+ nuclei colocalizing near vessels and/or nerves in the GG muscle also increased following 17 days of tongue exercise (F1,20 = 7.631, p = 0.012). H Pax7 content increased following 17 days of exercise group in the SG muscle (F1,20 = 6.889, p = 0.016). I An increase in the percentage of Pax7+ myonuclei colocalizing near MyHC type IIb and IIx was observed following 17 days of exercise (F1,20 = 8.692, p = 0.008). J While a reduction in the percentage of Pax7+ myonuclei colocalizing around MyHC type IIb, IIx, and IIa was observed following exercise (F1,20 = 6.901, p = 0.016). M A significant interaction effect was observed for the percentage of Pax7+ nuclei colocalizing near vessels and/or nerves in the SG (F1,20 = 4.971, p = 0.037). (*p-value < 0.05; sample size indicated in individual bar-plots.)
We also determined the percentage of centralized nuclei within GG and SG muscle myofibers (Fig. 6A). With both increasing age (Fig. 6B, C) and following 17 days of tongue exercise (Fig. 6D), the percentage of centralized nuclei significantly increased. This may be suggestive of an active state of myofiber repair and remodeling, and be related to observed increases in SC differentiation.
Fig. 6.
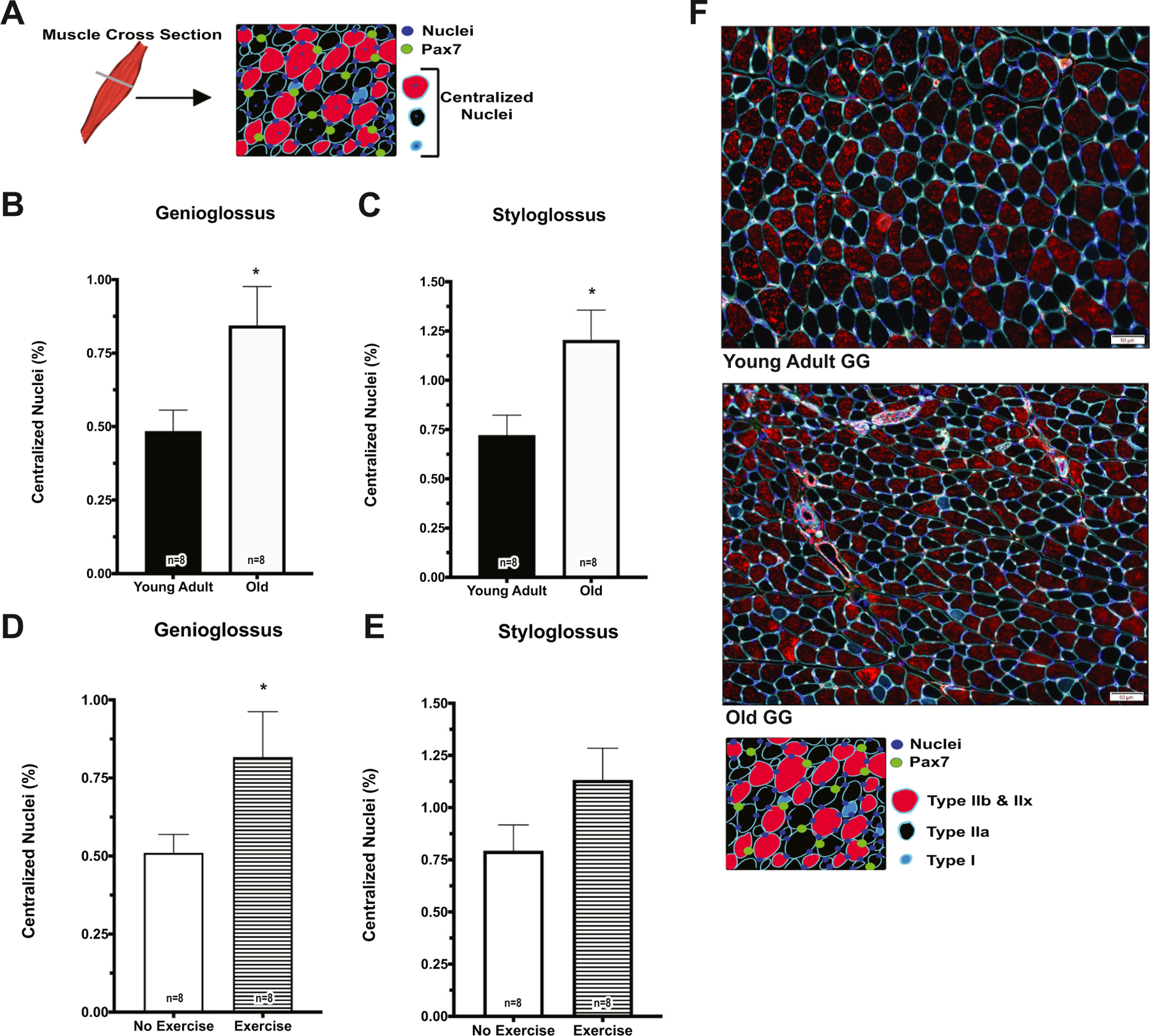
Centralized myofiber nuclei increased with age and following tongue exercise. A Schematic of tissue cross section. B, C Percentage of centralized nuclei increased in the old group in GG (F1,20 = 7.204, p = 0.014) and SG (F1,20 = 8.178, p = 0.001). D Following 17 days of exercise, percentage of centralized nuclei increased in GG (p = 0.033). F Representative images from the GG of a young adult and old rat. (*p-value < 0.05; sample size indicated in individual bar-plots.)
3.6. SC marker expression is predictive of MVTF
As shown in Fig. 7A, a strong, significant relationship (r2 = 0.953) was observed between the protein expression of SC markers in all of the tongue muscles and MVTF on multiple regression. MyoD, myogenin, and p16INK4a protein expression in the tongue muscles and time were significant predictors of MVTF.
Fig. 7.
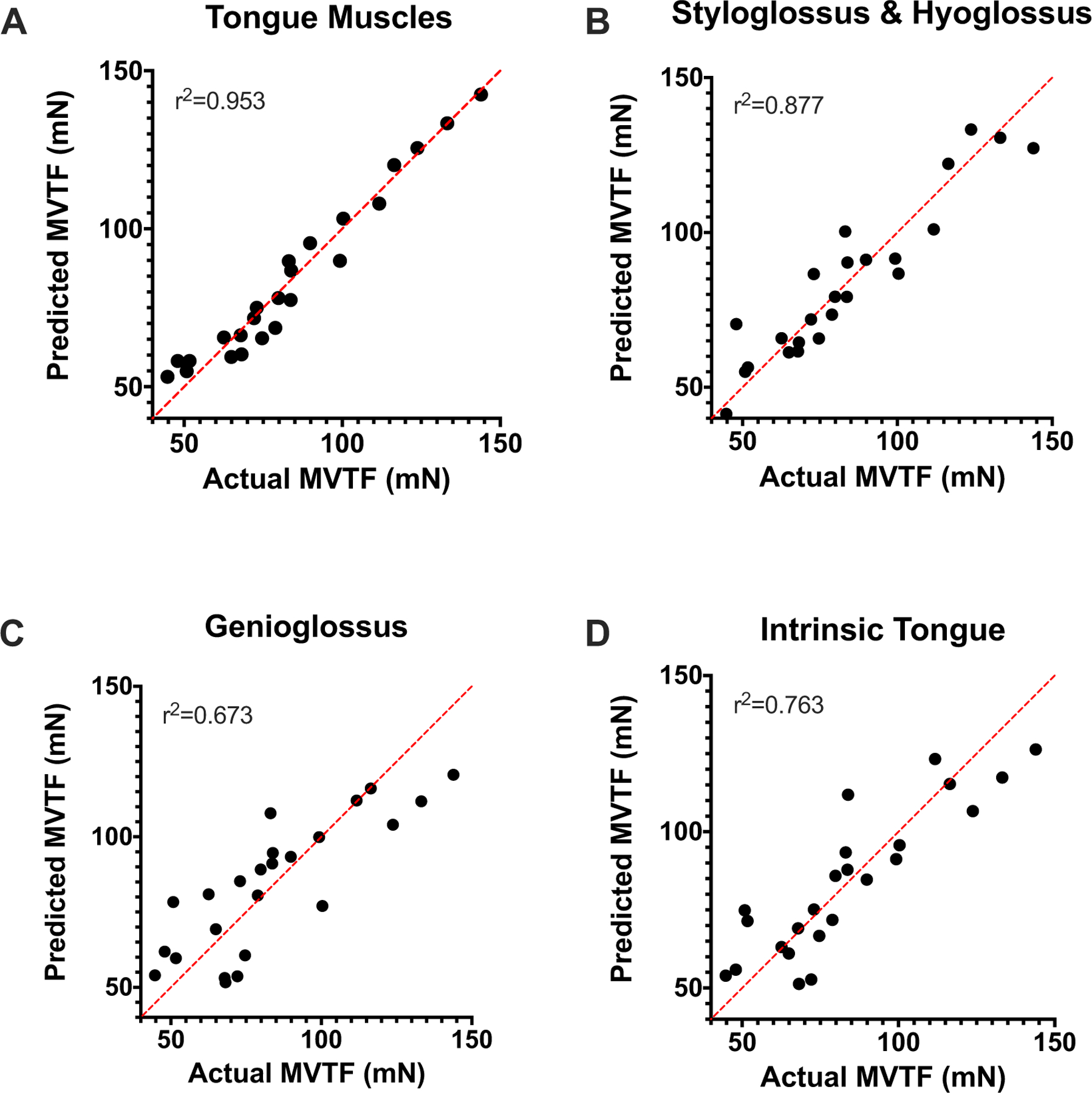
SC marker expression in tongue is predictive of MVTF. A A strong, significant relationship was observed on multiple linear regression between MVTF and SC protein markers (R2 = 0.953; F14,9 = 12.96, p < 0.001) in the tongue muscles. Specifically, time (p < 0.001), and MyoD (p = 0.019), myogenin (p = 0.030), and p16INK4a (p = 0.036) protein expression in tongue muscles were significant predictors of MVTF following exercise. B A strong, significant relationship in the SG and HG was observed between MVTF and SC markers (R2 = 0.877; F6,17 = 20.16, p < 0.001). Time (p < 0.001), and Pax7 (p = 0.048), MyoD (p = 0.013), myogenin (p = 0.010), and p16INK4a (p = 0.003) protein expression were significant predictors of MVTF. C, D Moderate, significant relationship was observed in the GG (R2 = 0.673; F6,17 = 5.818, p = 0.002) and IT (R2 = 0.763; F6,17 = 9.098, p < 0.001).
Following tongue exercise, in the combined SG and HG Pax7, MyoD, myogenin, and p16INK4a protein expression and time were strong, significant predictors of MVTF (r2 = 0.877; Fig. 7B). A moderate, significant relationship was observed between the SC markers and MVTF in the GG (r2 = 0.673; Fig. 7C) and IT (r2 = 0.763; Fig. 7D). Taken together, these findings indicate MVTF is significantly related to the protein expression of the SC markers and p16INK4a in tongue.
3.7. SC marker expression changes with time
We next examined whether expression of the SC markers changed over time in muscles of the tongue. In whole muscle, Pax7 protein expression was significantly upregulated in GG at d 17, and in IT at d 59 (Fig. 8A). In contrast, the percentage of Pax7+ SCs isolated from the combined SG and HG, and IT muscles was significantly reduced at d 17 (Fig. 8B). In the IT, whole muscle (Fig. 8D) and cellular (Pax7+ SCs; Fig. 8E) expression of p16INK4a was significantly increased at d 17, and could be related to the observed reduction of Pax7. At d 17, expression of p16INK4a in Pax7+ SCs was also significantly increased in the SG and HG muscles (Fig. 8E) MyoD protein expression was significantly reduced at d 59 in SG and HG muscles, and was elevated at d 3 in IT (Fig. 8G). Myogenin protein expression was significantly reduced in the IT at d 3 (Fig. 8H). Because SC marker expression changed throughout the 59-day experimental duration, these findings may be indicative of high regenerative turnover of tongue SCs.
Fig. 8.
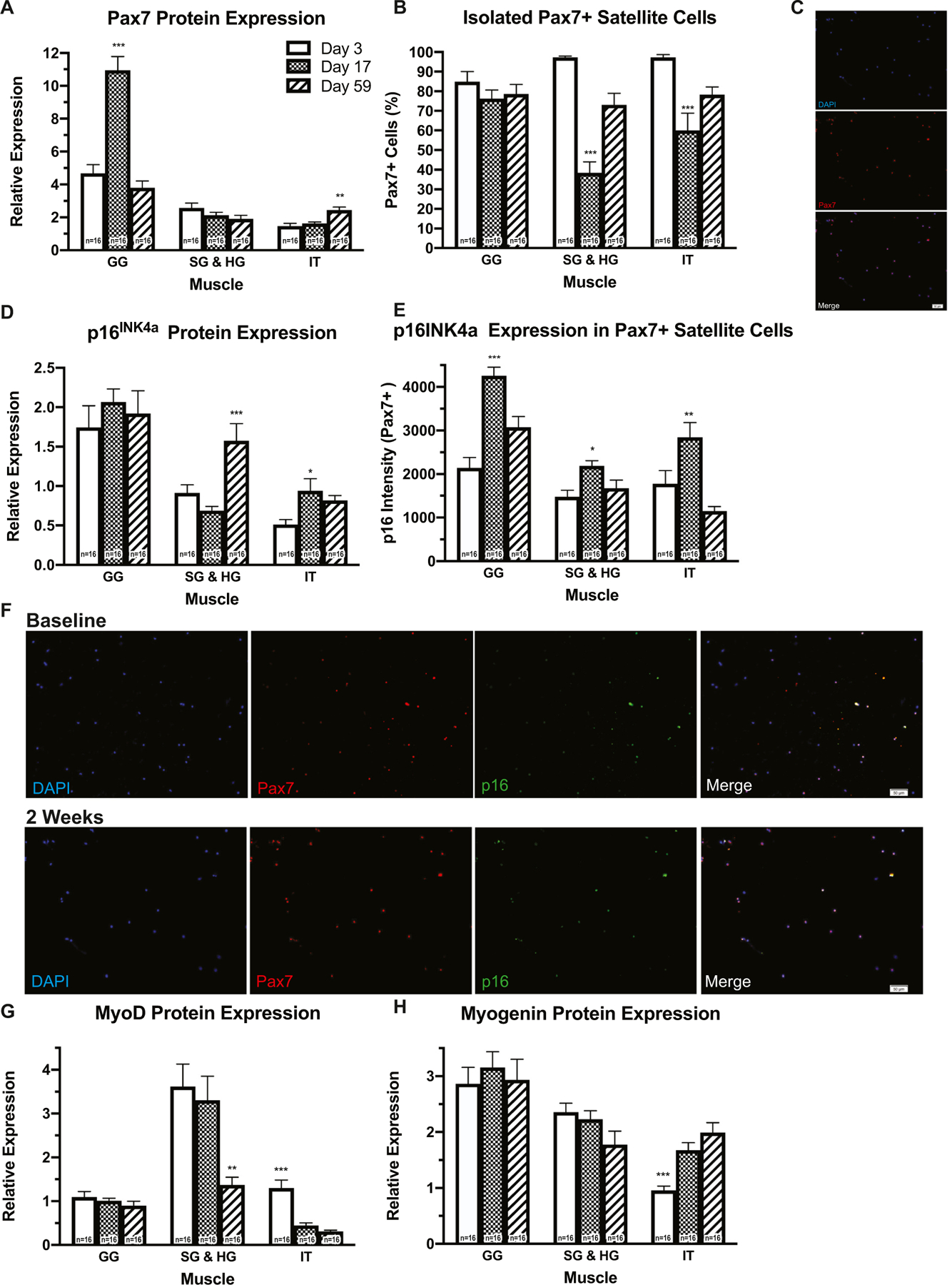
Tongue SCs have a high regenerative turnover across time. A In GG (F2,36 = 39.13, p < 0.001) at d 17, and in IT (F2,36 = 9.105, p < 0.001) at d 59, Pax7 protein expression was upregulated in whole muscle. B However at d 17, the percentage of Pax7+ SCs isolated from SG and HG (F2,36 = 37.52, p < 0.001), and IT muscles was reduced (F2,36 = 14.22, p < 0.0001). D, E At d 17 in the IT, expression of p16INK4a in whole muscle (F2,36 = 11.96, p = 0.0001) and in Pax7+ SCs was increased (F2,36 = 11.12, p = 0.0002). At d 17, expression of p16INK4a in Pax7+ SCs was also significantly increased in the SG and HG (F2,36 = 3.50, p = 0.041), and IT muscles (F2,36 = 11.12, p = 0.002). G Expression of the MyoD protein was reduced at d 59 in SG and HG muscles (F2,36 = 9.21, p = 0.0006), and elevated at d 3 in the IT (F2,36 = 18.72, p < 0.0001.) H Myogenin protein expression was reduced in the IT at d 3 (F2,36 = 12.66, p < 0.0001). C, F Representative ICC images from tongue muscles. (*p-value < 0.05; **p-value < 0.001; ***p-value < 0.0001; sample size indicated in individual bar-plots).
3.8. SC expression profiles differ between tongue and limb muscles
To determine whether there is differential expression of SCs among muscles of the tongue and limb, we compared whole muscle protein expression of Pax7, MyoD, myogenin, and p16INK4a in the tongue (GG, SG, HG, IT) and limb (extensor digitorum longus; EDL) muscles in the no exercise group. As shown in Fig. 9 significant differences in the expression of the aforementioned markers were observed among the tongue and limb muscles. Pax7, myogenin, and p16INK4a protein expression was significantly greatest in the GG (Fig. 9A, C, D), and myoD protein expression was greatest in the combined SG and HG (Fig. 9B). In comparison to the EDL, Pax7 protein expression in the GG was increased 19-fold, MyoD protein expression in the SG and HG was increased 3-fold, and myogenin protein expression in the GG was increased 4-fold. These data suggest that the expression of SC markers in the tongue muscles is increased compared to a representative limb muscle (EDL).
Fig. 9.
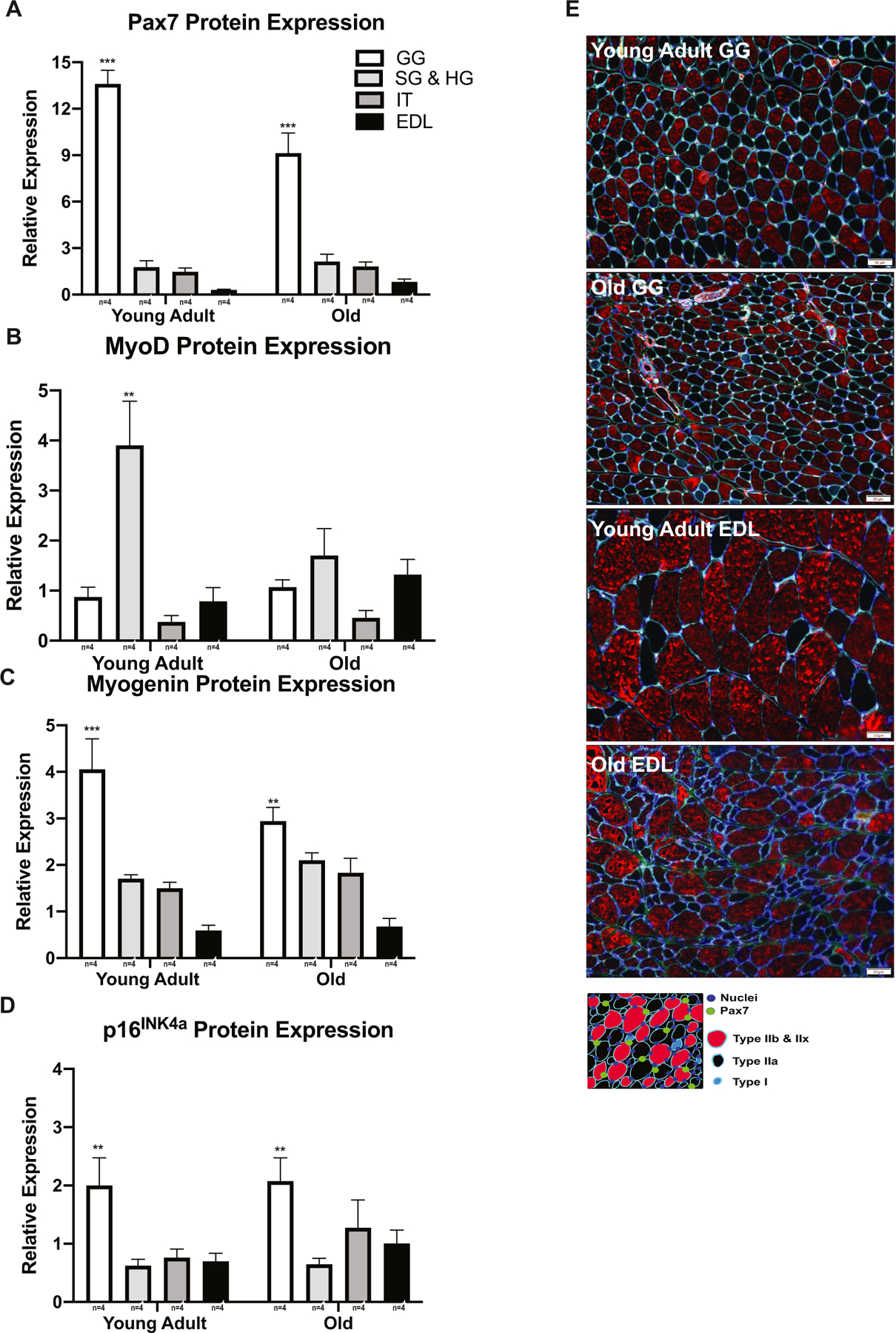
SC marker profiles are differentially expressed among tongue and limb muscles. A Pax7 protein expression was greatest in the GG (F3,24 = 7.888, p < 0.0001). B MyoD protein expression was upregulated in the SG and HG of the young adult group (F3,24 = 4.718, p = 0.0003). C Myogenin protein expression was upregulated in the GG (F3,24 = 31.18, p < 0.0001 [Young Adult], p=0.0004 [Old]). D p16INK4a protein expression was greatest in GG (F3,24 = 8.305, p =0.0006). E Representative images from young adult and old tongue (GG) and limb (EDL) muscles. (*p-value < 0.05; **p-value < 0.001; ***p-value < 0.0001; sample size indicated below individual bar-plots).
4. Discussion
Cellular mechanisms of age-related changes in lingual muscle are unknown. A putative mechanism is decline in the ability of SCs to repair and remodel damaged myofibers. Accordingly, the purpose of this study was to examine the manner in which aging contributes to deficits in SC expression and to examine the potential for a clinically relevant tongue exercise program to improve and activate the regenerative function of SCs in aging tongue musculature. Our findings indicate that SCs are impaired with age in the genioglossus muscle. Further, our results suggest that clinical rehabilitation strategies that strengthen the tongue may increase SC regenerative processes within the extrinsic tongue muscles and are associated with improved maximum voluntary tongue muscle strength.
The results of our study suggest the expression of SC markers in the tongue is distinct and muscle specific. Gene and protein expression profiles of the myogenic regenerative markers mediating quiescence, activation, and differentiation were differentially expressed in GG, combined SG and HG, and IT muscles with age and following exercise. The GG muscle was most susceptible to age-associated changes in that both whole muscle Pax7 gene and protein expression decreased with age. This may be evidence of an age-related loss of the resident, quiescent SC population and an overall, global decline in the regenerative potential of aged SCs (Schultz and Lipton, 1982). However, when we examined SC content in the mid-belly of the GG muscle, no age-related alterations in Pax7 SCs were observed, that indicates age-related changes in Pax7 SC content may be region dependent and reduced in the anterior or posterior regions of the GG. We also observed an age-related upregulation of whole muscle p16INK4a gene expression in the GG, and increased protein expression of p16INK4a in isolated, aged Pax7+ SCs from the GG. The upregulation of this cell cycle inhibitor could be indicative of a state of tissue-wide cellular senescence. Expression of p16INK4a blocks the transition from the G0 to G1 phase of the cell cycle and inhibits entry into the S phase, inducing a state of senescence and promoting age-related tissue dysfunction and degeneration (Kim and Sharpless, 2006; Sharpless and Depinho, 2007; Sousa-Victor et al., 2014). Increased p16INK4a expression may contribute to age-related reductions in Pax7 expression observed in the GG, impacting the ability of Pax7+ SCs to be activated by an exercise stimulus. This finding is consistent with previous studies that found increased levels of p16INK4a expression in numerous aged tissue types and within aging, cultured cells (Baker et al., 2016; Kim and Sharpless, 2006; LaPak and Burd, 2014; Naylor et al., 2013; Sharpless and Depinho, 2007; Sousa-Victor et al., 2014). The inability of aged SCs to progress through the cell cycle significantly impacts their capacity to initiate a regenerative response in response to injury, exercise, or disease.
Unlike the GG, we did not observe any age-related reductions in the expression of the SC markers or of p16INK4a within the SG and HG, or IT muscles, suggesting that SCs from these muscles may be spared from aging in a rat model. Even though, age-related increases in p16INK4a expression were not observed in aging SG, HG, IT, and EDL muscles this is consistent with recent studies that observed a low abundance of senescent cells and no significant changes in p16INK4a expression in gastrocnemius, heart, and quadricep muscles from aging and progeroid mouse models, and in aged human vastus lateralis muscles (Dungan et al., 2020; Jeyapalan et al., 2007; Wang et al., 2009; Yousefzadeh et al., 2020). The age-related expression of p16INK4a in skeletal muscle and SCs remains inconsistent, and controversial across numerous aging studies, and may indicate the need for better and more specific senescent markers. In previous studies from our laboratory, age-related alterations in the contractile properties and in the myofiber composition have been found in the tongue muscles (Cullins and Connor, 2017; Kletzien et al., 2018a, 2018b; Kletzien et al., 2013). Because the MyHC isoform and myofiber type composition of the tongue muscles change with age, we also examined the location of Pax7+ SCs according to myofiber type. No age-related alterations in the location of Pax7+ SCs were found in the tongue, contrary to what has been reported in the limb (Verdijk et al., 2007). However, studies in the limb only examined the location of Pax7+ SCs in proximity to either type II or type I myofibers, and did not take into consideration myofiber subtypes as was performed in the current study. Data from the present study suggest that the expression of regenerative myogenic markers of the SG, HG, and IT does not change with age. Thus, other mechanisms within the aged systemic or local microenvironment, likely contribute to the age-related changes in muscle physiology and composition previously observed in the SG, HG, and IT muscles.
Recent emphasis has been placed on the development of novel therapies that improve the regenerative potential of SCs and restore muscle function in the aging population. Regenerative medicine strategies to combat age-related deficits in muscle strength and function have taken many forms in the limb musculature (Almada and Wagers, 2016; Madl et al., 2018; Oh et al., 2014). They include endogenous delivery of stem cells, implantation of bioscaffolds, pharmacologic treatment, and exercise. Although exercise has been suggested as a potential intervention to improve muscle strength, performance, and function in elderly individuals, few studies have examined the effects of exercise on SC regenerative capacity over time (Bazgir et al., 2017; Bellamy et al., 2014; Cermak et al., 2013; Kadi et al., 2005; Smith and Merry, 2012). Progressive resistance weight training and high intensity endurance exercises have been shown to stimulate SC regenerative processes in the limb musculature in both humans and mice (Bazgir et al., 2017; Bellamy et al., 2014; Cermak et al., 2013; Kadi et al., 2005; Smith and Merry, 2012). In this study, we examined the regenerative ability of tongue muscle SCs in young adult and old rats following a tongue strengthening exercise modeled after a current clinical treatment for the rehabilitation of dysphagia in elderly people.
Our progressive resistance tongue exercise treatment affected the expression of the SC factors mediating differentiation. Consistent with findings in limb literature (Bazgir et al., 2017; Bellamy et al., 2014; Cermak et al., 2013; Kadi et al., 2005; Smith and Merry, 2012), we also observed following exercise increased SC content, SC activation and differentiation, and gains in strength in both early (3 & 17 days) and late (59 days) phases of the progressive resistance tongue exercise program. The combined SG and HG muscles were the most impacted by tongue exercise as evidenced by increases in the percentage of Pax7+ SCs (d 17) and the upregulation of myogenin expression (d 3, 17, & 59) throughout the duration of the tongue exercise program. The location of Pax7+ SCs in the SG muscle also changed following exercise, and could be related to exercise-induced muscle angiogenesis (Mounier et al., 2011), shifts in the myofiber phenotype (Cullins et al., 2017; Kletzien et al., 2013) and hypertrophy, (Damas et al., 2018), and increased MVTF. Further, protein expression of MyoD, myogenin, and p16INK4a in SG and HG muscles was predictive of MVTFs obtained at all time points, suggesting that SC activation and differentiation in these muscles are strongly related to gains in tongue force. Results from our study indicate that tongue exercise has the capacity to activate the SC pool of tongue muscles. The upregulation of SC markers following tongue exercise may be a mechanism related to observed improvements following rehabilitation using this strategy in elderly people with dysphagia.
The differences we observed in SC marker expression in the tongue with age and exercise may be related to the specific function of the tongue muscles. Exercise intensity required to elicit a regenerative response in SCs may also vary by muscle. In particular, the GG muscle has a primary cross system role in swallowing, speech, and respiratory actions. During the swallow the GG is the major force generator for propulsion of the bolus. The GG is persistently active and has a significant role during speech tasks. For respiratory actions, the GG is crucial to the dilation and/or narrowing of the pharynx during breathing, and is involved in opening the oropharynx and reducing resistance to breathing. Due to the high demand placed on the GG, it may be more susceptible to age-related changes than the SG, HG, and IT muscles.
Our results suggest tongue exercise had differential effects on SC activation and differentiation in the extrinsic and intrinsic tongue muscles. Most surprising was the diminished SC regenerative response of the GG compared to that of the SG and HG muscles. Following an exercise task that required protrusion of the tongue and activation of the GG muscle, we hypothesized the SC response would be most upregulated in the GG. Because of the constant activation required of the GG for swallowing, speech, and respiratory actions, a more intense exercise stimulus may have been required to invoke a SC response in the GG compared with the other tongue muscles. However, upregulation of SC markers in the SG and HG following our exercise program suggests this form of exercise is sufficient in sustaining a more severe muscle injury and in provoking a SC response in the SG and HG muscles, due to its intensity and perhaps unfamiliarity (Armstrong, 1990). During this exercise task, the SG and HG muscles likely played a crucial role in posturing the tongue and propelling the water bolus. The lack of a pronounced SC response in the GG and IT muscles, may indicate that the tongue exercise treatment used in this study results in minimal damage to those myofibers.
The results of our study also suggest the regenerative function of SCs in tongue and limb are distinct and muscle specific. The only change that occurred with aging in the tongue muscles was an age-related decrease in Pax7 gene and protein expression in the GG. Interestingly, the SG, HG, and IT muscles were spared from an age-associated functional decline in the SC pool that is consistently observed within aging limb muscles. However, the most striking difference between the muscles of the tongue (GG, SG, HG, IT) and the limb (EDL) was the elevated relative protein expression of Pax7, MyoD, and myogenin in the tongue compared to limb. The protein expression profiles of the SC markers between the IT and EDL were most similar. Our data demonstrate unique profiles of SC markers in the GG, combined SG and HG, and IT compared to the EDL. The differences we observed in Pax7, MyoD, and myogenin expression may be related to the diverse functional requirements and embryological origins of the tongue and limb muscles.
Because the muscles of the tongue are critical for swallowing, speech, and respiratory actions, the functional and regenerative demand on these muscles may be higher than in the limb muscles, which are primarily active for locomotion, balance, and postural tasks. The increased activity of tongue muscles for these critical life functions throughout a given day may contribute to an elevated regenerative turnover of tongue SCs in comparison to limb SCs. We observed over the 59-day study period, upregulated expression of SC markers in the tongue compared limb. Elevated protein expression of these factors in the tongue muscles may be indicative of a state of chronic SC activation, similar to the increased state of activation observed in extraocular and pharyngeal muscles. Alterations in SC regenerative capacity have also been observed in the trunk, diaphragm, intrinsic muscles of the larynx, pharynx, and masseter in comparison to the muscles of the limb (Randolph and Pavlath, 2015). Further, it has been suggested that differences in SC marker expression among muscles are also related to differences in their embryological development. Limb skeletal muscles originate developmentally from the dermomyotome of the somatic mesoderm, while the tongue muscles arise from both the cranial mesoderm and the somatic mesoderm (Buckingham et al., 2003; Parada and Chai, 2015). The regulatory factors involved in limb and tongue myogenesis also differ drastically. Differences in function, embryologic origin, and myogenic regulatory factors may contribute to the diverse SC regenerative profiles we observed among the tongue and limb muscles studied.
5. Conclusion
The characterization of the myogenic regenerative marker expression of lingual SCs with age and following a clinically relevant exercise treatment is a necessary first step to determine the therapeutic potential of SCs as a future treatment for degenerative muscle diseases that affect swallowing function. This study serves as a foundation for future work in the development of cell- and tissue-based regenerative therapies targeting the tongue musculature for the prevention and treatment of dysphagia and for translational studies in humans. Better understanding of the underlying extrinsic (systemic and local) and cell-intrinsic mechanisms of age-related degeneration and exercise-induced regeneration will guide future clinical studies and treatment choices.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institutes of Health (F31AG054315, T32DC009401, R01DC005935, R01DC008149, R01DC014358, R01DC018071, R37CA225608, R21DC016135). We would like to thank members of the Connor and Suzuki labs, in particular SA Stevenson, JA Russell, MJ Cullins, BN Krekeler, EM Lynch, J Jeffrey, and S Tey. We would also like to thank G Leverson for statistical consulting.
Abbreviations:
- MyHC
myosin heavy chain
- SC
satellite cell
- MVTF
maximal voluntary tongue force
- GG
genioglossus
- SG
styloglossus
- HG
hyoglossus
- IT
intrinsic tongue
- EDL
extensor digitorum longus
Footnotes
Declaration of competing interest
Authors declare no competing interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.exger.2020.111104.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
- Almada AE, Wagers AJ, 2016. Molecular circuitry of stem cell fate in skeletal muscle regeneration, ageing and disease. Nat. Rev. Mol. Cell Biol 17 (5), 267–279. 10.1038/nrm.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R, 1990. Initial events in exercise-induced muscular injury. Med. Sci. Sports Exerc 22 (4), 429–435. [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, … van Deursen JM, 2016. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530 (7589). 10.1038/nature16932 184–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazgir B, Fathi R, Valojerdi MR, Mozdziak P, Asgari A, 2017. Satellite cells contribution to exercise mediated muscle hypertrophy and repair. Cell Journal (Yakhteh) 18 (4), 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker BJ, Russell JA, Connor NP, 2015. Effects of aging on evoked retrusive tongue actions. Arch. Oral Biol 60 (6), 966–971. 10.1016/j.archoralbio.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy LM, Joanisse S, Grubb A, Mitchell CJ, McKay BR, Phillips SM, … Parise G, 2014. The acute satellite cell response and skeletal muscle hypertrophy following resistance training. Plos One 9 (10), e109739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau HM, Cosgrove BD, Ho ATV, 2015. The central role of muscle stem cells in regenerative failure with aging. Nat. Med 21 (8), 854–862. 10.1038/nm.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M, Bajard L, Chang T, Daubas P, Hadchouel J, Meilhac S, … Relaix F, 2003. The formation of skeletal muscle: from somite to limb. Journal of anatomy 202 (1), 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhead LM, Sapienza CM, Rosenbek JC, 2007. Strength-training exercise in dysphagia rehabilitation: principles, procedures, and directions for future research. Dysphagia 22 (3), 251–265. 10.1007/s00455-006-9074-z. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Whishaw IQ, Schallert T, 2002. Animal models of neurological deficits: how relevant is the rat? Nat. Rev. Neurosci 3 (7), 574–579. 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- Cermak NM, Snijders T, McKay BR, Parise G, Verdijk LB, Tarnopolsky MA, … van Loon LJC, 2013. Eccentric Exercise Increases Satellite Cell Content in Type II Muscle Fibers. Medicine and science in sports and exercise 45 (2), 230–237. 10.1249/MSS.0b013e318272cf47. [DOI] [PubMed] [Google Scholar]
- Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K, 2009. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. Journal of Speech Language and Hearing Research 52 (3), 732–744. 10.1044/1092-4388(2008/08-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullins MJ, Connor NP, 2017. Alterations of intrinsic tongue muscle properties with aging. Muscle Nerve 56 (6), E119–E125. 10.1002/mus.25605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullins MJ, Krekeler BN, Connor NP, 2017. Differential impact of tongue exercise on intrinsic lingual muscles. Laryngoscope 10.1002/lary.27044. [DOI] [PMC free article] [PubMed]
- Damas F, Libardi CA, Ugrinowitsch C, Vechin FC, Lixandrão ME, Snijders T, … Tricoli V, 2018. Early-and later-phases satellite cell responses and myonuclear content with resistance training in young men. Plos One 13 (1), e0191039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan CM, Peck BD, Walton RG, Huang Z, Bamman MM, Kern PA, Peterson CA, 2020. In vivo analysis of γH2AX+ cells in skeletal muscle from aged and obese humans. FASEB J 34 (5), 7018–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Yi ES, Ryu JH, 2014. Aspiration-related deaths in 57 consecutive patients: autopsy study. PLoS One 9 (7). 10.1371/journal.pone.0103795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U, 2007. Accumulation of senescent cells in mitotic tissue of aging primates. Mech. Ageing Dev 128 (1), 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AM, Connor NP, 2011. Effects of electrical stimulation on neuromuscular junction morphology in the aging rat tongue. Muscle Nerve 43 (2), 203–211. 10.1002/mus.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi F, Charifi N, Denis C, Lexell J, Andersen JL, Schjerling P, … Kjaer M, 2005. The behaviour of satellite cells in response to exercise: what have we learned from human studies? Pflugers Archiv-European Journal of Physiology 451 (2), 319–327. 10.1007/s00424-005-1406-6. [DOI] [PubMed] [Google Scholar]
- Kim WY, Sharpless NE, 2006. The regulation of INK4/ARF in cancer and aging. Cell 127 (2), 265–275. 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Kletzien H, Russell JA, Leverson GE, Connor NP, 2013. Differential effects of targeted tongue exercise and treadmill running on aging tongue muscle structure and contractile properties. J. Appl. Physiol 114 (4), 472–481. 10.1152/japplphysiol.01370.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien H, Hare AJ, Leverson G, Connor NP, 2018a. Age-related effect of cell death on fiber morphology and number in tongue muscle. Muscle Nerve 57 (1), E29–E37. 10.1002/mus.25671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien H, Russell JA, Leverson G, Connor NP, 2018b. Effect of neuromuscular electrical stimulation frequency on muscles of the tongue. Muscle Nerve 58 (3), 441–448. 10.1002/mus.26173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien H, Cullins MJ, Connor NP, 2019. Age-related alterations in swallowing biomechanics. Exp. Gerontol 118, 45–50. 10.1016/j.exger.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPak KM, Burd CE, 2014. The molecular balancing act of p16(INK4a) in cancer and aging. Mol. Cancer Res 12 (2), 167–183. 10.1158/1541-7786.mcr-13-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus C, Logemann JA, Huang CF, Rademaker AW, 2003. Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatrica Et Logopaedica 55 (4), 199–205. 10.1159/000071019. [DOI] [PubMed] [Google Scholar]
- Lever TE, Brooks RT, Thombs LA, Littrell LL, Harris RA, Allen MJ, … Robbins KL, 2015. Videofluoroscopic Validation of a Translational Murine Model of presbyphagia. Dysphagia 30 (3), 328–342. 10.1007/s00455-015-9604-7. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH, 2000. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. Journal of Speech Language and Hearing Research 43 (5), 1264–1274. 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- Madl CM, Heilshorn SC, Blau HM, 2018. Bioengineering strategies to accelerate stem cell therapeutics. Nature 557 (7705), 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Akagi J, 2015. Decreased tongue pressure is associated with sarcopenia and sarcopenic dysphagia in the elderly. Dysphagia 30 (1), 80–87. 10.1007/s00455-014-9577-y. [DOI] [PubMed] [Google Scholar]
- Martin NR, Lewis MP, 2012. Satellite cell activation and number following acute and chronic exercise: a mini review. Cellular and Molecular Exercise Physiology 1 (1), e3. [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, … Buckingham M, 2005. Direct isolation of satellite cells for skeletal muscle regeneration. Science 309 (5743), 2064–2067. [DOI] [PubMed] [Google Scholar]
- Mounier R, Chrétien F, Chazaud B, 2011. Blood vessels and the satellite cell niche Curr. Top. Dev. Biol 96, 121–138 (Elsevier; ). [DOI] [PubMed] [Google Scholar]
- Nagai H, Russell JA, Jackson MA, Connor NP, 2008. Effect of aging on tongue protrusion forces in rats. Dysphagia 23 (2), 116–121. 10.1007/s00455-007-9103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor RM, Baker DJ, van Deursen JM, 2013. Senescent cells: a novel therapeutic target for aging and age-related diseases. Clinical Pharmacology & Therapeutics 93 (1), 105–116. 10.1038/clpt.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, Robbins J, 2000. Age effects on the temporal evolution of isometric and swallowing pressure. Journals of Gerontology Series a-Biological Sciences and Medical Sciences 55 (11),M634–M640. [DOI] [PubMed] [Google Scholar]
- Nogueira D, Reis E, 2013. Swallowing disorders in nursing home residents: how can the problem be explained? Clin. Interv. Aging 8, 221–227. 10.2147/cia.s39452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Lee YD, Wagers AJ, 2014. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat. Med. 20 (8), 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota F, Connor NP, Konopacki R, 2005. Alterations in contractile properties of tongue muscles in old rats. Annals of Otology Rhinology and Laryngology 114 (10), 799–803 Retrieved from ://WOS:000232427200010. [DOI] [PubMed] [Google Scholar]
- Parada C, Chai Y, 2015. Mandible and tongue development Curr. Top. Dev. Biol 115, 31–58 (Elsevier; ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph ME, Pavlath GK, 2015. A muscle stem cell for every muscle: variability of satellite cell biology among different muscle groups. Front. Aging Neurosci 7 10.3389/fnagi.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J, Humpal NS, Banaszynski K, Hind J, Rogus-Pulia N, 2016. Age-related differences in pressures generated during isometric presses and swallows by healthy adults. Dysphagia 31 (1), 90–96. 10.1007/s00455-015-9662-x. [DOI] [PubMed] [Google Scholar]
- Rogus-Pulia N, Connor NP, 2016. Muscle strengthening approaches to dysphagia rehabilitation. Current Physical Medicine and Rehabilitation Reports 4 (4), 277–286. [Google Scholar]
- Rogus-Pulia N, Rusche N, Hind JA, Zielinski J, Gangnon R, Safdar N, Robbins J, 2016. Effects of device-facilitated isometric progressive resistance oropharyngeal therapy on swallowing and health-related outcomes in older adults with dysphagia. J. Am. Geriatr. Soc 64 (2), 417–424. 10.1111/jgs.13933. [DOI] [PubMed] [Google Scholar]
- Roy N, Stemple J, Merrill RM, Thomas L, 2007. Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Annals of Otology Rhinology and Laryngology 116 (11), 858–865 Retrieved from ://WOS:000251353400012. [DOI] [PubMed] [Google Scholar]
- Russell JA, Connor NP, 2014. Effects of age and radiation treatment on function of extrinsic tongue muscles. Radiat. Oncol 9 10.1186/s13014-014-0254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Ciucci MR, Hammer MJ, Connor NP, 2013. Videofluorographic assessment of deglutitive behaviors in a rat model of aging and Parkinson disease. Dysphagia 28 (1), 95–104. 10.1007/s00455-012-9417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM, 2008. Self-renewal and expansion of single transplanted muscle stem cells. Nature 456 (7221), 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaser AJ, Wang H, Volz LM, Connor NP, 2011. Biochemistry of the anterior, medial, and posterior genioglossus in the aged rat. Dysphagia 26 (3), 256–263. 10.1007/s00455-010-9297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaser AJ, Stang K, Connor NP, Behan M, 2012. The effect of age and tongue exercise on BDNF and TrkB in the hypoglossal nucleus of rats. Behav. Brain Res 226 (1), 235–241. 10.1016/j.bbr.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaser AJ, Ciucci MR, Connor NP, 2015. Cross-activation and detraining effects of tongue exercise in aged rats. Behav. Brain Res 297, 285–296. 10.1016/j.bbr.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz E, Lipton BH, 1982. Skeletal muscle satellite cells: changes in proliferation potential as a function of age. Mech. Ageing Dev 20 (4), 377–383. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Depinho RA, 2007. How stem cells age and why this makes us grow old. Nat. Rev. Mol. Cell Biol 8 (9), 703–713. 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- Smith H, Merry T, 2012. Voluntary resistance wheel exercise during post-natal growth in rats enhances skeletal muscle satellite cell and myonuclear content at adulthood. Acta Physiol 204 (3), 393–402. [DOI] [PubMed] [Google Scholar]
- Sousa-Victor P, Munoz-Canoves P, 2016. Regenerative decline of stem cells in sarcopenia. Mol. Asp. Med 50, 109–117. [DOI] [PubMed] [Google Scholar]
- Sousa-Victor P, Gutarra S, Garcia-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, … Munoz-Canoves P, 2014. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506 (7488). 10.1038/nature13013 316–+. [DOI] [PubMed] [Google Scholar]
- Steele CM, 2012. Exercise-based approaches to dysphagia rehabilitation In: Cichero J, Clave P (Eds.), Stepping Stones to Living Well with Dysphagia vol. 72 pp. 109–117. [DOI] [PubMed] [Google Scholar]
- Steele CM, 2013. Optimal approaches for measuring tongue-pressure functional reserve. J Aging Res 2013, 542909 10.1155/2013/542909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treuting PM, Dintzis SM, Montine KS, 2018. Comparative anatomy and histology: a mouse, rat and human atlas Retrieved from. http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=1204881.
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW, 1999. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. Journals of Gerontology Series A-Biological Sciences and Medical Sciences 54 (11), B492–B501. 10.1093/gerona/54.11.B492. [DOI] [PubMed] [Google Scholar]
- Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ, 2007. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. American Journal of Physiology-Endocrinology and Metabolism 292 (1), E151–E157. [DOI] [PubMed] [Google Scholar]
- Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, Von Zglinicki T, 2009. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 8 (3), 311–323. [DOI] [PubMed] [Google Scholar]
- Yousefzadeh MJ, Zhao J, Bukata C, Wade EA, McGowan SJ, Angelini LA, … Calubag MF, 2020. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell 19 (3), e13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article (and its supplementary information files).


