Abstract
For almost three decades, the measurement of circulating IGF-I has constituted a highly important biochemical tool in the management of GH disorders. In fact, in acromegaly the importance of circulating IGF-I has increased following the introduction of the GH receptor antagonist pegvisomant, as the use of this drug makes it impossible to use circulating GH as a monitor of disease activity. In addition, determination of circulating IGF-I constitutes a valuable scientific tool in various research areas, from epidemiological investigations through clinical trials and experimental studies.
The multiple facets of IGF-I physiology and patho-physiology may explain why numerous endocrine laboratories have invested in IGF-I assays, by means of either in-house assays or commercial kits. However, despite its widespread use, the measurement of IGF-I is by no means trivial. On the contrary, the pronounced binding of IGF-I to the high-affinity IGF-binding proteins (IGFBPs) constitutes a notorious source of error, which has necessitated the development of methods that more or less successfully circumvent interference from the IGFBPs. Furthermore, there are some unsolved issues with the international standardization of the different IGF-I assays and there is no consensus regarding the procedures used when collecting and storing samples for measurement of circulating IGF-I.
The aim of this review is to discuss the current state of the art of IGF-I immunoassays and to present the current analytical problems with IGF-I measurements. Finally, we would like to suggest an agenda that may be used when trying to produce internationally accepted uniform requirements for future IGF-I assays.
Keywords: IGF-I immunoassays, Assay standardization, Reference populations, Sample collection
1. Introduction
The discovery of IGF-I was founded on two independent observations: its ability to stimulate uptake of radio-labelled sulphate in cultured cartilage (sulphation factor; SF), and its ability to mimic the metabolic effects of insulin in the presence of neutralizing insulin antibodies (non-suppressible insulin-like activity; NSILA) [1]. Since these pioneering studies some 50 years ago, the knowledge of the biological aspects of IGF-I has expanded dramatically and today, IGF-I is considered a true multi-potent growth factor, controlling cell proliferation, differentiation and apoptosis, tissue growth and organ-specific functions throughout the body. This fascinating diversity of functions explains the comprehensive scope of IGF-I research, which covers areas as distinct as cancer, metabolic disorders, cardiovascular disease, neurodegenerative and neurological diseases and of course growth and GH disorders [2–7].
Originally, IGF-I was quantitated by laborious and relatively nonspecific cell-based bioassays or by competitive membrane binding assays. Therefore, it was a major breakthrough when the first specific radioimmunoassay for IGF-I was developed more than 30 years ago, thereby enabling much more accurate and specific results [8]. Other major breakthroughs were the mapping of the amino acid sequence of IGF-I by Rinderknecht and Humbel [9], as well as the discovery and cloning of the IGF-binding proteins (IGFBPs) and the realization that they interfered in immunoassays, making it necessary to separate IGF-I from the IGFBPs prior to assay [10,11]. Since then, the number of assays for IGF-I and its IGFBPs has expanded dramatically, and in particular the worldwide distribution of commercial assays has enabled virtually every endocrine laboratory to perform measurements of immunoreactive IGF-I levels. However, as outlined in this survey, the measurement of IGF-I is still not a routine biochemical procedure.
The aim of this review is to discuss the current state of the art of IGF-I immunoassays and to describe the analytical problems that are involved in measuring IGF-I. Finally, we would like to suggest an agenda that may be used when trying to produce internationally accepted uniform guidelines for future IGF-I assays.
2. Technical information regarding IGF-I immunoassays
Traditionally, the endogenous bioactivity of IGF-I has been estimated by immunoreactive IGF-I levels, obtained after inactivation or removal of the IGFBPs. Thus, immunoreactive IGF-I equals the total peptide concentration within the circulation and accordingly, this measurement does not allow any discrimination between unbound IGF-I and IGF-I carried within ternary or binary complexes. More sophisticated assays for the measurement of complex-associated IGF-I, free IGF-I and bioactive IGF-I have been published within the last decade, but generally, although data for some of the newer assays are sparse [12–15], they do not appear to perform any better than the traditional IGF-I assays in the diagnosis and management of GH disorders [16]. Therefore, this review will focus on immunoreactive IGF-I assays.
2.1. Competitive vs. non-competitive immunoassays
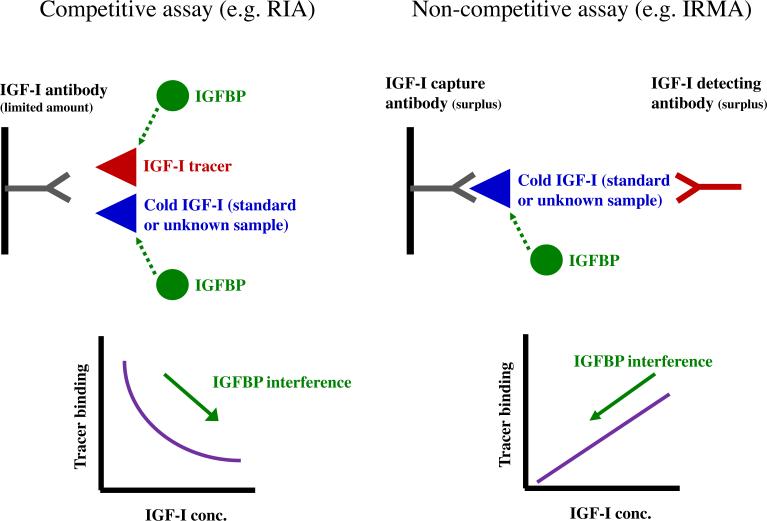
The competitive immunoassay technique was originally developed by Berson and Yalow in 1968 [17], and relies on the use of a fixed amount of specific antibody, competing for binding between a fixed amount of labelled antigen (i.e. tracer) and a variable amount of antigen from a standard solution (i.e. assay calibrator) or an unknown sample (Fig. 1, left panel). As both the tracer and the antibody concentrations are fixed, the assay signal is inversely related to the concentration of unlabelled ligand. Any interference from the IGFBPs depends upon the relative concentrations of IGF-I tracer vs. cold peptide, the affinity of the antibody and the concentration of the IGFBPs. However, as the IGFBPs are capable of binding the tracer, their presence may result in falsely elevated levels.
Fig. 1.
Illustration of the principles of competitive (left panel) and non-competitive immunoassays (right panel). In competitive assays, unlabelled antigen (from either standard solutions or unknown samples) competes with a fixed amount of labelled antigen for binding to a specific antibody. If there is no unlabelled antigen added, this will allow for maximal binding between labelled antigen and antibody. Conversely, as the concentration of unlabelled antigen is increased, this will reduce by competitive binding the ability of the labelled antigen to bind to the antibody. Consequently, the binding of tracer will approach zero (i.e. nonspecific binding). Thus, there is an inverse relationship between the unlabelled ligand concentration and the assay signal. In a competitive system, the introduction of IGFBPs may yield falsely elevated IGF-I levels. This phenomenon has been reported following addition of IGFBP-3 to samples that were analyzed using a commercial competitive assay [20]. In non-competitive immunoassays the ligand is bound between a capture antibody and a labelled antibody, and consequently, this type of assay yields a positive relationship between the assay signal and the ligand concentration. As compared to competitive assays, the introduction of two antibodies may often increase ligand specificity and sensitivity as well as the working range. In contrast to competitive assays the introduction of IGFBPs will result in falsely reduced IGF-I levels.
Non-competitive assays are usually based on two different antibodies (or sometimes one polyclonal antibody), directed against different epitopes on the antigen. The two antibodies are present in molar excess of the antigen and therefore, all added antigen becomes captured in a “sandwich” between the two antibodies, of which the first is immobilized on a solid phase (test tubes, micro-titer wells, beads etc.) and the second labelled with a signal (iodine, dye, europium etc.). In contrast to competitive assays, non-competitive assays yield a positive correlation between the concentration of antigen and the signal (Fig. 1, right panel). The use of two different antibodies, added in excess of the antigen, generally increases the quality of the results because non-competitive assays possess a wider operational range (2–3 vs. 1–2 orders of magnitude), are more sensitive (approximately 1 vs. 10 ng IGF-I per reaction tube) and are usually more specific than competitive assays [18]. Finally, due to shorter incubation times, most non-competitive assays may be finalized within one working day, whereas the majority of competitive assays require overnight incubation. In the non-competitive assay the introduction of IGFBPs will result in falsely low values.
2.2. IGFBP-interference
Soon after the publication of the first immunoassay for IGF-I [8] it became apparent that the presence of the IGFBPs interfered with the antibody recognition of IGF-I, and consequently, various techniques were developed to circumvent this interaction. Size-exclusion gel chromatography performed at low pH, e.g. 3.0 (preferentially by fast protein liquid chromatography; FPLC) is considered the gold standard, but its laborious nature makes this separation procedure unsuitable for most purposes. Therefore, simpler techniques have been employed. The most commonly used alternative is acid–ethanol extraction, during which the IGFBPs are dissociated by the low pH, precipitated by ethanol and separated by centrifugation, leaving unbound IGF-I in the supernatant. However, this method suffers from two problems. First, not all IGFBPs are precipitated: substantial amounts of IGFBP-1, IGFBP-3 and IGFBP-4 have been detected in the supernatant [11]. Even in non-competitive assays that use an excess of antibody, residual IGFBPs may cause problems, in particular in samples from patients with an abnormal IGFBP-profile such as type 1 diabetes, liver cirrhosis or chronic renal failure (CRF), where the IGFBPs are disproportionally elevated compared to IGF-I. Accordingly, various means to further minimize the interference of residual IGFBPs have been employed, including addition of excess IGF-II, cryoprecipitation, and the use of IGF-I analogues with reduced IGFBP-interaction [19]. An additional problem may be the possibility of co-precipitation of IGF-I during the centrifugation process [11].
As can be seen from Table 1, most commercial assays utilize an acidification procedure, which liberates IGF-I from the IGFBPs, followed by the addition of an IGFBP-blocking agent. Although the latter step is usually not described in detail, this most often includes the addition of excess IGF-II. Obviously, bypassing the need for centrifugation simplifies the separation procedure and circumvents problems with co-precipitation of IGF-I. Furthermore, as elegantly shown by Krebs et al. [20], these assays are only affected by IGFBPs when added in very high concentrations. However, the quality of the results is dependent upon the quality of the reagents that are used and some commercial assays are not completely resistant to changes in the IGFBPs. Therefore, cautiousness is still imperative when analyzing samples with an elevated IGFBP to IGF-I ratio. In this context it should be noted that assay validation data performed in patients with CRF, type 1 diabetes or liver cirrhosis are usually not provided by kit manufacturers.
Table 1.
Commonly used commercial assays for IGF-I. Data are derived from the kit insert, or as stated below.
| Manufacturer | Product no | Assay principle (capture + detection antibody) |
WHO 87/518 calibration |
Standard range (zero not included) (μg/l) |
Sensitivity in unknown samples (μg/l) |
Intra-assay CV(%) |
Inter-assay CV (%) |
Sample pre-treatment | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Nichols Institute Diagnostics | Nichols Advantage | Chemiluminescent Immunometric assay | Yes | - | 6 | 3.5-5.2 (a) | 5.7-7.1% (a) 1.9.-9.8 (b) |
Acid + neutral + IGF-II blocking of IGFBPs | Considered gold standard of IGF-I assays until its withdrawal in 2006 |
| DSL/Beckman Coulter | DSL-10-5600 | One step ELISA | Yes | 10-600 | 1.2 | 4.5-7.1 | 4.8-8.8 | Acid ethanol extraction | Insufficient reference material: 67 children and adolescents and 67 adults of both genders |
| DSL/Beckman Coulter | DSL-10-2800 | Two step non-extraction ELISA | Yes | 10-600 | 1 | 4.5-8.6 | 3.3-6.8 | Dissociation buffer to avoid IGFBP interference | Reference material: 284 samples (both genders) ranging from 0 to +60 years |
| Immunotech (Beckman Coulter) | A15729 | One-step IRMA with MAbs | Yes | 30-1200 | 2 | 6.3 (c) | 6.8 (c) | Acid + neutral + and IGF-II blocking of IGFBPs | Reference material covers the whole age span but number of subjects not given |
| Diagnostic Research Group (DRG) | IGF-I ELISA 600 (EIA-4140) | One step EIA with MAb (competitive) | Yes | 5-600 | 1.3 | 4.7-6.6 | 7.2-7.8 | Acid + neutral + blocking of IGFBPs | Insufficient reference material |
| R&D Systems | DG 100 | ELISA (MAb + PAb) | Yes (see comments) | 9.4-600 | 2.6 | 3.5-4.3 | 7.5-8.3 | Acid + neutral + blocking of IGFBPs | No reference material supplied. Multiply levels by 0.816 to obtain 87/518 calibrated values |
| Mediagnost | E20 | ELISA | WHO 02/254 | 42-1050 | 1.9 | 5.1-6.7 | 2.3-6.8 | Acid + neutral + and IGF-II blocking of IGFBPs | Extensive reference material included. TS 1-5 ranges also stated |
| Siemens | Immulite 2500 | Automated chemiluminescent assay | Yes | 25-1600 (d) | 20 (d) | 1.7-2.2 (d) 2.1-5.6 (e) |
1.9-3.6 (d) 10.8 (f) |
Non-extraction acidification and neutralization | Extensive reference ranges: 85 neonatal samples and 1,500 children, adolescents and adults. TS 1-5 ranges also stated |
| Immunodiagnostic Systems (IDS) | AC-27F1 | ELISA (PAb + MAb) | Yes | 16-1137 | 3.1 | 4.6-7.2 | 4.3-6.5 | Dissociation buffer to avoid IGFBP interference | Reference material covers the whole age span based on 231 subjects |
Abbreviations: acid (acidification); neutral (neutralization); MAb (monoclonal antibody); PAb (polyclonal antibody); ELISA (enzyme-linked immunosorbent assay); EIA (enzyme immunoassay); IRMA (immunoradiometric assay); TS (Tanner stage). Most of the information is derived from the kit inserts produced by the manufacturer. However, some data are derived from (a) Massart and Poirier [51],(b) Brabant et al. [53],(c) Cowan and Bartlett [21],(d) Krebs et al. [20],(e) Elmlinger et al. [65] and (f) Glaser et al. [66]. Not all kit inserts do explicitly state that unknown samples are acidified and blocked with excess IGF-II; however, this is most likely the case.
2.3. Head-to-head comparison of IGF-I assays
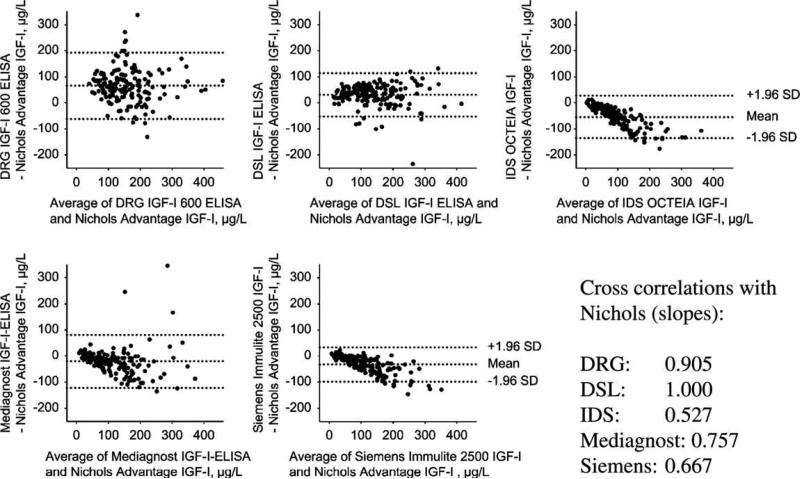
As seen in Table 1, many of the commercial assays for IGF-I are strikingly similar. They are calibrated against the WHO 87/518 IGF-I preparation, and they have similar sensitivities, calibrator ranges and CVs. Nevertheless, head-to-head comparisons have revealed important non-linear differences between assays. Krebs et al. [20] recently compared 5 currently available assays against the Nichols Advantage assay, which until its withdrawal in 2006 was considered the gold standard IGF-I assay. Altogether 173 patients from the German KIMS cohort were included in the study. Bland–Altman analyses of data demonstrated the presence of systematic deviations between assays (Fig. 2), and furthermore, the slopes of the regression lines varied from 0.527 to 1.00, despite the fact that all assays were calibrated against the same WHO reference preparation. Although the authors presented no data on the relationship between the 5 currently available assays, their relationship with the Nichols Advantage differed. This may be taken as a strong indication that a mutual comparison between the 5 currently available assays will show a similar result, at least for some of the assays. Cowan and Bartlett compared the DSL and the Immunotech assay (Table 1) in samples from 124 adolescent subjects and found a cross correlation of 0.78. Although a Bland–Altman plot was not shown, this comparison demonstrates some discrepancy, particularly for high values [21].
Fig. 2.
Bland–Altman plots based on a head to head comparison of 5 different commercial IGF-I immunoassays, using the Nichols Advantage IGF-I as reference. The study population was composed of 173 serum samples from GH deficient patients contained in the German KIMS cohort (Pfizer International Metabolic Database). Samples were collected either at baseline (i.e. as GH deficient) or during GH replacement therapy. As can be seen from the correlation analyses and the Bland–Altman plots, there were marked differences between some of the assays, despite the fact that all assays were calibrated against the IGF-I WHO international reference reagent 87/518. Reprinted from Krebs et al. [20] with permission from The Walter de Gruyter publishing group.
The reason for the observed discrepancies between apparently similar IGF-I assays is not clear, but may in part reflect differences in the sensitivity to the IGFBPs. Whatever the reason, however, these data emphasize that one cannot rely on a simple conversion factor when comparing IGF-I levels determined by different assays.
2.4. IGF-I assay standardization
As seen in Table 1, all commercial assays have been calibrated against the WHO International Reference Reagent (IRR) 87/518. However, the stock of the IRR 87/518 is almost depleted, and furthermore, its purity has been questioned. Therefore, Burns and colleagues initiated an international collaborative study with the purpose of establishing a new IRR for IGF-I. This goal was achieved, and by now, the Expert Committee on Biological Standardization of WHO has formally adopted the new IRR, which is named 02/254. Ampoules containing 8.50 μg are now available on request from the National Institute for Biological Standards and Control (see www.nibsc.ac.uk). Data on the preparation and calibration of the new IGF-I IRR preparation performed by Burns and colleagues has very recently been published [22].
2.5. IGF-I assay validation
There are numerous steps required to sufficiently validate an immunoassay [11,23]. In regards to IGF-I, we believe the following steps are mandatory. (i) Recovery of exogenous IGF-I when added to unknown samples prior to pre-treatment (i.e. before acid ethanol extraction or acidification and IGFBP-blocking). The amount of added peptide should be within the physiologically relevant range, and should be tested in patient samples with abnormal IGFBP profiles (i.e. CRF, liver cirrhosis or type 1 diabetes). (ii) Linearity, by which it can be tested whether the measured peptide in unknown samples dilutes in parallel with the IGF-I standards of the assay (i.e. that similar levels of IGF-I are obtained in different dilutions). (iii) Cross-reactivity and interference from IGF-II and IGFBPs. Cross-reactivity can be tested by measuring buffer solutions containing increasing amounts of each peptide. Ideally, the signal should remain in the range of the nonspecific signal. Interference can be investigated by adding increasing amounts of peptide to samples with known IGF-I concentrations. A deviation of the results from that obtained in control samples (no addition) represents interference. Both IGF-I standards and unknown samples should undergo this test. (iv) Reproducibility, which may be estimated by within assay and between assay CVs (SD/MEAN × 100%), calculated for IGF-I standards, unknown samples and long-term assay control samples. Long-term assay control samples are required to detect and correct assay drift, a phenomenon which is usually caused by loss of IGF-I standard potency, resulting in falsely increasing IGF-I levels in unknown samples. (v) Detection limit. It is common to state the detection limit of an assay, defined as the average plus three standard deviations of the signal corresponding to nonspecific binding. However, we believe it is more useful to state the lowest IGF-I concentration that can be measured in serum/plasma using a standard dilution.
Other validation steps that are related to the unknown samples include a comparison of various types of samples (serum, EDTA plasma, lithium heparin plasma and other biological fluids if relevant) – differences between results in for instance serum and plasma are often referred to as matrix effects. Also the effect of repetitive freezing and thawing on the signal obtained in the assay should be investigated. IGF-I is considered to be almost undegradable, whereas repetitive freezing and thawing is known to induce IGFBP-proteolysis that may indirectly affect the measurement of IGF-I [24]. Finally, each laboratory should determine the stability of processed samples following various storage periods (i.e. after samples have been extracted by acid–ethanol or treated by IGFBP-blocking reagents). In principle, all IGF-I assay developers (researchers as well as companies) should comply to this common set of assay validation steps, and we believe validation data should be made readily available in either publications or in the kit inserts.
3. Precautions in relation to collection of samples for IGF-I
IGF-I is considered to be a very stable peptide, both in vivo and after collection, and therefore it is generally believed that the endogenous levels of circulating IGF-I can be estimated by a single, randomly collected serum or plasma sample. However, collection and storage may affect the concentration of immunoreactive IGF-I levels and it is therefore important to discuss the procedures concerning sample collection and storage in order to optimize the quality of IGF-I measurements.
3.1. Circadian changes
In accordance with the pulsatile nature of its pituitary release, circulating GH levels show a marked circadian variation. Furthermore, the secretion of GH is highly influenced by numerous factors (body temperature, meal intake, physical activity, etc.) [25]. Consequently, the endogenous capacity to secrete GH can only be estimated accurately by repetitive sampling under standardized sample collection circumstances (e.g. during an oral glucose tolerance test, an insulin tolerance test, or by overnight profiling) [26–28]. In comparison to GH, circulating IGF-I levels demonstrate only minor circadian fluctuations. The majority of studies agree that circulating IGF-I levels remain relatively stable during the day time, being unaffected by meal intake as well as the type of meal [29–31], and similar observations have been made for IGF-II [32,33]. By contrast, circulating IGFBP-3 appears to be acutely influenced by meal intake as well as by the composition of carbohydrates [29,30,33]. The reason that IGFBP-3, but neither IGF-I nor IGF-II are affected by meal intake is unsolved, and it remains unknown whether circulating ALS levels can vary as do those of IGFBP-3.
There is evidence from some, but not all studies that circulating IGF-I level undergoes a nocturnal decline from midnight to 04:00 h, whereafter levels return to baseline [31,34]. These nocturnal fluctuations in circulating IGF-I may in part be explained by shifts in plasma volume. Accordingly, in healthy subjects the significant circadian fluctuations recorded for circulating IGF-I, ALS and IGFBP-3 all vanished after adjustment for serum total protein [32]. In summary, it is generally agreed that in daily clinical practice, no strict standardization of the sampling condition (time of day, fasting vs. non-fasting or type of meal) is required for collection of blood for IGF-I measurement. On the other hand, the nocturnal changes observed in some studies indicate that plasma IGF-I levels may be influenced by shifts in plasma volume, and this may be of importance in for instance patients treated with diuretics. Furthermore, there are data indicating that the day-time stability of IGF-I does not extend to IGFBP-3.
3.2. Short-term day to day variations in IGF-I levels
The lack of any major diurnal variation in circulating IGF-I levels, combined with the long half-life of ternary bound IGF-I and the absence of any major seasonal variation [31] has led to the concept that a single measurement of IGF-I is representative for an individual IGF-I level. Indeed, in two minor studies, repetitive samples collected six weeks (n = 24) [35] and eight weeks (n = 16) [36] apart have shown no significant difference in the individual IGF-I levels, although in the latter study the agreement of the two measurements as estimated by the correlation coefficient was as low as 0.65. However, the validity of a single IGF-I determination has been questioned in recent studies. Milani et al. compared overnight fasting plasma IGF-I levels collected at a two-week interval in healthy elderly subjects aged 50–90 years [37]. Despite highly correlated levels, the intra-individual variability was considerable, exceeding 10% in 29 subjects, 20% in 12 subjects and 30% in 5 subjects. Furthermore, in 40% of the study cohort the levels changed from one quartile to another. As this variability exceeded the assay imprecision (Nichols Advantage, intra-assay and inter-assay CV < 10%), these findings suggested the presence of a genuine biological within subject variability of IGF-I [37]. This idea has been confirmed in two recent studies. Nguyen et al. included more than 1100 elite athletes, who over a 2- to 3-week period donated three non-standardized serum samples for measurement of IGF-I, IGFBP-3 and ALS [38]. For all three GH dependent peptides, the between subject variation was estimated to constitute about 2/3 and the within subject variability about 1/3 of the total variability. It is noteworthy that most of the within subject variability appeared to be subject to biological variation rather than assay imprecision. When expressed as CVs, IGF-I, IGFBP-3 and ALS showed a within subject variability averaging 21%, 11% and 12%, respectively, whereas the corresponding assay CVs averaged 6%, 5% and 6%. Interestingly, a regression to the mean was observed: i.e. individuals with extreme values in the first samples tended to normalize in their subsequent samples [38]. Recently, Erotokritou-Mulligan et al. published a study that included 381 athletes (303 elites and 78 amateurs, and up to 4 fasting serum samples collected from each athlete over a period of up to one year) from four different centers. In that study, the within subject variability as estimated by the CV ranged from 14% to 16%, whereas the between subject variability ranged from 44% to 71% [39]. Fluctuations in IGF-I may be observed in even small study populations. In 10 healthy subjects having blood samples collected in the morning (fasting) and in the afternoon (non-fasting) for five consecutive weeks, the CVs of the 10 individual samples averaged 11% and 9% for IGF-I and IGF-II, respectively, whereas the corresponding assay CVs were <4% (data derived from [29]). The long-term within subject variability is nicely illustrated by Borofsky et al., [40] who followed two males for a 16-year period (Fig. 3). During that period an individual correlation analysis revealed an age-related decline in serum IGF-I, but as illustrated this decline cannot explain an up to twofold difference which was observed within a much shorter period of time.
Fig. 3.
IGF-I levels in two healthy male subjects, who were followed for 16 years with repetitive sampling [40]. Both subjects demonstrated a significant inverse relationship between serum IGF-I and age. However, as indicated by the dashed red and blue lines, there was considerable day-to-day variation that cannot be explained by the age-related decline. Thus, on top of the age-related decline, marked increases and reductions in serum IGF-I were observed within an interval of only a few years. Overall, IGF-I levels varied up to twofold in both subjects. Reprinted from Borofsky et al. [40] with permission from American Association for Clinical Chemistry.
In summary, there is no doubt that the inter-individual variability of circulating IGF-I is greater than the intra-individual variability, and therefore, circulating IGF-I fulfills an important criteria for being used as a biomarker of GH disorders and other health conditions. On the other hand, there is evidence of a significant biological variation of IGF-I, IGFBP-3 and ALS, at least in healthy subjects, and this should be kept in mind, in particular in cases with borderline IGF-I values. To what extent this variability is present in various patient populations remains to be investigated, but we believe that it is of at least a similar magnitude.
3.3. Pre-analytical storage conditions
Several studies have investigated the impact of pre-analytical sample handling and storage on immunoreactive IGF-I levels. These studies can be divided in two groups: (1) studies that have been conducted to illustrate how different ways of handling blood samples after collection at the bedside may influence the subsequent measurement of immunoreactive IGF-I and IGFBP-3; (2) studies that have been conducted to investigate the impact of long-term storage of frozen samples. Table 2 summarizes the current data on the “from patient to IGF-laboratory” handling of samples. As can be seen, there is a general agreement between the studies that immunoreactive IGF-I levels are fairly stable for several days, in particular when samples are centrifuged and serum/plasma separated shortly after collection (please compare Evans et al. [41] and Hartog et al. [42]). By contrast, in whole blood samples left without centrifugation, IGF-I levels appear to increase, as shown in two studies [42,43]. The mechanisms responsible for this increase in immunoreactive IGF-I levels are uncertain; release of IGF-I from blood cells has been suggested but this hypothesis remains unproven [42]. As regards IGFBP-3, the current data indicate that EDTA-plasma is preferred as storage media. Finally, it is evident that most studies of the pre-analytical handling of unfrozen samples for IGF-I determinations have been performed in rather small cohorts of healthy subjects, and consequently, we believe there is a demand for larger studies investigating samples from various patho-physiological conditions.
Table 2.
The effect of pre-analytical storage on immunoreactive IGF-I levels; unfrozen samples.
| Reference | Study population | Experimental protocol | Assay | Findings and comments |
|---|---|---|---|---|
| Holt et al. [67] | 9-10 HS | Samples (serum, LiH plasma or EDTA plasma) were (i) centrifuged and stored for up to 5 days at 4 °C or RT, or (ii) left un-centrifuged and stored for up to 5 days at 4 °Cor RT | IGF-I by Nichols IRMA or DSL IRMA | No significant changes. Overall mean intra-individual CV 5.5% vs. a CV of 3.9% when samples were immediately centrifuged and stored at −80 °C |
| Jane et al. [68] | 10 HS | EDTA blood samples kept at 4 °C or 24 °C for 0.5, 6 and 24 h before centrifugation and separation of plasma | IGF-I by in-house RIA IGFBP-3 by Nichols RIA | No significant changes |
| Evans et al. [41] | 6 HS | Whole blood collected in serum, EDTA plasma and LiH plasma tubes, centrifuged shortly after collection and stored at 4 °C or 30 °C for up to 120 h | IGF-I by in-house RIA, IGFBP-3 by Nichols RIA | IGF-I was stable (<10% change) for ≥114 h at 4 °C and ≥120 h at 30 °C. IGFBP-3 was stable ≥120 h at both temperatures |
| Harris et al. [43] | 11 subjects undergoing diagnostic CAG and 1 HS | Tubes containing blood were centrifuged immediately and serum frozen, or stored at RT for 2, 4, 10 or 24 h before freezing. One tube was kept at RT for 24 h before centrifugation and freezing | IGF-I, free IGF-I and IGFBP-3 by DSL ELISAs | IGF-I and IGFBP-3 stable in serum when centrifuged immediately after collection. In whole blood kept for 24 h IGF-I increased by +7% and IGFBP-3 by +20% Free IGF-I increased with increasing incubation length |
| Kristal et al. [69] | 40 HS | EDTA plasma samples were either immediately centrifuged or centrifuged after combined storage at 5 °C and packing in shipping containers equipped with frozen gel packs for 8 + 32 h, 24 + 48 h and 72 + 72 h. The temperature in the containers increased gradually to 21 °C at the end of the experiment | IGF-I and IGFBP-3 by DSL ELISAs | Significantly decreased levels at 172 h; IGF-I by 6%, IGFBP-3 by 3% |
| Elmlinger et al. [44] | 12 HS | LiH whole blood, serum and plasma incubated at 4, 22 and 3 °C, respectively, for up to 72 h | Immulite IGF-I and IGFBP-3 assays | LiH whole blood: IGF-I stable for 72 h at 4 and 22 °C, but only for 24 h at 37 °C. Serum: IGF-I stable for 72 h at all temperatures IGFBP-3 stable in LiH whole blood and serum for 72 h irrespective of temperature |
| Hartog et al. [42] | 10 HS | Whole blood collected in serum, EDTA and LiH tubes, and stored at RT for 15 min (control) and for 2, 8, 72, 168 and 336 h (i.e. up to 14 days) before processing (centrifugation and storage at –80 °C) | Immulite IGF-I and IGFBP-3 assays | IGF-I was stable in EDTA tubes, but increased in serum and LiH tubes by ~15% after 24 h, and by ~40% at end of study. IGFBP-3 levels fluctuated more in all three media, but were fairly stable in EDTA and LiH tubes for up to 7 days |
| Kouanda et al. [70] | 11 infants | Blood samples collected on filter paper for 1-2 weeks at 5 °C or at RT were compared to serum stored at –20 °C | In-house RIA | Significant positive correlations between freshly collected samples and samples stored on filter paper at 4 °C(r2 = 0.70) and at RT (r2 = 0.90) |
Abbreviations: HS (healthy subjects); RT (room temperature); LiH (lithium heparin); CAG (coronary angiography). Only data in serum, EDTA plasma and LiH plasma have been included.
IGF-I appears to be rather stable during long-term storage. Studies in healthy subjects have shown stability of IGF-I and IGFBP-3 levels within the same individuals when freshly collected serum samples were compared with samples stored at −25 °C for up to 12 months [44]. Even longer storage stability has been indicated by a Japanese study, which found no changes in IGF-I, IGF-II or IGFBP-3 levels in samples stored at −80 °C for 8 years [45]. However, different assays may yield highly variable results in long-term stored samples. Thus, by comparing different commercial assays, Khosravi et al. found highly correlated IGF-I levels in freshly collected samples from normal subjects (n = 61) and pregnant women (n = 20) assayed within one week after collection and storage at −70 °C, and in EDTA plasma samples stored for 2 years at −80 °C, but poorly correlated levels in serum samples (n = 84) that had been repetitively thawed and refrozen during ~8 years of storage at −20 °C. This variability was also present in freshly collected samples incubated for 28 days at 4 °C in the absence, but not in the presence of enzyme inhibitors, and therefore, it was suggested that IGF-I was susceptible to post-sampling degradation [24]. Although IGF-I is indeed degradable when exposed to intestinal enzymes [46], the authors never proved the existence of degraded IGF-I protein and therefore, it cannot be excluded that the lack of agreement between the assays reflects IGFBP-proteolysis. Nevertheless, the finding by Khosravi et al. [24] illustrated that long-term storage at −20 °C combined by repetitive thawing and freezing may affect IGF-I levels in an assay-dependent manner.
3.4. Serum versus plasma samples
There is no consensus regarding the preferred blood carrier medium when it comes to determination of circulating IGF-I levels. However, the most common carrier media appears to include serum, EDTA plasma and lithium heparin plasma. This raises the question whether levels obtained in one carrier medium are fully comparable to levels obtained in another media. So far, this issue has been investigated in only a few studies. Yu et al. compared serum, EDTA plasma and lithium heparin in 26 healthy subjects, and found strongly correlated IGF-I levels (correlation coefficient ≥ 0.95), although serum yielded significantly (+10%) higher IGF-I and IGF-II levels than the two corresponding plasma media [47]. Similarly, in 12 healthy subjects, Elmlinger et al. found 11% higher IGF-I and 8% higher IGFBP-3 levels in serum than in lithium heparin plasma [44]. However, as pointed out by Renehan et al., the appropriate way to compare agreement between assays is by the Bland–Altman method, which they employed in a comparison of freshly collected serum, EDTA plasma and lithium heparin samples from 47 healthy subjects [48]. For both IGF-I and IGF-II, the correlation analysis showed highly significant correlations when comparing levels in the three types of samples. However, Bland–Altman plots revealed significant discrepancies when comparing EDTA samples with either serum or lithium heparin, a finding which points to the necessity of taking the carrier medium into account, and avoiding the comparison of different types of samples within the same study cohort [49].
3.5. Repetitive freezing and thawing of IGF-I
IGF-I and IGFBP-3 are relatively stable peptides and no changes have been observed following repeated thawing and freezing of serum samples up to 7 times [47,50].
3.6. The requirements for generating IGF-I reference ranges
Comprehensive reference ranges for IGF-I are equally important as the availability of validated, high-quality IGF-I assays. Still, the requirements for generating an acceptable reference material for circulating IGF-I have never been uniformly defined [11]. As outlined below, there are various factors that should be considered when collecting samples for the ideal reference material.
Number of subjects
As mentioned above, circulating IGF-I values demonstrate considerable variation between individuals and clearly this degree of variability increases the need for an adequate number of subjects within the reference population. This issue has been highlighted by previous findings: Massart and Poirier [51] showed that in treated acromegalic patients, the interpretation of a single IGF-I measurement as either normal or abnormal was highly dependent on the number of subjects that was used to formulate the reference ranges rather than on a specific method. A similar dependence on the number of subjects included in the reference ranges has also been made for the utility of IGF-I in the diagnosis of GH deficiency [52].
Age relationship
It is well-recognized that circulating IGF-I is closely associated with age. During childhood, mean IGF-I levels slowly increase. At the onset of puberty, circulating IGF-I increases in parallel with the pubertal growth spurt and mean levels may increase more than fivefold when compared to early childhood. After adolescence, serum IGF-I slowly declines reaching childhood values in aged subjects (>80 years) [31,53]. Accordingly, there is a consensus that it is mandatory to use age-matched IGF-I reference ranges when employing IGF-I in the management of GH disorders [26,27,54]. However, as recently reviewed by Clemmons, a uniform standard for the optimal age range has never been established. Nevertheless, it is generally appreciated that the marked changes in circulating IGF-I taking place in the years surrounding puberty necessitates narrow age-intervals, whereas in adults of 30 years and more, grouping by decade may be sufficient [11].
Gender differences
The GH Research Society recommends the use of gender-specific IGF-I reference ranges [26,27,54]. When addressing levels of IGF-I in children and adolescents, this appears as a reasonable requirement, as IGF-I levels in girls are slightly higher during childhood and peak approximately 1 year earlier than in boys (14½ vs. 15½ years) [31]. In children aged between 1 and 17 years, a direct comparison between genders revealed that mean IGF-I levels are approximately 42 μg/l higher in girls than in boys of comparable age [53]. However, in adults the impact of gender on circulating IGF-I is less settled. Many smaller studies have failed to show any major impact of gender [31], and only when a large number of individuals are included, a significant gender difference is uncovered. Thus, in the Nichols Advantage reference material containing almost 2500 adults (≥20 years), Brabant et al. were able to show that mean levels of IGF-I were on average 12 μg/l higher in men than in women (Fig. 4) [53].
Fig. 4.
Age- and gender-specific IGF-I levels in healthy subjects. Serum IGF-I as measured by the Nichols Advantage IGF-I in a multicenter cohort including more than 3,900 healthy subjects (~2200 males and 1700 males). This study illustrates that serum IGF-I peaks earlier in girls than in boys, and that gender-specific reference ranges are necessary in children. However, in adults (+20 years) the gender difference in IGF-I was modest, averaging 12 μg/l. Reprinted from Brabant et al. [53] with permission from Karger AG.
Ethnicity
Recent data have indicated that ethnicity should be taken into account when generating reference ranges for IGF-I. This is not surprising when considering the relationship between genetics and circulating IGF-I levels. Twin studies have estimated that genetics may explain 38–63% of the circulating IGF-I levels [55,56], and several studies have reported a positive association between circulating IGF-I and height, which is considered to be genetically controlled [57].
Many studies have confirmed that ethnicity may indeed influence circulating levels of IGF-I and IGFBP-3, among others [31,58,59]. In particular two recent studies are of interest. In a longitudinal study, Casazza et al. compared African-American (AA) and European-American (EA) children from Tanner Stage (TS) 1 through 5 and found higher levels of IGFBP-3 in EA children throughout puberty as compared to AA children, whereas their IGF-I levels only differed at TS 1 (Fig. 5) [60]. In adults, ethnic differences appear to persist. Thus, a recent large cross sectional study based on almost 6000 subjects from the NHANES cohort found 14–18% higher levels of IGF-I in white and black Non-Hispanic American males as compared to Mexican–American males, whereas in women, Non-Hispanic females had 22% higher IGF-I levels than Mexican–Americans [61] (Fig. 6). In that study differences in IGFBP-3 were also noted (Fig. 6). In summary, we believe ethnicity needs to be taken into account, when generating reference ranges, and ideally, the ethnic composition of the reference material should reflect the society from where the patients originate.
Fig. 5.
The effect of ethnicity on serum IGF-I and IGFBP-3. In a longitudinal design, 162 African-American (AA, n = 72, black columns) and European-American (EA, n = 90, gray columns) were followed from Tanner Stage (TS) 1 through TS 5. Ethnic differences were evident for IGF-I in TS 1, and in all five TS for IGFBP-3, thereby illustrating the existence of genuine ethnic differences in the IGF-system. Reprinted from Casazza et al. [60] with permission from Endocrine Society, US.
Fig. 6.
The effect of ethnicity on serum IGF-I and IGFBP-3 in males (A, C) and females (B, D). Data are based on overnight fasting levels determined in 6061 adults of different ethnicity (Non-Hispanic whites, Hispanics, Non-Hispanic Blacks) contained within the US National Health and Nutrition Examination Survey III (NHANES III) study cohort. Reprinted from Berrigan et al. [61] with permission from Elsevier.
Body mass index
Obesity has marked effects on the GH-IGF-I axis. Thus, obesity suppresses the secretion of GH, but increases at the same time the hepatic sensitivity to GH [62]. The latter may be considered as a compensatory mechanism that enables maintenance of normal IGF-I levels despite a GH secretion which approaches hypopituitary levels. However, normal levels of IGF-I can only be maintained within certain BMI limits. This is clear from at least two studies including 250 subjects [63] and close to 4000 subjects [64], which both have shown a curvilinear relationship between BMI and circulating IGF-I levels. The findings of the latter study are depicted in Fig. 7. As can be seen, serum IGF-I starts to decrease at a BMI exceeding 32.5 kg/m2 in women and the same tendency, albeit less pronounced, is observed in males. Similarly, in the low BMI range, where chronic under-nutrition may be a problem, levels of IGF-I are decreased, and again the most predominant change was in women. Most likely, the gender differences observed in the extreme BMI groups are reflecting the number of participants with either high or low BMIs (893 women vs. 250 men) rather than a genuine gender difference.
Fig. 7.
The effect of obesity as estimated by body mass index (BMI) on serum IGF-I levels. Cross-sectional samples from 3983 subjects (2377 females and 1606 males) contained in the DETECT cohort. Subjects had no history of cancer, diabetes, kidney or liver diseases, and received no sex hormone therapy. Date are IGF-I levels expressed as BMI specific IGF-I mean standard deviation score (SDS) using the group with the highest IGF-I SDS mean as reference (ref). Data are separated in females (red lines) and males (blue lines). The asterisks denote statistically differences when compared to the gender-specific reference group. Data are derived from Table 2 in Schneider et al. [64] and reprinted with permission from European Journal of Endocrinology.
Numerous factors other than age, gender, ethnicity and BMI may impact on circulating IGF-I levels, e.g. thyroid hormones, cortisol, sex steroids, insulin sensitivity and level of fitness [11]. However, one needs a simple approach in order to be able to establish large population based reference ranges, and therefore we believe it is more important to restrict the inclusion criteria to simple but highly relevant parameters such as age, gender, ethnicity and BMI, which can all be determined at bedside without additional biochemical measurements.
4. Recommendations for future IGF-I immunoassays – suggestions by the authors
The interest in IGF-I has increased dramatically since its discovery more than 50 years ago in highly specialized research laboratories, and today, clinicians as well as researchers share a worldwide interest in having access to reliable and uniform IGF-I determinations. Therefore, it is important to agree on internationally accepted guidelines for the determination of IGF-I. In our view such guidelines should include all aspects of the measurement of IGF-I, from collection of blood through its analysis, i.e. from bedside to lab bench; only in this way will it be possible to obtain more uniform data. Table 3 contains an agenda for issues that should be considered when developing the guidelines.
Table 3.
Suggested points to be discussed when considering guidelines for IGF-I measurements.
| Issue | Points to discuss |
|---|---|
| Technical aspects of IGF-I assay |
The assay validation should include public and readily accessible data on – Preferred methods to eliminate IGFBP interference – Recovery – Linearity – Cross-reactivity and interference – Reproducibility (with-in assay and between assay CVs) – Operational range using standard dilutions – Calibration accordingly to the novel WHO IRR 02/254 – Comparability of serum and different types of plasma – Stability of processed samples (i.e. IGFBP-blocked or IGFBP-extracted samples) |
| Collection of blood |
Standardization in regards to collection of blood – What time of the day should blood be collected? – Is fasting required or indicated? – How quickly after collection should blood be centrifuged? – What are the requirements for transport of blood samples to the laboratory? |
| Generation of reference ranges |
Requirements for valid reference ranges – How narrow should the age ranges be in children, adolescents and adults? – How many subjects are required in each of the age ranges? – Are gender differences considered important after puberty? – Should ethnicity be considered? – What BMI ranges are acceptable? |
Footnotes
5. Disclosures
The authors have nothing to disclose.
References
- 1.Van den Brande JL. A personal view on the early history of the insulin-like growth factors. Horm. Res. 1999;51(Suppl. 3):149–175. doi: 10.1159/000053178. [DOI] [PubMed] [Google Scholar]
- 2.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 3.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr. Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 4.Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat. Rev. Drug Discov. 2007;6:821–833. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- 5.LeRoith D, Yakar S. Mechanisms of disease: metabolic effects of growth hormone and insulin-like growth factor 1. Nat. Clin. Pract. Endocrinol. Metab. 2007;3:302–310. doi: 10.1038/ncpendmet0427. [DOI] [PubMed] [Google Scholar]
- 6.Carro E, Torres-Aleman I. Serum insulin-like growth factor I in brain function. Keio J. Med. 2006;55:59–63. doi: 10.2302/kjm.55.59. [DOI] [PubMed] [Google Scholar]
- 7.Clemmons DR. Value of insulin-like growth factor system markers in the assessment of growth hormone status. Endocrinol. Metab. Clin. North Am. 2007;36:109–129. doi: 10.1016/j.ecl.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Furlanetto RW, Underwood LE, Van Wyk JJ, D'Ercole AJ. Estimation of somatomedin-C levels in normals and patients with pituitary disease by radioimmunoassay. J. Clin. Invest. 1977;60:648–657. doi: 10.1172/JCI108816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J. Biol. Chem. 1978;253:2769–2776. [PubMed] [Google Scholar]
- 10.Daughaday WH, Parker KA, Borowsky S, Trivedi B, Kapadia M. Measurement of somatomedin-related peptides in fetal, neonatal, and maternal rat serum by insulin-like growth factor (IGF) I radioimmunoassay, IGF-II radioreceptor assay (RRA), and multiplication-stimulating activity RRA after acid-ethanol extraction. Endocrinology. 1982;110:575–581. doi: 10.1210/endo-110-2-575. [DOI] [PubMed] [Google Scholar]
- 11.Clemmons DR. IGF-I assays: current assay methodologies and their limitations. Pituitary. 2007;10:121–128. doi: 10.1007/s11102-007-0032-z. [DOI] [PubMed] [Google Scholar]
- 12.Khosravi J, Diamandi A, Mistry J, Krishna RG. The high molecular weight insulin-like growth factor-binding protein complex: epitope mapping, immunoassay, and preliminary clinical evaluation. J. Clin. Endocrinol. Metab. 1999;84:2826–2833. doi: 10.1210/jcem.84.8.5914. [DOI] [PubMed] [Google Scholar]
- 13.Frystyk J. Free insulin-like growth factors – measurements and relationships to growth hormone secretion and glucose homeostasis. Growth Horm. IGF Res. 2004;14:337–375. doi: 10.1016/j.ghir.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Chen JW, Ledet T, Ørskov H, et al. A highly sensitive and specific assay for determination of IGF-I bioactivity in human serum. Am. J. Physiol. Endocrinol. Metab. 2003;284:E1149–E1155. doi: 10.1152/ajpendo.00410.2002. [DOI] [PubMed] [Google Scholar]
- 15.Lotton C, Rodrigue D, Elie C, et al. Akt phosphorylation in lymphocytes provides an index of in vitro insulin-like growth factor I sensitivity associated with growth hormone-induced growth. J. Clin. Endocrinol. Metab. 2008;93:1458–1463. doi: 10.1210/jc.2007-2575. [DOI] [PubMed] [Google Scholar]
- 16.Frystyk J. Utility of free IGF-I measurements. Pituitary. 2007;10:181–187. doi: 10.1007/s11102-007-0025-y. [DOI] [PubMed] [Google Scholar]
- 17.Berson SA, Yalow RS. General principles of radioimmunoassay. Clin Chim. Acta. 2006;369:125–143. doi: 10.1016/j.cca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Frystyk J, Dinesen B, Ørskov H. Non-competitive time-resolved immunofluorometric assays for determination of human insulin-like growth factor I and II. Growth Reg. 1995;5:169–176. [PubMed] [Google Scholar]
- 19.Khosravi MJ, Diamandi A, Mistry J, Lee PD. Noncompetitive ELISA for human serum insulin-like growth factor-I. Clin. Chem. 1996;42:1147–1154. [PubMed] [Google Scholar]
- 20.Krebs A, Wallaschofski H, Spilcke-Liss E, et al. Five commercially available insulin-like growth factor I (IGF-I) assays in comparison to the former Nichols Advantage IGF-I in a growth hormone treated population. Clin. Chem. Lab. Med. 2008;46:1776–1783. doi: 10.1515/CCLM.2008.349. [DOI] [PubMed] [Google Scholar]
- 21.Cowan DA, Bartlett C. Laboratory issues in the implementation of the marker method. Growth Horm. IGF. Res. 2009;19:357–360. doi: 10.1016/j.ghir.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Burns C, Rigsby P, Moore M, Rafferty B. The first international standard for insulin-like growth factor i (IGF-I) for immunoassay: preparation and calibration in an international collaborative study. Growth Horm. IGF Res. 2009;19:457–462. doi: 10.1016/j.ghir.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Braggio S, Barnaby RJ, Grossi P, Cugola M. A strategy for validation of bioanalytical methods. J. Pharma. Biomed. Anal. 1996;14:375–388. doi: 10.1016/0731-7085(95)01644-9. [DOI] [PubMed] [Google Scholar]
- 24.Khosravi J, Diamandi A, Bodani U, Khaja N, Krishna RG. Pitfalls of immunoassay and sample for IGF-I: comparison of different assay methodologies using various fresh and stored serum samples. Clin. Biochem. 2005;38:659–666. doi: 10.1016/j.clinbiochem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr. Rev. 1998;19:717–797. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- 26.Ho KKY, on behalf of the 2007 GH Deficiency Consensus Workshop Participants Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH research society in association with the European society for pediatric endocrinology, Lawson Wilkins society, European society of endocrinology, Japan endocrine society, and endocrine society of Australia. Eur. J. Endocrinol. 2007;157:695–700. doi: 10.1530/EJE-07-0631. [DOI] [PubMed] [Google Scholar]
- 27.Melmed S, Casanueva FF, Cavagnini F, et al. Guidelines for acromegaly management. J. Clin. Endocrinol. Metab. 2002;87:4054–4058. doi: 10.1210/jc.2002-011841. [DOI] [PubMed] [Google Scholar]
- 28.Giustina A, Barkan A, Chanson P, et al. Guidelines for the treatment of growth hormone excess and growth hormone deficiency in adults. J. Endocrinol. Invest. 2008;31:820–838. doi: 10.1007/BF03349263. [DOI] [PubMed] [Google Scholar]
- 29.Belobrajdic DP, Priebe IK, Forbes B, et al. Assessing the potential usefulness of IGF-related peptides and adiponectin for predicting disease risk. Growth Horm. IGF Res. 2008;18:198–204. doi: 10.1016/j.ghir.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Brand-Miller JC, Liu V, Petocz P, Baxter RC. The glycemic index of foods influences postprandial insulin-like growth factor-binding protein responses in lean young subjects. Am. J. Clin. Nutri. 2005;82:350–354. doi: 10.1093/ajcn.82.2.350. [DOI] [PubMed] [Google Scholar]
- 31.Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm. IGF Res. 2003;13:113–170. doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 32.Frystyk J, Nyholm B, Skjærbæk C, Baxter RC, Schmitz O, Ørskov H. The circulating IGF-system and its relationship with 24-hour glucose regulation and insulin sensitivity in healthy subjects. Clin. Endocrinol. (Oxf) 2003;58:777–784. doi: 10.1046/j.1365-2265.2003.01791.x. [DOI] [PubMed] [Google Scholar]
- 33.Yeoh SI, Baxter RC. Metabolic regulation of the growth hormone independent insulin-like growth factor binding protein in human plasma. Acta Endocrinol. (Copenh) 1988;119:465–473. doi: 10.1530/acta.0.1190465. [DOI] [PubMed] [Google Scholar]
- 34.Skjærbæk C, Frystyk J, Kaal A, et al. Circadian variation in serum free and total insulin-like growth factor (IGF)-I and IGF-II in untreated and treated acromegaly and growth hormone deficiency. Clin. Endocrinol. (Oxf) 2000;52:25–33. doi: 10.1046/j.1365-2265.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- 35.Goodman-Gruen D, Barrett-Connor E. Epidemiology of insulin-like growth factor-I in elderly men and women. The Rancho Bernardo study. Am. J. Epidemiol. 1997;145:970–976. doi: 10.1093/oxfordjournals.aje.a009065. [DOI] [PubMed] [Google Scholar]
- 36.Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 37.Milani D, Carmichael JD, Welkowitz J, et al. Variability and reliability of single serum IGF-I measurements: impact on determining predictability of risk ratios in disease development. J. Clin. Endocrinol. Metab. 2004;89:2271–2274. doi: 10.1210/jc.2003-032150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen TV, Nelson AE, Howe CJ, et al. Within-subject variability and analytic imprecision of insulin-like growth factor axis and collagen markers: implications for clinical diagnosis and doping tests. Clin. Chem. 2008;54:1268–1276. doi: 10.1373/clinchem.2008.105726. [DOI] [PubMed] [Google Scholar]
- 39.Erotokritou-Mulligan I, Eryl BE, Cowan D, et al. The use of growth hormone (GH)-dependent markers in the detection of GH abuse in sport: physiological intra-individual variation of IGF-I, type 3 pro-collagen (P-III-P) and the GH-2000 detection score. Clin. Endocrinol. (Oxf) 2009 Jul 24; doi: 10.1111/j.1365-2265.2009.03668.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Borofsky ND, Vogelman JH, Krajcik RA, Orentreich N. Utility of insulin-like growth factor-1 as a biomarker in epidemiologic studies. Clin. Chem. 2002;48:2248–2251. [PubMed] [Google Scholar]
- 41.Evans MJ, Livesey JH, Ellis MJ, Yandle TG. Effect of anticoagulants and storage temperatures on stability of plasma and serum hormones. Clin. Biochem. 2001;34:107–112. doi: 10.1016/s0009-9120(01)00196-5. [DOI] [PubMed] [Google Scholar]
- 42.Hartog H, van der Graaf WTA, Wesseling J, van der Veer E, Boezen HM. Measurement of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 after delayed separation of whole blood samples. Clin. Biochem. 2008;41:636–639. doi: 10.1016/j.clinbiochem.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Harris TG, Strickler HD, Yu H, et al. Specimen processing time and measurement of total insulin-like growth factor-I (IGF-I), free IGF-I, and IGF binding protein-3 (IGFBP-3) Growth Horm. IGF Res. 2006;16:86–92. doi: 10.1016/j.ghir.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Elmlinger MW, Zwirner M, Kuhnel W. Stability of insulin-like growth factor (IGF)-I and IGF binding protein (IGFBP)-3 measured by the IMMULITE automated chemiluminescence assay system in different blood specimens. Clin. Lab. 2005;51:145–152. [PubMed] [Google Scholar]
- 45.Ito Y, Nakachi K, Imai K, et al. Stability of frozen serum levels of insulin-like growth factor-I, insulin-like growth factor-II, insulin-like growth factor binding protein-3, transforming growth factor beta, soluble Fas, and superoxide dismutase activity for the JACC study. J. Epidemiol. 2005;15(Suppl. 1):S67–S73. doi: 10.2188/jea.15.S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderle P, Langguth P, Rubas W, Merkle HP. In vitro assessment of intestinal IGF-I stability. J. Pharm. Sci. 2002;91:290–300. doi: 10.1002/jps.10013. [DOI] [PubMed] [Google Scholar]
- 47.Yu H, Mistry J, Nicar MJ, et al. Insulin-like growth factors (IGF-I, free IGF-I and IGF-II) and insulin-like growth factor binding proteins (IGFBP-2, IGFBP-3, IGFBP-6, and ALS) in blood circulation. J. Clin. Lab. Anal. 1999;13:166–172. doi: 10.1002/(SICI)1098-2825(1999)13:4<166::AID-JCLA5>3.0.CO;2-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renehan AG, Jones J, Dwyer ST, Shalet SM. Determination of IGF-I, IGF-II, IGFBP-2, and IGFBP-3 levels in serum and plasma: comparisons using the Bland–Altman method. Growth Horm. IGF Res. 2003;13:341–346. doi: 10.1016/s1096-6374(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 49.Renehan AG, Zwahlen M, Minder PC, O'Dwyer ST, Shalet PS, Egger PM. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. The Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 50.Baxter RC, Martin JL. Radioimmunoassay of growth hormone-dependent insulinlike growth factor binding protein in human plasma. J. Clin. Invest. 1986;78:1504–1512. doi: 10.1172/JCI112742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Massart C, Poirier JY. Serum insulin-like growth factor-I measurement in the follow-up of treated acromegaly: comparison of four immunoassays. Clin. Chim. Acta. 2006;373:176–179. doi: 10.1016/j.cca.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 52.de Boer H, Blok GJ, Popp-Snijders C, van der Veen EA. Diagnosis of growth hormone deficiency in adults. The Lancet. 1994;343:1645–1646. doi: 10.1016/s0140-6736(94)93104-6. [DOI] [PubMed] [Google Scholar]
- 53.Brabant G, von zur MA, Wuster C, et al. Serum insulin-like growth factor I reference values for an automated chemiluminescence immunoassay system: results from a multicenter study. Horm. Res. 2003;60:53–60. doi: 10.1159/000071871. [DOI] [PubMed] [Google Scholar]
- 54.Giustina A, Barkan A, Casanueva FF, et al. Criteria for cure of acromegaly: a consensus statement. J. Clin. Endocrinol. Metab. 2000;85:526–529. doi: 10.1210/jcem.85.2.6363. [DOI] [PubMed] [Google Scholar]
- 55.Hong Y, Pedersen NL, Brismar K, Hall K, de FU. Quantitative genetic analyses of insulin-like growth factor I (IGF-I), IGF-binding protein-1, and insulin levels in middle-aged and elderly twins. J. Clin. Endocrinol Metab. 1996;81:1791–1797. doi: 10.1210/jcem.81.5.8626837. [DOI] [PubMed] [Google Scholar]
- 56.Harrela M, Koistinen H, Kaprio J, et al. Genetic and environmental components of interindividual variation in circulating levels of IGF-I, IGF-II, IGFBP-1, and IGFBP-3. J. Clin. Invest. 1996;98:2612–2615. doi: 10.1172/JCI119081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaessen N, Heutink P, Janssen JA, et al. A polymorphism in the gene for IGF-I: functional properties and risk for type 2 diabetes and myocardial infarction. Diabetes. 2001;50:637–642. doi: 10.2337/diabetes.50.3.637. [DOI] [PubMed] [Google Scholar]
- 58.DeLellis K, Rinaldi S, Kaaks RJ, Kolonel LN, Henderson B, Le ML. Dietary and lifestyle correlates of plasma insulin-like growth factor-I (IGF-I) and IGF binding protein-3 (IGFBP-3): the multiethnic cohort. Cancer Epidemiol. Biomarkers. Prev. 2004;13:1444–1451. [PubMed] [Google Scholar]
- 59.Cruickshank JK, Heald AH, Anderson S, et al. Epidemiology of the insulin-like growth factor system in three ethnic groups. Am. J. Epidemiol. 2001;154:504–513. doi: 10.1093/aje/154.6.504. [DOI] [PubMed] [Google Scholar]
- 60.Casazza K, Higgins PB, Fernandez JR, Goran MI, Gower BA. Longitudinal analysis of the insulin-like growth factor system in African-American and European American children and adolescents. J. Clin. Endocrinol. Metab. 2008;93:4917–4923. doi: 10.1210/jc.2008-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berrigan D, Potischman N, Dodd KW, et al. Race/ethnic variation in serum levels of IGF-I and IGFBP-3 in US adults. Growth Horm. IGF Res. 2009;19:146–155. doi: 10.1016/j.ghir.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gleeson HK, Lissett CA, Shalet SM. Insulin-like growth factor-I response to a single bolus of growth hormone is increased in obesity. J. Clin. Endocrinol. Metab. 2005;90:1061–1067. doi: 10.1210/jc.2004-0501. [DOI] [PubMed] [Google Scholar]
- 63.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab. 2006;17:328–336. doi: 10.1016/j.tem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Schneider HJ, Saller B, Klotsche J, et al. Opposite associations of age-dependent insulin-like growth factor-I standard deviation scores with nutritional state in normal weight and obese subjects. Eur. J. Endocrinol. 2006;154:699–706. doi: 10.1530/eje.1.02131. [DOI] [PubMed] [Google Scholar]
- 65.Elmlinger MW, Kuhnel W, Weber MM, Ranke MB. Reference ranges for two automated chemiluminescent assays for serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 (IGFBP-3) Clin. Chem. Lab. Med. 2004;42:654–664. doi: 10.1515/CCLM.2004.112. [DOI] [PubMed] [Google Scholar]
- 66.Gläser S, Friedrich N, Ewert R, et al. Association between serum insulin-like growth factor (IGF) I and IGF binding protein-3 and lung function. J. Clin. Endocrinol. Metab. 2009;94:2452–2458. doi: 10.1210/jc.2008-2662. [DOI] [PubMed] [Google Scholar]
- 67.Holt RI, Erotokritou-Mulligan I, Ridley SA, et al. A determination of the pre-analytical storage conditions for insulin like growth factor-I and type III procollagen peptide. Growth Horm. IGF Res. 2009;19:43–50. doi: 10.1016/j.ghir.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Jane EM, Livesey JH, Evans MJ. Hormone stability in human whole blood. Clin. Biochem. 2003;36:109–112. doi: 10.1016/s0009-9120(02)00440-x. [DOI] [PubMed] [Google Scholar]
- 69.Kristal AR, King IB, Albanes D, et al. Centralized blood processing for the selenium and vitamin E cancer prevention trial: effects of delayed processing on carotenoids, tocopherols, insulin-like growth factor-I, insulin-like growth factor binding protein 3, steroid hormones, and lymphocyte viability. Cancer Epidemiol. Biomarkers. Prev. 2005;14:727–730. doi: 10.1158/1055-9965.EPI-04-0596. [DOI] [PubMed] [Google Scholar]
- 70.Kouanda S, Tonglet R, De C, et al. Reference values of IGF-I in children from birth to 5 years of age, in Burkina Faso, using blood samples on filter paper. Growth Horm. IGF Res. 2008;18:345–352. doi: 10.1016/j.ghir.2008.01.008. [DOI] [PubMed] [Google Scholar]