Abstract
Background
One health is a flexible concept with many facets, including the environment, community, and the nosocomial super-bacteria resistance network. We investigated the molecular prevalence of extended-spectrum-β-lactamase-producing Escherichia coli (ESBL-EC) in workers, livestock, and the farm environment in Korea.
Methods
ESBL-EC isolates were obtained from samples from 19 swine farms, 35 retail stores, seven slaughterhouses, and 45 related workers throughout Korea from August 2017 to July 2018, using ChromID ESBL (BioM?rieux, Marcy l’Etoile, France) agar and enrichment broth. The presence of ESBL and mobilized colistin resistance (mcr) genes and antimicrobial resistance were determined. Clonality was evaluated with pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST).
Results
In total, 232 ESBL-EC isolates were obtained from 1,614 non-duplicated samples (14.4% positive rate). The ESBL-EC isolates showed regional and source-related differences. blaCTX-M-55 (N=100), blaCTX-M-14 (N=65), blaCTX-M-15 (N=33), and blaCTX-M-65 (N=23) were common ESBL types. The ESBL-EC isolates showed high resistance rates for various antimicrobial classes; however, all isolates were susceptible to carbapenem. One swine-originating colistin-resistant isolate did not carry any known mcr gene. PFGE was successful for 197 of the 232 isolates, and most PFGE types were heterogeneous, except for some dominant PFGE types (O, R, T, U, and V). MLST of 88 isolates was performed for representative PFGE types; however, no dominant sequence type was observed.
Conclusions
The proportion of ESBL-EC in swine industry-related samples was significant, and the isolates harbored common clinical ESBL gene types. These molecular epidemiologic data could provide important evidence for antimicrobial-resistance control through a one health approach.
Keywords: Extended-spectrum-β-lactamase, Escherichia coli, Antimicrobial resistance, One health, Swine, Mobilized colistin resistance
INTRODUCTION
Antimicrobial resistance (AMR) is a major challenge that requires multi-sectional efforts, such as those relating to human and veterinary medicine, agricultural sciences, epidemiology, and economics [1]. One health is a broad and flexible concept with various facets, such as the environment, community, and nosocomial super-bacteria AMR network [2]. Bacteria from livestock may be carriers of clinically relevant resistance genes for veterinary and human antimicrobials. The exposure to multidrug-resistant bacteria during farming, slaughtering, and distribution may cause transmission to occupationally exposed workers. Therefore, elucidating the current epidemiology and transmission mode of antimicrobial resistant bacteria among humans, animals, and environments is very important to establish an effective strategy for the controlling AMR.
Although a recent domestic study reported the isolation of CTX-M-55 or CTX-M-65-producing Escherichia coli from duck fecal and carcass samples [3], limited information is available on the current molecular epidemiology of extended-spectrum-βlactamase (ESBL)-producing E. coli (ESBL-EC) in the Korean veterinary sector. This information on ESBL-EC among workers, livestock, animal products, and farm environment would be useful for identifying sources of AMR.
A study was launched in 2017 with support from the Korea Centers for Disease Control and Prevention wherein we evaluated the prevalence of third generation-cephalosporinand colistin-resistant E. coli in workers, animals (swine, cows, and chickens), and farm environments. This study aimed to provide information on how the AMR transmission mechanisms among the three sectors (human-animal-environment) could be used to introduce a monitoring system and develop an effective control strategy This is the first study to evaluate the current molecular epidemiology of AMR and cover all steps, from production to sales, in the swine industry.
MATERIALS AND METHODS
Sample collection
A total of 1,614 non-duplicated samples were prospectively collected from 19 swine farms, 35 retail stores, and seven slaughterhouses in five administrative districts throughout Korea from August 2017 to July 2018. The samples included swabs from the following: nose, skin (groin region), rectum, and stool of swine; a pigsty fence, floor, and ventilation fan and soil surrounding a pigsty; pooled human stool in a toilet; knives, cutting boards, and slaughterhouse floor. Additionally, 0.5–1 L of livestock wastewater was collected; 10 g of pork meat was sampled in the slaughterhouses. Pork for sale, knives, cutting boards, and showcases in retail stores were also sampled using a swab. We also obtained swab samples from the nose, groin, axillary, antecubital fossa, inter-finger spaces (both hands), and stool of 45 workers after obtaining written informed consent.
The feces of livestock and workers were collected in stool boxes, and the soil was collected in sterilized bags. Swabbed samples were collected with sterilized cotton swabs and put into a transport medium (Copan diagnostics, Murrieta, CA, USA). Analysis was performed at Research Institute of Bacterial Resistance, Yonsei University College of Medicine. Samples were stored at 4°C before analysis and inoculated in culture media within 24-h of sampling. This study was approved by the Institutional Review Board of National Health Insurance Service Ilsan Hospital, Goyang, Korea (NHIMC 2017-07-041).
Microbiological study
A total of 25 g of soil surrounding a pigsty was mixed with 225 mL of buffered peptone water (BD Biosciences, San Jose, CA, USA) and incubated at 36 ± 1°C for 18 hours. After the sewage was filtered with a 0.2 μm filter, the content on the surface of the filter was inoculated into Mueller-Hinton (MH) broth and incubated at 36 ± 1°C for 18–24 hours. Other environmental samples were inoculated into 10 mL of MH broth and incubated at 36 ± 1°C for 18–24 hours. In addition, 10 g of pork meat was placed in 10 mL of MH broth and incubated at 36 ± 1°C for 18– 24 hours. After enrichment, 10–100 μL of the liquid culture was used to inoculate ChromID ESBL (BioMérieux, Marcy l’Etoile, France) agar to screen for ESBL-EC. Other swab samples from swine and workers were directly inoculated onto ChromID ESBL agar and incubated at 36 ± 1°C for 18–24 hours. E. coli was identified using the matrix-assisted laser desorption/ionization Biotyper system (Bruker Daltonik, Bremen, Germany).
The disk diffusion method and broth microdilution (BMD) were performed for testing AMR. Briefly, fresh grown E. coli, at a turbidity of 0.5 McFarland standard, was used to swab inoculate the surface of an MH agar plate (Oxoid, Basingstoke, UK). After the plate was allowed to stand for 15 minutes at 20–22°C, antimicrobial disks (Becton Dickinson, Franklin Lakes, NJ, USA) were placed in it and incubated overnight. Tested disks contained ampicillin, piperacillin, ampicillin-sulbactam, cefazolin, cefoxitin, cefepime, cefotaxime, ceftazidime, azteronam, imipenem, meropenem, ertapenem, amikacin, gentamicin, and ciprofloxacin. The inhibition zone diameter was interpreted according to the CLSI criteria [4]. To detect colistin resistant isolates, test organisms were screened on MH agar plate containing colistin (0, 1, 2, and 4 μg/mL) with the E. coli ATCC25922 strain. If minimal inhibitory concentration (MIC) was > 2 mg/L, the isolate was regarded as a colistin-resistant organism according to the CLSI breakpoints for P. aeruginosa and Acinetobacter spp., because there are no CLSI breakpoints for Enterobacteriaceae [4].
Molecular study
All isolates resistant to cefotaxime or ceftazidime were analyzed by PCR and sequencing for ESBL genes (blaTEM, blaSHV, and blaCTX-M), according to previous methods [5]. We used PCR annealing temperatures of 59°C (TEM), 61°C (SHV), and 56°C (CTX-M) to perform PCR using DNA Engine Tetrad 2 Peltier Thermal Cycler (Bio-Rad, Hercules, CA, USA). The PCR products were then subjected to direct sequencing using an automatic sequencer (ABI PRISM 3730XL analyzer, Applied Biosystems, Norwalk, CT, USA) and BigDye terminator v3.1 cycle sequencing kit (Applied Biosystems). Isolates resistant to colistin were analyzed by PCR and sequencing of the mobilized colistin resistance genes (mcr-1, mcr-2, mcr-3, mcr-4, and mcr-5), following previous methods [6, 7].
Pulsed-field gel electrophoresis (PFGE) was performed as described previously [8], and the patterns were analyzed using InfoQuest FP software (Bio-Rad) with stored isolates (-70°C in skim milk). The dendrogram was generated based on the unweighted pair group method, with an arithmetic average from Dice’s coefficient with 1% band position tolerance and 0.5% optimization settings. The sequence types (STs) were determined by multilocus sequence typing (MLST) of representative isolates [9].
Statistical analysis
For categorical variables (source, province, ESBL type), we used count and percentages of the group from which they were derived. AMR rate was calculated as the percentage of isolates that showed resistance to certain antimicrobials. The relative ratio of ESBL genotypes was defined as the percentage of the total. The prevalence or positive rate of ESBL-EC isolates was derived by comparing the number of samples with ESBL-EC with the total number of samples studied and was expressed as a percentage. Multiple samples from the same swine were calculated as one sample, and the swine was defined to be ESBLpositive even if only one sample from it was positive (nose, skin at groin region, rectum, and stool). Chi-square test was used for the comparative analysis of categorical variables using IBM SPSS Statistics for Windows software version 23.0 (IBM Corp., Armonk, NY, USA). Statistical significance of the results was defined at P < 0.05.
RESULTS
Prevalence of ESBL-EC isolates
The overall ESBL-EC positive rate was 14.4% (232/1,614), with the rates being 8.9% in pork, 18.4% in swine, 9.0% in workers, and 20.6% in the environment. ESBL-EC positive rate varied according to sample source and geographic location (Table 1). The ESBL-EC positive rate of pork samples from Seoul/Gyeonggi was significantly higher than that of samples from Chungcheong, Gyeongsang, and Jeolla (P = 0.003, P = 0.004 and P = 0.01). The ESBL-EC positive rate of swine samples from Gangwon, Jeolla, and Gyeongsang was significantly higher than that of samples from Chungcheong (P = 0.001, P = < 0.0001 and P = 0.001).
Table 1.
Prevalence and genotypes of ESBL-EC isolates
| Source* | Province | Positive rate (%, N) | P† | ESBL types (N) |
|---|---|---|---|---|
| Pork | Seoul/Gyeonggi | 18.4% (18/98) | 0.003‡ | CTX-M-55 (11), CTX-M-15 (2), CTX-M-14 (2), CTX-M-65 (2), CTX-M-27 (1) |
| Gangwon | 7.3% (6/82) | CTX-M-55 (3), CTX-M-14 (2), SHV-12 (1) | ||
| Jeolla | 7.5% (13/174) | 0.01§ | CTX-M-55 (10), CTX-M-14 (3) | |
| Chungcheong | 5.3% (5/95) | CTX-M-15 (5) | ||
| Gyeongsang | 5.5% (5/91) | 0.004∥ | CTX-M-55 (3), CTX-M-14 (2) | |
| Subtotal | 8.9% (48/540) | < 0.0001¶ | CTX-M-55 (27), CTX-M-14 (9), CTX-M-15 (8), CTX-M-65 (2), CTX-M-27 (1), SHV-12 (1) | |
| Swine | Seoul/Gyeonggi | 15.8% (19/120) | CTX-M-55 (16), CTX-M-102 (2), CTX-M-14 (1) | |
| Gangwon | 23.8% (19/80) | 0.001** | CTX-M-65 (18), CTX-M-55 (1) | |
| Jeolla | 22.5% (45/200) | < 0.0001†† | CTX-M-14 (21), CTX-M-15 (12), CTX-M-55 (11), CTX-M-28 (1) | |
| Chungcheong | 8.8% (14/160) | 0.001‡‡ | CTX-M-55 (12), CTX-M-15 (1), CTX-M-14 (1) | |
| Gyeongsang | 21.5% (43/200) | CTX-M-55 (21), CTX-M-14 (21), CTX-M-15 (1) | ||
| Subtotal | 18.4% (140/760) | 0.002§§ | CTX-M-55 (61), CTX-M-14 (44), CTX-M-65 (18), CTX-M-15 (14), CTX-M-102 (2), CTX-M-28 (1) | |
| Worker | Seoul/Gyeonggi | 11.5% (6/52) | CTX-M-15 (3), CTX-M-55 (1), CTX-M-14 (1), CTX-M-27 (1) | |
| Gangwon | 18.8% (3/16) | CTX-M-55 (2), CTX-M-65 (1) | ||
| Jeolla | 9.5% (4/42) | CTX-M-14 (2), CTX-M-55 (1), CTX-M-17 (1) | ||
| Chungcheong | 0.0% (0/44) | - | ||
| Gyeongsang | 12.5% (3/24) | CTX-M-15 (2), CTX-M-3 (1) | ||
| Subtotal | 9.0% (16/178) | 0.003∥∥ | CTX-M-15 (5), CTX-M-55 (4), CTX-M-14 (3), CTX-M-3 (1), CTX-M-17 (1), CTX-M-27 (1), CTX-M-65 (1) | |
| Environment | Seoul/Gyeonggi | 25.0% (6/24) | CTX-M-55 (3), CTX-M-102 (2), CTX-M-69 (1) | |
| Gangwon | 15.4% (2/13) | CTX-M-65 (2) | ||
| Jeolla | 20.5% (8/39) | CTX-M-14 (5), CTX-M-55 (2), CTX-M-15 (1) | ||
| Chungcheong | 22.2% (6/27) | CTX-M-15 (4), CTX-M-55 (2) | ||
| Gyeongsang | 18.2% (6/33) | CTX-M-14 (4), CTX-M-15 (1), CTX-M-55 (1) | ||
| Subtotal | 20.6% (28/136) | < 0.0001¶¶ | CTX-M-14 (9), CTX-M-55 (8), CTX-M-15 (6), CTX-M-65 (2), CTX-M-102 (2), CTX-M-69 (1) | |
| Total | 14.4% (232/1,614) | CTX-M-55 (100), CTX-M-14 (65), CTX-M-15 (33), CTX-M-65 (23), CTX-M-102 (4), CTX-M-27 (2), | ||
| CTX-M-3 (1), CTX-M-28 (1), CTX-M-69 (1), CTX-M-17 (1), SHV-12 (1) |
*Pork samples were collected from slaughterhouses and retail stores. Worker and environment samples were collected from swine farms, slaughterhouses, and retail stores; †Chi-square test was used to compare categorical variables; comparison of positive rates between ‡pork samples from Seoul/Gyeonggi and Chungcheong, §pork samples from Seoul/Gyeonggi and Jeolla, and llpork samples from Seoul/Gyeonggi and Gyeongsang; comparison of positive rates between ¶pork and swine samples; comparison of positive rates between **swine samples from Gangwon and Chungcheong, ††swine samples from Chungcheong and Jeolla, and ‡‡swine samples from Chungcheong and Gyeongsang; comparison of positive rates between §§swine and worker samples, llllworker and environment samples, and ¶¶environment and pork samples.
Abbreviations: ESBL, extended-spectrum-β-lactamase; ESBL-EC, Extended-spectrum-β-lactamase-producing Escherichia coli.
AMR of ESBL-EC isolates
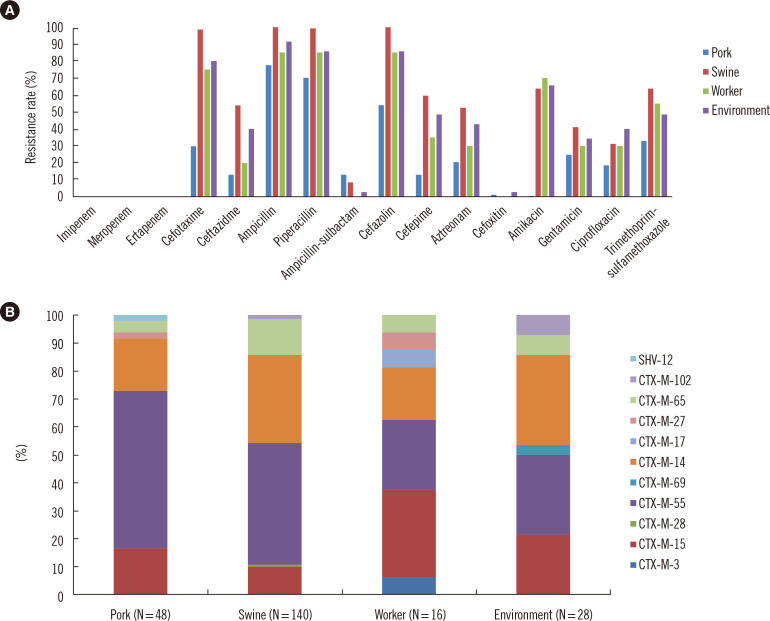
The ESBL-EC isolates showed AMR to gentamicin (41.1%), amikacin (51.1%), ciprofloxacin (32.5%), and trimethoprimsulfamethoxazole (57.6%), in addition to β-lactams. All isolates were susceptible to imipenem, meropenem, or ertapenem. The AMR rates differed according to sample source. Isolates from swine showed higher resistance rates for β-lactams, amikacin, gentamicin, and ciprofloxacin than isolates from pork samples (Fig. 1A).
Fig. 1.
AMR characterization of ESBL-EC isolates recovered from swine industry-related samples. (A) AMR rates. (B) The relative ratio of ESBL genotypes.
Abbreviations: AMR, antimicrobial resistance; ESBL, extended-spectrum-β-lactamase; ESBL-EC, extended-spectrum-β-lactamase-producing Escherichia coli.
ESBL genes
All ESBL-EC isolates contained a single CTX-M type ESBL gene. Common genotypes included blaCTX-M-55 (N = 100), blaCTX-M-14 (N = 65), blaCTX-M-15 (N = 33), and blaCTX-M-65 (N = 23). The relative proportion of ESBL-EC isolates indicated some differences in ESBL genotype among swine industry-related isolates, depending on the source (Fig. 1B). However, most of the isolates shared ESBL genotypes common to clinical isolates, such as CTX-M-14, 15, and 55. One swine-originating E. coli strain showed a MIC of 4 μg/mL for colistin (according to BMD analysis) but had no mcr genes.
Molecular epidemiology
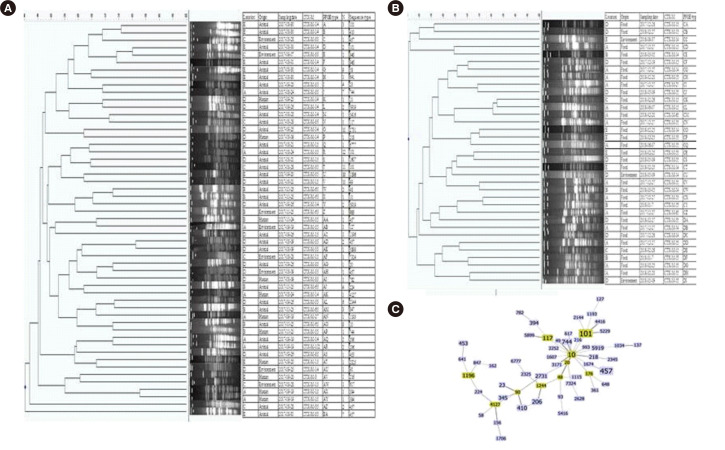
PFGE was successful in 197 of the 232 isolates, and most of PFGE types were heterogeneous (total 105 PFGE types) with some dominant PFGE types (O, R, T, U, and V), which were detected with a cut-off level of 5% (if more than 10 isolates had the same PFGE type). Thirty-five isolates failed to grow in repeated subculture of stored isolates (-70°C in skim milk) or could not be analyzed by repeated PFGE.
MLST of 88 isolates was performed with representative PFGE types (Fig. 2A and 2B), and they showed heterogeneous STs, with ST101 (N = 8), ST457 (N = 7), ST10 (N = 5), ST117 (N = 3), ST206 (N = 3), ST410 (N = 3), and ST744 (N = 3). No dominant ST was detected by MLST with representative PFGE types (Fig. 2C). ST131 was not detected in this study.
Fig. 2.
Clonal traits of ESBL-EC. (A) Samples from swine farms, (B) Samples from slaughterhouses or retail houses, (C) The minimum spanning tree was constructed using the goeBURST algorithm, with the Phyloviz software v2.0 (http://www.phyloviz.net/). The allelic profiles were downloaded from the MLST website (http://pubmlst.org/escherichia/), which included the E. coli STs. The Group founder is colored in green, and the related STs are in blue.
Abbreviations: ESBL, extended-spectrum-β-lactamase; ESBL-EC, extended-spectrum-β-lactamase-producing Escherichia coli; STs, sequence types; MLST, multilocus sequence typing.
DISCUSSION
The isolation of ESBL-EC with blaCTX-M variants from livestock is a constant problem [14]. In the present study, the overall positive rate of ESBL-EC was 14.4%, and the ESBL-EC isolates were less frequent in pork (8.9%) and worker (9.0%) samples than in swine (18.4%) and environment (20.6%) samples. The positive rate also varied according to geographic location. For example, 18% of pork samples from Seoul/Gyeonggi had ESBL-EC; which is much higher than in other regions. Common ESBL genotypes included blaCTX-M-55, blaCTX-M-14, and blaCTX-M-15, which are prevalent among ESBL-EC isolated from clinical samples in Korea [15, 16]. This provides indirect evidence of the resistance transfer from swine to workers. However, definite evidence of transfer from swine to workers was not found based on whole genome analysis of four blaCTX-M-55-carrying E. coli isolates highly suspected of dissemination in one swine farm [17]. PFGE types were heterogeneous, except for the some dominant PFGE types (O, R, T, U, and V). MLST types of isolates with representative PFGE types were very heterogeneous, without dominant clones, suggesting sporadic rather than clonal spread in the study groups.
The community prevalence of ESBL increased gradually in the mid-2000s, owing to the wide spread of ST131 with blaCTXM-15 [18]. The success of this clone might be due to its specific traits, including multidrug resistance, high virulence, and efficient transmission [19]. ST131 E. coli isolates have been reported from non-human sources [20, 21]; however, to the best of our knowledge, there have been no reports that have examined ST131 E. coli isolates in livestock or companion animals in Korea. ST131 ESBL-EC isolates were not detected in this study, suggesting a low prevalence of ST131 ESBL-EC in swine-related samples in Korea. However, continuous monitoring is needed to prevent the spread of ST131 E. coli in the community, considering its competency to capture carbapenemase genes [22].
This study also included the monitoring of carbapenemaseproducing and colistin-resistant E. coli in livestock or related industries in Korea; however, we could not detect these isolates in swine-related samples. Although there are several reports regarding carbapenemase-producing Enterobacteriaceae (CPE) in livestock [23, 24], including those that co-produce MCR-1 and carbapenemase [23], to the best of our knowledge, no studies examining CPE in livestock have been conducted in Korea. Recently, two New Delhi metallo-β-lactamase (NDM-5)-producing E. coli isolates were isolated from a dog and a cat in Korea [25]. The spread of CPE from livestock or the environment to humans is a global public health concern. To date, the prevalence of mcr genes in E. coli isolates from livestock has been very low in Korea [26–28].
The extensive use of antimicrobials has resulted in the generation of antimicrobial concentration gradients in humans and livestock, thus accelerating the emergence and spread of antimicrobial-resistant bacteria among humans and animals [10, 11]. Environmental contamination and livestock production systems have been implicated as likely reservoirs of AMR and promote AMR transmission to humans via the colonization of commensal bacteria, such as E. coli [12]. Huge amounts of antimicrobials of the same classes as those used for human clinical treatment have been used for growth promotion and infection treatment in livestock [14]. According to a report on antimicrobial usage in livestock in Korea [13], the largest number of antimicrobials sold was for use in swine (48–57%), followed by those for use in poultry (18–24%), fisheries (11–25%), and cattle (5– 8%). Therefore, the correlation of antimicrobial use in swine farms and AMR rates of colonized E. coli in swine could be an important evidence for the control of antimicrobial usage in livestock.
In summary, the current nationwide molecular epidemiology of major antimicrobial-resistant organisms was characterized in swine-related industries. The proportion of ESBL-EC in swine industry-related samples was high, and a number of dominant PFGE types and clinically common ESBL genes were observed in these samples. The spread of resistant bacteria to humans and animals via foodstuffs needs to be decreased, and the concentrations of antimicrobials and antimicrobial-resistant bacteria introduced into the environment need to be minimized. In this regard, our epidemiological data could be useful for developing evidence-based policies for the control of antimicrobial-resistant bacteria in livestock to improve animal and human health in line with the “one health” concept.
The limitation of this study is that evidences of the spread of ESBL-EC among workers, livestock, the environment, and slaughterhouses were not documented and the prevalence of ESBLEC in livestock was not evaluated in a longitudinal study. Further studies are needed on samples from swine at various breeding stages.
ACKNOWLEDGEMENTS
I thank So Ra Yoon, PhD for the statistics from research Institute of national health insurance Ilsan hospital.
Footnotes
AUTHOR CONTRIBUTIONS
Kim YA and Lee K conceived the experiments. Seo YH and Park GE conducted the experiments. Kim H and Lee H analyzed the results. Kim YA and Kim H wrote the manuscript. All authors reviewed the manuscript.
CONFLICTS OF INTEREST
No potential conflicts of interest relevant to this paper were reported.
RESEARCH FUNDING
This study was supported by research funds from the Korea Centers for Disease Control and Prevention (Project No. 2017NER54060 and 2020ER540500).
REFERENCES
- 1.Lee H, Yoon EJ, Kim D, Jeong SH, Won EJ, Shin JH, et al. Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017: first one-year report from Kor-GLASS. Euro Surveill. 2018;23:1800047. doi: 10.2807/1560-7917.ES.2018.23.42.1800047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell N, Davidson I, Yelling P, Collinson A, Pollard A, Johnson L, et al. Developing a local antimicrobial resistance action plan: the Cornwall One Health Antimicrobial Resistance Group. J Antimicrob Chemother. 2017;72:2661–5. doi: 10.1093/jac/dkx164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Na SH, Moon DC, Choi MJ, Oh SJ, Jung DY, Sung EJ, et al. Antimicrobial resistance and molecular characterization of extended-spectrum β-lactamase-producing Escherichia coli isolated from ducks in South Korea. Foodborne Pathog Dis. 2019;16:799–806. doi: 10.1089/fpd.2019.2644. [DOI] [PubMed] [Google Scholar]
- 4.CLSI. CLSI M100. 27th ed. Clinical and Laboratory Standards Institute; Wayne, PA: 2017. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 5.Ryoo NH, Kim E-C, Hong SG, Park YJ, Lee K, Bae IK, et al. Dissemination of SHV-12 and CTX-M-type extended-spectrum β-lactamases among clinical isolates of Escherichia coli and Klebsiella pneumoniae and emergence of GES-3 in Korea. J Antimicrob Chemother. 2005;56:698–702. doi: 10.1093/jac/dki324. [DOI] [PubMed] [Google Scholar]
- 6.Rojas LJ, Salim M, Cober E, Richter SS, Perez F, Salata RA, et al. Colistin resistance in carbapenem-resistant Klebsiella pneumoniae: laboratory detection and impact on mortality. Clin Infect Dis. 2017;64:711–8. doi: 10.1093/cid/ciw805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon EJ, Hong JS, Yang JW, Lee KJ, Lee H, Jeong SH. Detection of mcr-1 plasmids in Enterobacteriaceae isolates from human specimens: comparison with those in Escherichia coli isolates from livestock in Korea. Ann Lab Med. 2018;38:555–62. doi: 10.3343/alm.2018.38.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park YS, Bae IK, Kim J, Jeong SH, Hwang SS, Seo YH, et al. Risk factors and molecular epidemiology of community-onset extended-spectrum β-lactamase-producing Escherichia coli bacteremia. Yonsei Med J. 2014;55:467–75. doi: 10.3349/ymj.2014.55.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EnteroBase. [Updated on Oct 2020];Escherichia coli MLST database. https://enterobase.warwick.ac.uk/species/ecoli/allele_st_search .
- 10.Andersson DI, Hughes D. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol. 2014;12:465–78. doi: 10.1038/nrmicro3270. [DOI] [PubMed] [Google Scholar]
- 11.Manyi-Loh C, Mamphweli S, Meyer E, Okoh A. Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules. 2018;23:795. doi: 10.3390/molecules23040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rousham EK, Unicomb L, Islam MA. Human, animal and environmental contributors to antibiotic resistance in low-resource settings: integrating behavioural, epidemiological and One Health approaches. Proc Biol Sci. 2018;285:20180332. doi: 10.1098/rspb.2018.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim S, Lee J, Lee H, Nam H, Moon D, Jang G, et al. Trends in antimicrobial sales for livestock and fisheries in Korea during 2003-2012. Korean J Vet Res. 2014;54:81–6. doi: 10.14405/kjvr.2014.54.2.81. [DOI] [Google Scholar]
- 14.Lazarus B, Paterson DL, Mollinger JL, Rogers BA. Do human extraintestinal Escherichia coli infections resistant to expanded-spectrum cephalosporins originate from food-producing animals? A systematic review. Clin Infect Dis. 2015;60:439–52. doi: 10.1093/cid/ciu785. [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Kim YA, Park YS, Choi MH, Lee GI, Lee K. Risk factors and molecular features of sequence type (ST) 131 extended-spectrum β-lactamase-producing Escherichia coli in community-onset bacteremia. Sci Rep. 2017;7:14640. doi: 10.1038/s41598-017-14621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YA, Kim JJ, Kim H, Lee K. Community-onset extended-spectrum-β-lactamase-producing Escherichia coli sequence type 131 at two Korean community hospitals: The spread of multidrug-resistant E. coli to the community via healthcare facilities. Int J Infect Dis. 2017;54:39–42. doi: 10.1016/j.ijid.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Kim YA, Kim H, Choi MH, Seo YH, Lee H, Lee K. Whole-genome analysis of blaCTX-M-55-carrying Escherichia coli among pigs, farm environment, and farm workers. Ann Lab Med. 2020;40:180–3. doi: 10.3343/alm.2020.40.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson JR, Urban C, Weissman SJ, Jorgensen JH, Lewis JS 2nd, Hansen G, et al. Molecular epidemiological analysis of Escherichia coli sequence type ST131 (O25:H4) and blaCTX-M-15 among extended-spectrum-β-lactamase-producing E. coli from the United States, 2000 to 2009. Antimicrob Agents Chemother. 2012;56:2364–70. doi: 10.1128/AAC.05824-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson JR, Clermont O, Johnston B, Clabots C, Tchesnokova V, Sokurenko E, et al. Rapid and specific detection, molecular epidemiology, and experimental virulence of the O16 subgroup within Escherichia coli Sequence Type 131. J Clin Microbiol. 2014;52:1358–65. doi: 10.1128/JCM.03502-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platell JL, Johnson JR, Cobbold RN, Trott DJ. Multidrug-resistant extraintestinal pathogenic Escherichia coli of sequence type ST131 in animals and foods. Vet Microbiol. 2011;153:99–108. doi: 10.1016/j.vetmic.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Zurfluh K, Hächler H, Nüesch-Inderbinen M, Stephan R. Characteristics of extended-spectrum β-lactamase- and carbapenemase-producing Enterobacteriaceae isolates from rivers and lakes in Switzerland. Appl Environ Microbiol. 2013;79:3021–6. doi: 10.1128/AEM.00054-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YA, Qureshi ZA, Adams-Haduch JM, Park YS, Shutt KA, Doi Y. Features of infections due to Klebsiella pneumoniae carbapenemase-producing Escherichia coli: emergence of sequence type 131. Clin Infect Dis. 2012;55:224–31. doi: 10.1093/cid/cis387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Zhang R, Li J, Wu Z, Yin W, Schwarz S, et al. Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat Microbiol. 2017;2:16260. doi: 10.1038/nmicrobiol.2016.260. [DOI] [PubMed] [Google Scholar]
- 24.Fischer J, San José M, Roschanski N, Schmoger S, Baumann B, Irrgang A, et al. Spread and persistence of VIM-1 carbapenemase-producing Enterobacteriaceae in three German swine farms in 2011 and 2012. Vet Microbiol. 2017;200:118–23. doi: 10.1016/j.vetmic.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Hong JS, Song W, Jeong SH. Molecular characteristics of NDM-5-producing Escherichia coli from a cat and a dog in South Korea. Microb Drug Resist. 2020;26:1005–8. doi: 10.1089/mdr.2019.0382. [DOI] [PubMed] [Google Scholar]
- 26.Lee JY, Lim SK, Choi Y, Moon DC, Shin J, Ko KS. Whole sequences and characteristics of mcr-1-harboring plasmids of Escherichia coli strains isolated from livestock in South Korea. Microb Drug Resist. 2018;24:489–92. doi: 10.1089/mdr.2017.0369. [DOI] [PubMed] [Google Scholar]
- 27.Belaynehe KM, Shin SW, Park KY, Jang JY, Won HG, Yoon IJ, et al. Emergence of mcr-1 and mcr-3 variants coding for plasmid-mediated colistin resistance in Escherichia coli isolates from food- producing animals in South Korea. Int J Infect Dis. 2018;72:22–4. doi: 10.1016/j.ijid.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Lim SK, Kang HY, Lee K, Moon DC, Lee HS, Jung SC. First detection of the mcr-1 gene in Escherichia coli isolated from livestock between 2013 and 2015 in South Korea. Antimicrob Agents Chemother. 2016;60:6991–3. doi: 10.1128/AAC.01472-16. [DOI] [PMC free article] [PubMed] [Google Scholar]