Abstract
The risk factors for the pulmonary infections after hypertensive cerebral hemorrhage remains unclear. We aimed to investigate the potential risk factors for the postoperative pulmonary infection in patients with hypertensive cerebral hemorrhage.
Patients with hypertensive cerebral hemorrhage undergone surgery from January 2018 to December 2019 were included. Related personal and medical information were collected. Univariate and multivariate logistic regression analyses were performed to identify the potential risk factors for the postoperative pulmonary infection.
A total of 264 patients were included, and the incidence of pulmonary infection for patients with hypertensive cerebral hemorrhage after surgery was 19.70%. Escherichia coli is the most common bacteria of pulmonary infection. Multivariate regression analysis revealed that the preoperative hypoalbuminemia (OR2.89, 1.67∼4.78), tracheotomy (OR5.31, 1.24∼11.79), diabetes (OR4.92, 1.32∼9.80), preoperative GCS (OR5.66, 2.84∼11.21), and the duration of mechanical ventilation (OR2.78, 2.32∼3.61) were the independent risk factors for the pulmonary infection in patients with hypertensive cerebral hemorrhage (all P < .05).
Patients with hypertensive intracerebral hemorrhage after surgery have a higher risk of postoperative pulmonary infections, and there are many related risk factors, which should be taken seriously in clinical practice.
Keywords: hypertension, intracerebral hemorrhage, neurosurgery, pulmonary infection, risk
1. Introduction
With the changes in the style of modern life, the incidence of hypertension is on the rise, and long-term hypertension will accelerate the atherosclerosis process, posing a great threat to the cardiovascular system.[1,2] Hypertensive cerebral hemorrhage is a common complication of hypertension.[3] It has the characteristics of sudden onset, rapid development, and many adverse complications.[4] It is been found[5] that it will reduce the oxygen supply of brain tissues and cause different degrees of damage to patients neurological functions. Reaching the indication for surgery after intracerebral hemorrhage requires surgical treatment. The purpose of surgical treatment is to remove intracranial hematoma, reduce intracranial pressure, and avoid the occurrence of cerebral hernia.[6] However, intracranial surgery often requires the indwelling of various drainage tubes, and the risk of complications such as rebleeding and pulmonary infection is high.[7,8] Therefore, the prevention and treatment of related complications after intracranial surgery is essential.
Pulmonary infection is one of the common complications of hypertensive cerebral hemorrhage, which will aggravate brain tissue hypoxia and cause second injury to brain, and even posing a serious threat to the life and health of patients.[9,10] Therefore, the prevention of pulmonary infection after surgery in patients with hypertensive cerebral hemorrhage is vital to the prognosis of patients. Currently, the risk factors for the pulmonary infections after hypertensive cerebral hemorrhage remains unclear, and understanding this do has positive significance for reducing the risk of related complications. Therefore, we conducted this study with attempt to investigate the potential risk factors for the postoperative pulmonary infection in patients with hypertensive cerebral hemorrhage, to providing insights into the treatment and management of hypertensive cerebral hemorrhage
2. Methods
2.1. Ethical consideration
This present study has been certified and approved by the ethics committee of Shenzhen People's Hospital (SX 20170086), and verbal informed consents had obtained from the included participants.
2.2. Participants
Patients with hypertensive cerebral hemorrhage undergone surgery in the department of neurosurgery of our hospital from January 2018 to December 2019 were included in this present study. The inclusion criteria were:
-
1.
It was the first acute cerebral hemorrhage for patient, and the diagnosis complied with the related guidelines.[11,12]
-
2.
The Glasgow coma score (GCS) at admission was between 3 and 12 points.
-
3.
The patient showed no signs of lung infection or pneumonia at the time of admission.
-
4.
After admission, surgery with hematoma removal or decompression of bone flaps was performed under general anesthesia for patients, and those with hematoma ruptured into the ventricle also underwent external drainage.
The exclusion criteria were:
-
1.
patients with congenital hematological disorders.
-
2.
The cerebral hemorrhage was caused by the malformations of cerebral vascular.
2.3. The diagnosis criteria for pulmonary infection
The diagnostic criteria for lung infections were based on the diagnostic standards.[13] if patient showed any 3 of the following 5 items, the diagnosis of pulmonary infection can be determined:
-
1.
respiratory symptoms such as cough, purulent sputum, and deep breathing
-
2.
Auscultation showed both lungs with dry and wet rales and/or signs of lung consolidation in varying degrees;
-
3.
Body temperature ≥38.5°C with an increase in peripheral white blood cell count ≥1.0 × 109/L;
-
4.
X-ray chest radiograph showed inflammatory changes;
-
5.
Pathogens were obtained from sputum or blood culture.
2.4. Data collections
The included patients with hypertensive cerebral hemorrhage after surgery were divided into infection group and no infection group according to the presence or absence of concurrent pulmonary infections. Following information and data were collected and analyzed: the age; gender; the concurrent diseases such as diabetes, hyperlipidemia et al; whether or not performed the tracheotomy; the hemorrhage localization; the preoperative GCS; the duration of operations; the duration of nasal feeding tube; length of hospital stay.
2.5. Statistical analysis
We processed the collected data with SPASS23.0 software. Categorical variables were analyzed using the χ2 test or Fishers exact test, and continuous variables were analyzed using Student t test or Mann–Whitney U test, and were generally presented as means and standard deviation. Multivariate logistic regression analyses were performed using the forward likelihood ratio selection method to identify independent risk factors and it is presented with an odds ratio (OR) and 95% confidence intervals (95%CI). Potential candidate variables were those with P < .05 in univariate analyses. And P < .05 was considered statistically significant in this present study.
2.6. The characteristics of included patients
A total of 264 patients with hypertensive cerebral hemorrhage were included in this present study, with 52 patients in the pulmonary infection group, and 212 patients in the no infection group. The incidence of pulmonary infection for patients with hypertensive cerebral hemorrhage after surgery was 19.70%. And 12 patients had ventilator associated pneumonia. The overall average age of included patients was (66.3 ± 8.71) year old. The characteristics of included patients in infection and no infection group were shown in Table 1. There were significant differences in the diabetes, preoperative hypoalbuminemia, the duration of mechanical ventilation, preoperative GCS, COPD, tracheotomy, length of operation, the duration of nasal feeding tube among infection and no infection group (all P < .05), no significant differences were detected in gender, age, hyperlipidemia, hemorrhage localization and length of hospital stay(all P > .05).
Table 1.
The characteristics and clinical information of included patients.
| Items | Infection (n = 52) | No infection (n = 212) | χ2/t | P |
| Age (years) | 46.5 ± 8.35 | 46.2 ± 9.73 | 11.351 | .085 |
| Gender | ||||
| Male | 38 | 169 | 1.044 | .105 |
| Female | 13 | 43 | ||
| Diabetes | ||||
| Yes | 22 | 46 | 3.289 | .043 |
| No | 30 | 166 | ||
| Hyperlipidemia | ||||
| Yes | 16 | 39 | 1.537 | .061 |
| No | 36 | 173 | ||
| Preoperative hypoalbuminemia | ||||
| Yes | 42 | 68 | 1.979 | .001 |
| No | 10 | 144 | ||
| Hemorrhage localization | ||||
| Basal ganglia | 20 | 87 | 5.484 | .122 |
| Thalamus | 13 | 51 | ||
| Cerebellum | 9 | 40 | ||
| Ventricle | 7 | 23 | ||
| Cerebral lobe | 3 | 11 | ||
| The duration of mechanical ventilation (days) | 2.3 ± 1.10 | 1.5 ± 0.85 | 8.250 | .037 |
| Preoperative GCS score | 6.8 ± 2.64 | 8.7 ± 3.29 | 11.073 | .009 |
| COPD | ||||
| Yes | 29 | 63 | 4.527 | .002 |
| No | 23 | 149 | ||
| Tracheotomy | ||||
| Yes | 30 | 47 | 2.185 | .011 |
| No | 22 | 165 | ||
| Length of operation (min) | ||||
| <60 | 39 | 179 | 22.063 | .009 |
| ≥60 | 13 | 33 | ||
| The duration of nasal feeding tube (days) | 3.9 ± 1.01 | 2.1 ± 0.93 | 1.425 | .025 |
| Length of hospital stay (days) | 5.21 ± 1.28 | 5.02 ± 1.14 | 1.755 | .106 |
Table 2.
Multivariate regression analysis on the risk factors of pulmonary infection in patients with hypertensive cerebral hemorrhage.
| Factors | β | S̄x | OR | 95%CI | P | Rank |
| Preoperative hypoalbuminemia | 0.06 | 0.279 | 2.89 | 1.67∼4.78 | .002 | 1 |
| Tracheotomy | 0.90 | 0.346 | 5.31 | 1.24∼11.79 | .035 | 2 |
| Diabetes | 1.03 | 0.445 | 4.92 | 1.32∼9.80 | .032 | 3 |
| Preoperative GCS | 0.97 | 0.325 | 5.66 | 2.84∼11.21 | .039 | 4 |
| The duration of mechanical ventilation | 1.13 | 0.117 | 2.78 | 2.32–3.61 | .045 | 5 |
2.7. Bacterial species distribution
The blood culture results of 52 infection patients indicated that there were 14 cases of Escherichia coli, 9 cases of Proteus mirabilis, 2 case of Pseudomonas aeruginosa, 1 case of Klebsiella oxytoca, 5 cases of Enterococcus faecalis, and 21 cases of negative results (Fig. 1).
Figure 1.
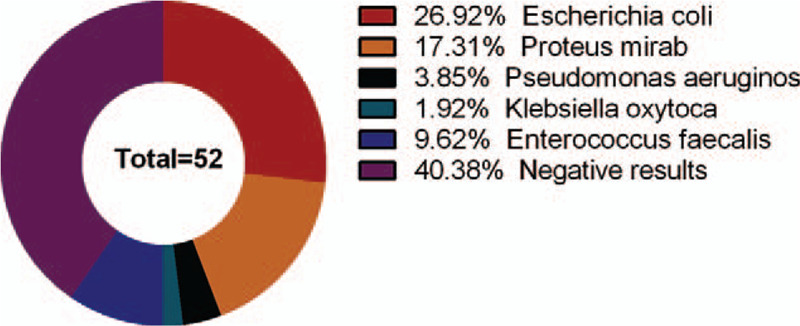
The bacterial distribution of blood culture of 52 patients with pulmonary infection.
The sputum culture results of 52 fever patients indicated that there were 11 cases of Escherichia coli, 6 cases of Proteus mirabilis, 3 cases of Enterococcus faecalis and 32 cases of negative results (Fig. 2).
Figure 2.
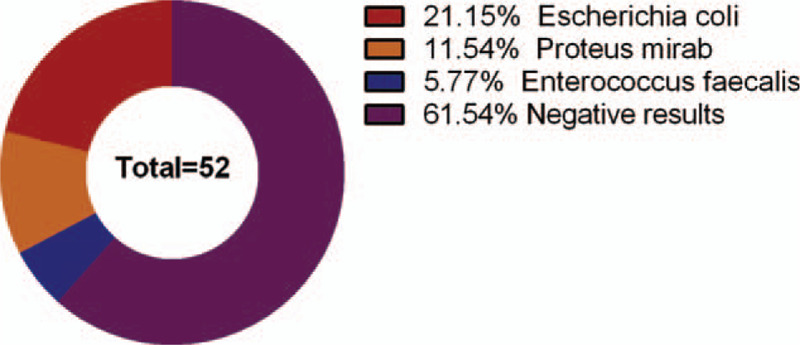
The bacterial distribution of sputum culture of 52 patients with pulmonary infection.
2.8. Multivariate regression analysis on the potential risk factors
We further performed the multivariate regression analysis on the following items with statistical significance in the univariate analysis: diabetes, preoperative hypoalbuminemia, the duration of mechanical ventilation, preoperative GCS, COPD, tracheotomy, length of operation, the duration of nasal feeding tube. And the results of multivariate regression analysis indicated the preoperative hypoalbuminemia (OR2.89, 1.67∼4.78), tracheotomy (OR5.31, 1.24∼11.79), diabetes (OR4.92, 1.32∼9.80), preoperative GCS (OR5.66, 2.84∼11.21), and the duration of mechanical ventilation (OR2.78,2.32∼3.61) were the independent risk factors for the pulmonary infection in patients with hypertensive cerebral hemorrhage (all P < .05).
3. Discussion
Pulmonary infection is a common complication after intracerebral hemorrhage. The incidence of pulmonary infection after intracerebral hemorrhage in this present study is 19.70%. It is been reported that the incidence of pulmonary infection in conservatively treated patients after intracerebral hemorrhage is 18%,[14] which is lower than that of our findings. It is understandable that postoperative patients are more likely to have pulmonary infections, the patients who have reached the surgical indication generally have larger hematomas and deeper injury of brain functions.[15] Furthermore, patients may need to retain various ducts after surgery to maintain the intracranial pressure in normal scope.[16,17] Therefore, postoperative patients are more likely to have pulmonary infections than those patients that did not undergo surgery treatment. Furthermore, our results have indicated that the preoperative hypoalbuminemia, tracheotomy, diabetes, preoperative GCS, and the duration of mechanical ventilation were the independent risk factors for the pulmonary infection in patients with hypertensive cerebral hemorrhage, more attentions should be paid and prophylaxis must be conducted for those patients.
In this study, gram-negative bacteria has accounted for the majority of bacterial infections. This may be caused by long-term resident bacteria in the lungs caused by secretion accumulations.[18] It may be related to bacterial imbalance caused by long-term use of antibacterial drugs.[19] Gram-positive bacteria mainly cause lung infection through invasion through invasive procedures and surgery.[20] The types of pathogenic bacteria in patients with hypertensive intracerebral hemorrhage combined with pulmonary infection are more complicated.[21] Therefore, accurate drug susceptibility identification has guiding significance in selecting the appropriate antibacterial drug. It is been reported[22–24] that Staphylococcus and Staphylococcus epidermidis are sensitive to vancomycin; Gram-negative bacteria Klebsiella pneumoniae and Acinetobacter baumannii are sensitive to cefoperazone. Antibiotics should be selected clinically based on the results of drug sensitivity tests.[25]
Patients with hypertensive intracerebral hemorrhage are in a state of high consumption and prone to hypoalbuminemia when the nutritional supply is not enough. Patients with hypoalbuminemia are prone to be infected due to decreased albumin and globulin.[26,27] At the same time, albumin is an important part of colloid osmotic pressure in blood vessels. Hypoalbuminemia can cause changes in colloid osmotic pressure and make extracellular fluid hypotonic, which can cause hypotonic dehydration and electrolyte disorders.[28,29] The results of this study have showed that the patients with hypoalbuminemia is more likely to get pulmonary infection than the patients with normal albumin. Therefore, patients with intracerebral hemorrhage should be supplemented with sufficient nutrients, and monitor the changes in albumins.[30] If necessary, supplement human albumin to keep the balance.
Tracheostomy is an important risk factor of pulmonary infection for patients with hypertensive cerebral hemorrhage.[31] In patients with severe conscious disturbance, the large amount of sputum in the lungs cannot be discharged spontaneously, tracheostomy may be performed to keep the airway open.[32] However, there are many disadvantages related to tracheotomy. For example, due to the change of natural breathing passages, the inhaled air lacks filtration and humidification of the nasal mucosa, bacteria may easily invade, it is prone to be infected.[33] Therefore, patients with tracheotomy should be given nebulization and moistened inhaled air, and those with thick sputum should be given suction treatment.[32] The sputum suction should be gentle to reduce the irritation of the tracheobronchial mucosa. If necessary, relevant antibiotics may be used for anti-infective treatment.[34]
In patients with diabetes, due to abnormal glucose metabolism, the bodys energy supply is unbalanced, and the immunity is reduced, thus infection is more likely to occur.[35] Previous studies[36] have shown that people with poor glycemic control are at higher risk of infection. GCS is an important indicator of the functions of the central nervous system. The hematomas formed after hypertensive intracerebral hemorrhage compress the adjacent nerve tissues and blood vessels, causing cerebral tissue ischemia and hypoxia, affecting the blood supply and metabolism of nervous tissues, and edema caused by hematoma aggravated the degree of cerebral ischemia and hypoxia, the occupation of hematoma and edema can cause intracranial hypertension.[37,38] With the impairment of patients consciousness, and the GCS decrease accordingly, and the patients cough reflex and swallowing function were impaired, which easily caused aspiration and sputum could not be excreted timely, thus increasing the chance of lung infection. Therefore, for patients with severe conscious disorders, intensify respiratory care after surgery are highlighted.
The duration of mechanical ventilation is also an important risk factor. Tracheal intubation can directly damage the tracheal mucosa, leading to a decrease in the defense capacity of airway.[39] At the same time, the oropharyngeal bacteria are directly brought into the airway during intubation, which increases the probability of lung infection.[40] Besides, repeated suctioning can also directly damage the tracheal mucosa. Tracheal intubation changes the natural airway, avoids the filtering of air by the nasal mucosa, increases the chance of bacteria entering the lungs.[41] It is been reported[42] that the sputum in the gap between the balloon and the glottis cannot be eliminated by suction, and it is easy to breed bacteria, leading to that biofilm attach to the tracheal tube. These bacteria can migrate down the respiratory tract, increase the chance of pulmonary infections. Previous studies[43,44] have confirmed that the subglottic secretion drainage is beneficial to combating the pulmonary infection. Study[45] has shown that pulmonary infections are prone to occur after mechanical ventilation, mainly because the bacteria that adhere to the upper respiratory tract and it can be blown to the lower respiratory tract by tracheal tube. When using the ventilator, it should be noted that the ventilator tube should be replaced and disinfected regularly to avoid breeding bacteria, and the gas should be completely humidified, this will reduce the bacterial blow-in and airway mucosal damage to a certain extent, thereby reducing incidence of pulmonary infections.[46]
It must be aware that there are several other factors may also be associated with the pulmonary infections in patients with hypertensive intracerebral hemorrhage. Patients with hypertensive intracerebral hemorrhage need to insert nasal feeding tubes for feeding to ensure enteral nutrition due to unconsciousness or difficulty in swallowing. Indwelling nasal feeding tubes for too long can increase the incidence of pneumonia.[47] We could not include those information for analysis with concerns that the collected records were incomplete. Furthermore, preoperative vomiting may be associated with the aspiration pneumonia.[48] Patients with cerebral hemorrhage vomit due to high intracranial pressure caused by hematoma and edema. And the decrease in cough reflex and swallowing function caused by damage of the central nervous system, the vomitus is easily sucked into the trachea in the oropharynx, causing aspiration related pneumonia. All those potential risks merits to be considered in the future.
Several limitations in this study must be considered. Only patients that underwent surgery. Firstly, we only included the patients that underwent surgery with consideration that it may contribute to great heterogeneity if we have included all the ICH patients, but several patients with large ICH are medically managed and have a similar length of stay. Secondly, the sample size of our study was small, future studies with larger sample size and multi-centers are needed in the future.
4. Conclusions
In conclusion, the incidence of postoperative pulmonary infection in patients with hypertensive cerebral hemorrhage is rather high, and the preoperative hypoalbuminemia, tracheotomy, diabetes, preoperative GCS, and the duration of mechanical ventilation were the independent risk factors for the pulmonary infection in patients with hypertensive cerebral hemorrhage. Corresponding preventive and therapeutic measures should be taken clinically based on these risk factors. At the same time, given the relatively small sample size and limited data collected in this study, future studies with larger sample are needed to further explore its related risk factors, thus providing evidences for the treatment of hypertensive cerebral hemorrhage.
Author contributions
S X, M X designed research; S X, B D, A S conducted research; S X, F S analyzed data; S X and ZJ W wrote the first draft of manuscript; M X had primary responsibility for final content. All authors read and approved the final manuscript.
Conceptualization: Shihai Xu, Fei Shi.
Formal analysis: Shihai Xu, Aijun Shan, Jin Wang, Manying Xie.
Investigation: Shihai Xu, Bo Du, Aijun Shan, Fei Shi, Jin Wang.
Methodology: Aijun Shan.
Project administration: Shihai Xu, Bo Du.
Resources: Shihai Xu, Fei Shi, Jin Wang, Manying Xie.
Software: Shihai Xu, Bo Du, Jin Wang, Manying Xie.
Supervision: Shihai Xu, Bo Du, Manying Xie.
Validation: Bo Du, Manying Xie.
Visualization: Bo Du.
Footnotes
Abbreviations: 95%CI = 95% confidence intervals, GCS = Glasgow coma score, OR = odds ratio.
How to cite this article: Xu S, Du B, Shan A, Shi F, Wang J, Xie M. The risk factors for the postoperative pulmonary infection in patients with hypertensive cerebral hemorrhage: a retrospective analysis. Medicine. 2020;99:51(e23544).
This study had been funded by Science and Technology Innovation Foundation of Shenzhen (JCYJ20190806150005453).
This present study has been certified and approved by the ethics committee of Shenzhen People's Hospital (SX 20170086), and verbal informed consents had obtained from the included participants with consideration that our study is a retrospective study, we cannot obtained the written informed consents, we had called every included participants to obtain the verbal informed consents, it had been approved by the ethics committee of Shenzhen People's Hospital.
The authors declare no conflicts of interest.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Gasparini S, Ferlazzo E, Sueri C, et al. Hypertension, seizures, and epilepsy: a review on pathophysiology and management. Neurol Sci 2019;40:1775–83. [DOI] [PubMed] [Google Scholar]
- [2].Pescatello LS, Buchner DM, Jakicic JM, et al. Physical activity to prevent and treat hypertension: a systematic review. Med Sci Sports Exerc 2019;51:1314–23. [DOI] [PubMed] [Google Scholar]
- [3].Gross BA, Jankowitz BT, Friedlander RM. Cerebral intraparenchymal hemorrhage: a review. JAMA 2019;321:1295–303. [DOI] [PubMed] [Google Scholar]
- [4].Zhang S, Zhang X, Ling Y, et al. Predicting recurrent hypertensive intracerebral hemorrhage: derivation and validation of a risk-scoring model based on clinical characteristics. World Neurosurg 2019;127:e162–71. [DOI] [PubMed] [Google Scholar]
- [5].Ziai WC, Thompson CB, Mayo S, et al. Intracranial hypertension and cerebral perfusion pressure insults in adult hypertensive intraventricular hemorrhage: occurrence and associations with outcome. Crit Care Med 2019;47:1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang Y, Song L, Zhao J. Role of scalp hypothermia in patients undergoing minimally invasive evacuation of hypertensive cerebral hemorrhage. Pak J Med Sci 2019;35:1451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ruiz-Sandoval JL, Chiquete E, Parra-Romero G, et al. Hypertensive thalamic hemorrhage: analysis of short-term outcome. Int J Neurosci 2019;129:189–94. [DOI] [PubMed] [Google Scholar]
- [8].Gui C, Gao Y, Hu D, et al. Neuroendoscopic minimally invasive surgery and small bone window craniotomy hematoma clearance in the treatment of hypertensive cerebral hemorrhage. Pak J Med Sci 2019;35:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tang Y, Yin F, Fu D, et al. Efficacy and safety of minimal invasive surgery treatment in hypertensive intracerebral hemorrhage: a systematic review and meta-analysis. BMC Neurol 2018;18:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang W, Zhou N, Wang C. Minimally invasive surgery for patients with hypertensive intracerebral hemorrhage with large hematoma volume: a retrospective study. World Neurosurg 2017;105:348–58. [DOI] [PubMed] [Google Scholar]
- [11].Ding W, Gu Z, Song D, et al. Development and validation of the hypertensive intracerebral hemorrhage prognosis models. Medicine (Baltimore) 2018;97:e12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Salvetti M, Paini A, Bertacchini F, et al. Therapeutic approach to hypertensive emergencies: hemorrhagic stroke. High Blood Press Cardiovasc Prev 2018;25:191–5. [DOI] [PubMed] [Google Scholar]
- [13].Connor KA. Management of nosocomial pneumonia. AACN Adv Crit Care 2018;29:5–10. [DOI] [PubMed] [Google Scholar]
- [14].Burgmann H, Hiesmayr JM, Savey A, et al. Impact of nosocomial infections on clinical outcome and resource consumption in critically ill patients. Intensive Care Med 2010;36:1597–601. [DOI] [PubMed] [Google Scholar]
- [15].Chen QH, Lin D, Yu QG, et al. Efficacy of lumbar cistern drainage combined with intrathecal antibiotherapy for the treatment of ventriculo-subarachnoid infections following surgery for hypertensive intracerebral hemorrhage. Neurochirurgie 2017;63:13–6. [DOI] [PubMed] [Google Scholar]
- [16].de Oliveira Manoel AL. Surgery for spontaneous intracerebral hemorrhage. Crit Care 2020;24:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hanley DF, Thompson RE, Rosenblum M, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet 2019;393:1021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Naidus EL, Lasalvia MT, Marcantonio ER, et al. The diagnostic yield of noninvasive microbiologic sputum sampling in a cohort of patients with clinically diagnosed hospital-acquired pneumonia. J Hosp Med 2018;13:34–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Russell CD, Koch O, Laurenson IF, et al. Diagnosis and features of hospital-acquired pneumonia: a retrospective cohort study. J Hosp Infect 2016;92:273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Messika J, Stoclin A, Bouvard E, et al. The challenging diagnosis of non-community-acquired pneumonia in non-mechanically ventilated subjects: value of microbiological investigation. Respir Care 2016;61:225–34. [DOI] [PubMed] [Google Scholar]
- [21].Lange N, Berndt M, Jorger AK, et al. Clinical characteristics and course of postoperative brain abscess. World Neurosurg 2018;120:e675–83. [DOI] [PubMed] [Google Scholar]
- [22].Shirley M. Ceftazidime-avibactam: a review in the treatment of serious gram-negative bacterial infections. Drugs 2018;78:675–92. [DOI] [PubMed] [Google Scholar]
- [23].Campion M, Scully G. Antibiotic use in the intensive care unit: optimization and de-escalation. J Intensive Care Med 2018;33:647–55. [DOI] [PubMed] [Google Scholar]
- [24].Wu M, Pan H, Leng W, et al. Distribution of microbes and drug susceptibility in patients with diabetic foot infections in Southwest China. J Diabetes Res 2018;2018:9817308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tumbarello M, Losito AR, Giamarellou H. Optimizing therapy in carbapenem-resistant Enterobacteriaceae infections. Curr Opin Infect Dis 2018;31:566–77. [DOI] [PubMed] [Google Scholar]
- [26].Zhang F, Liu X, Tan Z, et al. Effect of postoperative hypoalbuminemia and supplement of human serum albumin on the development of surgical site infection following spinal fusion surgery: a retrospective study. Eur Spine J 2020;29:1483–9. [DOI] [PubMed] [Google Scholar]
- [27].Kishawi D, Schwarzman G, Mejia A, et al. Low preoperative albumin levels predict adverse outcomes after total joint arthroplasty. J Bone Joint Surg Am 2020;102:889–95. [DOI] [PubMed] [Google Scholar]
- [28].Michelis R, Sela S, Zeitun T, et al. Unexpected normal colloid osmotic pressure in clinical states with low serum albumin. PLoS One 2016;11:e0159839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Caraceni P, Domenicali M, Tovoli A, et al. Clinical indications for the albumin use: still a controversial issue. Eur J Intern Med 2013;24:721–8. [DOI] [PubMed] [Google Scholar]
- [30].Limaye K, Yang JD, Hinduja A. Role of admission serum albumin levels in patients with intracerebral hemorrhage. Acta Neurol Belg 2016;116:27–30. [DOI] [PubMed] [Google Scholar]
- [31].Bosel J. Use and timing of tracheostomy after severe stroke. Stroke 2017;48:2638–43. [DOI] [PubMed] [Google Scholar]
- [32].Dasenbrock HH, Rudy RF, Gormley WB, et al. The timing of tracheostomy and outcomes after aneurysmal subarachnoid hemorrhage: a nationwide inpatient sample analysis. Neurocrit Care 2018;29:326–35. [DOI] [PubMed] [Google Scholar]
- [33].Divani AA, Hevesi M, Pulivarthi S, et al. Predictors of nosocomial pneumonia in intracerebral hemorrhage patients: a multi-center observational study. Neurocrit Care 2015;22:234–42. [DOI] [PubMed] [Google Scholar]
- [34].Yaghi S, Moore P, Ray B, et al. Predictors of tracheostomy in patients with spontaneous intracerebral hemorrhage. Clin Neurol Neurosurg 2013;115:695–8. [DOI] [PubMed] [Google Scholar]
- [35].Bjerkreim AT, Thomassen L, Waje-Andreassen U, et al. Hospital readmission after intracerebral hemorrhage. J Stroke Cerebrovasc Dis 2016;25:157–62. [DOI] [PubMed] [Google Scholar]
- [36].Tseng CH, Muo CH, Lin MC, et al. Association between reduced risk of intracerebral hemorrhage and pelvic inflammatory disease. Medicine (Baltimore) 2016;95:e2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cao D, Li Q, Fu P, et al. Early hematoma enlargement in primary intracerebral hemorrhage. Curr Drug Targets 2017;18:1345–8. [DOI] [PubMed] [Google Scholar]
- [38].Wilkinson DA, Keep RF, Hua Y, et al. Hematoma clearance as a therapeutic target in intracerebral hemorrhage: from macro to micro. J Cereb Blood Flow Metab 2018;38:741–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lioutas VA, Marchina S, Caplan LR, et al. Endotracheal intubation and in-hospital mortality after intracerebral hemorrhage. Cerebrovasc Dis 2018;45:270–8. [DOI] [PubMed] [Google Scholar]
- [40].Busl KM. Nosocomial infections in the neurointensive care unit. Neurosurg Clin N Am 2018;29:299–314. [DOI] [PubMed] [Google Scholar]
- [41].Hinduja A, Dibu J, Achi E, et al. Nosocomial infections in patients with spontaneous intracerebral hemorrhage. Am J Crit Care 2015;24:227–31. [DOI] [PubMed] [Google Scholar]
- [42].Mathieu A, Guillon A, Leyre S, et al. Aerosolized lidocaine during invasive mechanical ventilation: in vitro characterization and clinical efficiency to prevent systemic and cerebral hemodynamic changes induced by endotracheal suctioning in head-injured patients. J Neurosurg Anesthesiol 2013;25:8–15. [DOI] [PubMed] [Google Scholar]
- [43].Wen Z, Zhang H, Ding J, et al. Continuous versus intermittent subglottic secretion drainage to prevent ventilator-associated pneumonia: a systematic review. Crit Care Nurse 2017;37:e10–7. [DOI] [PubMed] [Google Scholar]
- [44].Wei HP, Yang K. Effects of different oral care scrubs on ventilator-associated pneumonia prevention for machinery ventilates patient: a protocol for systematic review, evidence mapping, and network meta-analysis. Medicine (Baltimore) 2019;98:e14923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kozka M, Sega A, Wojnar-Gruszka K, et al. Risk factors of pneumonia associated with mechanical ventilation. Int J Environ Res Public Health 2020;17:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mosier JM, Hypes CD. Mechanical ventilation strategies for the patient with severe obstructive lung disease. Emerg Med Clin North Am 2019;37:445–58. [DOI] [PubMed] [Google Scholar]
- [47].Wu H, Zhao X, Ting Kung B, et al. Effect of nasogastric tube on salivagram result in paediatric patients. Nucl Med Commun 2019;40:894–7. [DOI] [PubMed] [Google Scholar]
- [48].Wang J, Zhang M, Pan W, et al. Management of recurrent tracheoesophageal fistula after esophageal atresia and follow-up. Dis Esophagus 2017;30:1–8. [DOI] [PubMed] [Google Scholar]


