Abstract
Context
The variety of tumor-seeking radiopharmaceuticals, which are currently in clinical use, may have a potential role as imaging agents for adrenal gland tumors, due to physiological characteristics of this organ.
Objective
The purpose of this study was to evaluate the diagnostic potential of 99mTc-HYNIC-TOC, 99mTc(V)-DMSA, and 99mTc-MIBI in the assessment of adrenal tumors, by correlating with imaging findings and histopathologic results.
Design
The research is designed as a cross-sectional prospective study.
Patients and method
The study included 50 patients with adrenal tumors (19 hormone-secreting and 31 nonfunctioning) and 23 controls without adrenal involvement. In all patients, single-photon emission computed tomography (SPECT) was performed, using qualitative and semiquantitative analysis. The tumor to non-tumor tracer uptake was conducted by using a region-of-interest technique. Adrenal to background (A/B) ratio was calculated in all cases.
Results
99mTc-HYNIC-TOC scintigraphy showed a high statistical significance between A/B ratios, while other two tracers resulted in a lower sensitivity, specificity and accuracy. Futhermore, 99mTc-HYNIC-TOC could have a high diagnostic yield to detect adrenal tumors (the receiver-operating-characteristic curve analysis, A/B ratio cut-off value of 8.40).
Conclusion
A semiquantitative SPECT analysis showed that 99mTc-HYNIC-TOC is a highly sensitive tumor-seeking agent for the accurate localization of adrenal tumors.
Keywords: scintigraphy, adrenal tumor, 99mTc(V)-DMSA, 99mTc-HYNIC-TOC, 99mTc-MIBI
INTRODUCTION
Benign or malignant primary adrenal tumors (AT) are derived from the adrenal medulla or cortex. Differences in embryological origin, structure and function of the adrenal cortex and medulla result in a diverse clinical presentation of these tumors, which is associated with high rates of morbidity and mortality (1-3).
Adrenal masses accidentally detected by imaging techniques, without any evidence of the impaired structure and function of the adrenal glands, are referred to as adrenal incidentalomas (AI) (2, 4, 5). Evidences, mostly obtained from imaging examination and autopsy, suggest an increased prevalence and incidence of AI in older people, as well as in female gender (2, 6, 7). According to recent studies up to 80% of AT are nonfunctioning adenomas, 20-30% secrete cortisol, while up to 10% are aldosterone secreting tumors (1, 4, 8-10). The prevalence of pheochromocytomas is 3-6%, while androgens or estrogens secreting masses and primary malignancies and adrenal metastases are extremely rare (4, 8-10, 11). Whether these tumors are hormone-secreting or not, their heterogeneity contributes not only to various clinical manifestations, but also leads to a more complex diagnostic approach (2, 3, 10).
In order to define functional activity of AT, the initial clinical assessment is followed by a laboratory evaluation of medulla or cortex (2, 3, 10). Computed tomography (CT) scans and magnetic resonance imaging (MRI) provide insight into detailed anatomy and potential determination of adrenal tumor nature (12, 13). According to comparative imaging studies, radionuclide imaging has a complementary and increasingly important role in the characterization of adrenal lesions. Radiotracers, synthesized as precursor-like compounds, can mimic metabolic pathways, showing affinity for adrenal gland tissue (14, 15). Although 131I- and 123I-metaiodobenzylguanidine (MIBG) scintigraphy supposed to be the sovereign method for the identification and localization of pheochromocytoma, this imaging modality showed particularly limited success in the evaluation of adrenocortical tumors (15, 16).
Various tumor-seeking radiopharmaceuticals are potent agents for tumour characterization and may also provide functional status of these tumors. On this basis, the growing tendency of using these tumor tropic radiolabeled agents in the assessment of adrenal tumors is completely understandable (15-17).
In this study, we emphasized the role of nuclear medicine imaging using Technetium-99m radiolabeled agents: hydrazinonicotinylacid-d-phenylalanyl-1-tyrosine 3-octreotide (99mTc-HYNIC-TOC), hexakis-2-methoxyisobutylisonitrile (99mTc-MIBI) and dimercaptosuccinic acid (99mTc(V)-DMSA), in the diagnostic evaluation of adrenal tumors.
MATERIAL AND METHODS
Study population
This cross-sectional study included 73 (58 females, 15 males) consecutive patients, of which 50 with confirmed diagnosis of AT. The control group comprised 23 patients with clinically diagnosed pituitary adenoma without adrenal involvement. The study was conducted during the years 2016-2019 at the Clinical Center Kragujevac, Serbia, in accordance with the Declaration of Helsinki (World Medical Association) and institutional ethical committee. Informed consent has been obtained for all subjects before the diagnostic procedures. Exclusion criteria were: age <18 years, pregnancy, breast feeding, diseases and/or drugs influencing hormonal secretion, disorders with a similar clinical presentation, systemic or infiltrative diseases potentially affecting the adrenal glands, history of malignant disease and other severe life-threatening diseases, no consent being given.
Biochemical parameters and imaging procedures
All patients underwent a standardized diagnostic evaluation of hypothalamic–pituitary–adrenal tumors, based on biochemical, clinical parameters and imaging criteria (2, 3, 10, 13, 14). Functional status of pituitary and adrenal glands was evaluated by baseline hormonal assessment which included serum cortisol (with overnight, low-dose and high-dose dexamethasone suppression test-DST), adrenocorticotropic hormone (ACTH), prolactin, potassium, aldosterone, progesterone, testosterone and β-estradiol, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), plasma free metanephrine and catecholamines.
The imaging evaluation of pituitary and adrenal glands was assessed with a 1.5-T closed magnet (Magnetom Symphony™, Siemens, Germany), which included T1- and T2- weighted images, plus chemical shift imaging (CSI) (in-phase and out-of-phase imaging) and/or dynamic-gadolinium sequences. Conventional morphological characteristics were also assessed with a 64-row multi-detector CT scanner (Aquilion™, Toshiba, Japan). The standard examination protocol comprised a pre-contrast and post-contrast (60sec/15min) scanning with iodinated contrast agent, with quantification of the percentage of absolute or relative contrast enhancement.
Nuclear medicine imaging
All patients, adrenal tumor group and controls, underwent radionuclide imaging with all three of the radiopharmaceuticals (99mTc-HYNIC-TOC, 99mTc(V)-DMSA and 99mTc-MIBI), within 4-6 week interval between each scan. All radiopharmaceuticals were prepared using a commercially available kit, with recommended administered activity according to the manufacturer’s instructions.
Acquisition protocol: the whole-body scans were performed in the anterior and posterior projections (256x1024 matrix, 12 cm/min), on dual-head Gamma camera (Syngo-E.cam™, Siemens, Germany), equipped with low energy high resolution collimators, at a window setting of 140 keV. Single-photon emission computed tomography (SPECT) scan of the region of interest was conducted with the following parameters: 360° noncircular orbit (body contour mode) step and shoot mode, at 30s per view, 1.23 zoom. The acquired data were collected in a 128x128 image matrix, and reconstructed in transverse, coronal and sagittal projections using an iterative ordered subset expectation (OSEM) algorithm.
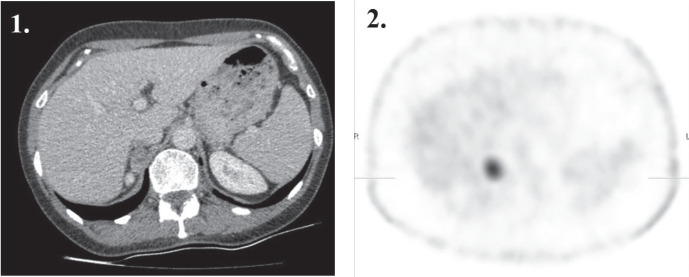
All images were first interpreted qualitatively (visually) by two experienced nuclear medicine physicians, who were unaware of the other imaging findings and/or other clinical information. A focal tracer uptake in the region of interest, greater than background activity, was considered a positive finding (Fig. 1). There was no inter-reader disagreement.
Figure 1.
A transaxial (1) contrast-enhanced CT image shows a 30 mm homogeneous circumscribed well delineated tumor in the right adrenal; (2) <sup>99m</sup>Tc-HYNIC-TOC SPECT image with a focal somatostatin-avid tracer uptake in the right adrenal mass, which was confirmed as a lipid-poor adenoma.
Further analysis of all visual findings was done to compare the tumor tracer uptake to non tumor tissue. All data were analyzed on a Syngo-E.cam™ system using a region-of-interest (ROI) technique for semiquantitative analysis of major organ and tumor uptake.
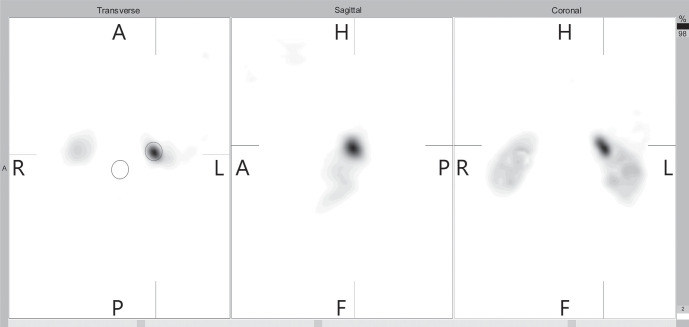
Semi-quantitative analysis was performed by calculation of tumor to background ratios using manually drawn ROI over the tumor as well as a contralateral ROI copied and mirrored in an axial (transverse sectional) slice (Fig. 2). An experienced nuclear medicine physician manually positioned the ROI on the transverse SPECT images of each participant without changing the size or shape of the ROIs. A set of standard SPECT ROI that defined the physiological adrenal uptake was also established. Total counts were measured for all slices correcting for background counts, which calculated the maximum pixel activity between the tumor’s ROI to the contralateral mirror ROI, respectively. The mean ROI values as total counts/total pixels were measured and the fraction was calculated as A/B ratios in all patients.
Figure 2.
Transverse, sagittal and coronal slices of the acquired SPECT, post processing using iterative reconstruction OSEM method. A ROI was drawn on transversal image around the tumor (red circle) while a mirror ROI was drawn above background tissue (blue circle).
Statistical analysis
All statistical analysis was performed using SPSS for Windows 20.0 (SPSS Inc. USA). Continuous variables are summed as arithmetic means, medians and standard deviations, and categorical variables as proportions (percentages of categories). The estimates of sensitivity, specificity, positive and negative predictive values, and accuracy, were obtained with the use of 2x2 contingency tables. The mean values and standard deviation were used for continuous variables, whereas the number and percentage were used for nominal variables. The alpha level for significance was set to p<0.05.
RESULTS
The study involved 73 (58 females, 15 males; mean age 52.68±12.43y; age range, 24–72 y) consecutive patients, of which 50 with clinical diagnosis of AT (mean age 56.03±11.53; age range, 31-72y). The control group (CG) were a consecutive series of 23 patients with pituitary tumor, without clinical and/or imaging evidence of adrenal involvement (2 women and 21 men; mean age 47.78±12.78, age range 24-69 y).
All patients (AT and controls) underwent the diagnostic protocol which included clinical, biochemical and hormonal parameters, CT/MR imaging as well as immunopathological findings after surgery. Distribution of demographic characteristics and clinical parameters of the patients with adrenal tumors are reported in Table 1. Androgen-secreting adrenal tumors have not been detected in study population. In all cases of the secreting forms (SF) of AT (n=19), a final diagnosis was confirmed histopathologically, after adrenalectomy. In 14 patients, a laparoscopic transperitoneal approach was used (73.7%), while 5 patients (27.3%) underwent open adrenalectomy. Despite the standard CT/MR imaging evaluation, the functional imaging of adrenal lesions was evaluated with 131I-MIBG scintigraphy. This tracer uptake was positive in all 4 cases of pheochromocytomas. For clinically silent adrenal masses with diameter less than 4cm (n=13), serial clinical follow-up examinations have been scheduled up to 2 years.
Table 1.
Demographic and clinical features of patients with adrenal tumors
| All adrenal tumors | Non-secreting tumors | Hormonally secreting tumors | |||
|---|---|---|---|---|---|
| Cortisol | Aldosterone | Catecholamines | |||
| No. of adrenal masses | 50 | 31 | 9 | 6 | 4 |
| Gender | |||||
| male | 12 | 7 | 0 | 4 | 1 |
| female | 38 | 24 | 9 | 2 | 3 |
| Age | 53.06±11.53 | 52.50±9.53 | 53.60±13.54 | ||
| Diagnosis | |||||
| imaging (CT/MRI) | 13 | 13 | |||
| histopathology | 37 | 18 | 9 | 6 | 4 |
| Diameter meana (cm) | 3.75±1.39 | 4.55±1.61 | 3.06±1.18 | ||
| Site | |||||
| right | 26 | 17 | 4 | 3 | 2 |
| left | 17 | 10 | 3 | 2 | 2 |
| bilateral | 7 | 4 | 2 | 1 | 0 |
| Metabolic characteristics | |||||
| hyperlipidemia | 26 | 18 | 4 | 3 | 1 |
| hypertension | 21 | 13 | 3 | 1 | 4 |
| elevated fasting glucose | 7 | 4 | 1 | 2 | 0 |
| abdominal obesity | 31 | 22 | 6 | 2 | 1 |
a. the largest diameters of adrenal lesions.
Based on this qualitative image analysis, the number of true positives, true negatives, false positives and false negatives findings, were used to calculate sensitivity, specificity, positive and negative predictive values and accuracy for 99mTc-HYNIC-TOC, 99mTc(V)-DMSA and 99mTc-MIBI scintigraphy (Table 2).
Table 2.
The qualitative scintigraphic analysis of adrenal tumors
| n=73 | Sensitivity (%) (95% CIa) |
Specificity (%) (95% CIa) |
PPV (%) (95% CIa) |
NPV (%) (95% CIa) |
Accuracy (%) |
|---|---|---|---|---|---|
| 99mTc-HYNIC-TOC | 77.78 (57.74-91.38) |
89.47 (66.86-98.70) |
91.30 (71.96-98.93) |
73.91 (51.59-89.77) |
82.60 |
| 99mTc(V)-DMSA | 59.26 (38.80-77.61) |
84.21 (60.42-96.62) |
84.21 (60.42-96.62) |
59.26 (38.80-77.61) |
69.56 |
| 99mTc-MIBI | 51.85 (31.95-71.33) |
89.47 (66.86-98.70) |
87.50 (61.65-98.45) |
56.57 (37.43-74.54) |
67.39 |
a. confidence interval; b-positive predictive value; c-negative predictive value.
Furthermore, the adrenal to background ratios with all three radiopharmaceuticals were compared using semiquantitative analysis. In SPECT studies, the A/B ratios with 99mTc-HYNIC-TOC were significantly higher in AT group (16.59±5.55) compared with controls (5.78±1.70) (t-test for independent samples* t(73)=6.61, p<0.001). The differences of A/B ratios between AT and control groups in subjects with 99mTc(V)-DMSA (Md=4.23 vs. Md=3.63 respectively) and 99mTc-MIBI (Md=4.74 vs. Md=4,34 respectively) were not statistically significant (p>0.05, Mann-Whitney U Test#). Distribution of these findings among different groups of patients is shown in Figure 3.
Figure 3.
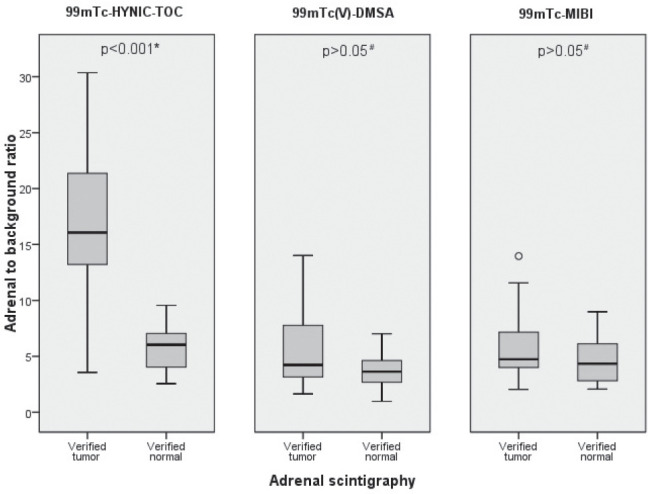
Distribution of adrenal/background ratios in the 3 studied groups revealing a statistically significant difference (t-test for independent samples*) only with 99mTc-HYNIC-TOC SPECT between adrenal tumors (n=50) and the control group (n=23).
According to these results, only 99mTc-HYNIC-TOC scintigraphy showed a high diagnostic potential in detecting adrenal tumors, while other two tracers resulted in a lower sensitivity, specificity, as well as accuracy.
The accuracy of semiquantitative 99mTc-HYNIC-TOC scintigraphy in classifying correctly the existence of AT was evaluated by using the receiver-operating-characteristic (ROC) analysis. The ROC curves analysis revealed that 99mTc-HYNIC-TOC scintigraphy provided the discriminating power in detecting AT, at the optimal A/B ratio cut-off value of 8.40 (area= 0.901, p<0.001, sensitivity 82.4%, specificity 95.5%) (Fig. 4).
Figure 4.
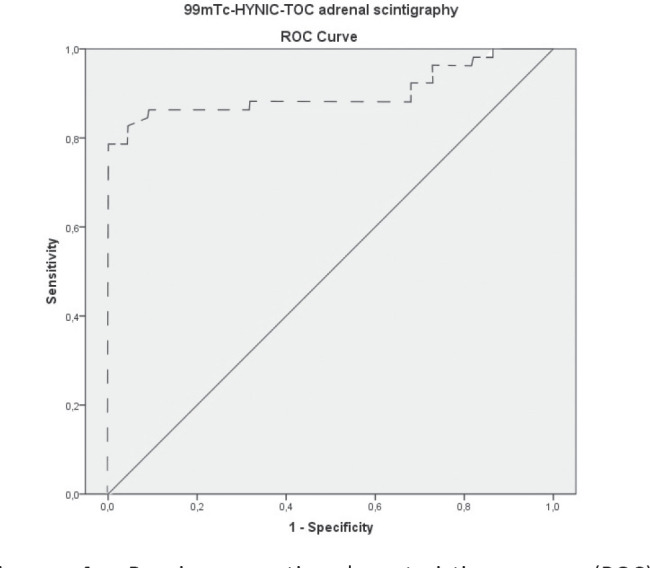
Receiver-operating-characteristic curve (ROC) for 99mTc-HYNIC-TOC SPECT uptake values. This curve was constructed from A/B ratios of adrenal tumors lesions and of normal adrenals.
DISCUSSION
The initial clinical workup for AT’s is to distinguish benign from malignant processes of the patients, harboring the lesion and the size of the lesion. A complete clinical and biochemical evaluation allows the clinician to distinguish tumors which require surgical removal, from those which should be subject to further investigation (2, 3, 18).
This commonly encountered clinical problem was well recognized in our study revealing that non-secreting AT’s, so-called incidentalomas, are most frequent (62%). Adrenal masses which resulted in the hypersecretion of hormones and metabolites were detected in 38% of patients, developing marked symptoms, including cortisol-secreting (18%), aldosterone-secreting (12%) and medullary tumors (8%). The main size of all AT’s was 3.75±1.39 (range, 1.0–15.0cm), with larger size of adrenal cortical tumors (4.55±1.61) than of medullary tumors (3.06±1.18). Other types of adrenal tumors, including malignant ones, have not been detected in study population.
Our study results partially matches with the observation of others regarding tumor type, while is in accordance with the facts that about 60-80% of AT’s are asymptomatic with a size less than 4 cm in the largest diameter (3, 10, 19-20).
Although gender distribution among patients with AT’s varies in different series, the females are most commonly affected predominantly in the fourth to sixth decade of life (6, 7, 14, 16). In the present study, the gender distribution of adrenal masses is in accordance with the majority of investigations with similar methodology. Female subjects were more commonly affected (76%), with mean age of 53.06±11.53.
The extent of diagnosis of AT’s may vary among different institutions, but most would agree that the clinical status is of primary concern. The main recommendation is hormonal screening, followed by specific morphology imaging (2, 3, 9, 13). The final diagnosis of AT in the present study was confirmed by initial clinical and follow-up examinations or and/or radiological imaging, as well as histopathological findings after surgery (Table 1).
During the past decades, the development of morphology imaging techniques has resulted in the diagnosis of unsuspected AT’s (incidentalomas) (12, 13, 21). Molecular imaging for diagnosis of AT’s using various radiopharmaceuticals (tracers) remains controversial, but most would agree that the norepinephrine analogue metaiodobenzylguanidine (MIBG) has high diagnostic accuracy only in detecting pheochromocytomas, with high sensitivity (83%-100%) and specificity (95–100%) (17, 22, 23).
Although many scintigraphy findings of AT have been reported, there are only few studies regarding comparative evaluation of other tumor-seeking agents for detecting adrenal masses (14, 15). The main purpose of this study was to evaluate the use of scintigraphic modality in the assessment of AT in order to allow the clinician to make a precise diagnosis and customize treatment accordingly.
Numerous studies have reported that somatostatin receptor scintigraphy (SRS) is equal or superior to other conventional imaging methods for sympathomedullary imaging especially when the MIBG is false negative. The visualization of neuroendocrine tumors is allowed on the basis of existing of somatostatin membrane receptors (SSTR) (24-26). Encouraged by the results of various studies that somatostatine radiolabelled analog 99mTc-HYNIC-TOC has a high sensitivity in localizing pheochromocytoma, we performed this scintigraphic modality in a group of patients with adrenal tumors. Results of the in vitro studies demonstrate that type 2, 3 and 5 SSTR are expressed in adrenal cortical tumors (27, 28), but SRS has not been widely used in clinical practice (27, 29, 30).
The results of this study revealed that 99mTc-HYNIC-TOC scintigraphy demonstrates high-grade visually uptake in AT compared to normal adrenal accumulation, with the sensitivity and specificity of 77.78% and 89.47 respectively (Table 1). Reports on the sensitivity of this technique have been variable, ranging from 77% to 88% (30-32). The diagnostic potential of qualitative scintigraphy with 99mTc(V)-DMSA and 99mTc-MIBI was lower, with overall sensitivities of 59.26% and 51.85% respectively (Table 1). Even though a previous study results demonstrated increased uptake of 99mTc(V)-DMSA and 99mTc-MIBI in various benign and malignant tumors (35-37), there are no published series involving these tracers in the AT’s.
In our series, the adrenal involvement was evaluated using simple semiquantitative index-A/B ratios between AT group and controls. The 99mTc-HYNIC-TOC A/B ratios on SPECT studies were higher with statistically significant difference between groups (p<0.001), comparing with 99mTc(V)-DMSA and 99mTc-MIBI (Fig. 3). To our knowledge there are no published data performed via this approach and this is the first study to evaluate adrenal masses, using the semiquantitative method, with all three radiopharmaceuticals. Our study results suggest that 99mTc-HYNIC-TOC semiquantitative method could have the highest diagnostic yield for detecting AT. Based upon the present results, SRS can be a front-line imaging modality of adrenocortical and adrenomedullary tumors respectively. 99mTc-HYNIC-TOC positive scintigraphy, accompanied with MIBG negative scan, highly suggests the existence adrenocortical adenoma.
Interestingly, using histology as the gold standard, combining SRS with radiology imaging identified 5 (13.5%) more patients with AT than were with CT/MRI alone. This suggests that SRS should be used in patients in whom conventional imaging methods fail to localize a possible primary tumor, with existing clinical symptoms.
The lower sensitivity of 99mTc(V)-DMSA and 99mTc-MIBI revealed that scintigraphy with these tracers was not sufficiently sensitive for evaluation of patients with AT. Accumulation of these tracers is generally related to the functional nature related to tumor pathophysiology (35, 37).
In conclusion, our findings suggest that somatostatin receptor scintigraphy is the single most sensitive method for localizing AT, compared with MRI and MDCT. Furthermore, the expression of high-affinity SSTR in most AT implies that the 99mTc-HYNIC-TOC may be of value for initial diagnosis and for the post-surgical detection of residual tumors, multifocal and metastatic disease, and therapeutic possibilities of radiolabeled somatostatin analogs.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The study was supported by the University of Kragujevac, Faculty of Medical Sciences, (Junior Project N° 07/15).
References
- 1.Lirov R, Else T, Lerario AM, Hammer GD. Adrenal Tumors. In: DeVita VT Jr, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg’s Cancer: principles & practice of oncology. 10th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2015. pp. 1195–1204. [Google Scholar]
- 2.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, Tabarin A, Terzolo M, Tsagarakis S, Dekkers OM. Management of adrenal incidentalomas: European society of endocrinology clinical practice guideline in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol. 2016;175(2):G1–G34. doi: 10.1530/EJE-16-0467. [DOI] [PubMed] [Google Scholar]
- 3.Terzolo M, Stigliano A, Chiodini I, Loli P, Furlani L, Arnaldi G, Reimondo G, Pia A, Toscano V, Zini M, Borretta G, Papini E, Garofalo P, Allolio B, Dupas B, Mantero F, Tabarin A, Italian Association of Clinical Endocrinologists AME position statement on adrenal incidentaloma. Eur J Endocrinol. 2011;164(6):851–870. doi: 10.1530/EJE-10-1147. [DOI] [PubMed] [Google Scholar]
- 4.Hanna FW, Issa BG, Lea SC, George C, Golash A, Firn M, Ogunmekan S, Maddock E, Sim J, Xydopoulos G, Fordham R. Adrenal lesions found incidentally: how to improve clinical and cost-effectiveness. BMJ Open Quality. 2020;9(1) doi: 10.1136/bmjoq-2018-000572. e000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young WF. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007;356(6):601–610. doi: 10.1056/NEJMcp065470. [DOI] [PubMed] [Google Scholar]
- 6.Tang YZ, Bharwani N, Micco M, Akker S, Rockall AG, Sahdev A. The prevalence of incidentally detected adrenal enlargement on CT. Clin Radiol. 2014;69(1):e37–42. doi: 10.1016/j.crad.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149:273–285. doi: 10.1530/eje.0.1490273. [DOI] [PubMed] [Google Scholar]
- 8.Reimondo G, Puglisi S, Pia A, Terzolo M. Autonomous hypercortisolism: definition and clinical implications. Minerva Endocrinol. 2019;44(1):33–42. doi: 10.23736/S0391-1977.18.02884-5. [DOI] [PubMed] [Google Scholar]
- 9.Morelli V, Palmieri S, Salcuni AS, Eller-Vainicher C, Cairoli E, Zhukouskaya V, Scillitani A, Beck-Peccoz P, Chiodini I. Bilateral and unilateral adrenal incidentalomas: biochemical and clinical characteristics. Eur J Endocrinol. 2013;168:235–241. doi: 10.1530/EJE-12-0777. [DOI] [PubMed] [Google Scholar]
- 10.Cvasciuc IT, Gull S, Oprean R, Lim KH, Eatock F. Changing pattern of pheochromocytoma and paraganglioma in a stable UK population. Acta Endocrinol (Buchar) 2020;16(1):78–85. doi: 10.4183/aeb.2020.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zawadzka-Leska SK, Radziszewski M, Malec K, Stadnik A, Ambroziak U. Predictive value of chromogranin A in a diagnosis towards Pheochromocytoma in adrenal incidentaloma. Acta Endocrinol (Buchar) 2016;12(4):437. doi: 10.4183/aeb.2016.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundin A. Imaging of adrenal masses with emphasis on adrenocortical tumors. Theranostics. 2012;2:516–522. doi: 10.7150/thno.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blake MA, Cronin CG, Boland GW. Adrenal imaging. AJR Am J Roentgenol. 2010;194(6):1450–1460. doi: 10.2214/AJR.10.4547. [DOI] [PubMed] [Google Scholar]
- 14.Maurea S, Mainenti PP, Ponsiglione A, Salvatore M. Advanced Adrenal Imaging: Comparison between Radionuclide and MR Techniques. J Adv Radiol Med Image. 2016;1(1):103. [Google Scholar]
- 15.Gross MD, Bui C, Shapiro B. Adrenocortical scintigraphy. In: Ell PJ, Gambhir SS, editors. Nuclear Medicine in Clinical Diagnosis and Treatment. 3rd ed. Edinburgh: Churchill and Livingstone; 2004. pp. 45–52. [Google Scholar]
- 16.McDermott S, McCarthy CJ, Blake MA. Images of pheochromocytoma in adrenal glands. Gland surg. 2015;4(4):350. doi: 10.3978/j.issn.2227-684X.2014.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundin A. Imaging of adrenal masses with emphasis on adrenocortical tumors. Theranostics. 2012;2:516–522. doi: 10.7150/thno.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeiger M, Thompson G, Duh QY, Hamrahian A, Angelos P, Elaraj D, Fishman E, Kharlip J. The American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocrine Practice. 2009;15:1–20. doi: 10.4158/EP.15.S1.1. [DOI] [PubMed] [Google Scholar]
- 19.Patel RD, Vanikar AV, Suthar KS, Kanodia KV. Primary Adrenal Tumors-Five Years Single Centre Experience. J Pathol. 2012;2(04):107–112. [Google Scholar]
- 20.Martucci VL, Pacak K. Pheochromocytoma and paraganglioma: diagnosis, genetics, management, and treatment. Curr Probl Cancer. 2014;38(1):7–41. doi: 10.1016/j.currproblcancer.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schieda N, Alrashed A, Flood TA, Samji K, Shabana W, McInnes MD. Comparison of quantitative MRI and CT washout analysis for differentiation of adrenal pheochromocytoma from adrenal adenoma. Am J Roentgenol. 2016;206(6):1141–1148. doi: 10.2214/AJR.15.15318. [DOI] [PubMed] [Google Scholar]
- 22.van Berkel A, Rao JU, Lenders JW, Pellegata NS, Kusters B, Piscaer I, Hermus AR, Plantinga TS, Langenhuijsen JF, Vriens D, Janssen MJ. Semiquantitative 123I-Metaiodobenzylguanidine scintigraphy to distinguish pheochromocytoma and paraganglioma from physiologic adrenal uptake and its correlation with genotype-dependent expression of catecholamine transporters. J Nucl Med. 2015;56(6):839–846. doi: 10.2967/jnumed.115.154815. [DOI] [PubMed] [Google Scholar]
- 23.Taïeb D, Timmers HJ, Hindié E, Guillet BA, Neumann HP, Walz MK, Opocher G, De Herder WW, Boedeker CC, De Krijger RR, Chiti A. EANM 2012 guidelines for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2012;39(12):1977–1995. doi: 10.1007/s00259-012-2215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasad V, Fetscher S, Baum RP. Changing role of somatostatin receptor targeted drugs in NET: nuclear medicine’s view. J Pharm Pharm Sci. 2007;10(2):321s–327s. [PubMed] [Google Scholar]
- 25.Maxwell JE, Howe JR. Imaging in neuroendocrine tumors: an update for the clinician. Int J Endocr Oncol. 2015;2:159–168. doi: 10.2217/ije.14.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bombardieri E, Ambrosini V, Aktolun C, Baum RP, Bishof-Delaloye A, Del Vecchio S, Maffioli L, Mortelmans L, Oyen W, Pepe G, Chiti A. 111In-pentetreotide scintigraphy: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging. 2010;37:1441–1448. doi: 10.1007/s00259-010-1473-6. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler CG, Brown JW, Schally AV, Erler A, Gebauer L, Treszl A, Young L, Fishman LM, Engel JB, Willenberg HS, Petersenn S. Expression of neuropeptide hormone receptors in human adrenal tumors and cell lines: antiproliferative effects of peptide analogues. Proc Natl Acad Sci USA. 2009;106(37):15879–15884. doi: 10.1073/pnas.0907843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pisarek H, Stêpieñ T, Kubiak R, Pawlikowski M. Somatostatin receptors in human adrenal gland tumors–immunohistochemical study. Folia Histochem Cytobiol. 2008;46(3):345–351. doi: 10.2478/v10042-008-0051-2. [DOI] [PubMed] [Google Scholar]
- 29.Blanchet EM, Martucci V, Pacak K. Pheochromocytoma and paraganglioma: current functional and future molecular imaging. Front Oncol. 2012;1:58. doi: 10.3389/fonc.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Li F, Zhuang H, Jing H, Du Y, Zeng Z. 99mTc-HYNIC-TOC scintigraphy is superior to 131I-MIBG imaging in the evaluation of extraadrenal pheochromocytoma. J Nuc Med. 2009;50(3):397–400. doi: 10.2967/jnumed.108.058693. [DOI] [PubMed] [Google Scholar]
- 31.Artiko V, Afgan A, Petrović J, Radović B, Petrović N, Vlajković M, Šobić-Šaranović D, Obradović V. Evaluation of neuroendocrine tumors with 99mTc-EDDA/HYNIC TOC. Nucl Med Rev Cent East Eur. 2016;19(2):99–103. doi: 10.5603/NMR.2016.0020. [DOI] [PubMed] [Google Scholar]
- 32.Garai I, Barna S, Nagy G, Forgacs A. Limitations and pitfalls of 99mTc-EDDA/HYNICTOC (Tektrotyd) scintigraphy. Nucl Med Rev Cent East Eur. 2016;19(2):93–98. doi: 10.5603/NMR.2016.0019. [DOI] [PubMed] [Google Scholar]
- 33.Shukla J, Mittal BR. Dimercaptosuccinic acid: A multifunctional cost effective agent for imaging and therapy. Indian J Nucl Med. 2015;30(4):295–302. doi: 10.4103/0972-3919.164015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Saeedi FJ, Mathew PM, Luqmani YA. Assessment of Tracer 99m Tc (V)-DMSA Uptake as a Measure of Tumor Cell Proliferation In Vitro. PloS One. 2013;8(1) doi: 10.1371/journal.pone.0054361. e54361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papantoniou V, Tsiouris S, Mainta E, Valotassiou V, Souvatzoglou M, Sotiropoulou M, Nakopoulou L, Lazaris D, Louvrou A, Melissinou M, Tzannetaki A. Imaging in situ breast carcinoma (with or without an invasive component) with technetium-99m pentavalent dimercaptosuccinic acid and technetium-99m 2-methoxy isobutyl isonitrile scintimammography. Breast Cancer Res. 2004;7(1) doi: 10.1186/bcr948. R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vukomanovic V, Matovic M, Doknic M, Ignjatovic V, Vukomanovic IS, Djukic S, Djukic A. Adrenocorticotropin-producing pituitary adenoma detected with 99mTc-hexakis-2-methoxy-isobutyl-isonitrile single photon emission computed tomography. A case report. Acta Endocrinol (Buchar) 2015;11:253–256. [Google Scholar]
- 37.Moretti JL, Hauet N, Caglar M, Rebillard O, Burak Z. To use MIBI or not to use MIBI? That is the question when assessing tumour cells. Eur J Nucl Med Mol Imaging. 2005;32(7):836–842. doi: 10.1007/s00259-005-1840-x. [DOI] [PubMed] [Google Scholar]