Abstract
Objectives
To assess the effects of electroacupuncture (EA) at the Zusanli (ST36), Guanyuan (CV4), Zhongwan (CV12), and Fenglong (ST40) acupoints on sirtuin 1 (SIRT1) and glucose transporter type 4 (GLUT4) expression in high-fat diet (HFD)-induced insulin-resistant (IR) rats.
Methods
Wistar rats were divided into normal control (NC), HFD, and HFD+EA groups. NC rats were fed a standard chow diet and did not receive EA. After being fed an HFD for eight weeks, rats in the HFD+EA group received EA at 2 Hz five times a week for eight weeks. Rats in the HFD group did not receive EA.
Results
In HFD-induced IR rats, EA inhibited body weight increase and water intake, which were observed in HFD rats. EA had no effect on fasting blood glucose and postprandial blood sugar levels. Intraperitoneal insulin tolerance testing revealed that EA enhanced insulin sensitivity in HFD-induced IR rats. Compared with NC rats, SIRT1 and GLUT4 were downregulated in the quadriceps femoris of HFD-fed rats but were increased after eight weeks of EA stimulation.
Conclusions
EA enhanced HFD-induced insulin resistance by activating SIRT1 and GLUT4 in the quadriceps femoris. These results provide powerful evidence supporting the beneficial effects of EA on HFD-induced insulin resistance.
Keywords: SIRT1, GLUT4, electroacupuncture, insulin resistance, high-fat diet
INTRODUCTION
Obesity and diabetes mellitus are becoming increasingly important medical issues. Insulin resistance (IR) is the most common factor in metabolic disorders such as hyperglycemia and hyperinsulinemia, which are caused by IR-related conditions such as obesity, type 2 diabetes mellitus, metabolic syndrome, hypertension, polycystic ovary syndrome, and non-alcoholic fatty liver diseases, as well as aging (1, 2). The incidence of IR-related diseases is increasing worldwide and is likely to continue rising in the coming decades, posing a significant threat to human health (3). IR involves the impaired ability of insulin to inhibit hepatic glucose output and stimulate glucose uptake into muscle and fat (4). Therefore, reducing IR is of critical importance.
Sirtuin 1 (SIRT1) is one of the seven homologs of silent information regulator 2 (Sir2) in mammals. Increasing evidence has suggested that SIRT1 is involved in regulating glucose and lipid metabolism (4-6). Moreover, SIRT1 overexpression exerted protection against insulin resistance in diabetic models (7) and high-fat diet-induced metabolic disorders (8). SIRT1 increases insulin sensitivity by regulating various transcription factors in endocrine signalling pathways, particularly glucose transporter 4 (GLUT4). Skeletal muscle is one of the most important peripheral target organs of insulin, and GLUT4 expression in skeletal muscle is extremely important for insulin sensitivity (9). As such, SIRT1 activation might become a novel target for the treatment of diseases associated with insulin resistance.
An increasing number of individuals manage obesity and diabetes mellitus by changing their daily habits and lifestyles. However, these methods have not been well integrated and are unsuccessful at reducing the incidence of obesity and insulin resistance in the general population (10). If acupuncture could reduce the impaired ability of insulin to inhibit hepatic glucose output, stimulate glucose uptake into muscle and fat, and enhance insulin sensitivity, its clinical use could be expanded greatly.
Experimental and clinical studies have demonstrated that acupuncture elicits multiple biological responses in obesity-related insulin resistance (2, 11, 12). In response to acupuncture, SIRT1 is upregulated, protecting β-cells and regulating gluconeogenesis and glycolysis to maintain glucose homeostasis (6, 13, 14). In addition, we recently found that electroacupuncture (EA) increased insulin sensitivity by activating SIRT1/PGC-1α in diabetic obese mice (15). Although acupuncture is effective for the treatment of insulin resistance, the mechanisms underlying its effects are unclear. Rodents are a valuable model of insulin resistance and provide important insights into the molecular mechanisms underlying insulin resistance associated with lifestyle modifications (12).
The aim of the present study was to investigate whether EA ameliorates the impaired insulin sensitivity in high-fat diet (HFD)-fed IR rats and to evaluate the effects of EA on glucose metabolism by examining the protein expression of SIRT1 and GLUT4 in the quadriceps femoris.
MATERIALS AND METHODS
Experimental animals
Forty specific-pathogen-free Wistar rats (7 weeks of age, 20 male and 20 female) were purchased from the Animal Diseases Control & Prevention Centre of Hubei Province, China (ADCPC, Wuhan, China). All animals were fed a standard chow diet for one week of acclimatization. The rats were housed in an air-conditioned room in a 12:12-h light:dark cycle (lights on at 07:30) and allowed ad libitum access to water and either a standard chow diet (70.0% carbohydrate, 20.0% protein, 10.0% fat; 380 kcal/100 g; ADCPC) or an HFD (38.5% carbohydrate, 15% protein, 46.5% fat; 540 kcal/100 g; SLAC Laboratory Animal, Shanghai, China) for eight weeks (16). All experimental procedures were carried out in accordance with the guidelines for the care and use of Laboratory Animals published by National Institutes of Health of the United States, and approved by the Animal Care and Use of the Model Animal Research Institute at Wuhan Myhalic Biotechnology Co., Ltd. Approval number is HLK-20190718-01.
Study
At eight weeks of age, the rats were divided randomly (50% male and 50% female) into three experimental groups: standard chow diet (normal control, NC, n = 16), HFD (n = 12), and HFD with EA (HFD+EA, n = 12). All animals were allowed ad libitum access to water and their respective diet until they were evaluated at 16 weeks of age. Eight, four, and four rats in the NC, HFD, and HFD+EA groups, respectively, were subjected to a hyperinsulinemic-euglycemic clamp at 16 weeks of age. Rats in the HFD+EA group were treated with EA for eight weeks. Body weight, food intake, water intake, fasting blood-glucose (FBG), and postprandial blood-glucose (PBG) were measured every two weeks during the EA stimulation period. Intraperitoneal glucose tolerance testing (IPGTT) and intraperitoneal insulin tolerance testing (IPITT) were performed at 22 and 23 weeks of age, respectively. The rats were sacrificed with an overdose of pentobarbital at the end of week 24, and the levels of SIRT1 and GLUT4 were measured using western blot and immunohistochemistry.
Hyperinsulinemic–euglycemic clamp
The hyperinsulinemic-euglycemic clamps were performed as described previously with some modifications (12, 17, 18). Rats were anesthetized with sodium pentobarbital (Somnotol; 30 mg/kg), and cannulated in the carotid artery for blood sampling (PE-50 cannula; Smiths medical) and the jugular vein for glucose and insulin infusions (dual PE-10 cannula encased in Silastic tubing; Taihua Silastic). After 4–5 days of recovery, the rats underwent an euglycemic-hyperinsulinemic clamp. The rats were subjected to fasting for 12 h, and human insulin (Humulin R, Novo Nordisk) was infused into the venous circulation at a rate of 2 mU/kg/min for 2 h. The carotid artery was assessed every 5 min using a blood glucose meter (One Touch SureStep Hospital, Lifescan, Inc, USA) throughout the infusion. The arterial (carotid) blood glucose concentration was clamped at the basal fasting level using a variable rate of 20% glucose infusion that was delivered via the jugular cannula. Whole-body insulin sensitivity was defined using the glucose infusion rate (GIR) from 60–120 min of the clamp (GIR60-120) (19). The rats were decapitated upon completion of the clamp procedure.
EA stimulation
EA was applied at the Zusanli (ST36), Fenglong (ST40), Zhongwan (CV12), and Guanyuan (CV4) acupuncture points using 0.30 × 25 mm needles (Suzhou Acupuncture & Moxibustion Appliance Co., China). The Zusanli acupoints are located 5 mm below and lateral to the anterior tubercle of the tibia, and the Fenglong acupoints are located at the midpoint between the depression lateral to the patella ligament and the lateral malleolus of the tibiofibula. Needles were inserted at these points perpendicularly at 3–5 mm. The Guanyuan acupoint is located three-fifths down the ventral midline connecting the umbilicus to the pubic tubercle; the needle at this point was inserted obliquely toward the xiphisternum at 3–5 mm. The Zhongwan acupoint is located at the mid-point of the ventral midline that connects the umbilicus to the sternum; the needle at this point was inserted obliquely toward the pubic tubercle at 3–5 mm. Needles at CV4 and ST36 on the one side, which were linked to ST36 on the other side on the following day, were linked with two electrodes of a Hans acupoint nerve stimulator (HANS-100A, Nanjing Jisheng Medical Technology Co., Ltd., Nanjing, China) EA apparatus. Needles at CV12 and ST40 on the one side, which were linked to ST40 on the other side on the following day, were linked with the other two electrodes of the electrostimulator. The points were stimulated electrically with successive low-frequency waves of 2 Hz and an intensity of 1 mA. Rats in the HFD+EA group received EA stimulation for 10 min each day, with five stimulations performed every week (15, 20, 21). EA stimulation lasted for eight weeks. During the EA stimulation period, all animals were fed a standard chow diet.
Body weight, food intake, water intake, fasting blood glucose, and postprandial blood sugar
Body weight, food intake, water intake, FBG, and PPG were analyzed after 0, 2, 4, 6, and 8 weeks of EA stimulation. Briefly, body weight, food intake, and water intake were measured using electronic scales, and tail-snip FBG and PBG levels were quantified using a glucometer (One Touch Sure Step, Lifescan).
Intraperitoneal glucose and insulin tolerance testing
IPGTT was performed after six weeks of treatment. Rats were injected intraperitoneally with glucose (2 g glucose/kg body mass) after an overnight fast. Blood samples were collected for glucose level determination from the tail vein 0, 30, 60, 90, and 120 min following glucose injection. IPITT were performed after seven weeks of EA treatment. Rats were injected intraperitoneally with regular human insulin (0.5 U/kg body mass) after an overnight fast. Blood samples were collected from the tail vein for glucose determination prior to insulin administration and after 0, 30, 60, 90, and 120 min.
Western blot
The protein expression of SIRT1 was analyzed in the quadriceps femoris of all experimental animals using western blot. Total protein lysates were extracted in radioimmunoprecipitation assay buffer containing protease inhibitors (Pierce, USA). Protein concentrations were determined using a bicinchoninic acid assay kit (Pierce). Protein lysates (40 μg) were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked with 5% skimmed milk at room temperature for 30 min and then incubated with polyclonal antibodies against SIRT1 (1:200) or β-tubulin (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. After three washes with TBST, the blots were incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000) at room temperature for 1 h. Protein bands were visualized using the super signal kit according to the manufacturer’s instructions (Pierce). SIRT1 immunoreactivity was normalized against β-tubulin. The experiment was repeated at least three times for each sample.
Immunohistochemistry
Immunohistochemical staining was performed to detect the expression of GLUT4 in the quadriceps femoris. The quadriceps femoris was removed from the leg of the rats and immersed in a 4% paraformaldehyde solution. Serial 4-μm sections of quadriceps femoris were incubated for 20 min with 3% hydrogen peroxide to quench endogenous peroxide. After several washes with phosphate-buffered saline (PBS), the sections were blocked with 10% normal goat serum in PBS for 30 min and incubated overnight with rabbit anti-rat GLUT4 (1:100; Santa Cruz) at 4°C. The sections were then washed and incubated at room temperature with biotinylated goat anti-rabbit IgG (1:200; Santa Cruz, CA, USA) for 30 min. Nickel-intensified biotinylated diaminobenzidine was used to visualize the signal, and positive expression in the cytoplasm was assessed by measuring the integrated optical density (IOD) using Image Pro Plus 6 software (22, 23).
Statistical analysis
All analyses were performed using SPSS17.0 analytical software. One-way analysis of variance (ANOVA) with subsequent least significant difference (LSD) tests were conducted to determine the significance of differences in multiple comparisons. Data are expressed as the mean ± SD, and P < 0.05 indicates statistical significance.
RESULTS
Hyperinsulinemic-euglycemic clamp
In this study, we evaluated the effects of HFD-induced IR by comparing rats fed standard chow (n = 8) and HFD (n = 8, four rats in each of the HFD and HFD+EA groups). The GIR was then measured during a hyperinsulinemic-euglycemic clamp. The mean GIR in the HFD-fed rats were significantly lower than that in the NC group (19.61 ± 1.47 vs. 26.33 ± 2.09 mg/kg/min, respectively; P < 0.01), indicating that the IR model was established successfully.
EA improved IPITT, but not IPGTT
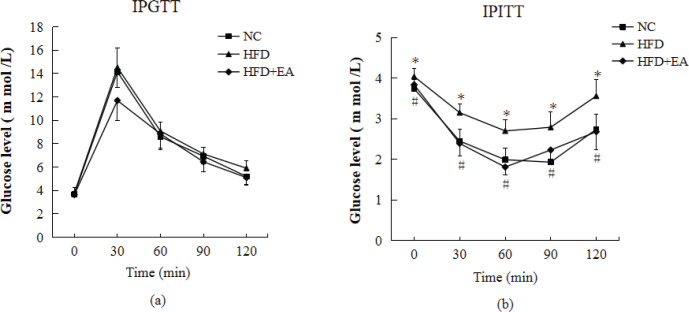
There were no significant differences in the blood glucose levels among groups according to the IPGTT. Compared with the NC group, the IPITT revealed that insulin intolerance was impaired in HFD-induced IR rats. However, the impaired insulin intolerance was ameliorated after EA intervention in HFD+EA rats (Fig. 1).
Figure 1.
Effect of EA on IPGTT and IPITT. (a) Intraperitoneal glucose tolerance test. Rats fasted overnight and were then injected intraperitoneally with glucose (2 g glucose/kg of body weight). Blood glucose levels were measured at the indicated times. (b) Intraperitoneal insulin tolerance test. Rats fasted overnight and were then injected with insulin solution (0.5 U/kg of body weight) intraperitoneally. Blood glucose levels were measured at the indicated time points. Each data point represents the mean ± SD of eight rats. Differences between means were determined using ANOVA followed by LSD’s multiple comparison test. *P < 0.05 vs. NC; #P < 0.05 vs. HFD.
EA reduced body weight gain, food intake, and water intake
The body weight of HFD-fed rats was higher than that of standard chow diet-fed rats at 16 weeks of age. The HFD-fed rats weighed 100 g more than standard chow-fed rats after 24 weeks. Low-frequency EA reduced body weight significantly in HFD-fed rats after six weeks of treatment, and these effects became more significant after eight weeks of EA intervention (Table 1).
Table 1.
Animal characteristics, blood analyses, and IOD
| Parameter (unit) | NC (n = 8) | HFD (n = 8) | HFD+EA (n = 8) |
|---|---|---|---|
| Body weight (g) | |||
| 0 weeks 2 weeks 4 weeks 6 weeks 8 weeks |
293.88 ± 32.91 303.75 ± 49.70 310.00 ± 47.23 312.50 ± 43.87 305.88 ± 62.72 |
356.50 ± 65.96 363.25 ± 76.26 386.43 ± 89.68 392.59 ± 57.31 401.63 ± 91.81 |
352.62 ± 65.40 332.75 ± 67.28 326.62 ± 53.59 307.12 ± 42.11# 294.13 ± 53.78# |
| Food intake (g) | |||
| 0 weeks 2 weeks 4 weeks 6 weeks 8 weeks |
25.77 ± 4.87 25.97 ± 4.25 26.85 ± 2.77 25.41 ± 4.83 21.81 ± 3.64 |
19.46 ± 4.47** 22.27 ± 5.50 22.80 ± 4.44* 20.61 ± 3.50* 18.00 ± 2.07* |
16.51 ± 2.25** 24.67 ± 2.68 20.92 ± 3.10** 19.62 ± 2.56** 18.21 ± 3.78* |
| Water intake (mL) | |||
| 0 weeks 2 weeks 4 weeks 6 weeks 8 weeks |
35.62 ± 8.24 41.62 ± 8.03 55.25 ± 8.73 29.50 ± 5.50 30.87 ± 5.76 |
40.75 ± 9.64 47.62 ± 8.51 66.37 ± 7.78** 36.12 ± 8.37 37.87 ± 5.66* |
32.62 ± 9.30 36.12 ± 6.46## 37.62 ± 9.15## 26.75 ± 6.04# 25.12 ± 7.71## |
| FBG (mmol/L) | |||
| 0 weeks 2 weeks 4 weeks 6 weeks 8 weeks |
3.40 ± 0.95 3.17 ± 0.55 3.86 ± 0.39 3.78 ± 0.38 3.41 ± 0.27 |
4.57 ± 0.92** 4.71 ± 0.43** 4.73 ± 0.59** 4.01 ± 0.30 3.71 ± 0.48 |
4.65 ± 0.66** 3.50 ± 0.69## 4.07 ± 0.55# 3.91 ± 0.30 3.60 ± 0.52 |
| PPG (mmol/L) | |||
| 0 weeks 2 weeks 4 weeks 6 weeks 8 weeks |
5.32 ± 0.66 4.48 ± 0.57 4.56 ± 0.47 4.78 ± 0.53 5.38 ± 0.43 |
6.31 ± 0.78** 5.22 ± 0.61* 5.27 ± 0.49* 5.47 ± 0.73* 5.64 ± 0.56 |
6.12 ± 0.59* 4.56 ± 0.59# 4.47 ± 0.66# 4.48 ± 0.79# 5.24 ± 0.55 |
| IOD of GLUT4 | 0.352 ± 0.018 | 0.297 ± 0.011* | 0.338 ± 0.015 # |
Data are expressed as the mean ± SD. Differences between means were determined using ANOVA followed by LSD multiple comparison test. *P < 0.05 vs. NC; **P < 0.01 vs. NC; # P < 0.05 vs. HFD; ## P < 0.01 vs. HFD. IOD: integrated optical density.
HFD-fed rats showed lower food intake than did standard chow diet-fed rats throughout the experimental period. EA did not reduce the food intake of HFD-fed rats (Table 1).
Water intake was higher in the HFD-fed rats compared with that in the standard chow diet-fed rats throughout the experimental period. However, EA reduced water intake in the HFD-fed rats significantly after two weeks, and the effect was maintained until the end of the experiment. The effect became more significant after eight weeks of EA intervention (Table 1).
FBG and PPG levels were not affected
As shown in Table 1, FBG and PPG were higher in HFD-fed rats than in the standard diet-fed rats at 16 weeks of age. However, the FBG and PPG levels show no significant difference at the end of the experimental period in EA-treated HFD-fed rats compared with those in untreated rats.
EA upregulated SIRT1 and GLUT4 in the quadriceps femoris
The effect of EA on SIRT1 expression was investigated because of the association between SIRT1 and metabolic activity and its critical role in insulin sensitivity. The expression of SIRT1 decreased significantly in HFD-fed IR rats compared with that in NC rats. In addition, EA significantly upregulated SIRT1 compared with that in HFD-fed rats (Fig. 2).
Figure 2.
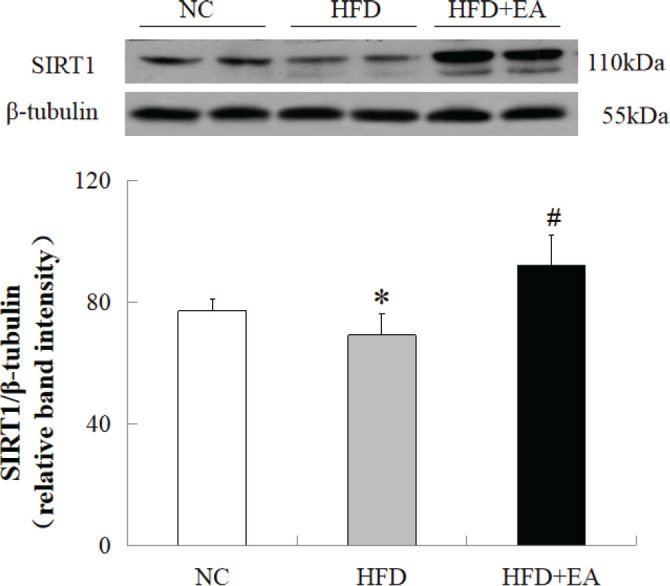
Effect of EA on SIRT1 expression in the skeletal muscle of HFD-fed IR rats. SIRT1 levels were decreased significantly in HFD-fed IR rats compared with those in the NC group. EA increased SIRT1 expression in HFD-fed IR rats. Total protein was obtained from the quadriceps muscles of IR rats and subjected to western blot for SIRT1. β-tubulin was used as the loading control. Data are presented as the mean ± SD of eight rats per group. *P < 0.05 vs. NC; #P < 0.01 vs. HFD.
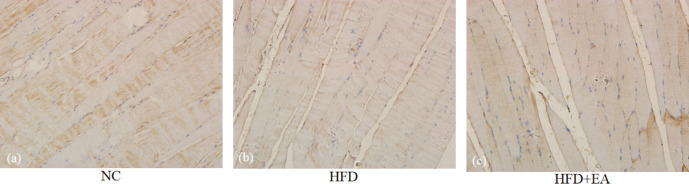
Immunohistochemistry revealed strong GLUT4-positive staining in the NC group, with large, dark brown particles. The pigmentation particles in the HFD group were lighter and smaller than those in the NC group. The particles in the HFD+EA group were lighter than those in the NC groups, but darker than those in the HFD group (Fig. 3). The positive regions were quantified (Table 1), revealing that the IOD of the HFD group was significantly lower than that of the NC (P < 0.05) and the HFD+EA groups (P < 0.05). There was no significant difference between the NC and HFD+EA groups.
Figure 3.
Immunohistochemical analysis of GLUT4 expression in the quadriceps femoris. (a) NC, (b) HFD-induced IR rats, and (c) HFD-induced IR rats treated with EA (×400). With reference to NC group, compared HFD+EA group with HFD group, EA increased GLUT4 expression in the quadriceps femoris significantly.
DISCUSSION
Acupuncture is a therapeutic technique that supports the harmonization and appropriate integration of traditional medicine with modern medicine and has been recognized by both the World Health Organization (WHO) and the National Institutes of Health (13, 24). The WHO lists approximately 100 diseases, symptoms, or conditions that are improved by acupuncture, including obesity and non-insulin-dependent diabetes mellitus (25), as supported by several studies (11, 20, 26).
In recent years, traditional Chinese medicine has turned its focus on IR. The term “insulin resistance” does not exist in traditional Chinese medicine, but its syndromes are related to hyperglycemia and hyperinsulinemia and involve phlegm-dampness, dampness-heat, and blood stasis. IR is caused by overeating greasy and sweet food and excessive drinking, which could lead to failure of the liver and gallbladder to maintain the normal flow of Qi, failure of the spleen and kidney to transport, convert, and distribute, and phlegm stasis. The principle of treatment focuses primarily on smoothing the liver to strengthen the spleen, reducing phlegm to eliminate dampness, clearing heat to remove dampness, and eliminating blood stasis to activate blood circulation, concurrently reinforcing the liver and kidney (23, 27, 28). Over the last 30 years, we have analyzed and evaluated the therapeutic effects and the mechanism of action of acupuncture on insulin resistance (2). We revealed that acupuncture enhanced insulin sensitivity significantly in both clinical studies (29) and animal experiments (11, 15, 30) .
An HFD induces whole-body and individual tissue insulin resistance in rats. The current methodology enables the effects of high-fat feeding in individual muscle tissue to be compared. The “gold standard” for assessing insulin sensitivity is hyperinsulinemic-euglycemic clamps, which investigate and quantify insulin secretion and resistance. Insulin is infused into the vein at a constant rate, resulting in a drop in blood glucose. To maintain blood glucose at a constant level, exogenous dextrose is infused into the venous circulation. Under the steady-state conditions of euglycemia, the glucose infusion rate equals glucose uptake by all tissues in the body and is indicative of tissue sensitivity to exogenous insulin (17, 30, 31). The present study indicates that the IR model was established successfully by feeding rats an HFD for eight weeks.
Low-frequency EA (1–15 Hz) is applied frequently for the treatment of IR, with beneficial results (11) including the attenuation of sympathetic nervous activity, regulation of inflammation, and increase in insulin signaling (2). Several studies suggested that low-intensity EA (1–3 mA) can be applied to the hindlimbs of rats in a conscious and comfortable state without causing stressful reactions (12, 32). Low-intensity EA (1 mA, 2 Hz) was selected for this study and was found to be sufficient to cause muscle contraction.
Several studies have confirmed that acupuncture can ameliorate IR. A previous report using IPITTs (33) found that EA enhanced insulin sensitivity by stimulating afferent fibers. The current results showed that EA reduced water intake and body weight gain and improved IPITT to increase insulin sensitivity in HFD-fed IR rats.
SIRT1 is an important member of the mammalian sirtuin family and is expressed widely in various tissues including muscle, liver, and fat. Recent evidence suggests that there is a close relationship between SIRT1 and insulin resistance (6). For example, SIRT1 is involved in secretory processes during glucose metabolism. Decreased SIRTI expression or activity might be a cause of insulin resistance (34). Increasing the expression of SIRT1 in IR could directly increase the insulin sensitivity of adipose, muscle, and liver tissues, promote insulin secretion, facilitate glucose utilization (35), and reduce plasma glucose levels, thereby ameliorating insulin resistance. In the current study, the expression of SIRT1 was decreased in the quadriceps femoris of rats in the HFD group compared with that in the NC group, suggesting that SIRT1 expression was decreased in IR rats. However, treatment with EA for eight weeks normalized SIRT1 levels in IR rats, which suggests that acupuncture might improve IR via SIRT1.
The transmembrane transport of glucose in skeletal muscle cells is the key rate-limiting step during glucose consumption and relies mainly on GLUT4. GLUT4 is an important signaling molecule in multiple signaling pathways and has been regarded as the switch cell translocation pathway. We demonstrated previously that EA upregulated GLUT4 significantly in the liver of diabetic rats (36). GLUT4 expression is regulated by PGC-1α, which is in turn regulated by SIRT1 (15). Therefore, the activation of SIRT1/PGC-1α could upregulate GLUT4 in the liver and skeletal muscle (37) to promote glucose transport in these tissues. The current results revealed that HFD induced GLUT4 expression in skeletal muscle, whereas EA increased the expression of GLUT4 significantly in the skeletal muscle of IR rats to improve insulin sensitivity. GLUT4 could then act as the downstream effector of the SIRT1/PGC-1α pathway.
This report builds upon previous studies to demonstrate that low-frequency EA enhanced the insulin sensitivity of HFD-induced IR rats. HFD-induced IR is the most suitable model of diabetes mellitus because of human dietary habits. More importantly, this study suggested a potential molecular mechanism whereby EA ameliorates insulin resistance in HFD-fed rats. EA increased the expression of SIRT1 and GLUT4, in turn enhancing glucose metabolism and ameliorating insulin resistance.
In conclusion, EA at the CV4, ST36, CV12, and ST40 acupoints in HFD-induced IR rats lowered the FBG and PPG and regulated the expression of SIRT1 and GLUT4. These results provide powerful evidence to suggest the beneficial effects of EA on HFD-induced insulin resistance in rats. In spite of the limitations of our conclusions, we will perform additional studies to assess the effect and mechanism of glucose metabolism regulation using acupuncture at a single acupoint, as well as SIRT1 transfection, gene transcription, gene knockout, and the translocation of GLUT4 disorders, gene transcription and its activity and other related aspects, to reveal the mechanism of action of acupuncture for the treatment of IR.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributors
FX and RC were involved in the study design and manuscript writing. CM and LT were involved in the literature review. WW and FY were involved in data analysis. LC and JL were involved in data collection and interpretation. FL and ZC critically revised the manuscript and gave constructive opinions on articles. All authors have approved the final version to be published.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81603699, No. 81473787, No. 81001557).
References
- 1.Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007;583(Pt 1):9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang F, Koya D. Acupuncture: is it effective for treatment of insulin resistance? Diabetes, obesity & metabolism. 2010;12(7):555–569. doi: 10.1111/j.1463-1326.2009.01192.x. [DOI] [PubMed] [Google Scholar]
- 3.Liang FX, Chen R, Wang H. Discussion on clinical research thinking of insulin resistance and its related diseases treated with acupuncture and moxibustion. Zhongguo zhen jiu = Chinese acupuncture & moxibustion. 2012;32(7):639–643. [PubMed] [Google Scholar]
- 4.Chen YR, Lai YL, Lin SD, Li XT, Fu YC, Xu WC. SIRT1 interacts with metabolic transcriptional factors in the pancreas of insulin-resistant and calorie-restricted rats. Mol Biol Rep. 2013;40(4):3373–3380. doi: 10.1007/s11033-012-2412-3. [DOI] [PubMed] [Google Scholar]
- 5.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 6.Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nature reviews. Endocrinology. 2009;5(7):367–373. doi: 10.1038/nrendo.2009.101. [DOI] [PubMed] [Google Scholar]
- 7.Banks AS, Kon N, Knight C, Matsumoto M, Gutiérrez-Juárez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8(4):333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 2008;105(28):9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiological reviews. 2013;93(3):993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 10.Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49(5):821–830. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YC, Li TM, Tzeng CY, Chen YI, Ho WJ, Lin JG, Chang SL. Electroacupuncture at the Zusanli (ST-36) Acupoint Induces a Hypoglycemic Effect by Stimulating the Cholinergic Nerve in a Rat Model of Streptozotocine-Induced Insulin-Dependent Diabetes Mellitus. Evid Based Complement Alternat Med. 2011;2011:650263. doi: 10.1093/ecam/neq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tominaga A, Ishizaki N, Naruse Y, Kitakoji H, Yamamura Y. Repeated application of low-frequency electroacupuncture improves high-fructose diet-induced insulin resistance in rats. Acupunct Med. 2011;29(4):276–283. doi: 10.1136/acupmed-2011-010006. [DOI] [PubMed] [Google Scholar]
- 13.Belivani M, Dimitroula C, Katsiki N, Apostolopoulou M, Cummings M, Hatzitolios AI. Acupuncture in the treatment of obesity: a narrative review of the literature. Acupunct Med. 2013;31(1):88–97. doi: 10.1136/acupmed-2012-010247. [DOI] [PubMed] [Google Scholar]
- 14.Zeng L, Chen R, Liang F, Tsuchiya H, Murai H, Nakahashi T, Iwai K, Takahashi T, Kanda T, Morimoto S. Silent information regulator, Sirtuin 1, and age-related diseases. Geriatr Gerontol Int. 2009;9(1):7–15. doi: 10.1111/j.1447-0594.2008.00504.x. [DOI] [PubMed] [Google Scholar]
- 15.Liang F, Chen R, Nakagawa A, Nishizawa M, Tsuda S, Wang H, Koya D. Low-Frequency Electroacupuncture Improves Insulin Sensitivity in Obese Diabetic Mice through Activation of SIRT1/PGC-1α in Skeletal Muscle. Evid Based Complement Alternat Med. 2011;2011:735297. doi: 10.1155/2011/735297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zecchin HG, Priviero FB, Souza CT, Zecchin KG, Prada PO, Carvalheira JB, Velloso LA, Antunes E, Saad MJ. Defective insulin and acetylcholine induction of endothelial cell-nitric oxide synthase through insulin receptor substrate/Akt signaling pathway in aorta of obese rats. Diabetes. 2007;56(4):1014–1024. doi: 10.2337/db05-1147. [DOI] [PubMed] [Google Scholar]
- 17.Hughey CC, Hittel DS, Johnsen VL, Shearer J. Hyperinsulinemic-euglycemic clamp in the conscious rat. J Vis Exp. 2011;48:2432. doi: 10.3791/2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang T, Zhao S, Li W, Ma L, Ding M, Li R, Liu Y. High-fat diet from perilla oil induces insulin resistance despite lower serum lipids and increases hepatic fatty acid oxidation in rats. Lipids Health Dis. 2014;13:15. doi: 10.1186/1476-511X-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraegen EW, James DE, Storlien LH, Burleigh KM, Chisholm DJ. In vivo insulin resistance in individual peripheral tissues of the high fat fed rat: assessment by euglycaemic clamp plus deoxyglucose administration. Diabetologia. 1986;29(3):192–198. doi: 10.1007/BF02427092. [DOI] [PubMed] [Google Scholar]
- 20.Ishizaki N, Okushi N, Yano T, Yamamura Y. Improvement in glucose tolerance as a result of enhanced insulin sensitivity during electroacupuncture in spontaneously diabetic Goto-Kakizaki rats. Metabolism. 2009;58(10):1372–1378. doi: 10.1016/j.metabol.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Mannerås L, Jonsdottir IH, Holmäng A, Lönn M, Stener-Victorin E. Low-frequency electro-acupuncture and physical exercise improve metabolic disturbances and modulate gene expression in adipose tissue in rats with dihydrotestosterone-induced polycystic ovary syndrome. Endocrinology. 2008;149(7):3559–3568. doi: 10.1210/en.2008-0053. [DOI] [PubMed] [Google Scholar]
- 22.Xu TJ, Liu Y, Yuan B. Effect of insulin in combination with selenium on Irs/PI3K-mediated GLUT4 expression in cardiac muscle of diabetic rats. Eur Rev Med Pharmacol Sci. 2011;15(12):1452–1460. [PubMed] [Google Scholar]
- 23.Lee FY, Huo ZJ, Zhang L, Guo J, Chen H, Liu T, Peng B, Hong PX, Peng YY, Fan YF, Chen YP. The Effects of Needling Fenglong (ST40) and Neiguan (PC6) on IL-17 of ApoE-Gene-Knockout Mice’s Liver. Evid Based Complement Alternat Med. 2014;2014:691863. doi: 10.1155/2014/691863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin CS, Shim BS, Lee H, Choi SH. Acupuncture in accomplishing ‘health for all’. Neurol Res. 2010;32(Suppl 1):18–21. doi: 10.1179/016164109X12537002793724. [DOI] [PubMed] [Google Scholar]
- 25.WHO Acupuncture: review and analysis of reports on controlled clinical trials. Parkinsonism & Related Disorders. 2003;(Suppl 2):S163. [Google Scholar]
- 26.Wang F, Tian DR, Han JS. Electroacupuncture in the treatment of obesity. Neurochem Res. 2008;33(10):2023–2027. doi: 10.1007/s11064-008-9822-6. [DOI] [PubMed] [Google Scholar]
- 27.Feng YL, Zheng GY, Ling CQ. The investigation of the correlation between metabolic syndrome and Chinese medicine constitution types in senior retired military personnel of the People’s Liberation Army. Chin J Integr Med. 2012;18(7):485–489. doi: 10.1007/s11655-011-0948-z. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Huang H, Yue W. [Regulatory functions of electroacupuncture at fenglong (ST40) on blood lipids and hepatic ABCA1 and PPARalpha in hyperlipidemia rats] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32(9):1245–1248. [PubMed] [Google Scholar]
- 29.Zhang Z, Li X, Ji X. Clinical Study on Effects of Acupuncture on Insulin Resistance in the Patient of Type II Diabetes. Chinese Acupuncture & Moxibustion. 2002 [Google Scholar]
- 30.Haupt DW. Differential metabolic effects of antipsychotic treatments. European neuropsychopharmacology. The journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 3):S149–155. doi: 10.1016/j.euroneuro.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 32.Iwa M, Matsushima M, Nakade Y, Pappas TN, Fujimiya M, Takahashi T. Electroacupuncture at ST-36 accelerates colonic motility and transit in freely moving conscious rats. Am J Physiol Gastrointest Liver Physiol. 2006;290(2):G285–292. doi: 10.1152/ajpgi.00068.2005. [DOI] [PubMed] [Google Scholar]
- 33.Higashimura Y, Shimoju R, Maruyama H, Kurosawa M. Electro-acupuncture improves responsiveness to insulin via excitation of somatic afferent fibers in diabetic rats. Auton Neurosci. 2009;150(1-2):100–103. doi: 10.1016/j.autneu.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450(7170):712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang ZZ, Liang FX, Wang H. Effect of “Shuanggu Yitong (tonifying congenital and acquired essence,eliminating pathogen)” Acupuncture Therapy with Different Intensity on Expression of GLUT4 mRNA in DM Rats. Chinese Archives of Traditional Chinese Medicine. 2008 [Google Scholar]
- 37.Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci USA. 2001;98(7):3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]