Abstract
Background:
Transcranial direct current stimulation (tDCS), in conjunction with language therapy, improves language-therapy outcomes in primary progressive aphasia (PPA). However, no studies show whether white-matter integrity predicts language therapy or tDCS effects in PPA.
Objective:
We aimed to determine whether white-matter integrity, measured by diffusion tensor imaging (DTI), predicts written naming/spelling language therapy effects (letter accuracy on trained and untrained words) with and without tDCS over the left inferior frontal gyrus (IFG) in PPA.
Methods:
Thirty-nine participants with PPA were randomly assigned to tDCS or sham condition, coupled with language therapy for 15 daily sessions. White-matter integrity was measured by mean diffusivity (MD) and fractional anisotropy (FA) in DTI scans before therapy. Written naming outcomes were evaluated before, immediately after, two weeks and two months post-therapy. To assess tDCS treatment effect, we used a mixed effects model with treatment evaluation and time interaction. We considered a forward model selection approach to identify brain regions/fasciculi of which white matter integrity can predict improvement in performance of word naming.
Results:
Both sham and tDCS groups significantly improved in trained items immediately after and at two months post-therapy. Improvement in the tDCS group was greater and generalized to untrained words. White-matter integrity of ventral language pathways predicted tDCS effects in trained items whereas white-matter integrity of dorsal language pathways predicted tDCS effects in untrained items.
Conclusions:
White-matter integrity influences both language therapy and tDCS effects. Thus, it holds promise as a biomarker for deciding which patients will benefit from language therapy and tDCS.
Keywords: white-matter integrity, primary progressive aphasia (PPA), transcranial direct current stimulation (tDCS), language therapy, white matter, electrical stimulation, neurodegeneration, prediction
Introduction
The emergence of neuromodulation techniques—especially transcranial direct current stimulation (tDCS)—has provided tools to augment language rehabilitation effects in post-stroke aphasia1 and recently in primary progressive aphasia (PPA)2–6. In neurodegenerative syndromes without pharmacological agents, there is a great need for treatments that maintain treatment outcomes for as long as possible. In PPA, patients primarily show language impairments. In recent years, studies of tDCS in PPA by different groups, including ours2, have shown significant augmentative tDCS effects in several language functions, such as oral naming, spelling, and story-telling3–7.
In our randomized, sham-controlled, double-blind, crossover design clinical trial of a tDCS intervention in 36 people with PPA2, electrical stimulation over the left inferior frontal gyrus (IFG), concurrent with lexical retrieval language therapy targeting written naming, significantly improved language therapy outcomes (letter accuracy) more than language therapy (written naming/spelling) alone (sham condition). In patients who did improve, therapy gains were sustained up to two months and generalized to untrained items. Given the variability in response to tDCS, the question of who may benefit from tDCS becomes even more pertinent for future interventions, especially from a clinical perspective.
In previous studies we identified cognitive, language and volumetric (gray matter) parameters that significantly predicted language therapy effects, as well as additional tDCS effects, in a written naming/spelling treatment in PPA8,9. In the present study we aimed to determine whether white-matter integrity is associated with tDCS and language therapy effects in PPA.
White matter integrity and language outcomes in aphasia
In the language domain, white-matter structural connections have been correlated with language deficits mostly in post-stroke aphasia10–13 and recently in PPA14–21. The degree of white-matter tract disruption has been correlated strongly and repeatedly with language deficits to an even greater extent than grey-matter lesions22,23. For example, the degree of lesion in the left arcuate fasciculus has been correlated with speech production impairment in post-stroke aphasia11,24. In written language, we have shown that the structural connections of the mid-fusiform gyrus, but not gray-matter volume or integrity, accounted for the spelling deficits22. In PPA, previous studies from several groups including ours, have shown that integrity of white-matter tracts correlate with language deficits in all PPA variants14–20.
With regard to language recovery and therapy, recent studies have identified structural integrity of the arcuate and superior longitudinal fasciculi as important parameters for anomia recovery in the first 3 or 6 months post-stroke and of language severity at the chronic stage25,26. Other studies have shown an association between white-matter integrity and language therapy outcomes in post-stroke aphasia26,27. Finally, two recent studies showed that language therapy outcomes may also correlate with white-matter reconstruction in post-stroke aphasia26,28. Therefore, white-matter integrity could be used as a biomarker of language therapy outcomes in post-stroke aphasia.
We are only aware of one study showing that tDCS induced white-matter changes in post-stroke rehabilitation, which occurred in descending motor tracts and correlated with improvements in motor impairments after tDCS coupled with physical therapy29.
To our knowledge, no studies have investigated the integrity of white-matter tracts as predictors of language therapy outcomes in PPA, with or without tDCS. The present study addresses these gaps. Given that electrical current distribution models assume that current may flow through white-matter tracts, it is pertinent, from a theoretical perspective, to determine whether there is any association between the integrity of white matter tracts and the effects of tDCS on language therapy outcomes. Clinically, it is very important to determine whether baseline white-matter integrity predicts language therapy outcomes so that tDCS can be preferentially given to those who are most likely to benefit.
The present study addressed the question of whether white-matter integrity predicts language therapy and tDCS effects in PPA. We first conducted an analysis of the behavioral results to determine whether tDCS treatment is beneficial. The goals of the behavioral analysis were threefold: (1) To determine whether patients benefited from tDCS in addition to language therapy and language therapy alone; (2) to identify whether therapeutic benefits maintained at different timepoints; and (3) to evaluate the effect of generalization, i.e., whether therapeutic benefits existed in both trained and untrained lists of items. The goals of the white-matter integrity analysis were twofold: (1) to determine which areas and tracts predict effects of tDCS in addition to language therapy and language therapy alone for trained items; (2) to determine which areas and tracts predict effects of tDCS in addition to language therapy and language therapy alone for untrained items (generalization effect). We hypothesized that the integrity of white-matter tracts between the stimulated area (left IFG) and the areas structurally connected to it in the ventral and dorsal tracts would predict tDCS in addition to language therapy and language therapy effects on the treatment outcome (letter accuracy).
Methods
Participants
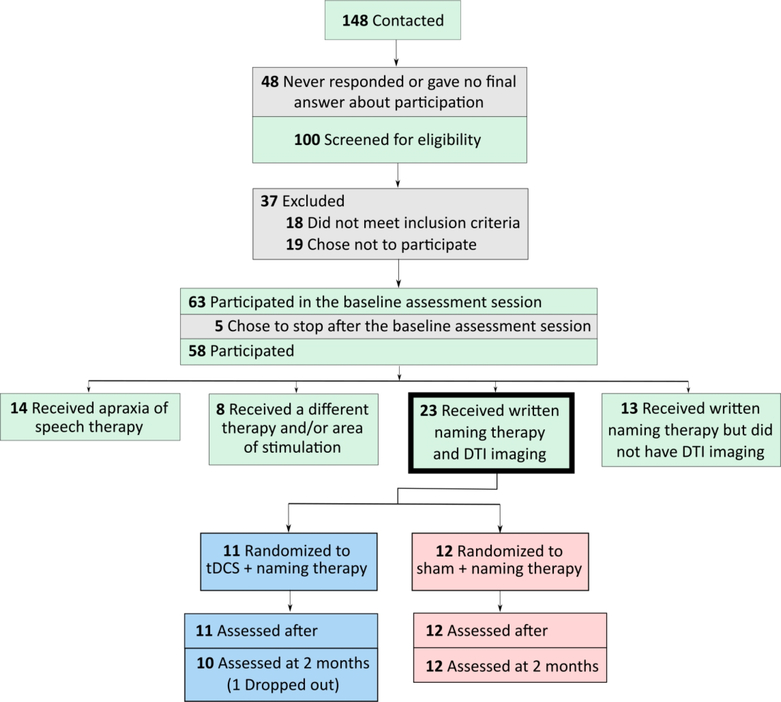
Thirty-nine participants with PPA were included in the study (see Figure 1). Twenty participants received tDCS and nineteen sham, both in conjunction with written naming/spelling therapy. One participant who received tDCS dropped out from the study after two weeks of the intervention—she had accompanying behavioral issues and was fatigued by daily travel. We used the same cohort of participants as in our previous study assessing the effects of tDCS on functional connectivity using resting-state fMRI (rsfMRI)30. The participants’ characteristics (demographic and clinical) are presented in Table 1.
Figure 1.
CONSORT diagram for the present study.
Table 1.
Demographics for each participant. Condition: s=sham, t=tDCS. Variant: n=nonfluent PPA, l= logopenic PPA, s=semantic PPA. Onset is the number of years it has been since each participant began experiencing language difficulties. LangSev refers to language severity, as measured by the Fronto-Temporal Dementia Clinical Dementia Rating scale (FTD-CDR); TotSev refers to total severity measured by the FTD-CDR scale, taking into account memory, orientation, judgement and problem-solving, community affairs, home and hobbies, personal care, behavior/comportment/personality, and language62. Mean and standard deviation (S.D.) were calculated within each treatment arm.
| Participant | Condition | Variant | Sex | Onset | Age | LangSev | TotSev | # of Sessions | Baseline Trained | Baseline Untrained |
|---|---|---|---|---|---|---|---|---|---|---|
| P01 | s | l | M | 3.5 | 69 | 1 | 3.5 | 10 | 50.33 | 50.00 |
| P02 | s | n | F | 3 | 66 | 1 | 5 | 10 | 56.12 | 48.72 |
| P03 | s | n | M | 8 | 73 | 2 | 5 | 12 | 88.39 | 70.55 |
| P04 | s | n | M | 6 | 64 | 3 | 15 | 10 | 31.25 | 69.33 |
| P05 | s | n | F | 8 | 66 | 3 | 19 | 10 | 41.61 | 49.38 |
| P06 | s | l | F | 3 | 71 | 2 | 5 | 12 | 77.40 | 77.65 |
| P07 | s | n | M | 1.5 | 78 | 2 | 5.5 | 14 | 40.00 | 30.00 |
| P08 | s | n | M | 2 | 77 | 2 | 12 | 11 | 80.36 | 93.75 |
| P09 | s | s | F | 3 | 68 | 2 | 6.5 | 10 | 55.33 | 58.05 |
| P10 | s | l | M | 7.5 | 74 | 1 | 3 | 13 | 45.59 | 56.19 |
| P11 | s | s | F | 9.5 | 68 | 3 | 18.5 | 10 | 50.00 | 50.00 |
| P12 | s | l | F | 1.5 | 69 | 3 | 17 | 12 | 30.67 | 19.21 |
| P13 | s | s | F | 5.5 | 75 | 2 | 7.5 | 13 | 2.14 | 10.68 |
| P14 | s | n | F | 4 | 68 | 2 | 15 | 49.00 | 47.00 | |
| P15 | s | n | M | 3.5 | 76 | 2 | 14 | 10 | 11.00 | 11.00 |
| P16 | s | n | M | 2.5 | 70 | 0.5 | 0.5 | 12 | 40.00 | 51.67 |
| P17 | s | s | M | 2.5 | 69 | 1 | 2.5 | 8 | 57.20 | 42.90 |
| P18 | s | l | M | 3 | 58 | 1 | 2.5 | 11 | 60.00 | 75.00 |
| P19 | s | l | F | 2.5 | 64 | 1 | 3.5 | 10 | 69.74 | 61.08 |
| Mean | 4.21 | 69.63 | 1.81 | 8.08 | 11.21 | 49.27 | 51.17 | |||
| SD | 2.44 | 5.06 | 0.80 | 6.09 | 1.72 | 21.82 | 22.08 | |||
| P20 | t | n | F | 6 | 60 | 2 | 8 | 12 | 29.47 | 16.88 |
| P21 | t | l | F | 1.5 | 53 | 1 | 9.5 | 13 | 90.10 | 86.20 |
| P22 | t | l | F | 10.5 | 75 | 3 | 12 | 9 | 30.00 | 35.00 |
| P23 | t | n | F | 4 | 69 | 2 | 6 | 10 | 88.52 | 80.78 |
| P24 | t | n | F | 9 | 74 | 2 | 3 | 15 | 45.00 | 38.33 |
| P25 | t | n | F | 74 | 1 | 1.5 | 15 | 74.17 | 68.33 | |
| P26 | t | n | F | 2 | 69 | 2 | 10 | 10 | 57.38 | 42.78 |
| P27 | t | s | M | 7.5 | 59 | 2 | 5.5 | 15 | 23.56 | 15.98 |
| P28 | t | l | M | 3 | 51 | 0.5 | 2 | 14 | 94.37 | 84.55 |
| P29 | t | l | F | 9.5 | 70 | 3 | 10 | 14 | 75.98 | 59.22 |
| P30 | t | n | M | 2.5 | 80 | 2 | 3 | 12 | 65.93 | 68.09 |
| P31 | t | n | M | 2 | 65 | 0.5 | 1 | 13 | 75.00 | 75.00 |
| P32 | t | n | F | 1.5 | 63 | 3 | 8 | 14 | 10.00 | 20.83 |
| P33 | t | l | M | 0.5 | 68 | 0.5 | 1 | 10 | 67.76 | 71.60 |
| P34 | t | s | F | 2.5 | 64 | 1 | 1 | 15 | 22.32 | 42.51 |
| P35 | t | l | M | 1 | 54 | 2 | 8 | 13 | 36.62 | 32.25 |
| P36 | t | l | M | 4 | 63 | 2 | 9.5 | 10 | 61.54 | 56.30 |
| P37 | t | l | F | 6.5 | 62 | 2 | 11.5 | 15 | 20.83 | 5.63 |
| P38 | t | s | M | 10 | 71 | 2 | 4 | 13 | 22.94 | 26.95 |
| P39 | t | n | M | 10.5 | 66 | 3 | 6 | 13 | 59.84 | 36.36 |
| Mean | 4.95 | 65.50 | 1.82 | 6.02 | 12.80 | 52.57 | 48.18 | |||
| SD | 3.56 | 7.69 | 0.83 | 3.76 | 2.01 | 26.50 | 24.96 | |||
| p-value | 0.817 | 0.876 | 0.463 | 0.055 | 0.972 | 0.226 | 0.017 | 0.673 | 0.694 | |
Timeline of subject enrollment:
Recruitment for the randomized, double-blinded, sham-controlled, crossover trial had started in 2013 under a Science of Learning Institute award by the Johns Hopkins University (ClinicalTrials.gov Identifier: NCT02606422). Recruitment with the same design continued under NIH/NIDCD R01 DC014475 in compliance with the new clinical trials definition imposed by NIH for NIH-funded research. Double blinding was secured by Dr. Argye Hillis in the first award period and by Dr. Kyrana Tsapkini in the second award period. Neither was involved in therapy or therapy evaluation concurrently.
DTI methods
MRI scans were acquired on a 3T Philips Achieva MRI scanner equipped with a 32-channel head coil at the Johns Hopkins University Kennedy Krieger Institute. Scans occurred within one day before the start of treatment.
MPRAGE:
We used T1 weighted images (TR/TE/TI=8.1/3.7/842 ms) with a 1×1×1 mm3 resolution, FOV=224×224 mm, acquired in the axial plane, SENSE acceleration factor=2, flip angle=8°, scan time = 4.5 min.
DTI:
We used DTI to measure white matter FA and MD. DTI was acquired with a multi-slice, single-shot, echo-planar imaging (EPI), SE sequence, TR/TE=7324/75 ms, SENSE factor=2.5, FOV=212×212, matrix=96×96, reconstructed to 256×256, 32 directions, b-value of 700 s/mm2, and a scan time of 4.27 min.
Image Analysis:
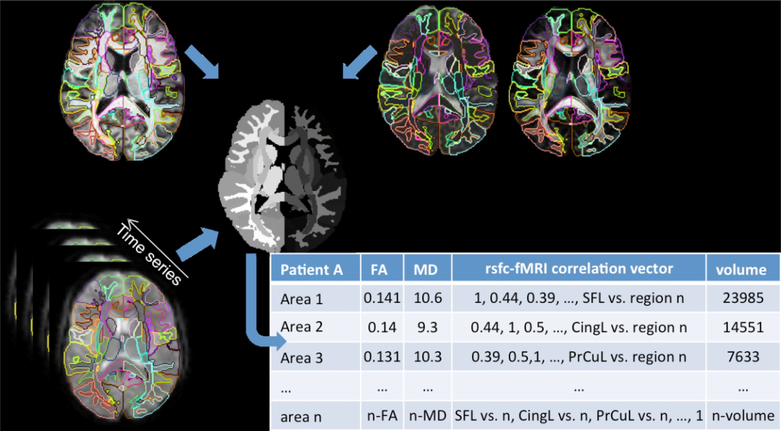
We automatically pre-processed and segmented the diffusion weighted images using MRICloud (www.mricloud.org)31. MRICloud is a web platform that calculates DTI scalars, such as FA and MD, using software-based pipelines (DTIStudio32), and segments them in 169 regions of interest (ROIs) by diffeomorphic mapping to multiple templates previously segmented (the “multi-atlas” approach)33. This approach34 facilitates the integration of image information in multiple domains and reduces the noise and information residing in the order of million voxel squares down to matrices of hundreds of structures, with high reproducibility35. In this study, we focused on the areas of the language network, particularly the peripheral white matter beneath the cortex examined in the previous rsfMRI study. We analyzed functional anisotropy (FA) and mean diffusivity (MD) which are complementary measures of white-matter integrity (see Figure 2).
Figure 2.
Schematic diagram of the multimodal analysis using a common parcellation map.
The nine ROIs included were: inferior frontal gyrus (IFG), angular gyrus (AG), supramarginal gyrus (SMG), cingulate gyrus (CG), middle frontal gyrus (MFG), fusiform gyrus (FuG), superior temporal gyrus (STG), inferior temporal gyrus (ITG) and middle temporal gyrus (MTG) on the left hemisphere.
TDCS methods
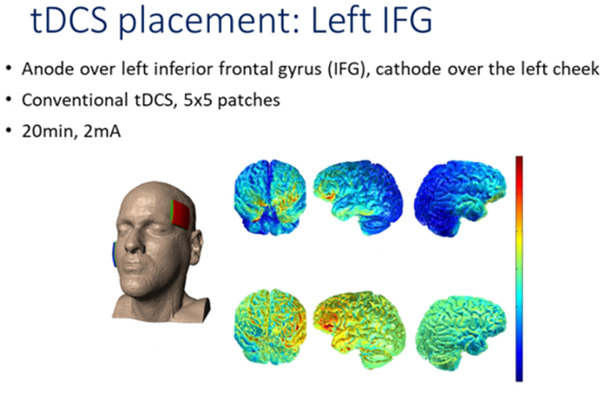
To deliver tDCS, we used the Soterix Transcranial Direct Current Stimulation 1×1 Clinical Trials device, Model 1500. tDCS setup is described in our main trial2. The anode was placed over the left frontal lobe, centered on F7 in the 10–20 electrode placement system36. The cathode was placed over the right cheek. We aimed to excite the left frontal lobe with anodal stimulation, shown to augment language rehabilitation in other studies37. Non-metallic, conductive rubber electrodes, fitted with saline-soaked sponges to limit skin-electrode reactions, were 5 cm x 5 cm; because of the size, the full left IFG was covered. Current was delivered with an intensity of 2 mA (estimated current density 0.08 mA/cm2) for a total of 20 minutes each tDCS session. Delivery of tDCS was simultaneous with the start of language therapy, which continued for an additional 20–25 minutes beyond the cessation of tDCS in each session. Sham consisted of 30 seconds of current ramping up to 2 mA and back down to 0 mA simultaneous with the start of language therapy. These procedures have successfully blinded participants to the stimulation condition38. The speech-language therapist administering tDCS and language therapy was also blind to the stimulation condition. To monitor any adverse effects, each participant was asked to rate his or her pain level once or twice during each session, using the Wong Baker FACES Pain Rating Scales (www.WongBakerFACES.org). Some participants reported tingling and itching that usually lasted for about 30 seconds. The protocol required 15 consecutive weekday sessions for each participant. We made every effort to keep this schedule, although some participants had to leave a few days earlier due to other life commitments.
Language therapy methods
We combined the spell-study-spell procedure39 in our previous PPA treatment studies3,40 with an oral and written naming paradigm41. Given the possibility of different spelling deficits in each variant, as both we and others have found42,43, we developed individualized trained and untrained word sets while maintaining the same procedures and outcome measures (letter accuracy).
The participant was shown a picture on a computer, asked to orally name it, and then to write the name. If the patient could not name the picture (orally or in writing), (s)he was asked to provide three characteristics of the item, to evaluate and reinforce semantic knowledge as in semantic feature analysis treatment41. If (s)he still could not produce the word orally, (s)he was provided with the correct word and asked to repeat it three times. Likewise, if the patient could not write it or wrote it incorrectly, the therapist provided the correct spelling in a spell-study-spell procedure, i.e., the clinician writes the correct word, reviews each letter’s sound, then asks the patient to copy the word three times.
Trained and untrained sets (10–30 words depending on individual severity) were matched in length and frequency. Four evaluations were administered for each therapy phase: before therapy, immediately after the end of therapy, two weeks post- and two months post-therapy. Letter accuracy was determined based on a scoring system44 that considered letter deletions, additions, substitutions, and movements. Letter accuracy was used as a more precise outcome instead of whole-word accuracy in order to capture the effects of the different types of errors. Each letter was evaluated with 1 point, 0.5 points for correct identification and 0.5 points for correct position. Scores for trained and untrained words were transformed to percentage points for each participant. Inter-rater reliability was managed as follows: each item was scored, then a second person performed ratings and noted discrepancies. Inter-rater reliability was 95%. Discrepancies were discussed to generate a consensus score. This was a double-blinded study, i.e., not only participants but also speech-language therapists providing the treatment, as well as technicians performing evaluations and scoring performance were blinded to the treatment condition.
Statistical analysis
1. Longitudinal analysis of tDCS and sham intervention(s) on written naming
After intervention, written naming improvement was evaluated with change in letter accuracy at three different time points: immediately after, two weeks post- and two months post-therapy, for both trained and untrained sets. In this study, we considered the relative change in behavior as the outcome to account for the variation in the initial performance across patients. The improvement of behavior was measured in the relative scale defined as:
where Zij is the language outcome (percentage of letter accuracy) at the jth time point of subject i, for j = 1,2 (immediately after and two-month post-intervention); and Zi0 is the language score pre-intervention. One advantage of using this relative change measure (proportional maximal gain) is that it addresses the possible ceiling treatment effects, especially for those subjects with a higher pre-intervention score, i.e., less progressed cases. This method has been advocated and used in previous reports of language therapy outcomes as well45. To track the treatment impact on the trajectory of improvement in behavior, the following mixed effects model was utilized:
where the treatment group (denoted by G with G = 1 for the tDCS group, and G = 0 for the sham group), the evaluation time indicator (denoted by T with T = 1 for the two months post measurement and T = 0 for the immediately after timepoint), as well as their interactions as fixed effects; the α’s are the model coefficients, ui is the random effect of subject i which is normally distributed with mean zero and variance τ2, εij is the model error term normally distributed with mean zero and variance σ2. With the inclusion of the interaction terms, we can compare the expected behavior (letter accuracy) change for different combinations of treatment group and time of evaluation, i.e., α0 represents the expected letter accuracy score change immediately after the intervention for the sham group and (α0+α1) for the tDCS group; the expected letter accuracy improvement after two months is demonstrated by (α0+α2) for the sham group and (α0+α1+α2+α3) for the tDCS group. In order to balance the two groups, inverse probability weighting was employed, where the weights were estimated from a logistic regression model with variant type, sex, age, number of treatment sessions and language severity as the predictors.
2. White matter integrity analyses to predict written naming (letter accuracy) changes
We considered a forward model selection approach to identify a group of brain regions/fasciculi that white matter integrity can predict the relative changes in written naming (letter accuracy). This analysis was conducted for sham and tDCS groups separately at each time point, as the set of predictors may vary by therapy groups and time. In total, 48 models were fitted (2 treatment group * 3 timepoints * 2 DTI outcomes (FA/MD) * 2 types of DTI metric (ROI/fasciculus) * 2 types of language outcome (trained/untrained) = 48). In the ROI analysis, we considered brain regions of inferior frontal gyrus left and right (IFG_L and IFG_R), cingulate gyrus left (CG_L), middle-frontal gyrus left (MFG_L), angular gyrus left (AG_L), fusiform gyrus left (FuG_L), superior temporal gyrus left (STG_L), middle temporal gyrus left (MTG_L), inferior temporal gyrus left (ITG_L) and supramarginal gyrus left (SMG_L); and in the fasciculi analysis, we considered superior longitudinal fasciculus (SLF_L and SLF_R), inferior frontal-occipital fasciculus (IFOF_L and IFOF_R), and uncinate fasciculus (UnF_L and UnF_R) for both hemispheres. We included variant type and language severity in the model to account for potential confounding effects. The forward stepwise selection includes the following steps: (1) from all the brain regions/fasciculi, we select the one with the largest increment in the leave-one-out cross-validated coefficient of determination (ΔR2), where the increment is significantly greater than zero; (2) repeat first step until no remaining factor has a significant ΔR2. The selected regions/fasciculi thus have a strong association with the changes in written naming performance. With the selected regions/fasciculi, we fit a multiple regression model and report the coefficient as well as the corresponding p-value.
Results
We first present the behavioral effects of tDCS and sham interventions on letter accuracy of trained and untrained words immediately after, two weeks post- and two-months post-intervention. Subsequently, we present results that address the question of which white matter areas and tracts moderate the effects of tDCS and sham interventions.
1. Improvement in trained items sustained longer with tDCS than sham and generalization to untrained items occurred only in the tDCS condition.
After controlling for the effects of variant type, sex, age, number of treatment sessions and language severity, we found that, for trained items, the treatment effect was significant for both tDCS and sham interventions at all three time points after intervention (see Tables 2(a) and 2(b) with averages and 95% confidence intervals). The improvement in the tDCS group is significantly higher than that in the sham group (immediately after intervention p-value = 0.010, two-week after intervention p-value = 0.019, and two-month after intervention p-value = 0.003). As time progressed, the treatment effect for trained items declined for both tDCS and sham groups. Notably, the treatment effect of tDCS over sham became greater.
Table 2.
Behavioral effects of interventions. Estimated tDCS effect over sham in letter accuracy change, standard error (SE), 95% confidence interval (CI), and p-value at each post-intervention time point (After=immediately after intervention; 2wp=two weeks post-intervention; 2mp=two months post-intervention) using inverse probability weighting (IPW).
| Time | Estimate (SE) | 95% CI | p-value | |
| Trained items | After | 0.74 (0.29) | (0.17, 1.32) | 0.010 |
| 2wp | 0.68 (0.29) | (0.11, 1.25) | 0.019 | |
| 2mp | 0.85 (0.29) | (0.28, 1.42) | 0.003 | |
| Untrained items | After | 0.37 (0.28) | (−0.18, 0.92) | 0.192 |
| 2wp | 0.23 (0.28) | (−0.32, 0.78) | 0.409 | |
| 2mp | 0.75 (0.28) | (0.19, 1.30) | 0.008 | |
| (a) sham group | ||||
| Time | Estimate (SE) | 95% CI | p-value | |
| Trained items | After | 0.80 (0.20) | (0.41, 1.19) | <0.001 |
| 2wp | 0.70 (0.20) | (0.31, 1.10) | <0.001 | |
| 2mp | 0.47 (0.20) | (0.08, 0.86) | 0.018 | |
| Untrained items | After | 0.25 (0.19) | (−0.13, 0.63) | 0.193 |
| 2wp | 0.34 (0.19) | (−0.04, 0.72) | 0.079 | |
| 2mp | −0.00 (0.19) | (−0.38, 0.38) | 0.994 | |
| (b) tDCS group | ||||
| Time | Estimate (SE) | 95% CI | p-value | |
| Trained items | After | 1.55 (0.21) | (1.13, 1.97) | <0.001 |
| 2wp | 1.39 (0.21) | (0.97, 1.80) | <0.001 | |
| 2mp | 1.32 (0.21) | (0.90, 1.74) | <0.001 | |
| Untrained items | After | 0.62 (0.20) | (0.21, 1.02) | 0.002 |
| 2wp | 0.57 (0.20) | (0.17, 0.97) | 0.005 | |
| 2mp | 0.74 (0.20) | (0.34, 1.15) | <0.001 | |
Similarly, after controlling for the effects of variant type, sex, age, number of treatment sessions and language severity, for the untrained items, we found that only the tDCS group maintained significant improvement at all three post-intervention time points (see Table 2(b)). At two-months post-intervention, the tDCS group scored significantly higher than the sham group on the outcome measure, (letter accuracy) (p-value = 0.008, see Table 2).
2. White matter integrity (MD and FA values) influences tDCS and sham interventions in trained items independently from the effects of variant type and language severity.
After controlling for variant type and language severity, for the trained items, in the tDCS group, we identified significant association between improvement in letter accuracy with white matter integrity in CG_L (positive correlation, p-value = 0.010 of MD value at immediately after intervention) and UnF_L (negative correlation, p-value = 0.035 of MD value at two weeks after intervention). In the sham group, CG_L (positive correlation, p-value = 0.040 of FA value at immediately after intervention), SLF_R (negative correlation, p-value = 0.012 of MD value at two weeks after intervention; and positive correlation p-value = 0.003 of FA value at immediately after intervention, andp-value < 0.001 at two weeks after intervention), SMG_L (positive correlation, p-value = 0.002 of FA value at two weeks after intervention), UnF_L (positive correlation, p-value = 0.034 of FA value at two months after intervention) areas/fasciculi showed a significant association with improvement in letter accuracy (see Table 3).
Table 3.
Association of white-matter areas with tDCS and sham on changes in letter accuracy for trained items at each point-intervention time point (After=immediately after intervention; 2wp=two weeks post-intervention; 2mp=two months post-intervention). Cross-validated increment of coefficient of determination (CV ΔR2), estimated model coefficients (β), standard error (SE) and p-values are shown.
| (a) MD values: ROIs | |||||
| Time | ROI | CV ΔR2 | β (SE) | p-value | |
| tDCS | After | CG_L | 0.13 | 77.93 (26.25) | 0.010 |
| SMG_L | 0.12 | −25.61 (14.01) | 0.089 | ||
| 2wp | - | - | - | - | |
| 2mp | - | - | - | - | |
| sham | After | - | - | - | - |
| 2wp | SMG_L | 0.12 | −24.48 (13.93) | 0.104 | |
| 2mp | - | - | - | - | |
| (b) MD values: fasciculi | |||||
| Time | Fasciculus | CV ΔR2 | β (SE) | p-value | |
| tDCS | After | - | - | - | - |
| 2wp | UnF_L | 0.11 | −29.06 (12.20) | 0.035 | |
| 2mp | UnF_L | 0.17 | −29.72 (15.13) | 0.075 | |
| sham | After | - | - | - | - |
| 2wp | SLF_R | 0.27 | −52.38 (17.68) | 0.012 | |
| 2mp | - | - | - | - | |
| (c) FA values: ROIs | |||||
| Time | ROI | CV ΔR2 | β (SE) | p-value | |
| tDCS | After | - | - | - | - |
| 2wp | - | - | - | - | |
| 2mp | - | - | - | - | |
| sham | After | CG_L | 0.02 | 32.72 (14.47) | 0.040 |
| 2wp | SMG_L | 0.22 | 24.82 (6.19) | 0.002 | |
| FuG_L | 0.06 | −16.20 (6.61) | 0.032 | ||
| 2mp | - | - | - | - | |
| (d) FA values: fasciculi | |||||
| Time | Fasciculus | CV ΔR2 | β (SE) | p-value | |
| tDCS | After | - | - | - | - |
| 2wp | - | - | - | - | |
| 2mp | - | - | - | - | |
| sham | After | SLF_R | 0.21 | 13.61 (3.80) | 0.003 |
| IFOF_R | 0.10 | 5.01 (2.57) | 0.073 | ||
| 2wp | SLF_R | 0.52 | 12.59 (2.31) | <0.001 | |
| IFOF_R | 0.03 | 2.46 (1.46) | 0.120 | ||
| 2mp | UnF_L | 0.22 | 5.75 (2.40) | 0.034 | |
| SLF_R | 0.13 | 7.76 (3.89) | 0.069 | ||
3. White matter integrity (MD and FA values) influences tDCS and sham interventions in untrained items independently from the effects of variant type and language severity.
After controlling for variant type and language severity, for the untrained items, in the tDCS group, we identified the following regions/fasciculi with significant correlations to improvements in letter accuracy: CG_L (positive correlation, p-value = 0.001 of MD value at two weeks after intervention), SMG_L (negative correlation and p-value = 0.001 in MD value at two week after intervention), MTG_L (positive correlation, p-value = 0.015 of MD value at two weeks after intervention, and negative correlation and p-value = 0.019 in FA value at two weeks after intervention), ITG_L (positive correlation, p-value = 0.020 of MD value at two months after intervention), IFG_R (positive correlation, p-value = 0.005 of FA value at two weeks after intervention) and SLF_L (negative correlation, p-value = 0.055 of MD value at two weeks after intervention); and in the language therapy only group, we identified the following regions/fasciculi including ITG_L (positive correlation, p-value = 0.037 of FA value at immediately after intervention), IFG_L (positive correlation, p-value = 0.042 of FA value at two months after intervention), UnF_L (positive correlation, p-value < 0.001 of FA value at immediately after intervention) and UnF_R (positive correlation, p-value < 0.001 of FA at immediately after intervention), although there were no significant behavioral effects on letter accuracy in sham.
Discussion
In the present study, we asked the question of whether white matter integrity, as measured by MD and FA values from DTI scans, may predict effects of tDCS and sham interventions, each coupled with language therapy (written naming/spelling), in PPA. Overall, the behavioral results of this study confirmed the results of the larger behavioral trial2 while statistically controlling for (i.e., independent from) variant type: (a) both tDCS and sham coupled with language therapy caused significant improvements in treatment outcomes; (b) effects of tDCS coupled with written naming of trained items sustained significantly longer than sham (language therapy alone); (c) only the tDCS intervention generalized to untrained items both immediately after and at two-months post-intervention. We interpret the predictions for the behavioral effects in terms of white matter integrity of the two language streams: dorsal (SMG_L and SLF_L) and ventral (MTG_L, ITG_L, UnF_L). We found that tDCS effects in trained words were predicted by higher white-matter integrity of specific areas of the ventral language stream (UnF_L) at baseline, whereas tDCS effects in untrained words were predicted by higher white-matter integrity of the dorsal language stream (SMG_L, SLF_L) at baseline, and, consequently, by lower white-matter integrity of the ventral stream (MTG_L and ITG_L).
Recent studies on the role of structural connectivity in rehabilitation have looked at white-matter connections both as a mechanism for language rehabilitation as well as a predictor26,46 (see also Forkel, 201447 for a review). In such short intervention periods (2–3 weeks) we did not expect to find changes in the white-matter, so we looked at white-matter integrity only as a predictor for effects of tDCS and language therapy alone (sham). Overall, although the tDCS effects on language outcomes were larger than sham (see Table 2), the amount of variance that was explained by white-matter integrity for tDCS effects was smaller than the variance explained by white matter integrity for language therapy alone (sham), where the amount of variance is estimated by the sum of CV ΔR2 in Table 3. Therefore, white-matter integrity was more predictive for language therapy alone than for tDCS effects.
With regard to the influence of white-matter integrity of specific areas/tracts, we found that, for trained words, tDCS effects were significantly influenced by the integrity of the UnF_L at both the two-week and two-month follow-up. Therefore, the integrity of the ventral language pathway predicted the sustainability of tDCS effects in trained items (lexical retrieval). Note that the left UnF here, that was our automated atlas, MRI studio, indication48, most probably represents the extreme capsule fasciculus. With the present methods, we can only capture the bundle that connects the left IFG to left temporal areas; the extreme capsule has also been found to be a probable anatomical correlate of lexical retrieval49,50 as we discuss later on. In contrast, for generalization in untrained items, we found that only tDCS effects were significantly influenced by the white matter integrity of dorsal but not ventral tracts and areas (SMG_L and SLF_L). However, the effects were short-lived, i.e., significant only up to two weeks post-intervention. These dorsal areas and tracts have been found to be responsible for rule-based learning of phoneme-to-grapheme correspondences, i.e., the sublexical route, as opposed to the ventral route, responsible for lexical retrieval49,50.
Interestingly, tDCS effects for both trained and untrained words were positively correlated with lower white-matter integrity in dementia-related areas (such as CG_L), indicating that tDCS may be particularly helpful in more progressed cases as previous studies have also suggested5. On the other hand, language therapy effects (sham) were positively correlated, i.e., significantly impacted by white-matter integrity in right hemisphere large dorsal bundles (SLF_R) and left hemisphere parietal and temporal bundles and areas (UnF_L, SMG_L and FuG_L) indicating that language therapy effects are impacted by several areas of the left hemisphere as well as the right hemisphere. Although the areas predicting treatment effects in language therapy alone cannot automatically be considered as potential candidates for tDCS, since the mechanisms may be different, it is important to stress the involvement of the right hemisphere in rehabilitation potential in PPA. Notably, even in the tDCS condition, the right-hemisphere homologue of our stimulation site, i.e., the IFG_R, predicted a small, albeit significant, amount of change in untrained items (7% change, p=0.005, see Table 4).
Table 4.
Association of white-matter areas with tDCS and sham on changes in letter accuracy for untrained items at each point-intervention time point (After=immediately after intervention; 2wp=two weeks post-intervention; 2mp=two months post-intervention). Cross-validated increment of coefficient of determination (CV ΔR2), estimated model coefficients (β), standard error (SE) and p-values are shown.
| (a) MD values: ROIs | |||||
| Time | ROI | CV ΔR2 | β (SE) | p-value | |
| tDCS | After | - | - | - | - |
| 2wp | IFG_R | 0.17 | 21.17 (17.76) | 0.267 | |
| CG_L | 0.09 | 97.44 (18.46) | 0.001 | ||
| SMG_L | 0.15 | −110.66 (20.97) | 0.001 | ||
| MTG_L | 0.17 | 31.57 (10.20) | 0.015 | ||
| STG_L | 0.05 | 21.85 (12.79) | 0.126 | ||
| 2mp | ITG_L | 0.15 | 61.21 (21.62) | 0.020 | |
| FuG_L | 0.05 | −29.57 (16.51) | 0.107 | ||
| STG_L | 0.09 | 34.88 (24.74) | 0.107 | ||
| sham | After | - | - | - | - |
| 2wp | - | - | - | - | |
| 2mp | - | - | - | - | |
| (b) MD values: fasciculi | |||||
| Time | Fasciculus | CV ΔR2 | β (SE) | p-value | |
| tDCS | After | - | - | - | - |
| 2wp | SLF_L | 0.15 | −32.97 (15.52) | 0.055 | |
| 2mp | - | - | - | - | |
| sham | After | - | - | - | - |
| 2wp | - | - | - | - | |
| 2mp | - | - | - | - | |
| (c) FA values: ROIs | |||||
| Time | ROI | CV ΔR2 | β (SE) | p-value | |
| tDCS | After | - | - | - | - |
| 2wp | IFG_R | 0.07 | 16.06 (4.60) | 0.005 | |
| MTG_L | 0.33 | −14.96 (5.43) | 0.019 | ||
| 2mp | - | - | - | - | |
| sham | After | ITG_L | 0.12 | 4.83 (2.09) | 0.037 |
| 2wp | - | - | - | - | |
| 2mp | IFG_L | 0.13 | 21.44 (9.51) | 0.042 | |
| (d) FA values: fasciculi | |||||
| Time | Fasciculus | CV ΔR2 | β (SE) | p-value | |
| tDCS | After | - | - | - | - |
| 2wp | - | - | - | - | |
| 2mp | - | - | - | - | |
| sham | After | UnF_L | 0.12 | 5.54 (0.76) | <0.001 |
| UnF_R | 0.47 | −5.34 (0.92) | <0.001 | ||
| 2wp | - | - | - | - | |
| 2mp | - | - | - | - | |
Towards a unified account of functional and structural connectivity in tDCS effects
This is the first study, to our knowledge, to show significant associations between white-matter integrity and language therapy outcomes with and without tDCS in PPA. The present findings align with our previous findings8 in gray matter volumes as predictors of tDCS effects in PPA. In that study, despite using absolute values for calculating therapy effects (in the present study we used proportional maximal gain), we found that gray-matter volumes of temporal areas responsible for storing lexical-semantic information49,50 predicted tDCS effects in trained items. Similarly, gray-matter volumes of parietal areas (particularly the left SMG) responsible for performing rule-based computations (e.g., the Phoneme-to-Grapheme conversion50) and for temporarily storing and encoding the sequencing of information51 predicted tDCS effects in untrained items. Temporal areas (MTG_L, ITG_L) correspond to the ventral language pathways of the present study, directly connected to the stimulation area, the left IFG50,52. Parietal areas (SMG_L) correspond to the dorsal language pathways of the present study, also directly connected to the stimulation area, the left IFG50,52.
The present findings also align with previous findings on functional connectivity as a mechanism for tDCS effects. White-matter integrity has not been shown to be a mechanism for tDCS effects because there are no detectable changes in white-matter after such short interventions (2–3 weeks). With regard to mechanism, we30 and others53,54 recently showed, using resting-state fMRI, that tDCS gains were correlated with decreases in long-distance functional connectivity between the stimulated area and ventral areas responsible for active retrieval55,56 (in our case, the left MTG, MTG_pole and ITG)30 of therapy-trained words. Functional connectivity also predicts tDCS effects and to a much greater extent than structural connectivity as found here (up to 42% in one of our recent studies). In light of those results, we hypothesize that the modulations of functional connectivity between left IFG and ventral temporal areas53,54 (i.e., the neural coactivation of those areas) may, at least partly, be due to structural connectivity between these areas57. Our results indicate that white-matter integrity may mediate the mechanism of tDCS effects, i.e., change in functional connectivity. A future mediation analysis combining the effects of both modalities would shed light on this important question.
Limitations of DTI methods in the present study
In the present study we focused on the language network. Therefore, connectivity of other areas that may have been implicated in important yet peripheral functions in therapy such as attention or learning were beyond the scope of the present study. Although interesting, including many areas as predictors, in addition to the other clinical variables such as variant type that we wanted to control for, would have made the analysis unstable from a statistical perspective due to the large number of predictors.
A word of caution is needed when we refer to tracts. In the present study we used a DTI method based on a region-of-interest analysis (ROI) rather than actual tractography. Although this method measures the uncinate fasciculus as the only detectable ventral tract that directly connects the left IFG and left temporal areas, this may be an approximation since it does not reflect the whole spectrum of structural connectivity between fronto-temporal areas. Besides the uncinate fasciculus, that actually does not connect IFG_L to temporal areas, a recently documented but difficult-to-identify fasciculus, the extreme capsule fasciculus, connects the IFG (triangularis) with superior, middle and inferior temporal areas49,50,52,58,59. With our methods we cannot be sure that we measured the uncinate and not the extreme capsule fasciculus as responsible for the tDCS effects because we measure only the volume of the white-matter bundle curve that could be either the UnF_L or the extreme capsule. In fact, it is difficult to identify single tract fasciculi with the methods employed here. Specific seeding in the extreme capsule and individual brain analyses are needed to determine this issue in subsequent studies. Whether the uncinate or the extreme capsule, these tracts are part of the ventral language stream49,50,60 indicating that electrical current distribution from the left IFG has, at least partly, travelled through the axons of the ventral-stream fasciculi60.
Finally, we would like to note some limitations about the DTI indices, FA and MD. Changes in DTI indices such as MD and FA are frequently interpreted as cellular loss, axonal degeneration, and defective myelination, among other factors. However, the seminal studies responsible for those interpretations, later translated and perpetuated in neuroscience, were mostly preformed in single-cell models that do not represent the complexity of the brain tissue. Also, they occurred in the absence of imaging nuisances such as noise, artifacts, and macroscopic resolution. Therefore, although DTI indices are sensitive markers of neuropathology, their physiopathological basis is far to be clear and MRI-based interpretations, although very commonly found in the literature, are far from definitive.
That being said, based in other disease models and methods, and post-mortem evaluation, FA can be considered a sensitive, non-specific, marker of microstructural architecture; while MD may be more sensitive to reveal diffusion changes related to de/dysmyelination, encephalomalacia, inflammation, or all sorts of edema61. Commonly, neuropathologies show inverse trends (i.e., decrease / increase) on FA / MD. The sensitivity of these metrics unclearly varies. Given this complex scenario, it is out of our scope to make assertions about the physiological basis of changes in FA and MD, as MRI does not offer the necessary resolution to do so. Therefore, we can only vaguely speculate that our findings are driven by the most common factors beyond DTI indices: changes in tissue architecture and intra/extra cellular composition.
Conclusions
Our study confirmed that tDCS over the left IFG augments written naming outcomes in PPA and increases sustainability and generalization to untrained words. However, regular language therapy (without tDCS) depends more on white-matter integrity than tDCS. White-matter integrity of specific tracts is needed for tDCS: (1) the extreme capsule/uncinate fasciculi connecting the left IFG with temporal areas are needed for lexical retrieval (as evidenced for trained items); (2) fronto-parietal fasciculi (e.g., superior longitudinal fasciculus III) are needed for rule-based computations that lead to generalization of treatment effects (as evidenced for untrained items). Together with previous findings8,30, the current study reveals the interplay between structural and functional connectivity in tDCS effects in PPA. Effects of tDCS may, thus, depend more on functional than structural connectivity since they explain more variance for functional connectivity. The fact, however, that there was an inverse correlation between tDCS effects and white-matter integrity of dementia-related areas such as the cingulate, shows that tDCS may help to circumvent white-matter damage in neurodegenerative disorders, probably by modulating functional connectivity. Understanding the contributions of white-matter integrity, cortical volume and functional connectivity is crucial in bringing the field of neuromodulation in rehabilitation of PPA from ‘imprecision’ to ‘precision’. Our results have important implications for clinical decisions for choosing which patients would benefit from language therapy or electrical stimulation in neurodegenerative disorders but also which behavioral treatments may be more appropriate or beneficial for each patient.
Supplementary Material
Figure 3.
Current flow for the montage of the tDCS montage used in the study: anode was placed over the left IFG and cathode over the right cheek.
Acknowledgements
We would like to thank our participants and referring physicians for their dedication and interest in our study. This work was supported by grants from the Science of Learning Institute at Johns Hopkins University and by the NIH (National Institute of Deafness and Communication Disorders) through award R01 DC014475 to KT. AH was supported by NIH (NIDCD) through awards R01 DC015366, R01 DC011317, R01 DC05375 and P50 DC014664.
Abbreviations
- AG
angular gyrus
- CG
cingulate gyrus
- FuG
fusiform gyrus
- IFG
inferior frontal gyrus
- IFOF
inferior fronto-occipital fasciculus
- ITG
inferior temporal gyrus
- MFG
middle frontal gyrus
- MTG
middle temporal gyrus
- SLF
superior longitudinal fasciculus
- SMG
supramarginal gyrus
- STG
superior temporal gyrus
- UnF
uncinate fasciculus
References
- 1.Fridriksson J, Rorden C, Elm J, Sen S, George MS, Bonilha L. Transcranial direct current stimulation vs sham stimulation to treat aphasia after stroke: a randomized clinical trial. JAMA Neurol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsapkini K, Webster KT, Ficek BN, et al. Electrical brain stimulation in different variants of primary progressive aphasia: A randomized clinical trial. Alzheimers Dement. Transl. Res. Clin. Interv 2018;4:461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsapkini K, Frangakis C, Gomez Y, Davis C, Hillis AE. Augmentation of spelling therapy with transcranial direct current stimulation in primary progressive aphasia: Preliminary results and challenges. Aphasiology. 2014;28(:1112–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung J, Bauer A, Grossman M, Hamilton RH, Coslett HB, Reilly J. Semantic Feature Training in Combination with Transcranial Direct Current Stimulation (tDCS) for Progressive Anomia. Front. Hum. Neurosci 2017;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McConathey EM, White NC, Gervits F, et al. Baseline performance predicts tdcs-mediated improvements in language symptoms in primary progressive aphasia. Front. Hum. Neurosci 2017;11:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotelli M, Manenti R, Petesi M, et al. Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. J. Alzheimers Dis. JAD 2014;39:799–808. [DOI] [PubMed] [Google Scholar]
- 7.Roncero C, Kniefel H, Service E, Thiel A, Probst S, Chertkow H. Inferior parietal transcranial direct current stimulation with training improves cognition in anomic Alzheimer’s disease and frontotemporal dementia. Alzheimers Dement. Transl. Res. Clin. Interv 2017;3:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Aguiar V, Zhao Y, Faria A, et al. Brain volumes as predictors of tDCS effects in primary progressive aphasia. Brain Lang. 2020;200:104707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Aguiar V, Zhao Y, Ficek BN, et al. Cognitive and language performance predicts effects of spelling intervention and tDCS in Primary Progressive Aphasia. Cortex. 2020;124:66–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hillis AE, Beh YY, Sebastian R, et al. Predicting recovery in acute poststroke aphasia. Ann. Neurol 2018;83:612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchina S, Zhu LL, Norton A, Zipse L, Wan CY, Schlaug G. Impairment of Speech Production Predicted by Lesion Load of the Left Arcuate Fasciculus. Stroke. 2011. Available at: https://www.ahajournals.org/doi/abs/10.1161/STROKEAHA.110.606103 Accessed October 9, 2018. [DOI] [PMC free article] [PubMed]
- 12.Chutinet A, Rost NS. White matter disease as a biomarker for long-term cerebrovascular disease and dementia. Curr. Treat. Options Cardiovasc. Med 2014;16:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright A, Tippett D, Saxena S, et al. Leukoaraiosis is independently associated with naming outcome in poststroke aphasia. Neurology. 2018;91:e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catani M, Piccirilli M, Cherubini A, et al. Axonal injury within language network in primary progressive aphasia. Ann. Neurol 2003;53:242–247. [DOI] [PubMed] [Google Scholar]
- 15.Odolil A, Wright AE, Keator LM, et al. Leukoaraiosis severity predicts rate of decline in primary progressive aphasia. Aphasiology. 2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandelli ML, Caverzasi E, Binney RJ, et al. Frontal white matter tracts sustaining speech production in primary progressive aphasia. J. Neurosci 2014;34:9754–9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galantucci S, Tartaglia MC, Wilson SM, et al. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain. 2011;134:3011–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwindt GC, Graham NL, Rochon E, et al. Whole-brain white matter disruption in semantic and nonfluent variants of primary progressive aphasia. Hum. Brain Mapp 2013;34:973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu S, Leyton CE, Hodges JR, Piguet O, Hornberger M. Divergent longitudinal propagation of white matter degradation in logopenic and semantic variants of primary progressive aphasia. J. Alzheimers Dis 2016;49:853–861. [DOI] [PubMed] [Google Scholar]
- 20.Mahoney CJ, Malone IB, Ridgway GR, et al. White matter tract signatures of the progressive aphasias. Neurobiol. Aging 2013;34:1687–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meier E, Breining BL, Sheppard SM, et al. White matter hyperintensities independently contribute to language deficits in primary progressive aphasia. Cognitive and Behavioral Neurology. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein-Peterson Z, Vasconcellos Faria A, Mori S, Hillis AE, Tsapkini K. Relatively normal repetition performance despite severe disruption of the left arcuate fasciculus. Neurocase. 2012;18:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonilha L, Rorden C, Fridriksson J. Assessing the clinical effect of residual cortical disconnection after ischemic strokes. Stroke J. Cereb. Circ 2014;45:988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breier JI, Hasan KM, Zhang W, Men D, Papanicolaou AC. Language Dysfunction After Stroke and Damage to White Matter Tracts Evaluated Using Diffusion Tensor Imaging. Am. J. Neuroradiol 2008;29:483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forkel SJ, Thiebaut de Schotten M, Dell’Acqua F, et al. Anatomical predictors of aphasia recovery: a tractography study of bilateral perisylvian language networks. Brain. 2014;137:2027–2039. [DOI] [PubMed] [Google Scholar]
- 26.Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca’s aphasia undergoing intense intonation-based speech therapy. Ann. N. Y. Acad. Sci 2009;1169:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohr B. Neuroplasticity and Functional Recovery after Intensive Language Therapy in Chronic Post Stroke Aphasia: Which Factors Are Relevant? Front. Hum. Neurosci 2017;11:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonilha L, Gleichgerrcht E, Nesland T, Rorden C, Fridriksson J. Success of Anomia Treatment in Aphasia Is Associated With Preserved Architecture of Global and Left Temporal Lobe Structural Networks. Neurorehabil. Neural Repair 2016;30:266–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng X, Schlaug G. Structural white matter changes in descending motor tracts correlate with improvements in motor impairment after undergoing a treatment course of tDCS and physical therapy. Front. Hum. Neurosci 2015;9:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ficek BN, Wang Z, Zhao Y, et al. The effect of tDCS on functional connectivity in primary progressive aphasia. NeuroImage Clin 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori S, Wu D, Ceritoglu C, et al. MRICloud: Delivering high-throughput MRI neuroinformatics as cloud-based software as a service. Comput. Sci. Eng 2016;18:21–35. [Google Scholar]
- 32.Jiang H, Van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput. Methods Programs Biomed 2006;81:106–116. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, van Zijl PC, Mori S. Image contrast using the secondary and tertiary eigenvectors in diffusion tensor imaging. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med 2006;55:439–449. [DOI] [PubMed] [Google Scholar]
- 34.Faria AV, Joel SE, Zhang Y, et al. Atlas-based analysis of resting-state functional connectivity: Evaluation for reproducibility and multi-modal anatomy-function correlation studies. NeuroImage. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rezende TJ, Campos BM, Hsu J, et al. Test–retest reproducibility of a multi-atlas automated segmentation tool on multimodality brain MRI. Brain Behav. 2019;9:e01363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Homan RW. The 10–20 electrode system and cerebral location. Am. J. EEG Technol 1988;28:269–279. [Google Scholar]
- 37.Fridriksson J, Richardson JD, Baker JM, Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham-controlled study. Stroke J. Cereb. Circ 2011;42:819–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 2006;117:845–850. [DOI] [PubMed] [Google Scholar]
- 39.Rapp B, Glucroft B. The benefits and protective effects of behavioural treatment for dysgraphia in a case of primary progressive aphasia. Aphasiology. 2009;23:236–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsapkini K, Hillis AE. Spelling intervention in post-stroke aphasia and primary progressive aphasia. Behav. Neurol 2013;26:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beeson PM, Egnor H. Combining treatment for written and spoken naming. J. Int. Neuropsychol. Soc. JINS 2006;12:816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shim H, Hurley RS, Rogalski E, Mesulam M. Anatomic, clinical, and neuropsychological correlates of spelling errors in primary progressive aphasia. Neuropsychologia. 2012;50:1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sepelyak K, Crinion J, Molitoris J, et al. Patterns of breakdown in spelling in primary progressive aphasia. Cortex. 2011;47:342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodman RA, Caramazza A. The Johns Hopkins University Dysgraphia Battery. Johns Hopkins University; Baltimore, MD; 1985. [Google Scholar]
- 45.Sage K, Hesketh A, Ralph MAL. Using errorless learning to treat letter-by-letter reading: contrasting word versus letter-based therapy. Neuropsychol. Rehabil 2005;15:619–642. [DOI] [PubMed] [Google Scholar]
- 46.Bonilha L, Hillis AE, Hickok G, Den Ouden DB, Rorden C, Fridriksson J. Temporal lobe networks supporting the comprehension of spoken words. Brain. 2017;140:2370–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forkel SJ, Thiebaut de Schotten M, Dell’Acqua F, et al. Anatomical predictors of aphasia recovery: a tractography study of bilateral perisylvian language networks. Brain. 2014;137:2027–2039. [DOI] [PubMed] [Google Scholar]
- 48.Mori S, Wu D, Ceritoglu C, et al. MRICloud: delivering high-throughput MRI neuroinformatics as cloud-based software as a service. Comput. Sci. Eng 2016;18:21–35. [Google Scholar]
- 49.Loh KK, Procyk E, Neveu R, et al. Cognitive control of orofacial motor and vocal responses in the ventrolateral and dorsomedial human frontal cortex. Proc. Natl. Acad. Sci 2020;117:4994–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saur D, Kreher BW, Schnell S, et al. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci 2008;105:18035–18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rapp B, Purcell J, Hillis AE, Capasso R, Miceli G. Neural bases of orthographic long-term memory and working memory in dysgraphia. Brain. 2015;139:588–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrides M Broca’s area in the human and the non-human primate brain. Broca’s Reg. 2006:31–46. [Google Scholar]
- 53.Meinzer M, Lindenberg R, Darkow R, Ulm L, Copland D, Floel A. Transcranial direct current stimulation and simultaneous functional magnetic resonance imaging. J. Vis. Exp. JoVE 2014;(86). doi: 10.3791/51730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meinzer M, Antonenko D, Lindenberg R, et al. Electrical brain stimulation improves cognitive performance by modulating functional connectivity and task-specific activation. J. Neurosci. Off. J. Soc. Neurosci 2012;32:1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc. Natl. Acad. Sci 1997;94:14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrides M, Alivisatos B, Evans AC. Functional activation of the human ventrolateral frontal cortex during mnemonic retrieval of verbal information. Proc. Natl. Acad. Sci. U. S. A 1995;92:5803–5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Margulies DS, Petrides M. Distinct Parietal and Temporal Connectivity Profiles of Ventrolateral Frontal Areas Involved in Language Production. J. Neurosci 2013;33:16846–16852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J. Neurosci. Off. J. Soc. Neurosci 2008;28:11435–11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rolheiser T, Stamatakis EA, Tyler LK. Dynamic processing in the human language system: synergy between the arcuate fascicle and extreme capsule. J. Neurosci. Off. J. Soc. Neurosci 2011;31:16949–16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. [DOI] [PubMed] [Google Scholar]
- 61.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knopman DS, Kramer JH, Boeve BF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131:2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.