Abstract
Activated B cells participate in either extrafollicular (EF) or germinal center (GC) responses. Canonical responses are composed of a short wave of unmutated plasma cells arising from EF sites, followed by GC producing somatically mutated memory B cells (MBC) and long-lived plasma cells. However, somatic hypermutation and affinity maturation can take place at both sites, and a substantial fraction of MBC are produced prior to GC formation. Infection responses range from GC responses that persist for months, to persistent EF responses with dominant suppression of GCs. Here we review the current understanding of the functional output of EF and GC responses and the molecular switches promoting them. We discuss the signals that regulate the magnitude and duration of these responses, and outline gaps in knowledge and important areas of inquiry. Understanding such molecular switches will be critical for vaccine development, interpretation of vaccine efficacy and the treatment for autoimmune diseases.
eTOC blurb
B cell responses track towards either germinal center or extrafollicular responses. The extrafollicular response is increasingly being appreciated as a dominant mode in certain infections and in autoimmunity. Elsner and Shlomchik review these two response types and discuss the implications for immunity, vaccine design and disease stratification and therapy.
Introduction
B cell responses to immunization or infection have broadly been divided into canonical responses that feature a germinal center (GC) reaction and non-canonical responses that lack GCs and feature B cell proliferation and differentiation into plasmablasts (PB) at extrafollicular (EF) sites. Canonical GC responses are often, but not always, preceded by a short phase of EF proliferation and differentiation while non-canonical reactions typically have prolonged responses at EF sites (MacLennan et al., 1991; MacLennan et al., 2003).
In animal models the type of B cell response to a particular immunization or pathogen has in some cases been documented, while in most cases the type of response in human infection and vaccination is not known. In general, it is not clear why certain stimuli lead to particular responses. Nor are the general mechanisms that direct one or another type of response well understood. Further, the differences in the types of cells produced by these two distinct types of B cell reactions are also not well-appreciated.
The goal of this review is to compare and contrast these two response types. We will first outline the sequential stages and processes common to B cell responses and cover the similarities and differences between classical and non-canonical responses. While according to most dogma the classical GC response exclusively creates isotype switched, affinity-matured, V region-mutated B cells that can seed both memory and long-lived PC compartments, we will see that relatively recent and emerging data demonstrates that many if not all of these processes also pertain to the EF response. We will then review examples of the different types of immunization and infection that lead to either response. Finally, we will discuss studies that have tried to delineate the molecular and cellular control of each type of B cell response. We conclude by discussing how the nature of the B cell response can be critical for determining acute protection, effective responses to vaccines and the designs thereof, and how this fits with recent reports that in severe infections with SARS-CoV-2 the B cell response is dominated by EF PB, with poor GC formation.
Overview of B cell response patterns
The classical description of the steps in a B cell immune response to a T cell-dependent antigen (Ag) derives mainly from rodent models. It has been well-reviewed (Cyster and Allen, 2019; Nutt et al., 2015; Weisel and Shlomchik, 2017). In brief, within two days, responding B cell and T cell blasts are observed at the T cell border. Around this time, some B cells migrate to EF regions and begin PB differentiation. PB both secrete antibody (Ab) and proliferate, and their numbers typically peak 4–6 days after immunization. Concurrently, some T and B cells from the initial proliferative focus migrate into B cell follicles, where they continue to proliferate and become committed to the GC fate. The GC ultimately will be the source of most if not all of the LLPC that migrate to bone marrow, as well as many (but not all) of the MBC that will be formed. GC responses peak approximately 2 weeks post immunization, and are a continuing source of antibody forming cells (AFCs); thus it is important that two time points must be assessed to analyze EF- versus GC-derived antibodies.
Responses that proceed primarily via the EF pathway and do not form GCs may begin similarly, but have a different trajectory. Instead of involuting, as is the case for immunizations, the initial activated B cell and PB reaction continues to expand. Depending on the context, the response can last weeks to months (e.g. Ehrlichia muris and Salmonella enterica serovar Typhimurium (STm) infection) or even for the life of the organism in the case of spontaneous autoimmunity (Cunningham et al., 2007; Jenks et al., 2019; Racine et al., 2010). In general, there is little or no detectable histologic GC response. In some settings, a small population with surface markers consistent with a GC phenotype can be detected by flow cytometry. However, even these appear hypomorphic with respect to expression of Bcl6, the GC master regulator transcription factor (Di Niro et al., 2015). Such cells may be located in small clusters that otherwise do not have features of GCs.
Comparison of B cell immune response processes in canonical and EF responses:
Both EF and canonical GC responses feature extensive proliferation of responding cells, including both PBs and B cell blasts at the early stages of all types of responses. B cell blasts are the precursors of PBs (Trivedi et al., 2019; William et al., 2005b). At early time points, proliferating B blasts and PB expand rapidly, such that the Salmonella response can yield a few million PB at its peak of 7–10 days post-infection (Di Niro et al., 2015). The half-life of PBs is only a few days, limiting the overall size of the response (Slifka et al., 1998; Smith et al., 1996). This implies that ongoing responses, such as during the early phase of the response to STm or E. muris, as well as chronic responses as seen in lupus-prone mice, are continually replenished by recruitment of new B cell blasts. Presumably, as pathogen or Ag is cleared, for example at approximately 4–6 weeks of STm infection (Cunningham et al., 2007), these precursors are no longer activated and the response involutes.
Similarly, there is extensive proliferation among GC (Allen et al., 2007; Mesin et al., 2016). However, a critical difference is that GC do not clonally expand in terms of overall cell number once reaching peak numbers (Faro et al., 2019; Mesin et al., 2016). While GCs develop rapidly, they reach peak cell numbers rather early (day 9–12 in a typical anti-hapten response), after which they start to involute. The lack of expansion may seem counterintuitive, since GC B cells (GCBC) divide 3–4 times per day and continuous BrdU administration will result in near complete labeling within a day (Allen et al., 2007; Mayer et al., 2017). Since the total numbers of long-lived progeny of the GC (i.e. MBC and LLPC) are rather small, most of the balance for cell division is cell death rather than differentiation. Indeed, at any given time substantial percentages of GC B cells can be observed undergoing apoptosis (Anderson et al., 2009; Mayer et al., 2017).
Interestingly, these two different modes of response are associated with two different metabolic programs. Activated B cell blasts, which undergo massive clonal expansion at the beginning of both classical and non-canonical responses, are very large cells that metabolize both glucose and fatty acids, consume oxygen (i.e. undertake oxidative phosphorylation) and also acidify culture media after short-term in vitro incubation, indicative of aerobic glycolysis (Weisel et al., 2020). Such metabolic programs are likely necessary to support the generation of new macromolecules to increase the net biomass associated with clonal expansion.
In contrast, after initial GC expansion, cell numbers remain constant or decline, so that no net additional biomass is created. Perhaps commensurate with maintenance rather than expansion, GCBC mainly metabolize fatty acids via oxidative phosphorylation, as revealed in recent studies from our lab (Weisel et al., 2020). GCBC did not detectably utilize glucose or glutamine, nor appreciably acidify the media, but did consume oxygen. These metabolic analyzer results were confirmed by 13C tracing as well as studies showing that GCBC take up fat but not glucose in vivo and in vitro. Originally, it was proposed that the GC was hypoxic, which might have suggested that they would not carry out oxidative phosphorylation (Cho et al., 2016). However, oxidative phosphorylation can occur even at very low oxygen tensions (Konigsberg et al., 2013). GCBC did not demonstrate expression of a HIF-1α gene expression program, suggesting that they were not experiencing functional hypoxia (Weisel et al., 2020).
GCs are sites for Ag-driven selection (Jacob et al., 1991; Jacob et al., 1993; Liu et al., 1989; Zhang et al., 1988). Mechanisms underlying this have been extensively reviewed elsewhere (Bannard and Cyster, 2017; Shlomchik et al., 2019). In addition, it appears that the LLPC compartment is especially enriched in higher affinity mutants (Phan et al., 2006; Smith et al., 1997). This finding has been interpreted as “instructional”, in that cells with higher affinity gain signals that cause them to differentiate towards the PC fate. However, the Brink group, which originally put forth the instructional interpretation, later developed evidence that prior to GC formation higher affinity cells underwent more net expansion, rather than were instructionally deviated towards PC differentiation (Chan et al., 2009). The instructional model nonetheless has significant credence in the literature (Ise and Kurosaki, 2019), though how it applies to PC differentiation from the GC has not been rigorously tested.
If affinity-dependent signals do direct precursor cells toward PC differentiation, then the question becomes which signals are involved and how they do it? It has been suggested that very strong BCR plus CD40 signals can induce the differentiation of PC precursors in the GC (Ise and Kurosaki, 2019). We showed that PCs tend to differentiate during the late phase of the GC at a time when there are more V region mutations and more affinity-based selection could have taken place (Weisel et al., 2016). This alone could account for the observed higher affinity of LLPC; in fact, there could be a connection as it remains possible that late in the GC, B cells reach an affinity that allows them to be directed to the PC fate, though we do not favor this interpretation.
At EF sites, selection for initial recruitment is promiscuous during a response to immunization with either a hapten-carrier (Dal Porto et al., 1998) or an infection. Both the STm and E. muris initial B cell response is very diverse and of low affinity (Di Niro et al., 2015; Trivedi et al., 2019), suggestive of a low affinity threshold for activation. Indeed, the great majority of responding AFCs in these settings cannot be assigned as specific to any given pathogen-derived Ag using standard assays. Yet, these responses are indeed specific, because in mice with BCRs restricted to irrelevant specificities, the immune response to either bacteria is markedly reduced. This indicates that BCR specificity matters. High-throughput V region sequencing also supports this conclusion (Di Niro et al., 2015; Trivedi et al., 2019).
After initial promiscuous clonal selection, it has been less clear whether affinity maturation occurs during the subsequent or sustained EF response. A priori, there is no reason to think that affinity selection would not occur, even in canonical EF, as B cells in both sites recognize Ag natively, and do not need to engage any particular APC for BCR-mediated activation. Presumably, the quality of BCR signals would remain important for promoting continued clonal expansion, providing a basis for affinity selection. However, direct evidence for this has been sparse. Early microdissection studies by Jacob et al analyzing the response to the NP hapten demonstrated that, initially, Vh regions encoding very low affinity Ab were prevalent (Jacob and Kelsoe, 1992). However, as the EF response progressed, there was already purifying selection for the higher affinity VH186.2, which dominates the early GC response as a more fit Vh region (Jacob and Kelsoe, 1992). Studies in lymphotoxin- or TNF-mutant mice, which lack normal lymphoid architecture and are unable to form GCs (Matsumoto et al., 1996b), demonstrated that mutation and affinity maturation could occur (Matsumoto et al., 1996a). However, these studies did not establish whether such events would occur in normal mice undergoing EF responses. A further possibility is that selection is possible in both canonical and prolonged EF responses, but a longer response allows for better selection due to more T cell contact, which would be hypothetically less efficiently obtained outside of the GC. SHM occurs in the GC, with the most definitive evidence coming from microdissection of GC structures on sections (Berek et al., 1991; Jacob et al., 1991; Jacob et al., 1993; Kuppers et al., 1993), and more recently from conceptually similar experiments that used photolabeling of individual GCs to collect cells for V region sequencing (Ersching et al., 2017; Victora et al., 2010).
It was originally thought that SHM does not occur in EF responses and is exclusively the provenance of GCBC. This misconception is unfortunately often repeated to this day, although much evidence refutes it. William et al. found extensive intraclonal somatic V region diversification using microdissection analysis of a persistent non-canonical EF response in lupus-prone mice (William et al., 2002). These data established that SHM could indeed take place at EF sites. Similar results were obtained in another autoimmune strain, with the same autoreactive B cell clonotype proliferating and mutating extrafollicularly and in GCs (Sang et al., 2014). The chronicity of the autoimmune response likely increased the number of mutations (and perhaps even the rate of mutation (William et al., 2002)), thus making the presence of ongoing mutation more easily detectible. The mutation rate in autoreactive EF responses in MRL/lpr mice was similar to that seen in peak GC (Kleinstein et al., 2003). The observation of ongoing SHM during an EF response was recapitulated in inducible systems (Herlands et al., 2007). Similarly, in human autoimmunity, where non-canonical responses have been seen in lymphoid infiltrates in synovium of Rheumatoid Arthritis (RA) patients (most of which do not have a GC-like structure), both SHM and isotype switching were observed (Schroder et al., 1996). Parallel observations were made in both Sjogren’s syndrome and among infiltrating B cells in ductal breast carcinoma (Nzula et al., 2003; Stott et al., 1998). Review of these papers reveals that at least some of the microdissected B cell clusters that yielded mutated V region sequences did not resemble GC structures and were most likely an EF-like response.
Nonetheless, after the initial discovery that SHM could occur robustly during chronic autoimmune responses, direct evidence that SHM could occur outside of GCs during anti-pathogen responses had been lacking. Critically, Cunningham et al reported that the B cell response to STm was exclusively EF and did not include GCs until over a month after infection (Cunningham et al., 2007). This key observation stimulated us to investigate whether SHM was occurring during this EF response. Microdissection of foci of PBs revealed ongoing SHM, as evidenced by intraclonal diversity among clonally related V regions, similar to observations in autoimmune-prone mice (Di Niro et al., 2015). This was further confirmed by isolation of B cell hybridomas that contained mutations, and by high throughput Vh region sequencing. We subsequently found SHM occurring among proliferating B cell clusters during E. muris infection (Trivedi et al., 2019).
In the case of the STm response, reversion analysis demonstrated that these V region mutations did lead to increased affinity, proving that affinity-based selection does happen during ongoing EF responses (Di Niro et al., 2015). Data from E. muris infection also argues for V region mutation and selection during EF responses (Li et al., 2002; Trivedi et al., 2019; Yates et al., 2013). Similarly, in the rheumatoid factor EF response in MRL/lpr lupus-prone mice, analysis of SHM patterns as well as direct measurement of autoAb affinity show selection upon V region mutations occurring outside GC (William et al., 2002; William et al., 2005b). In these cases, it appears that not all clones turn on mutation and selection, suggesting that overall the EF response is less efficient at generating more fit B cells. However, in the rheumatoid factor response, in clones that do undergo mutation, both rate and selection appear comparable to that in the GC (Kleinstein et al., 2003; William et al., 2002).
It has also been clear that isotype switch occurs readily in EF responses, both during this phase in canonical responses (Marshall et al., 2011; Toellner et al., 1998) and during non-canonical responses (Cunningham et al., 2007). In each of the cases that SHM was observed, isotype switching was also readily observed to downstream IgG and IgA isotypes (Bergqvist et al., 2006; Di Niro et al., 2015; Shlomchik et al., 1987; Trivedi et al., 2019); this confirmed the induction of AID, the enzyme required for both SHM and isotype switching (Muramatsu et al., 2000), and its efficacy in mediating DNA modifications. Given that AID is expressed and active in non-GC proliferating B cells (Cattoretti et al., 2006; Pape et al., 2003), it would stand to reason that SHM could also be occurring.
While for many years it was assumed that isotype switch occurs in the GC (and nearly every diagram and review of the GC claims this), recent work has posited that this may not occur as frequently as previously assumed. (Roco et al., 2019). This area remains controversial and definitive precursor-product experiments to test this idea are lacking, as are mechanistic explanations as to why AID in GCBC could efficiently target V regions but somehow not cause switching.
It is now appreciated that adaptive immune responses can occur within tissues, driven by local Ag recognition and proliferation, resulting in in situ differentiation of Ag-specific lymphocytes. Tissue responses can occur at mucosal sites as well as within parenchymal organs, provided that they are infected or under autoimmune attack. A consequence of prolonged infection at tissue sites can be the formation of tertiary lymphoid tissue (TLT) (Dieu-Nosjean et al., 2014; Silva-Cayetano and Linterman, 2020). Such aggregations are variable in composition and structure, but typically resemble T and B cell zones of lymph nodes, with differentiation of high endothelial venules and in some cases stromal cells such as FDCs. It must be noted that FDC are not indicative of GC responses per se (as they exist in resting follicles) but may be needed in order to permit GC responses (Haberman and Shlomchik, 2003; Hannum et al., 2000; Kosco-Vilbois, 2003). TLT are not typically seen at the earliest stages of infection but require time to differentiate (Luo et al., 2019), evidently dependent on lymphotoxin signals that can be provided by B cells (Lorenz et al., 2003). In mice TLT can form in lungs during influenza infection (Moyron-Quiroz et al., 2004). In humans TLT and sometimes GC are observed in chronic hepatitis C infection of the liver (de Ruiter and van der Laan, 2015; Murakami et al., 1999), as well as in chronic organ rejection post-transplantation (Koenig and Thaunat, 2016). Previously-induced TLT can host immunization-dependent responses to typical immunogens (Nacionales et al., 2009; Weinstein et al., 2008). Spontaneous or induced responses in TLT can, but do not have to, include GCs (Nacionales et al., 2009). Thus, fully induced TLT may be functionally similar to secondary lymphoid tissue in terms of supporting immune responses.
Chronic autoimmunity can also result in organized lympho-myeloid infiltrates in target organs that may be distinct from TLT (Clark et al., 2015). Infiltrates in kidneys of lupus patients can range from disorganized clusters of T and B cells, to delineated T and B cell zones, to—occasionally—organized TLT that appear to have follicular structure (Chang et al., 2011; Liarski et al., 2014). Whether these structures are just responses to the inflammatory environment or harbor specific autoimmune responses to local tissues remains unclear.
Acute tissue responses can also resemble EF responses, with clusters of T and B cells, along with scattered myeloid cells forming proliferative foci, in which B cell differentiation can be observed. These tissue responses are devoid of GC-like structures and can range from small clusters of proliferating lymphocytes to larger sheets of cells in which T and B cells are admixed. Examples include infection of the liver with E. muris (Trivedi et al., 2019) as well as hepatitis B and C in humans (Farci et al., 2010; Freni et al., 1995). At least some cases of lupus nephritis might be included in this category (Chang et al., 2011).
The nature of lymphoid aggregates and local responses is variable, with one extreme being small clusters of acutely responding cells and another the fully developed TLT associated with long-term infection or autoimmunity. However, in our view, unless a defined GC structure is seen, with Bcl6-expressing GCBC and TFH, then the responses observed should not be considered as GC-like but rather EF-like, and thus represent another situation in which EF responses dominate. In either case, microdissection of cells followed by V region sequencing is the preferred method to determine where SHM and potential Ag-driven selection are taking place.
Classically, the generation of both MBC and LLPC has been attributed exclusively or mostly to the GC process. However, it has become apparent that many and maybe even most MBC are formed prior to the onset of GCs during protein immunizations (Inamine et al., 2005; Takemori et al., 2014; Toyama et al., 2002; Weisel and Shlomchik, 2017; Weisel et al., 2016). Hence, GCs are not needed to develop MBC. Whether EF responses per se generate MBC is much less clear. Winslow and colleagues presented direct evidence that MBC are generated by E. muris infection, which predominantly if not exclusively elicits an EF response (Yates et al., 2013). Our lab confirmed and extended these results by characterizing the MBC and showing that liver was a generative site of B cell responses to E. muris, as well as a location harboring MBC (Trivedi et al., 2019). Malaria, which favors an EF response but does induce some GC response, generates an abundant population of IgM MBC, suggestive of an extra-GC origin (Krishnamurty et al., 2016). Subsequent to B. burgdorferi infection, little evidence was seen for MBC development, despite short-lived GC responses that ensued (Elsner et al., 2015). This was attributed to dominant suppression of B cell responses during the early infection; however there was evidence for some MBC at one year post-infection, though Ag-specific MBC could not be directly enumerated (Elsner et al., 2015). Perhaps due to the difficulty in tracking MBC combined with the difficulty in excluding a role for a residual GC response, there is still relatively little knowledge about formation of MBC during infections in which the EF response dominates.
With regard to LLPC, the majority of evidence suggests that these are almost exclusively derived from an authentic GC reaction. Mice lacking Bcl6 expression in B cells generated IgM MBC readily, but failed to generate any long-lived serum IgG or BM AFC (Toyama et al., 2002). Similarly, disrupting the GC with anti-CD40L abrogated the great majority of IgG1 BM AFC measured at later time points (Takahashi et al., 1998). LLPC are notable for substantially higher levels of SHM and affinity maturation, compared to MBC, both hallmarks of prolonged mutation and selection within the GC (Smith et al., 1997; Takahashi et al., 2001). Commensurate with this, bacterial infections that have limited or no GC reaction, such as STm, E. muris and B. burgdorferi, produce IgG Abs of limited lifespan and few LLPCs (Elsner et al., 2015; Manne et al., 2019; Racine et al., 2010). Despite this, evidence exists in systems of experimental immunization with T cell-independent Ags, which presumably do not elicit GCs, that lead to long-lived Ab responses (Allman et al., 2019; Foote et al., 2012). The mechanisms by which long-lived Ab is generated and sustained in these systems bears further investigation.
Nonetheless, EF responses can lead to very large isotype-switched AFC responses in secondary lymphoid tissues that can result in acutely high titers of IgG Abs. Such Abs can be modestly affinity-matured. While desirable for acute protection, these EF-derived Abs would not afford long-term neutralizing type immunity to reinfection (a defect seen in B. burgdorferi infection, for example (Elsner et al., 2015)) and thus are not desirable outcomes of vaccination. As vaccine research often seeks to measure titers at relatively early time points and after multiple boosts, perhaps as a means to quicken the process of evaluation, it may not distinguish GC responses that lead to gradual accumulation of LLPC (Good-Jacobson and Shlomchik, 2010; Good-Jacobson et al., 2010; Weisel et al., 2016) from strong EF responses that give high titers that will eventually wane due to a dearth of LLPC. This in turn suggests the need to better develop and employ biomarkers of GC responses in order to monitor vaccines that are being designed to create long-lived neutralizing Ab titers.
What types of responses promote EF or GC reactions?
The relative dominance of EF or GC during the immune response to different infections exists along a spectrum. Hapten immunization systems have defined the canonical response as an early wave of EF response, ending by approximately day 6, followed by a GC phase lasting a few weeks. Infection systems show great diversity in the dominance, and length, of each response (summarized in Table 1).
Table 1.
Infections driving EF or GC dominant responses along with known associated mechanisms.
| Pathogen | EF or GC dominant | GC phenotype, other effects on architecture | Known contributing mechanisms | References |
|---|---|---|---|---|
| Salmonella enterica serovar Typhimurium | EF | GC suppression | IL-12 blocks TFH and GC | (Elsner and Shlomchik, 2019) (Cunningham et al., 2007) (Nanton et al., 2015) (Di Niro et al., 2015) |
| Loss of T and B zones in lymph nodes, some disruption in spleen | LPS and TLR4 in lymph nodes, but TLR4 KO doesn’t restore splenic GC | (Di Niro et al., 2015) (St John and Abraham, 2009) |
||
| Ehrlichia muris | EF | GC suppression | Unknown | (Popescu et al., 2019) (Racine et al., 2010) |
| Disrupted splenic architecture | High TNFα, blocking restores some GC | (Popescu et al., 2019) | ||
| Borrelia burgdorferi | EF | GC suppression | Unknown | (Elsner et al., 2015) (Hastey et al., 2012) |
| Loss of T and B zones in lymph nodes | Unknown (MyD88, TRIF & IFNAR independent) | (Hastey et al., 2012) (Hastey et al., 2014) |
||
| Plasmodium sp. (malaria) | Both | GC suppression | IFNγ & TNFα block TFH, Excess PB are nutrient sink | (Ryg-Cornejo et al., 2016) (Wilmore et al., 2013) (Vijay et al., 2020) |
| Disrupted splenic architecture | Unknown (TLR2, TLR4, TLR9 & MyD88 independent) | (Achtman et al., 2003) (Cadman et al., 2008) |
||
| LCMV clone 13 | Both | Partial GC suppression | Early Type 1 IFN wave kills activated B cells | (Fallet et al., 2016) |
| Disrupted splenic architecture | Unknown | (Sullivan et al., 2011) | ||
| HIV | Both | GC suppression | Reduced TFH helper function | (Cubas et al., 2013) |
| Lymph node architecture disruption and fibrosis | Multifactorial | Reviewed in (Estes, 2013) | ||
| Influenza virus | GC | Long-lasting GC | Unknown cause | (Rothaeusler and Baumgarth, 2010) |
| Normal lymph node architecture | (Elsner et al., 2012) | |||
| Vesicular stomatitis virus | GC | Fast, long-lasting GC | IL-6 promotes early GC by promoting TFH and blocking TH1 | (De Giovanni et al., 2020) |
| Normal splenic architecture | (Bachmann et al., 1996) |
Viruses that induce high levels of cellular damage (cytopathic), such as Vesicular Stomatitis Virus (VSV) or Influenza virus, tend to induce both EF and GC responses, and produce neutralizing Abs much more quickly than the non-cytopathic virus LCMV (Fink et al., 2007; Hangartner et al., 2006). VSV and influenza-induced GCs persist beyond 100 days post infection, far longer than the 3–4 weeks for NP-immunization, despite undetectable viral replication after a week of infection (Bachmann et al., 1996; Rothaeusler and Baumgarth, 2010). Influenza infection generates high affinity Abs and life-long immunity (Baumgarth, 2013; Sangster et al., 2019), potentially as a result of the length of the GC response. Consistent with this, influenza-specific GC can be blocked with the mTOR inhibitor rapamycin, with the resulting Abs being lower affinity but more broadly protective (Keating et al., 2013). The lengthy GC response could contribute to the ability of influenza infection to induce life-long protective Abs. Similar findings have been reported for West Nile virus, where MBC containing fewer mutations can more effectively neutralize viral escape mutants (Purtha et al., 2011). Why influenza in particular induces such persistent GCs remains unknown. Insight into control of GC-longevity could benefit HIV vaccine design, as broadly neutralizing HIV Abs are of the most highly mutated Abs described, arising after months of infection (Burton and Hangartner, 2016; Havenar-Daughton et al., 2017).
At the other end of the spectrum are infections that induce prolonged EF responses, some also dominantly suppressing GCs. These include the extracellular spirochete B. burgdorferi, the obligate intracellular bacteria E. muris, and the facultative intracellular bacteria STm (Cunningham et al., 2007; Elsner et al., 2015; Elsner and Shlomchik, 2019; Hastey et al., 2012; Nanton et al., 2015; Racine et al., 2010). During B. burgdorferi infection, GCs peak around 2 weeks of infection but then quickly collapse due to an unknown suppressive mechanism. These early GCs fail to induce a long-lived Ab response, and the infection dominantly suppresses the long-lived immunity generated by a concurrently administered influenza immunization (Elsner et al., 2015). E. muris and STm infections both fail to induce GC for 2–4 weeks, and when immunized with the hapten NP-CGG after infection, both suppress NP-specific GC responses (Elsner and Shlomchik, 2019; Racine et al., 2010). STm specifically inhibits GC, and suppresses neither T-dependent (NP-CGG) nor T-independent (NP-Ficoll) NP-specific EF responses (Elsner and Shlomchik, 2019). Approximately 4 weeks post infection, the T cell response gains control over the infection, bacterial burden is reduced, and the GC response begins (Cunningham et al., 2007; Di Niro et al., 2015). The precise impacts of this early GC suppression on long-lived immunity are still unknown. However molecular typing has shown that people in endemic regions can be reinfected with Salmonella in the same season (Okoro et al., 2012), implying poor or delayed immunity. Plasmodium chabaudi also suppress T-dependent, but not T-independent B cell responses (Wilmore et al., 2013), and induces an unusually large and prolonged EF response, though it does not completely suppress GC (Achtman et al., 2003).
A characteristic of EF-dominant responses is disruption of normal splenic or lymph node architecture (Table 1). This encompasses loss of distinct B cell follicles and T cell zones, loss of marginal zone B cells, and sometimes a loss of T cells from T cell zones. After foot-pad inoculation with STm, draining lymph nodes lose their normal architecture by a TLR4/LPS dependent mechanism (St John and Abraham, 2009). However, splenic architecture disruption after STm infection occurs even in the absence of MyD88, TLR2/4, ASC or IL-1R, suggesting multiple independent mechanisms (Di Niro et al., 2015). During B. burdorferi infection, neither deletion of MyD88, MyD88 and TRIF, nor IFNAR, restores lymph node architecture (Hastey et al., 2014). For E. muris infection, TNF neutralization improves splenic organization, particularly T cell zones (Popescu et al., 2019). Efficient GC formation relies on many migration cues and cell contact events (Lu and Cyster, 2019), therefore architecture disruption is expected to contribute to GC suppression, though establishing a direct relationship is difficult. Architecture disruption could be a host adaptation to optimize the EF response by intermixing activated B and T cells, facilitating contacts that would promote B cell differentiation.
What factors mechanistically drive the predominant response towards EF vs GC?
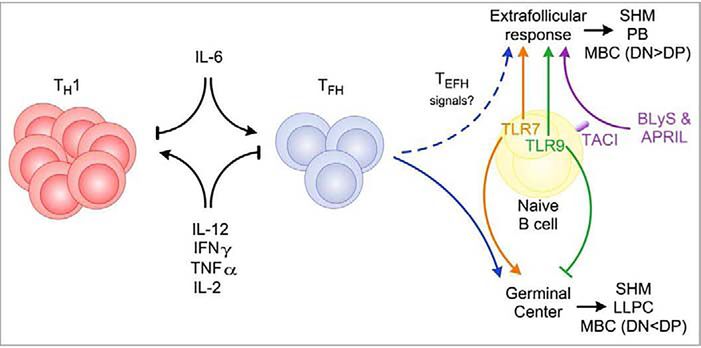
An early step in determining the direction of the B cell response is sensing of pathogen-derived signals by innate immune receptors, such as TLRs. Cells may also receive signals from pathogen-derived factors that interact with host cell receptors. The nature of these innate and pathogen-derived signals in turn can drive specific types of host adaptive B cell responses. A common response to sensing such signals is cytokine secretion. The types and quantities of the elicited cytokines in turn can tune the immune response to fit the infection. Some cytokines, such as IL-21, have been shown to promote both EF and GC responses (Lee et al., 2011; Linterman et al., 2010; Zotos et al., 2010), and therefore can be regarded as augmenting B cell responses more broadly. Here, we will focus on cytokines and other host factors that preferentially direct one response over the other (Figure 1).
Figure 1. Effectors driving the switch between EF or GC responses.
Effector molecules can influence the dominance of EF or GC responses by directly acting on B cells, or indirectly by promoting or repressing TFH differentiation. IL-6 drives TFH differentiation, thereby promoting GC, while pro-inflammatory molecules (IL-12, IFNγ, TNFα, and IL-2) repress TFH and downstream GC. IL-12 also promotes T cell proliferation, therefore a balance of proliferation and repression of TFH differentiation must be struck for optimal TFH/GC formation. Additional unknown signals can drive the development of T extrafollicular helper cells (TEFH), which promote EF responses. BLyS and APRIL act directly on B cells through the receptor TACI to promote EF responses. TLR7 promotes GC while TLR9 inhibits GCs, although the mechanisms behind these divergent effects are unclear. EF and GCBC both undergo somatic hypermutation (SHM), hence the main difference between them is likely the efficiency of obtaining T cell help that induces further rounds of proliferation and mutation. EF and GC responses both generate MBC. However, subsets of MBC have different origins. CD80- PD-L2- (double negative, DN) MBC are primarily formed prior to GC, and CD80+ PD-L2+ (double positive, DP) are predominantly from GC. LLPC are the latest to form and arise almost entirely, if not exclusively, from GCs.
IL-12 is a major regulator of T cell immunity, driving effector T cell differentiation in both CD4 and CD8 T cells (Tait Wojno et al., 2019; Vignali and Kuchroo, 2012; Xin et al., 2016). Recently, we found that IL-12 regulates humoral immunity by strongly inhibiting TFH differentiation (Elsner and Shlomchik, 2019). Though it has been suggested that human and mouse cells respond differently to IL-12, in review of this literature we determined that the in vitro response to IL-12 from both organisms is actually quite similar, as detailed in the Discussion of Elsner and Shlomchik, 2019. IL-12 induces cells secreting both IFNγ and IL-21 in vitro from both mouse and human T cells (Ma et al., 2009; Nakayamada et al., 2011; Schmitt et al., 2009). However, IL-21 production is not unique to TFH (Liu and King, 2013; Spolski and Leonard, 2014), and these in vitro-differentiated cells are unlikely to represent true TFH as only a very small frequency of cells express CXCR5, PD-1, or Bcl-6, all of which are the well-accepted markers of TFH. In vivo, we found that IL-12 was both necessary and sufficient to suppress the GC response (Elsner and Shlomchik, 2019). IL-12R deficiency augmented proportional TFH differentiation among responding T cells to levels nearly the same as in non-infected mice. However, we also found that IL-12R was necessary for expansion of activated T cells, thus affecting both quality and magnitude of the response. Similar outcomes were observed with T-bet-deficient cells during LCMV infection (Sheikh et al., 2019; Weinstein et al., 2018). Interestingly, the effects of T-bet expression are context dependent, as during influenza infection T-bet deficiency increased TFH by both frequency and number (Sheikh et al., 2019). This may be related to the amount of T-bet expressed, with LCMV and STm inducing much higher T-bet expression, and thus much stronger proliferation and proportional suppression of TFH differentiation than influenza (Elsner and Shlomchik, 2019; Sheikh et al., 2019). We found that the suppression of TFH by IL-12 was only observed in high IL-12 conditions, either in the form of injected recombinant IL-12 or that induced by STm infection. Typical immunizations may not induce high IL-12 production, potentially explaining why humans with genetic deficiencies in the IL-12 pathway do not have consistent changes in serum antibody levels to vaccinations (Schmitt et al., 2013). Clearly more studies are necessary to determine the effects of IL-12 on humoral immunity in humans, and whether common vaccines even induce enough IL-12 to have any effect, but the possibility remains open that IL-12 plays a similar role as in mice.
Importantly, simply administering recombinant IL-12 during NP-CGG immunization also suppressed TFH differentiation and reduced NP-specific GC B cells (Elsner and Shlomchik, 2019), confirming that IL-12 per se suppresses TFH development. Earlier studies had shown that injection of recombinant IL-12 promoted serum Ab responses to both DNP-OVA and DNP-Ficoll, and enhanced the Ab response to a T-independent polysaccharide vaccine (Buchanan et al., 1998), but these Abs were not maintained long-term. This suggests that IL-12 directs humoral immunity toward short-lived EF responses independently of its effects on T cells; whether this is due to effects on B cells or other bystander cells remains unclear. Together, these findings implicate IL-12 as a molecular switch between EF and GC B cell responses.
In murine lupus models (Jacobson et al., 1995; Roark et al., 1995; Sang et al., 2014; William et al., 2002; William et al., 2005a) and perhaps in many SLE patients (Jenks et al., 2019; Jenks et al., 2018), there is a dominant EF response associated with production of anti-nuclear and rheumatoid factor Abs. We suggest that such responses are supported by IL-12 and that IL-12 is a driving cytokine in lupus. Polymorphisms in genes associated with IL-12 signaling are risk alleles for SLE (Bentham et al., 2015). IL-12 promotes nephritis and the accumulation of anti-dsDNA Abs in mice (Kikawada et al., 2003; Nakajima et al., 1997). In humans, treatment with ustekinumab, an IL-12/23 blocking Ab, improved outcomes when added to standard-of-care (van Vollenhoven et al., 2018). IL-23 specific blockade did not affect accumulation of anti-dsDNA Abs in MRL/lpr mice (Kyttaris et al., 2013). With a model of immunization-induced autoantibody production, recombinant IL-12 administered at days −1, 0 and 1 of immunization promoted splenic Ab secreting cell accumulation at 25 days of immunization (Kim et al., 2008). While the role of IL-12 in driving human lupus remains uncertain, studies with IL-12-specific blockade will help clarify this point, possibly connecting an EF-driving mechanism with overall disease pathology.
In a model of severe malaria infection with Plasmodium berghei ANKA, IFNγ and TNFα were found to suppress TFH differentiation, and their neutralization increased TFH differentiation and GCBC numbers (Ryg-Cornejo et al., 2016). T cell expression of T-bet was required for TFH and GC suppression. Similar results were observed with IFNγ neutralization and in IFNγ KO mice, indicating that in this system IFNγ alone was sufficient to suppress TFH. In contrast, during STm infection we found that IFNγ receptor deficient T cells were not able to form TFH (Elsner and Shlomchik, 2019). The difference between the infections may be that the TH1 response to STm is much stronger. Malaria did not induce complete repression of GCs. Rather, there was some TFH development, and these TFH expressed some T-bet. Furthermore, this infection induced both a poor PB and GC/TFH response, indicating a reduced B cell response overall. This is again in contrast to STm, which does not suppress PB responses (Cunningham et al., 2007; Elsner and Shlomchik, 2019; Ryg-Cornejo et al., 2016). Plasmodium chabaudi infection, a less severe murine malaria model, suppressed NP-OVA induced GCs and serum Ab, but augmented the response to the T-independent Ag NP-Ficoll (Wilmore et al., 2013). Therefore, in malaria infection models, IFNγ seems to have a context-dependent role in TFH and GC suppression.
TNFα suppressed GC development in mouse E. muris infection. TNFα KO mice had increased GC and TFH phenotype cells, but also more PBs and IgM AFC with, reciprocally, fewer IgG AFCs (Popescu et al., 2019). However, in these TNFα-deficient mice, histologic GCs were not well formed, possibly due to the overall distortion of splenic architecture due to TNFα-deficiency per se (Matsumoto et al., 1996b; Pasparakis et al., 1996). These opposing effects of constitutive loss of TNFα make this study somewhat hard to interpret. In this system the lack of TNFα enhanced both GC and AFC responses, indicating that TNFα might be generally suppressing B cell responses, rather than selectively switching the response from GC to EF AFC mode. This axis deserves further investigation.
IL-6 is a pleiotropic cytokine (Hunter and Jones, 2015). IL-6 deficient mice have dramatically smaller GCs and reduced IgG isotypes after T-dependent immunization (Kopf et al., 1998), and reduced IgA secreting cells after mucosal challenge (Ramsay et al., 1994). IL-6 is thought to mainly promote differentiation of Ag specific B cells to PBs (Gonzalez-Garcia et al., 2006) but not to fully differentiated plasma cells (Jego et al., 1999), and to support long-lived plasma cell survival in the bone marrow (Minges Wols et al., 2002). Activated B cells secrete IL-6 (Hirano et al., 1986; Yoshizaki et al., 1984), which acts in an autocrine fashion to promote Ab secretion (Rieckmann et al., 1991), and also indirectly promotes IL-21 production from CD4 T cells, which further promotes Ab secretion (Dienz et al., 2009).
Though IL-6 promotes B cell differentiation, in the larger picture it enhances GC in many contexts, including autoimmune disease and infections. Using a murine model of SLE where only B cells lack the Wiskott-Aldrich syndrome (WAS) protein, B cell derived IL-6 was necessary for the maturation of TFH and development of spontaneous autoimmune GC (Arkatkar et al., 2017). IL-6 seems to mainly promote GC indirectly, via stimulating TFH differentiation (Crotty, 2019; Vinuesa et al., 2016). During chronic infection of mice with LCMV clone 13, Harker et. al. identified two phases of IL-6 production, with strong peaks at day 3 and day 25 (Harker et al., 2011). This second spike of IL-6 was required on T cells, but not B cells, to promote TFH and GC, and clear the virus. VSV infection, which induces GC much earlier than LCMV clone 13, induces GC via IL-6 which in turn depends on a wave of type I IFN production peaking at 8 hours of infection that is necessary and sufficient to promote a switch toward TFH and reduce Th1 differentiation (De Giovanni et al., 2020). Type I IFN acted on DCs to induce IL-6 production, which then acted on responding T cells to promote TFH differentiation. Blocking IL-6 prior to VSV infection switched the response from TFH dominant to Th1 dominant; this effect was no longer seen after 24hr of infection, indicating that IL-6 had to act early to promote TFH commitment. Administering Poly-IC, a TLR3 ligand that induces type I IFN production, at the same time as LCMV infection increased TFH differentiation. This demonstrated a method to engage this TFH-promoting pathway, which has obvious potential for benefitting vaccine design. Despite that IL-6 can promote TFH differentiation, IL-6 is not always essential for TFH differentiation (Nurieva et al., 2008; Poholek et al., 2010); other signals, such as IL-21 (Eto et al., 2011), could compensate for the lack of IL-6 in different systems.
Mechanistically, T cell-intrinsic IL-6R signaling is necessary for downregulation of the low affinity IL-2 receptor (CD122) (Papillion et al., 2019). IL-2 directly and potently represses TFH differentiation (Ballesteros-Tato et al., 2012) through STAT5 induction of BLIMP-1 (Nurieva et al., 2012; Yang et al., 2011). BLIMP-1 antagonizes Bcl6, which is necessary for TFH differentiation (Johnston et al., 2009; Nurieva et al., 2012). IL-6-induced CD122 downregulation was required to complete differentiation into GC TFH cells in a high IL-2 environment. TFH produce IL-2 (Papillion et al., 2019; Yu et al., 2009), and IL-2 production seems to be an early predictor of TFH differentiation (DiToro et al., 2018), highlighting why the IL-6 signal must come early. IL-6 directly inhibited CD122 upregulation by inducing STAT3 and blocking STAT5 binding at the Il2rb locus (Papillion et al., 2019; Yang et al., 2011). These studies collectively reveal that IL-6 acts as a molecular switch promoting TFH differentiation over Th1 differentiation, effectively playing the opposite role of IL-12.
The ligands BLyS (also called BAFF) and APRIL, are key regulators at multiple stages of B cell development and differentiation. Both ligands bind the receptors TACI and BCMA, while only BLyS binds the BAFF-R. Details of their functions outside of regulating canonical and non-canonical responses are reviewed elsewhere (Goenka et al., 2014b; Vincent et al., 2013).
BLyS is a survival factor for mature naïve B cells and BLyS-deficient mice have severely reduced mature B cell compartments. BLyS is unlikely to play a major role in GCs however, as BLyS-deficient mice make GCs, albeit smaller ones, most likely due to markedly reduced overall numbers of B cells (Rahman et al., 2003). GCBC downregulate TACI, the major activation-induced receptor for BLyS (Goenka et al., 2014a). Further, T cells were found to be the main source of BLyS in the GC, and T cell-restricted loss of BLyS only had a minor effect on GCs (Goenka et al., 2014a).
Increasing the concentration of BLyS in vivo promotes Ab secretion. Since BLyS plays only a small role in GCs, it is likely that BLyS primarily promotes EF responses. In support of this, a vaccine strain of rabies virus modified to express murine BLyS (there referred to as BAFF) from infected cells produced higher titers of rabies specific and virus neutralizing Abs, without significantly affecting GC (Haley et al., 2017). Mice expressing excess BLyS/BAFF through an integrated transgene display autoimmunity, characterized by large numbers of PBs and high autoantibody titers (Khare et al., 2000; Mackay et al., 1999); moreover, this phenotype is in large part T cell-independent (Groom et al., 2007). These data also suggest that BLyS tends to promote the EF response by acting directly on responding B cells.
Since BCMA is dispensable for mounting responses to both T-dependent and -independent Ags (Schneider et al., 2001; Xu and Lam, 2001), BLyS most likely promotes EF responses through its interaction with the receptor TACI. That APRIL—which only binds TACI and BCMA—has similar effects further supports this concept. TACI is upregulated upon B cell activation, and it is required for optimal Ab responses to T-independent type II Ags. Patients with TACI mutations and TACI deficient mice both show impaired responses to the T-independent Streptococcus pneumoniae polysaccharide vaccine Pneumovax, but normal responses to T-dependent tetanus vaccine (human) and KLH immunization (mice) (Castigli et al., 2005; Salzer et al., 2005; von Bulow et al., 2001; Zhang et al., 2007). APRIL is also required for optimal T-independent Ab responses (Castigli et al., 2004; Hardenberg et al., 2008). Since APRIL and TACI have a clear role in augmenting T-independent plasma cell responses, and as TACI is downregulated on GCBC, the combination of APRIL and TACI could be interpreted as primarily promoting EF B cell responses. Whether the role of TACI is to enhance clonal expansion of B cells in the EF pathway, or whether it specifically directs EF pathway differentiation, remains unclear.
This family of cytokines and receptors is often modulated during infection (Sakai and Akkoyunlu, 2017). Elevated serum BLyS has been observed during infection with HIV, tuberculosis, and malaria, among others. BLyS production can be induced from myeloid cells and epithelial cells by cytokines such as type I and II interferons (Kato et al., 2006; Nardelli et al., 2001) and by TLR ligands (Kato et al., 2006). TLR stimulation also upregulates TACI expression in PBs (Treml et al., 2007). Hence, via multiple mechanisms, innate responses to pathogens can influence this axis to determine the quality of the B cell response.
TLR ligands are strong adjuvants of Ab responses in both canonical and non-canonical B cell responses (DeFranco et al., 2012). Whether they can also help direct the type of B cell response remains an open question, largely because many studies have not distinguished between EF and GC derived Abs and/or responses.
The B cell-intrinsic effects of TLR ligation on the nature and magnitude of the response can depend on which ligands are present, as well as the concentration and context. B cell intrinsic LPS sensing was required for optimal Ab and GC responses to the combination of OVA and LPS (Pasare and Medzhitov, 2005). Subsequently, it was shown that the physical form of the TLR ligands in the immunizing protocol is crucial in determining the outcome. B cell intrinsic MyD88 signaling was not required for optimal serum Ab titers to a protein Ag mixed with the TLR9 ligand CpG, but was required if the TLR9 ligand was contained in a virus-like particle (Hou et al., 2011). Additionally, simply conjugating NP-CGG to a TLR9 ligand increased GC affinity maturation and the magnitude of the secondary B cell response (Rookhuizen and DeFranco, 2014). Engineering BCR-mediated uptake of Ag with a linked TLR9 ligand also promoted EF responses in an immunization setting (Eckl-Dorna and Batista, 2009). Conversely, in the setting of autoimmune disease, B cell-intrinsic TLR9 restricts spontaneously arising GCs whereas TLR7 is necessary for their spontaneous accumulation (Jackson et al., 2014; Soni et al., 2014). It is interesting that TLR7 and TLR9 seem to have different roles in the spontaneous GC process, particularly as these TLRs are thought to signal similarly and both are expressed in B cells.
Fewer studies address the effects of TLR ligands on EF responses, which occur early after immunization. B cells lacking MyD88 made both fewer PC at day 2 and reduced GC at day 11 (Tian et al., 2018) in response to virus-like particles, though the contribution of individual TLR’s was not examined. Adding TLR9 ligand to Ag-coated beads enhanced proliferation and PB differentiation of Ag-specific B cells as early as 4 days after immunization (Eckl-Dorna and Batista, 2009). This effect required TLR9 expression in B cells, and clearly demonstrated that TLR9 in this context stimulated EF responses. In the absence of T cell help, TLR’s are necessary and sufficient for formation of EF responses by autoreactive B cells, though both TLR7 and TLR9 were required for maximal responses (Herlands et al., 2008). Collectively, TLR stimuli appear to in general promote GC and EF responses, though they may not be required (Browne, 2012; Cerutti et al., 2011; DeFranco et al., 2012; Lanzavecchia and Sallusto, 2007; Palm and Medzhitov, 2009). TLR7 may promote GC responses selectively in some contexts while TLR9 seems to favor EF responses, though the differential mechanisms for this remain elusive. Further insight into TLR influence on B cell response quality would be important to consider in vaccine design, as TLR ligands are often used as adjuvants.
CONCLUDING REMARKS
Here we have reviewed two major modes of B cell responses: EF and GC. Although they differ fundamentally in terms of output—with the EF response generating early, lower affinity effector Ab responses and the GC response generating delayed but higher affinity and longer-lasting Ab responses—we emphasized how there are in fact many similarities, despite dogmas that ascribe certain features to one or the other. For example, both responses feature MBC generation, somatic V region hypermutation, and affinity maturation. Certain infections and immunopathologic states favor one or the other response, and in turn the direction of the response can explain the immunity that develops or how to influence immunopathologic responses. While the question of which factors direct the quality of the response and how they do it is far from settled, we have discussed several factors and mechanisms that are implicated. At an evolutionary level, our studies in STm suggest that the EF dominant response may be an adaptation to most effectively control severe infections where pathogen burden is high. In this system, IL-12 works to shunt all available resources to immediate effector T cell expansion and antibody production to control the infection, which would be critical for the survival of the animal. There could be a parallel to this in autoimmune diseases, where the antigenic target of the immune response is either abundant (such as DNA or DNA-containing immune complexes as in SLE), triggering an EF response, versus localized (insulin as in type I diabetes), triggering a GC response. Another factor that could control the nature of the autoimmune response is the degree of inflammation and cytokine production, particularly that of IL-12 and related cytokines. It is likely that, as in responses to infection, autoimmune responses exist along a spectrum from GC- to EF-dominant, and may even evolve over time with the severity of disease in an individual.
Having an understanding of the differences between response modes and learning how to manipulate them has theoretical and practical implications. On a theoretical level, it gives us a holistic view of how the humoral immune response tunes itself to the infectious challenge; as such, it is a defining axis of the host-pathogen interaction. Similarly, it helps to explain different autoimmune scenarios and perhaps to stratify patients within a given autoimmune disease (Jenks et al., 2019), with the benefit that this may help guide appropriate biological therapies. Perhaps most important, at both a theoretical and practical level, it has implications for vaccine design and evaluation. Emergency vaccination for toxin exposure or acute exposure prophylaxis may benefit from a robust EF response, as would local vaccination to contain an emerging novel (pandemic-risk) pathogen. Long-term Ab-based protection is predicated on generating LLPC, which must come from a GC reaction, but will take time to develop. Robust EF responses in the absence of GC responses can be confused for effective immune responses, as high amounts of IgG can be made and the half-life of IgG can be several weeks. Clear biomarkers are needed to track each type of response, other than simply IgG levels.
As this review is being completed, the world is enveloped in a pandemic outbreak of a novel coronavirus, SARS-CoV-2. Infections in some people are characterized by a dramatic inflammatory type response (Noroozi et al., 2020; Sallenave and Guillot, 2020) and, based on other examples as discussed in this review, one might suspect that an EF response is being generated. Indeed, patients that succumb to SARS-CoV-2 infection lack of GC and TFH and exhibit a concurrent expansion of TH1 and PB in lymph nodes and spleen (Kaneko et al., 2020). An expanded EF PB response with low SHM that positively correlates with disease severity, with B cell phenotypes that were similar to active disease SLE patients (Woodruff et al., 2020). Ab—including neutralizing Ab—is seen rather early (Huang et al., 2020; Kellam and Barclay, 2020; Suhandynata et al., 2020); yet, concerningly, there are reports that IgG Abs are not long lasting (Long et al., 2020). This too is indicative of an EF response and may indicate that for a substantial subset of patients, at least, Ab-mediated protection may not be long-lasting. The lack of lasting Ab has led some to be pessimistic about vaccination, but after all, as the content of this review has emphasized, the same Ags presented in a different context—using the right adjuvants to promote GC responses—could likely generate LLPC and thus long-term Ab-mediated protection, regardless of the case with the natural infection.
Acknowledgments
This work was supported by NIH grants T32 AI060525, T32 AI089443, R01 AI105018, and R01 AI043603.
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achtman AH, Khan M, MacLennan IC, and Langhorne J (2003). Plasmodium chabaudi chabaudi infection in mice induces strong B cell responses and striking but temporary changes in splenic cell distribution. J Immunol 171, 317–324. [DOI] [PubMed] [Google Scholar]
- Allen CD, Okada T, Tang HL, and Cyster JG (2007). Imaging of germinal center selection events during affinity maturation. Science 315, 528–531. [DOI] [PubMed] [Google Scholar]
- Allman D, Wilmore JR, and Gaudette BT (2019). The continuing story of T-cell independent antibodies. Immunol Rev 288, 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SM, Khalil A, Uduman M, Hershberg U, Louzoun Y, Haberman AM, Kleinstein SH, and Shlomchik MJ (2009). Taking advantage: high-affinity B cells in the germinal center have lower death rates, but similar rates of division, compared to low-affinity cells. J Immunol 183, 7314–7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkatkar T, Du SW, Jacobs HM, Dam EM, Hou B, Buckner JH, Rawlings DJ, and Jackson SW (2017). B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity. J Exp Med 214, 3207–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, Odermatt B, Hengartner H, and Zinkernagel RM (1996). Induction of long-lived germinal centers associated with persisting antigen after viral infection. J Exp Med 183, 2259–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, and Randall TD (2012). Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 36, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannard O, and Cyster JG (2017). Germinal centers: programmed for affinity maturation and antibody diversification. Curr Opin Immunol 45, 21–30. [DOI] [PubMed] [Google Scholar]
- Baumgarth N (2013). How specific is too specific? B-cell responses to viral infections reveal the importance of breadth over depth. Immunol Rev 255, 82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, Martin J, Fairfax BP, Knight JC, Chen L, et al. (2015). Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet 47, 1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek C, Berger A, and Apel M (1991). Maturation of the immune response in germinal centers. Cell 67, 1121–1129. [DOI] [PubMed] [Google Scholar]
- Bergqvist P, Gardby E, Stensson A, Bemark M, and Lycke NY (2006). Gut IgA class switch recombination in the absence of CD40 does not occur in the lamina propria and is independent of germinal centers. J Immunol 177, 7772–7783. [DOI] [PubMed] [Google Scholar]
- Browne EP (2012). Regulation of B-cell responses by Toll-like receptors. Immunology 136, 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RM, Arulanandam BP, and Metzger DW (1998). IL-12 enhances antibody responses to T-independent polysaccharide vaccines in the absence of T and NK cells. J Immunol 161, 5525–5533. [PubMed] [Google Scholar]
- Burton DR, and Hangartner L (2016). Broadly Neutralizing Antibodies to HIV and Their Role in Vaccine Design. Annu Rev Immunol 34, 635–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadman ET, Abdallah AY, Voisine C, Sponaas AM, Corran P, Lamb T, Brown D, Ndungu F, and Langhorne J (2008). Alterations of splenic architecture in malaria are induced independently of Toll-like receptors 2, 4, and 9 or MyD88 and may affect antibody affinity. Infect Immun 76, 3924–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, Mizoguchi E, and Geha RS (2004). Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A 101, 3903–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, and Geha RS (2005). TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet 37, 829–834. [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Buttner M, Shaknovich R, Kremmer E, Alobeid B, and Niedobitek G (2006). Nuclear and cytoplasmic AID in extrafollicular and germinal center B cells. Blood 107, 3967–3975. [DOI] [PubMed] [Google Scholar]
- Cerutti A, Puga I, and Cols M (2011). Innate control of B cell responses. Trends Immunol 32, 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TD, Gatto D, Wood K, Camidge T, Basten A, and Brink R (2009). Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol 183, 3139–3149. [DOI] [PubMed] [Google Scholar]
- Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, Kaverina N, Utset TO, Meehan SM, Quigg RJ, et al. (2011). In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol 186, 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Raybuck AL, Stengel K, Wei M, Beck TC, Volanakis E, Thomas JW, Hiebert S, Haase VH, and Boothby MR (2016). Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature 537, 234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MR, Trotter K, and Chang A (2015). The Pathogenesis and Therapeutic Implications of Tubulointerstitial Inflammation in Human Lupus Nephritis. Semin Nephrol 35, 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S (2019). T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 50, 1132–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G Jr., et al. (2013). Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 19, 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AF, Gaspal F, Serre K, Mohr E, Henderson IR, Scott-Tucker A, Kenny SM, Khan M, Toellner KM, Lane PJ, and MacLennan IC (2007). Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol 178, 6200–6207. [DOI] [PubMed] [Google Scholar]
- Cyster JG, and Allen CDC (2019). B Cell Responses: Cell Interaction Dynamics and Decisions. Cell 177, 524–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Porto JM, Haberman AM, Shlomchik MJ, and Kelsoe G (1998). Antigen drives very low affinity B cells to become plasmacytes and enter germinal centers. J Immunol 161, 5373–5381. [PubMed] [Google Scholar]
- De Giovanni M, Cutillo V, Giladi A, Sala E, Maganuco CG, Medaglia C, Di Lucia P, Bono E, Cristofani C, Consolo E, et al. (2020). Spatiotemporal regulation of type I interferon expression determines the antiviral polarization of CD4(+) T cells. Nat Immunol 21, 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter PE, and van der Laan LJ (2015). Evidence of B-cell follicles with germinal centers in chronic hepatitis C patients. Eur J Immunol 45, 1570–1571. [DOI] [PubMed] [Google Scholar]
- DeFranco AL, Rookhuizen DC, and Hou B (2012). Contribution of Toll-like receptor signaling to germinal center antibody responses. Immunol Rev 247, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Niro R, Lee SJ, Vander Heiden JA, Elsner RA, Trivedi N, Bannock JM, Gupta NT, Kleinstein SH, Vigneault F, Gilbert TJ, et al. (2015). Salmonella Infection Drives Promiscuous B Cell Activation Followed by Extrafollicular Affinity Maturation. Immunity 43, 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienz O, Eaton SM, Bond JP, Neveu W, Moquin D, Noubade R, Briso EM, Charland C, Leonard WJ, Ciliberto G, et al. (2009). The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J Exp Med 206, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu-Nosjean MC, Goc J, Giraldo NA, Sautes-Fridman C, and Fridman WH (2014). Tertiary lymphoid structures in cancer and beyond. Trends Immunol 35, 571–580. [DOI] [PubMed] [Google Scholar]
- DiToro D, Winstead CJ, Pham D, Witte S, Andargachew R, Singer JR, Wilson CG, Zindl CL, Luther RJ, Silberger DJ, et al. (2018). Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckl-Dorna J, and Batista FD (2009). BCR-mediated uptake of antigen linked to TLR9 ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood 113, 3969–3977. [DOI] [PubMed] [Google Scholar]
- Elsner RA, Ernst DN, and Baumgarth N (2012). Single and coexpression of CXCR4 and CXCR5 identifies CD4 T helper cells in distinct lymph node niches during influenza virus infection. J Virol 86, 7146–7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner RA, Hastey CJ, Olsen KJ, and Baumgarth N (2015). Suppression of Long-Lived Humoral Immunity Following Borrelia burgdorferi Infection. PLoS Pathog 11, e1004976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner RA, and Shlomchik MJ (2019). IL-12 Blocks Tfh Cell Differentiation during Salmonella Infection, thereby Contributing to Germinal Center Suppression. Cell Rep 29, 2796–2809 e2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersching J, Efeyan A, Mesin L, Jacobsen JT, Pasqual G, Grabiner BC, Dominguez-Sola D, Sabatini DM, and Victora GD (2017). Germinal Center Selection and Affinity Maturation Require Dynamic Regulation of mTORC1 Kinase. Immunity 46, 1045–1058 e1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes JD (2013). Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues. Immunol Rev 254, 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, and Crotty S (2011). IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One 6, e17739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallet B, Narr K, Ertuna YI, Remy M, Sommerstein R, Cornille K, Kreutzfeldt M, Page N, Zimmer G, Geier F, et al. (2016). Interferon-driven deletion of antiviral B cells at the onset of chronic infection. Sci Immunol 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farci P, Diaz G, Chen Z, Govindarajan S, Tice A, Agulto L, Pittaluga S, Boon D, Yu C, Engle RE, et al. (2010). B cell gene signature with massive intrahepatic production of antibodies to hepatitis B core antigen in hepatitis B virus-associated acute liver failure. Proc Natl Acad Sci U S A 107, 8766–8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faro J, von Haeften B, Gardner R, and Faro E (2019). A Sensitivity Analysis Comparison of Three Models for the Dynamics of Germinal Centers. Front Immunol 10, 2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K, Manjarrez-Orduno N, Schildknecht A, Weber J, Senn BM, Zinkernagel RM, and Hengartner H (2007). B cell activation state-governed formation of germinal centers following viral infection. J Immunol 179, 5877–5885. [DOI] [PubMed] [Google Scholar]
- Foote JB, Mahmoud TI, Vale AM, and Kearney JF (2012). Long-term maintenance of polysaccharide-specific antibodies by IgM-secreting cells. J Immunol 188, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freni MA, Artuso D, Gerken G, Spanti C, Marafioti T, Alessi N, Spadaro A, Ajello A, and Ferrau O (1995). Focal lymphocytic aggregates in chronic hepatitis C: occurrence, immunohistochemical characterization, and relation to markers of autoimmunity. Hepatology 22, 389–394. [PubMed] [Google Scholar]
- Goenka R, Matthews AH, Zhang B, O’Neill PJ, Scholz JL, Migone TS, Leonard WJ, Stohl W, Hershberg U, and Cancro MP (2014a). Local BLyS production by T follicular cells mediates retention of high affinity B cells during affinity maturation. J Exp Med 211, 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenka R, Scholz JL, Sindhava VJ, and Cancro MP (2014b). New roles for the BLyS/BAFF family in antigen-experienced B cell niches. Cytokine Growth Factor Rev 25, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Garcia I, Ocana E, Jimenez-Gomez G, Campos-Caro A, and Brieva JA (2006). Immunization-induced perturbation of human blood plasma cell pool: progressive maturation, IL-6 responsiveness, and high PRDI-BF1/BLIMP1 expression are critical distinctions between antigen-specific and nonspecific plasma cells. J Immunol 176, 4042–4050. [DOI] [PubMed] [Google Scholar]
- Good-Jacobson KL, and Shlomchik MJ (2010). Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. J Immunol 185, 3117–3125. [DOI] [PubMed] [Google Scholar]
- Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, and Shlomchik MJ (2010). PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol 11, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, Smyth MJ, Mackay CR, and Mackay F (2007). BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med 204, 1959–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman AM, and Shlomchik MJ (2003). Reassessing the function of immune-complex retention by follicular dendritic cells. Nat Rev Immunol 3, 757–764. [DOI] [PubMed] [Google Scholar]
- Haley SL, Tzvetkov EP, Meuwissen S, Plummer JR, and McGettigan JP (2017). Targeting Vaccine-Induced Extrafollicular Pathway of B Cell Differentiation Improves Rabies Postexposure Prophylaxis. J Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangartner L, Zinkernagel RM, and Hengartner H (2006). Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol 6, 231–243. [DOI] [PubMed] [Google Scholar]
- Hannum LG, Haberman AM, Anderson SM, and Shlomchik MJ (2000). Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J Exp Med 192, 931–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardenberg G, van Bostelen L, Hahne M, and Medema JP (2008). Thymus-independent class switch recombination is affected by APRIL. Immunol Cell Biol 86, 530–534. [DOI] [PubMed] [Google Scholar]
- Harker JA, Lewis GM, Mack L, and Zuniga EI (2011). Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science 334, 825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastey CJ, Elsner RA, Barthold SW, and Baumgarth N (2012). Delays and diversions mark the development of B cell responses to Borrelia burgdorferi infection. J Immunol 188, 5612–5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastey CJ, Ochoa J, Olsen KJ, Barthold SW, and Baumgarth N (2014). MyD88- and TRIF-independent induction of type I interferon drives naive B cell accumulation but not loss of lymph node architecture in Lyme disease. Infect Immun 82, 1548–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenar-Daughton C, Lee JH, and Crotty S (2017). Tfh cells and HIV bnAbs, an immunodominance model of the HIV neutralizing antibody generation problem. Immunol Rev 275, 49–61. [DOI] [PubMed] [Google Scholar]
- Herlands RA, Christensen SR, Sweet RA, Hershberg U, and Shlomchik MJ (2008). T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity 29, 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlands RA, William J, Hershberg U, and Shlomchik MJ (2007). Anti-chromatin antibodies drive in vivo antigen-specific activation and somatic hypermutation of rheumatoid factor B cells at extrafollicular sites. Eur J Immunol 37, 3339–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A, and et al. (1986). Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 324, 73–76. [DOI] [PubMed] [Google Scholar]
- Hou B, Saudan P, Ott G, Wheeler ML, Ji M, Kuzmich L, Lee LM, Coffman RL, Bachmann MF, and DeFranco AL (2011). Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity 34, 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AT, Garcia-Carreras B, Hitchings MDT, Yang B, Katzelnick LC, Rattigan SM, Borgert BA, Moreno CA, Solomon BD, Rodriguez-Barraquer I, et al. (2020). A systematic review of antibody mediated immunity to coronaviruses: antibody kinetics, correlates of protection, and association of antibody responses with severity of disease. medRxiv, 2020.2004.2014.20065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CA, and Jones SA (2015). IL-6 as a keystone cytokine in health and disease. Nat Immunol 16, 448–457. [DOI] [PubMed] [Google Scholar]
- Inamine A, Takahashi Y, Baba N, Miyake K, Tokuhisa T, Takemori T, and Abe R (2005). Two waves of memory B-cell generation in the primary immune response. Int Immunol 17, 581–589. [DOI] [PubMed] [Google Scholar]
- Ise W, and Kurosaki T (2019). Plasma cell differentiation during the germinal center reaction. Immunol Rev 288, 64–74. [DOI] [PubMed] [Google Scholar]
- Jackson SW, Scharping NE, Kolhatkar NS, Khim S, Schwartz MA, Li QZ, Hudkins KL, Alpers CE, Liggitt D, and Rawlings DJ (2014). Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J Immunol 192, 4525–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, and Kelsoe G (1992). In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J Exp Med 176, 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Kelsoe G, Rajewsky K, and Weiss U (1991). Intraclonal generation of antibody mutants in germinal centres. Nature 354, 389–392. [DOI] [PubMed] [Google Scholar]
- Jacob J, Przylepa J, Miller C, and Kelsoe G (1993). In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J Exp Med 178, 1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson BA, Panka DJ, Nguyen KA, Erikson J, Abbas AK, and Marshak-Rothstein A (1995). Anatomy of autoantibody production: dominant localization of antibody-producing cells to T cell zones in Fas-deficient mice. Immunity 3, 509–519. [DOI] [PubMed] [Google Scholar]
- Jego G, Robillard N, Puthier D, Amiot M, Accard F, Pineau D, Harousseau JL, Bataille R, and Pellat-Deceunynck C (1999). Reactive plasmacytoses are expansions of plasmablasts retaining the capacity to differentiate into plasma cells. Blood 94, 701–712. [PubMed] [Google Scholar]
- Jenks SA, Cashman KS, Woodruff MC, Lee FE, and Sanz I (2019). Extrafollicular responses in humans and SLE. Immunol Rev 288, 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, Tomar D, Woodruff MC, Simon Z, Bugrovsky R, et al. (2018). Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity 49, 725–739 e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, and Crotty S (2009). Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N, Kuo HH, Boucau J, Farmer JR, Allard-Chamard H, Mahajan VS, Piechocka-Trocha A, Lefteri K, Osborn M, Bals J, et al. (2020). Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell 183, 143–157 e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, and Schleimer RP (2006). Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J Immunol 177, 7164–7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating R, Hertz T, Wehenkel M, Harris TL, Edwards BA, McClaren JL, Brown SA, Surman S, Wilson ZS, Bradley P, et al. (2013). The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nat Immunol 14, 1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellam P, and Barclay W (2020). The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. Journal of General Virology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare SD, Sarosi I, Xia XZ, McCabe S, Miner K, Solovyev I, Hawkins N, Kelley M, Chang D, Van G, et al. (2000). Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci U S A 97, 3370–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikawada E, Lenda DM, and Kelley VR (2003). IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol 170, 3915–3925. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Caton M, Wang C, Khalil M, Zhou ZJ, Hardin J, and Diamond B (2008). Increased IL-12 inhibits B cells’ differentiation to germinal center cells and promotes differentiation to short-lived plasmablasts. J Exp Med 205, 2437–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinstein SH, Louzoun Y, and Shlomchik MJ (2003). Estimating hypermutation rates from clonal tree data. J Immunol 171, 4639–4649. [DOI] [PubMed] [Google Scholar]
- Koenig A, and Thaunat O (2016). Lymphoid Neogenesis and Tertiary Lymphoid Organs in Transplanted Organs. Front Immunol 7, 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigsberg M, Perez VI, Rios C, Liu Y, Lee S, Shi Y, and Van Remmen H (2013). Effect of oxygen tension on bioenergetics and proteostasis in young and old myoblast precursor cells. Redox Biol 1, 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M, Herren S, Wiles MV, Pepys MB, and Kosco-Vilbois MH (1998). Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J Exp Med 188, 1895–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosco-Vilbois MH (2003). Are follicular dendritic cells really good for nothing? Nat Rev Immunol 3, 764–769. [DOI] [PubMed] [Google Scholar]
- Krishnamurty AT, Thouvenel CD, Portugal S, Keitany GJ, Kim KS, Holder A, Crompton PD, Rawlings DJ, and Pepper M (2016). Somatically Hypermutated Plasmodium-Specific IgM(+) Memory B Cells Are Rapid, Plastic, Early Responders upon Malaria Rechallenge. Immunity 45, 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppers R, Zhao M, Hansmann ML, and Rajewsky K (1993). Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J 12, 4955–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyttaris VC, Kampagianni O, and Tsokos GC (2013). Treatment with anti-interleukin 23 antibody ameliorates disease in lupus-prone mice. Biomed Res Int 2013, 861028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A, and Sallusto F (2007). Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr Opin Immunol 19, 268–274. [DOI] [PubMed] [Google Scholar]
- Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL, Ramiscal RR, Chan TD, Gatto D, Brink R, et al. (2011). B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J Exp Med 208, 1377–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JS, Chu F, Reilly A, and Winslow GM (2002). Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J Immunol 169, 1419–1425. [DOI] [PubMed] [Google Scholar]
- Liarski VM, Kaverina N, Chang A, Brandt D, Yanez D, Talasnik L, Carlesso G, Herbst R, Utset TO, Labno C, et al. (2014). Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci Transl Med 6, 230ra246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, and Vinuesa CG (2010). IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med 207, 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SM, and King C (2013). IL-21-producing Th cells in immunity and autoimmunity. J Immunol 191, 3501–3506. [DOI] [PubMed] [Google Scholar]
- Liu Y-J, Joshua DE, Williams GT, Smith CA, Gordon J, and MacLennan ICM (1989). Mechanism of antigen-driven selection in germinal centres. Nature 342, 929–931. [DOI] [PubMed] [Google Scholar]
- Long Q-X, Tang X-J, Shi Q-L, Li Q, Deng H-J, Yuan J, Hu J-L, Xu W, Zhang Y, Lv F-J, et al. (2020). Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nature Medicine. [DOI] [PubMed] [Google Scholar]
- Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, and Newberry RD (2003). Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J Immunol 170, 5475–5482. [DOI] [PubMed] [Google Scholar]
- Lu E, and Cyster JG (2019). G-protein coupled receptors and ligands that organize humoral immune responses. Immunol Rev 289, 158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Zhu R, Yu T, Fan H, Hu Y, Mohanta SK, and Hu D (2019). Chronic Inflammation: A Common Promoter in Tertiary Lymphoid Organ Neogenesis. Front Immunol 10, 2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, Deenick EK, and Tangye SG (2009). Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol 87, 590–600. [DOI] [PubMed] [Google Scholar]
- Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, and Browning JL (1999). Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med 190, 1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan IC, Johnson GD, Liu YJ, and Gordon J (1991). The heterogeneity of follicular reactions. Res Immunol 142, 253–257. [DOI] [PubMed] [Google Scholar]
- MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, and Vinuesa CG (2003). Extrafollicular antibody responses. Immunol Rev 194, 8–18. [DOI] [PubMed] [Google Scholar]
- Manne C, Takaya A, Yamasaki Y, Mursell M, Hojyo S, Wu TY, Sarkander J, McGrath MA, Cornelis R, Hahne S, et al. (2019). Salmonella SiiE prevents an efficient humoral immune memory by interfering with IgG(+) plasma cell persistence in the bone marrow. Proc Natl Acad Sci U S A 116, 7425–7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JL, Zhang Y, Pallan L, Hsu MC, Khan M, Cunningham AF, Maclennan IC, and Toellner KM (2011). Early B blasts acquire a capacity for Ig class switch recombination that is lost as they become plasmablasts. European journal of immunology 41, 3506–3512. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Lo SF, Carruthers CJ, Min J, Mariathasan S, Huang G, Plas DR, Martin SM, Geha RS, Nahm MH, and Chaplin DD (1996a). Affinity maturation without germinal centres in lymphotoxin-alpha-deficient mice. Nature 382, 462–466. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Mariathasan S, Nahm MH, Baranyay F, Peschon JJ, and Chaplin DD (1996b). Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science 271, 1289–1291. [DOI] [PubMed] [Google Scholar]
- Mayer CT, Gazumyan A, Kara EE, Gitlin AD, Golijanin J, Viant C, Pai J, Oliveira TY, Wang Q, Escolano A, et al. (2017). The microanatomic segregation of selection by apoptosis in the germinal center. Science 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesin L, Ersching J, and Victora GD (2016). Germinal Center B Cell Dynamics. Immunity 45, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minges Wols HA, Underhill GH, Kansas GS, and Witte PL (2002). The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol 169, 4213–4221. [DOI] [PubMed] [Google Scholar]
- Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, and Randall TD (2004). Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 10, 927–934. [DOI] [PubMed] [Google Scholar]
- Murakami J, Shimizu Y, Kashii Y, Kato T, Minemura M, Okada K, Nambu S, Takahara T, Higuchi K, Maeda Y, et al. (1999). Functional B-cell response in intrahepatic lymphoid follicles in chronic hepatitis C. Hepatology 30, 143–150. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, and Honjo T (2000). Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563. [DOI] [PubMed] [Google Scholar]
- Nacionales DC, Weinstein JS, Yan XJ, Albesiano E, Lee PY, Kelly-Scumpia KM, Lyons R, Satoh M, Chiorazzi N, and Reeves WH (2009). B cell proliferation, somatic hypermutation, class switch recombination, and autoantibody production in ectopic lymphoid tissue in murine lupus. J Immunol 182, 4226–4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Hirose S, Yagita H, and Okumura K (1997). Roles of IL-4 and IL-12 in the development of lupus in NZB/W F1 mice. J Immunol 158, 1466–1472. [PubMed] [Google Scholar]
- Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, Sun HW, Vahedi G, Hakim O, Handon R, et al. (2011). Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity 35, 919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanton MR, Lee SJ, Atif SM, Nuccio SP, Taylor JJ, Baumler AJ, Way SS, and McSorley SJ (2015). Direct visualization of endogenous Salmonella-specific B cells reveals a marked delay in clonal expansion and germinal center development. Eur J Immunol 45, 428–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli B, Belvedere O, Roschke V, Moore PA, Olsen HS, Migone TS, Sosnovtseva S, Carrell JA, Feng P, Giri JG, and Hilbert DM (2001). Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood 97, 198–204. [DOI] [PubMed] [Google Scholar]
- Noroozi R, Branicki W, Pyrc K, Labaj PP, Pospiech E, Taheri M, and Ghafouri-Fard S (2020). Altered cytokine levels and immune responses in patients with SARS-CoV-2 infection and related conditions. Cytokine 133, 155143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, and Dong C (2008). Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Podd A, Chen Y, Alekseev AM, Yu M, Qi X, Huang H, Wen R, Wang J, Li HS, et al. (2012). STAT5 protein negatively regulates T follicular helper (Tfh) cell generation and function. J Biol Chem 287, 11234–11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SL, Hodgkin PD, Tarlinton DM, and Corcoran LM (2015). The generation of antibody-secreting plasma cells. Nat Rev Immunol 15, 160–171. [DOI] [PubMed] [Google Scholar]
- Nzula S, Going JJ, and Stott DI (2003). Antigen-driven Clonal Proliferation, Somatic Hypermutation, and Selection of B Lymphocytes Infiltrating Human Ductal Breast Carcinomas. Cancer Research 63, 3275–3280. [PubMed] [Google Scholar]
- Okoro CK, Kingsley RA, Quail MA, Kankwatira AM, Feasey NA, Parkhill J, Dougan G, and Gordon MA (2012). High-resolution single nucleotide polymorphism analysis distinguishes recrudescence and reinfection in recurrent invasive nontyphoidal Salmonella typhimurium disease. Clin Infect Dis 54, 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, and Medzhitov R (2009). Immunostimulatory activity of haptenated proteins. Proc Natl Acad Sci U S A 106, 4782–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape KA, Kouskoff V, Nemazee D, Tang HL, Cyster JG, Tze LE, Hippen KL, Behrens TW, and Jenkins MK (2003). Visualization of the genesis and fate of isotype-switched B cells during a primary immune response. J Exp Med 197, 1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papillion A, Powell MD, Chisolm DA, Bachus H, Fuller MJ, Weinmann AS, Villarino A, O’Shea JJ, Leon B, Oestreich KJ, and Ballesteros-Tato A (2019). Inhibition of IL-2 responsiveness by IL-6 is required for the generation of GC-TFH cells. Sci Immunol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, and Medzhitov R (2005). Control of B-cell responses by Toll-like receptors. Nature 438, 364–368. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Alexopoulou L, Episkopou V, and Kollias G (1996). Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med 184, 1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, and Brink R (2006). High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med 203, 2419–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, et al. (2010). In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol 185, 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu M, Cabrera-Martinez B, and Winslow GM (2019). TNF-alpha Contributes to Lymphoid Tissue Disorganization and Germinal Center B Cell Suppression during Intracellular Bacterial Infection. J Immunol 203, 2415–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtha WE, Tedder TF, Johnson S, Bhattacharya D, and Diamond MS (2011). Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J Exp Med 208, 2599–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine R, Jones DD, Chatterjee M, McLaughlin M, Macnamara KC, and Winslow GM (2010). Impaired germinal center responses and suppression of local IgG production during intracellular bacterial infection. J Immunol 184, 5085–5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman ZS, Rao SP, Kalled SL, and Manser T (2003). Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. J Exp Med 198, 1157–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay AJ, Husband AJ, Ramshaw IA, Bao S, Matthaei KI, Koehler G, and Kopf M (1994). The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science 264, 561–563. [DOI] [PubMed] [Google Scholar]
- Rieckmann P, D’Alessandro F, Nordan RP, Fauci AS, and Kehrl JH (1991). IL-6 and tumor necrosis factor-alpha. Autocrine and paracrine cytokines involved in B cell function. J Immunol 146, 3462–3468. [PubMed] [Google Scholar]
- Roark JH, Kuntz CL, Nguyen KA, Mandik L, Cattermole M, and Erikson J (1995). B cell selection and allelic exclusion of an anti-DNA Ig transgene in MRL-lpr/lpr mice. J Immunol 154, 4444–4455. [PubMed] [Google Scholar]
- Roco JA, Mesin L, Binder SC, Nefzger C, Gonzalez-Figueroa P, Canete PF, Ellyard J, Shen Q, Robert PA, Cappello J, et al. (2019). Class-Switch Recombination Occurs Infrequently in Germinal Centers. Immunity 51, 337–350 e337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rookhuizen DC, and DeFranco AL (2014). Toll-like receptor 9 signaling acts on multiple elements of the germinal center to enhance antibody responses. Proc Natl Acad Sci U S A 111, E3224–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothaeusler K, and Baumgarth N (2010). B-cell fate decisions following influenza virus infection. Eur J Immunol 40, 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryg-Cornejo V, Ioannidis LJ, Ly A, Chiu CY, Tellier J, Hill DL, Preston SP, Pellegrini M, Yu D, Nutt SL, et al. (2016). Severe Malaria Infections Impair Germinal Center Responses by Inhibiting T Follicular Helper Cell Differentiation. Cell Rep 14, 68–81. [DOI] [PubMed] [Google Scholar]
- Sakai J, and Akkoyunlu M (2017). The Role of BAFF System Molecules in Host Response to Pathogens. Clin Microbiol Rev 30, 991–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallenave JM, and Guillot L (2020). Innate Immune Signaling and Proteolytic Pathways in the Resolution or Exacerbation of SARS-CoV-2 in Covid-19: Key Therapeutic Targets? Front Immunol 11, 1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, Peter HH, Rockstroh JK, Schneider P, Schaffer AA, et al. (2005). Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet 37, 820–828. [DOI] [PubMed] [Google Scholar]
- Sang A, Niu H, Cullen J, Choi SC, Zheng YY, Wang H, Shlomchik MJ, and Morel L (2014). Activation of rheumatoid factor-specific B cells is antigen dependent and occurs preferentially outside of germinal centers in the lupus-prone NZM2410 mouse model. J Immunol 193, 1609–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster MY, Nguyen PQT, and Topham DJ (2019). Role of Memory B Cells in Hemagglutinin-Specific Antibody Production Following Human Influenza A Virus Infection. Pathogens 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N, Bustamante J, Bourdery L, Bentebibel SE, Boisson-Dupuis S, Hamlin F, Tran MV, Blankenship D, Pascual V, Savino DA, et al. (2013). IL-12 receptor beta1 deficiency alters in vivo T follicular helper cell response in humans. Blood 121, 3375–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, and Ueno H (2009). Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity 31, 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Takatsuka H, Wilson A, Mackay F, Tardivel A, Lens S, Cachero TG, Finke D, Beermann F, and Tschopp J (2001). Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J Exp Med 194, 1691–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder AE, Greiner A, Seyfert C, and Berek C (1996). Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci U S A 93, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh AA, Cooper L, Feng M, Souza-Fonseca-Guimaraes F, Lafouresse F, Duckworth BC, Huntington ND, Moon JJ, Pellegrini M, Nutt SL, et al. (2019). Context-Dependent Role for T-bet in T Follicular Helper Differentiation and Germinal Center Function following Viral Infection. Cell Rep 28, 1758–1772 e1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik MJ, Luo W, and Weisel F (2019). Linking signaling and selection in the germinal center. Immunol Rev 288, 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, and Weigert MG (1987). The role of clonal selection and somatic mutation in autoimmunity. Nature 328, 805–811. [DOI] [PubMed] [Google Scholar]
- Silva-Cayetano A, and Linterman MA (2020). Stromal cell control of conventional and ectopic germinal centre reactions. Curr Opin Immunol 64, 26–33. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Antia R, Whitmire JK, and Ahmed R (1998). Humoral immunity due to long-lived plasma cells. Immunity 8, 363–372. [DOI] [PubMed] [Google Scholar]
- Smith KG, Hewitson TD, Nossal GJ, and Tarlinton DM (1996). The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol 26, 444–448. [DOI] [PubMed] [Google Scholar]
- Smith KG, Light A, Nossal GJ, and Tarlinton DM (1997). The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J 16, 2996–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni C, Wong EB, Domeier PP, Khan TN, Satoh T, Akira S, and Rahman ZS (2014). B cell-intrinsic TLR7 signaling is essential for the development of spontaneous germinal centers. J Immunol 193, 4400–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolski R, and Leonard WJ (2014). Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov 13, 379–395. [DOI] [PubMed] [Google Scholar]
- St John AL, and Abraham SN (2009). Salmonella disrupts lymph node architecture by TLR4-mediated suppression of homeostatic chemokines. Nat Med 15, 1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott DI, Hiepe F, Hummel M, Steinhauser G, and Berek C (1998). Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjogren’s syndrome. J Clin Invest 102, 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhandynata RT, Hoffman MA, Kelner MJ, McLawhon RW, Reed SL, and Fitzgerald RL (2020). Longitudinal Monitoring of SARS-CoV-2 IgM and IgG Seropositivity to Detect COVID-19. J Appl Lab Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BM, Emonet SF, Welch MJ, Lee AM, Campbell KP, de la Torre JC, and Oldstone MB (2011). Point mutation in the glycoprotein of lymphocytic choriomeningitis virus is necessary for receptor binding, dendritic cell infection, and long-term persistence. Proc Natl Acad Sci U S A 108, 2969–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait Wojno ED, Hunter CA, and Stumhofer JS (2019). The Immunobiology of the Interleukin-12 Family: Room for Discovery. Immunity 50, 851–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Dutta PR, Cerasoli DM, and Kelsoe G (1998). In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J Exp Med 187, 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Ohta H, and Takemori T (2001). Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity 14, 181–192. [DOI] [PubMed] [Google Scholar]
- Takemori T, Kaji T, Takahashi Y, Shimoda M, and Rajewsky K (2014). Generation of memory B cells inside and outside germinal centers. European Journal of Immunology 44, 1258–1264. [DOI] [PubMed] [Google Scholar]
- Tian M, Hua Z, Hong S, Zhang Z, Liu C, Lin L, Chen J, Zhang W, Zhou X, Zhang F, et al. (2018). B Cell-Intrinsic MyD88 Signaling Promotes Initial Cell Proliferation and Differentiation To Enhance the Germinal Center Response to a Virus-like Particle. J Immunol 200, 937–948. [DOI] [PubMed] [Google Scholar]
- Toellner KM, Luther SA, Sze DM, Choy RK, Taylor DR, MacLennan ICM, and Acha-Orbea H (1998). T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J Exp Med 187, 1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama H, Okada S, Hatano M, Takahashi Y, Takeda N, Ichii H, Takemori T, Kuroda Y, and Tokuhisa T (2002). Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells. Immunity 17, 329–339. [DOI] [PubMed] [Google Scholar]
- Treml LS, Carlesso G, Hoek KL, Stadanlick JE, Kambayashi T, Bram RJ, Cancro MP, and Khan WN (2007). TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol 178, 7531–7539. [DOI] [PubMed] [Google Scholar]
- Trivedi N, Weisel F, Smita S, Joachim S, Kader M, Radhakrishnan A, Clouser C, Rosenfeld AM, Chikina M, Vigneault F, et al. (2019). Liver Is a Generative Site for the B Cell Response to Ehrlichia muris. Immunity 51, 1088–1101 e1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vollenhoven RF, Hahn BH, Tsokos GC, Wagner CL, Lipsky P, Touma Z, Werth VP, Gordon RM, Zhou B, Hsu B, et al. (2018). Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet 392, 1330–1339. [DOI] [PubMed] [Google Scholar]
- Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, and Nussenzweig MC (2010). Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 143, 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali DA, and Kuchroo VK (2012). IL-12 family cytokines: immunological playmakers. Nat Immunol 13, 722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay R, Guthmiller JJ, Sturtz AJ, Surette FA, Rogers KJ, Sompallae RR, Li F, Pope RL, Chan JA, de Labastida Rivera F, et al. (2020). Infection-induced plasmablasts are a nutrient sink that impairs humoral immunity to malaria. Nat Immunol 21, 790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA, and Mackay F (2013). The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev 24, 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG, Linterman MA, Yu D, and MacLennan IC (2016). Follicular Helper T Cells. Annu Rev Immunol 34, 335–368. [DOI] [PubMed] [Google Scholar]
- von Bulow GU, van Deursen JM, and Bram RJ (2001). Regulation of the T-independent humoral response by TACI. Immunity 14, 573–582. [DOI] [PubMed] [Google Scholar]
- Weinstein JS, Laidlaw BJ, Lu Y, Wang JK, Schulz VP, Li N, Herman EI, Kaech SM, Gallagher PG, and Craft J (2018). STAT4 and T-bet control follicular helper T cell development in viral infections. J Exp Med 215, 337–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JS, Nacionales DC, Lee PY, Kelly-Scumpia KM, Yan XJ, Scumpia PO, Vale-Cruz DS, Sobel E, Satoh M, Chiorazzi N, and Reeves WH (2008). Colocalization of antigen-specific B and T cells within ectopic lymphoid tissue following immunization with exogenous antigen. J Immunol 181, 3259–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel F, and Shlomchik M (2017). Memory B Cells of Mice and Humans. Annu Rev Immunol 35, 255–284. [DOI] [PubMed] [Google Scholar]
- Weisel FJ, Mullett SJ, Elsner RA, Menk AV, Trivedi N, Luo W, Wikenheiser D, Hawse WF, Chikina M, Smita S, et al. (2020). Germinal center B cells selectively oxidize fatty acids for energy while conducting minimal glycolysis. Nat Immunol 21, 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel FJ, Zuccarino-Catania GV, Chikina M, and Shlomchik MJ (2016). A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity 44, 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William J, Euler C, Christensen S, and Shlomchik MJ (2002). Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science 297, 2066–2070. [DOI] [PubMed] [Google Scholar]
- William J, Euler C, Leadbetter E, Marshak-Rothstein A, and Shlomchik MJ (2005a). Visualizing the onset and evolution of an autoantibody response in systemic autoimmunity. J Immunol 174, 6872–6878. [DOI] [PubMed] [Google Scholar]
- William J, Euler C, and Shlomchik MJ (2005b). Short-lived plasmablasts dominate the early spontaneous rheumatoid factor response: differentiation pathways, hypermutating cell types, and affinity maturation outside the germinal center. J Immunol 174, 6879–6887. [DOI] [PubMed] [Google Scholar]
- Wilmore JR, Maue AC, Lefebvre JS, Haynes L, and Rochford R (2013). Acute Plasmodium chabaudi infection dampens humoral responses to a secondary T-dependent antigen but enhances responses to a secondary T-independent antigen. J Immunol 191, 4731–4739. [DOI] [PubMed] [Google Scholar]
- Woodruff MC, Ramonell RP, Nguyen DC, Cashman KS, Saini AS, Haddad NS, Ley AM, Kyu S, Howell JC, Ozturk T, et al. (2020). Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin A, Masson F, Liao Y, Preston S, Guan T, Gloury R, Olshansky M, Lin JX, Li P, Speed TP, et al. (2016). A molecular threshold for effector CD8(+) T cell differentiation controlled by transcription factors Blimp-1 and T-bet. Nat Immunol 17, 422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, and Lam KP (2001). B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses. Mol Cell Biol 21, 4067–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, et al. (2011). Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol 12, 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JL, Racine R, McBride KM, and Winslow GM (2013). T cell-dependent IgM memory B cells generated during bacterial infection are required for IgG responses to antigen challenge. J Immunol 191, 1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki K, Nakagawa T, Fukunaga K, Tseng LT, Yamamura Y, and Kishimoto T (1984). Isolation and characterization of B cell differentiation factor (BCDF) secreted from a human B lymphoblastoid cell line. J Immunol 132, 2948–2954. [PubMed] [Google Scholar]
- Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, et al. (2009). The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468. [DOI] [PubMed] [Google Scholar]
- Zhang J, MacLennan ICM, Liu Y-J, and Lane PJL (1988). Is rapid proliferation in B centroblasts linked to somatic mutation in memory B cell clones? Immunol. Let 18, 197–300. [DOI] [PubMed] [Google Scholar]
- Zhang L, Radigan L, Salzer U, Behrens TW, Grimbacher B, Diaz G, Bussel J, and Cunningham-Rundles C (2007). Transmembrane activator and calcium-modulating cyclophilin ligand interactor mutations in common variable immunodeficiency: clinical and immunologic outcomes in heterozygotes. J Allergy Clin Immunol 120, 1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, et al. (2010). IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med 207, 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]