Abstract
Objective:
Low-dose testosterone has been shown to improve depression symptom severity, fatigue and sexual function in small studies in women not formally diagnosed with major depressive disorder (MDD). We sought to determine whether adjunctive low-dose transdermal testosterone improves depression symptom severity, fatigue, and sexual function in women with antidepressant-resistant MDD. A functional MRI (fMRI) substudy examined effects on activity in the anterior cingulate cortex (ACC), a brain region important in mood regulation.
Methods:
Randomized, double-blind, placebo-controlled, 8-week trial of adjunctive testosterone cream (AndroFeme®, Lawley Pharmaceuticals, Australia) in 101 women, ages 21–70, with antidepressant-resistant MDD. Primary outcome measure was depression severity by Montgomery-Asberg Depression Rating Scale (MADRS). Secondary endpoints included fatigue, sexual function, and safety measures. fMRI substudy (n=20) primary outcome was change in ACC activity.
Results:
Mean age was 47±14 (SD) years and mean baseline MADRS score was 26.6±5.9. Eighty-seven (86%) participants completed 8-weeks of treatment. MADRS scores decreased in both arms [testosterone: 26.8±6.3 to 15.3±9.6; placebo: 26.3±5.4 to 14.4±9.3 (baseline to 8 weeks, respectively)], with no difference between groups (p=0.91). Improvement in fatigue and sexual function did not differ between groups, nor did side effects. fMRI results showed a relationship between ACC activation and androgen levels pretreatment, but no difference in ACC activation with testosterone vs placebo.
Conclusions:
Adjunctive transdermal testosterone, though well tolerated, was not more effective than placebo in improving depression, fatigue or sexual dysfunction symptom severity. Imaging in a subset of participants demonstrated that testosterone did not result in greater activation of the ACC.
TRIAL REGISTRATION:
clinicaltrials.gov identifier: NCT01783574
Plain Language Summary:
Low-dose testosterone has been shown to improve mood, fatigue and sexual function in small studies of women not formally diagnosed with depression. The authors administered low-dose testosterone or placebo cream to women with depression that was resistant to traditional antidepressant therapy. Both the testosterone and placebo groups experienced improvements in mood, fatigue, sexual function, with no differences between the groups. They concluded that testosterone cannot be recommended for antidepressant-resistant major depressive disorder based on the results of this study, and that additional studies may still be warranted given the large placebo effect observed.
Introduction
In patients with major depressive disorder (MDD), nonresponse to selective serotonin reuptake inhibitor (SSRI) and serotonin norepinephrine reuptake inhibitor (SNRI) treatment is common, particularly in women, occurring in about 70% of patients despite adequate dosing.1 Additional, well-tolerated augmentation strategies are needed -- particularly ones that do not cause or exacerbate symptoms such as fatigue and sexual dysfunction. Transdermal testosterone, in doses designed to raise levels within or near those that are physiologically normal for women (10–20% of male levels), is a candidate for such a therapy based on modest preclinical and clinical data.
Preclinical and animal models are consistent with the hypothesis that androgens are modulators of mood.2–4 Testosterone affects brain function directly as well as by aromatization of testosterone to estradiol and conversion to the potent androgen, dihydrotestosterone (DHT).5–10 Additionally, the testosterone metabolite 5α-androstane, 3α,17β-diol (3α-diol) is a neuroactive steroid that has been shown to exert important GABAergic-related effects on affective symptoms. Preclinical models also suggest androgen effects on central serotonin neurotransmission, which is relevant to the modulation of core depression symptoms.11
There are also clinical data in hypogonadal men with refractory depression12 and from small, randomized, placebo-controlled trials in women not selected for MDD that suggest a beneficial effect of testosterone on mood. The latter were conducted in women post-bilateral oophorectomy with sexual dysfunction13 or in women with hypopituitarism14, low-dose testosterone administration improved mood, fatigue14 and sexual dysfunction.15 Our preliminary data in women with treatment-resistant depression (n=9) treated with transdermal testosterone in an 8-week open-label trial demonstrated that 67% achieved categorical response [defined as a decrease in Montgomery-Asberg Depression Rating Scale (MADRS) score ≥ 50% from baseline], and 33% achieved remission (MADRS score ≤ 10) after 8 weeks of therapy.16 Moreover, in our studies and in other investigations, evaluating in total over 2000 women followed for up to a year, low-dose transdermal testosterone was extremely well tolerated, without significant hyperandrogenic or metabolic side effects.17
Based on these data, we hypothesized that low-dose adjunctive testosterone would result in greater improvement in depression symptom severity in women with antidepressant-resistant MDD compared with placebo. We additionally hypothesized that testosterone would be well-tolerated and, compared with placebo, would improve fatigue and sexual dysfunction – specific symptoms that are commonly associated with MDD and with many medications used to treat MDD. In addition, because use of compounded and male-branded testosterone products by women is common,18 we sought to establish whether adjunctive transdermal testosterone was safe and well-tolerated for women with MDD. Finally, we also explored whether adjunctive low-dose testosterone would increase activation of the subgenual and dorsal anterior cingulate cortex (ACC), a brain region important in the regulation of mood.
Methods
Participants
The protocol was approved by the Partners Human Research Committee and the Butler Hospital Institutional Review Board, and written informed consent was obtained from all participants prior to any procedures being performed. Inclusion criteria included females between 21 and 75 years old, with primary diagnosis of MDD by DSM-IV criteria using the Structured Clinical Interview for DSM-IV (SCID-IV),19 depression severity reflected by MADRS score ≥ 12, and current treatment with an antidepressant (1st, 2nd or 3rd trial in the current depressive episode) at an adequate dose for at least 8 weeks, with sufficient source documentation to confirm a high level of confidence in treatment details using the MGH Antidepressant Treatment Response Questionnaire (ATRQ).20 In addition, baseline free testosterone level of no higher than the third quartile of the normal range was required. Exclusion criteria included significant suicide or homicide risk, history of psychotic features, or bipolar disorder as assessed by the SCID-IV. Medical exclusions included untreated hypothyroidism, current use of androgens, and history of a hormone-responsive cancer. Sixty-five participants were recruited and studied at the Massachusetts General Hospital site and 36 at the Butler Hospital site.
Design
The study utilized a 2-site, 8-week, randomized, placebo-controlled, parallel-groups trial design. Participants were randomized in a 1:1 ratio to low-dose adjunctive testosterone cream (AndroFeme 1%, Lawley Pharmaceuticals, West Leederville, Australia)21 or identical placebo also manufactured by Lawley Pharmaceuticals. The starting dose of 10 mg daily was chosen to target the upper-normal range for young women.21 Dose titration was performed by an unblinded study monitor based on serum free testosterone levels. Placebo sham dose adjustments were made to maintain investigator blinding. Testosterone cream was applied as an adjunct to ongoing, stable pharmacotherapy.
Psychiatric assessments, hormone levels and dose adjustments were performed as outlined in Table 1. To assure reliability and quality control, ratings at MGH were supervised by psychiatrists and psychologists at MGH and Butler Hospital, using the same standards and principles to ensure consistency across the two sites. Psychiatric evaluators at both sites were extensively trained in the use of the SCID and MADRS by “gold standard” videos; prior assessments of inter-rater reliability in these measures yielded kappa coefficients greater than 0.75 and intra-class correlation coefficients greater than 0.8.
Table 1.
Study schema and drug dosing schedule.
| Screening | Baseline | Week 2 | Week 4 | Week 6 | Week 8 | |
|---|---|---|---|---|---|---|
| Diagnostic Screening | ||||||
| SCID-IV and MGH ATRQ | x | |||||
| Depression Symptom Severity | ||||||
| MADRS | x | x | x | x | x | x |
| CGI-S | x | x | x | x | x | |
| IDS-SR | x | x | x | x | x | |
| Fatigue and Sleepiness | ||||||
| BFI | x | x | x | x | x | |
| ESS | x | x | x | x | x | |
| FSS | x | x | x | x | x | |
| Sexual Function | ||||||
| DISF | x | x | x | x | x | |
| Quality of Life | ||||||
| SF-36 | x | x | x | x | x | |
| Safety Measures | ||||||
| CHRT | x | x | x | x | x | |
| SAFTEE-SI | x | x | x | x | x | |
| Hormone Assessments | x | x | x | x | x | x |
| Drug Dosing | ||||||
| Randomized to starting dose of 10 mg/d (1 mL) testosterone or 1 mL placebo gel | x | |||||
| Dose Titrations | x | x | x | |||
| ||||||
| Drug Discontinuation | x | |||||
Abbreviations: SCID: Structured Clinical Interview for DSM-IV; MGH ATRQ: Massachusetts General Hospital Antidepressant Treatment Response Questionnaire; MADRS: Montgomery-Asberg Depression Rating Scale; CGI-S: Clinician Global Impressions-Severity Scale; IDS-SR: Inventory of Depressive Symptomology-Self-Report; BFI: Brief Fatigue Inventory; ESS: Epworth Sleepiness Scale; FSS: Fatigue Severity Scale; DISF: Derogatis Interview for Sexual Function; SF-36: Short Form-36; CHRT: Concise Health Risk Tracking (Self-Report); SAFTEE-SI: Systematic Assessment for Treatment Emergent Events Systematic Inquiry.
Hormone Assessment
Samples were collected before 10 a.m., stored at −80° C and batched for analysis. Serum testosterone, free testosterone, cortisol, estradiol and estrone concentrations were assayed by Mayo Medical Laboratories (Rochester, MN). Serum testosterone was measured using liquid chromatography with tandem mass spectrometry (LC-MS/MS), and free testosterone by equilibrium dialysis.
Statistical Analysis
The primary efficacy endpoint was change in MADRS score. Chi-square test or t-tests were used as appropriate to compare the distributions of baseline variables and evaluate baseline group equivalence. Outcome variables were measured at 5 time points: pre-treatment baseline and at weeks 2, 4, 6, and 8. The efficacy analysis used a repeated measures analysis of variance, with both treatments set to placebo at baseline, and to their respective groups at follow-up. The treatment effect using this model measures the average difference between the treatments at weeks 2 through 8, corrected for baseline values. The variance covariance matrix was left unspecified. With 50 subjects in each treatment group, we predicted greater than 80% power to detect a 5-point difference in the change in MADRS scores (baseline to 8-weeks) between the testosterone and placebo group.22,23
The same procedure was used for analysis of change in hormones, except that for all hormone levels other than testosterone and free testosterone, there were only 2 measurements available: baseline and week 8. We tested for an interaction between baseline free testosterone level and treatment by introducing an interaction term and a main effect of testosterone into this model. Interaction testing between menopausal status and treatment was performed using the same methods. Additionally, within group analyses were performed using paired t-tests. Response and remission rates were compared across the two groups using a Fisher Exact Test. Data are presented as mean ± standard deviation (SD).
The Systematic Assessment for Treatment Emergent Events Systematic Inquiry (SAFTEE-SI) questionnaire categorizes adverse event severity as 0 (none), 1 (mild), 2 (moderate), and 3 (severe). Treatment emergent side effects were defined as an increase by 2 or more levels of severity from pre-treatment baseline assessment. The proportion of participants in each of the two treatment groups who reported threshold side effects at any time during the treatment period were compared using a Fisher Exact Test.
fMRI Substudy
Thirty-one participants at the Massachusetts General Hospital (MGH) site were evaluated to enroll 20 subjects in the fMRI substudy. Eleven of these 31 subjects declined (n=8) or were not eligible (n=3). fMRI scans were performed at baseline and at week 8. In pre-menopausal women, all testing was performed in the follicular phase of the menstrual cycle.
fMRI data were acquired using a 3.0-T whole-body scanner (Skyra-System), equipped for echo planar imaging (Siemens Medical Systems, Iselin NJ) with a 3-axis gradient head coil. Images were projected using a rear projection system and E-Prime (2.0) stimulus presentation software. Following automated scout and shimming procedures and two high-resolution 3D MPRAGE sequences, fMRI images (i.e., blood oxygenation level dependent signal [BOLD]) were acquired using T2*-weighted sequence (39 horizontal slices aligned perpendicular, 3.1 mm thickness, TE= 28 ms, TR= 2.0, flip angle= 90°).
Participants completed a rapid event-related emotional conflict paradigm24,25 in which faces with fearful and happy expressions were presented with the words “happy” or “fear” written across them and participants’ task was to identify the emotional expression of the faces while ignoring the words, which were either congruent or incongruent with the facial expression.
fMRI data were processed using SPM8 software (Wellcome Department of Cognitive Neurology, London, UK; www.fil.ion.ucl.ac.uk/spm). fMRI images were motion corrected, spatially normalized to the standardized space established by the Montreal Neurologic Institute (MNI; www.bic.mni.mcgill.ca). Condition effects were modeled with regressors representing the occurrence of each trial type (incongruent vs. congruent). For each participant (first-level analysis), condition effects were estimated at each voxel, and statistical parametric maps (SPMs; i.e., contrast images) were produced for each condition (incongruent; congruent). To estimate conditions on the group level (second-level analysis) individual participants’ SPM contrast images were entered into a second-level random-effects analysis, using a flexible factorial model with subject as the first factor and condition (incongruent vs. congruent) as the second factor. The a priori specified region of interest was the ACC, with a secondary pre-specified region of interest of the posterior cingulate cortex (PCC). For each, we adopted a statistical threshold of p<0.05 uncorrected. The ACC and PCC were defined with masks provided by Anatomical Automatic Labeling tool26 implemented in the WFU PickAtlas http://www.ansir.wfubmc.edu).27,28
Results
Sample
Baseline clinical characteristics for 101 randomized participants are presented in Table 2. Sixty-six percent had one, 25% had two, and 9% had three failed antidepressant trials prior to study start. Mean age was 47±14 years and mean MADRS score 26.6±5.9, with no significant differences between the active testosterone and placebo groups. The percent of premenopausal women in the testosterone (59%) and placebo groups (48%) was not significantly different (p=0.32). Eighty-seven (86%) participants completed the 8-week study. Five subjects in the testosterone group (8%) and 9 in the placebo group (18%) dropped out before the 8-week visit (p=0.26). Reasons for discontinuation in the testosterone group included: lack of efficacy (n=2), physician decision (n=1), acne (n=1), and personal reasons (n=1). Reasons for discontinuation in the placebo group included: lack of efficacy (n=3), lost to follow-up (n=4), protocol violation (n=1), and personal reasons (n=1). Two of these participants returned for end-of-study visits (1 in the testosterone group and 1 in the placebo group); their data were included in the 8-week (completers) dataset. Mean final testosterone dose in the treatment group was 12.2±5.6 mg/day (median dose 10 mg/day, range 2.5–25 mg/day).
Table 2.
Baseline clinical characteristics.
| Testosterone (n=51) | Placebo (n=50) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 46 | 14 | 48 | 14 |
| BMI (kg/m2) | 28.2 | 7.6 | 30.3 | 7.9 |
| Premenopausal (n) | 30 | 24 | ||
| Number of Failed Trials | 1.5 | 0.7 | 1.4 | 0.6 |
| Total Testosterone (ng/dL) | 19 | 11 | 18 | 9 |
| Free Testosterone (ng/dL) | 0.3 | 0.2 | 0.3 | 0.2 |
| Montgomery-Asberg Depression Rating Scale (MADRS) Score | 26.8 | 6.3 | 26.3 | 5.4 |
Hormone Levels
Mean total testosterone and free testosterone levels increased significantly over time within the group of women receiving testosterone and also compared to the group receiving placebo. Total testosterone levels increased from 19±11 to 105±70 ng/dL in the testosterone group and from 18±9 to 18±10 ng/dL in the placebo group (p<0.0001, within and between groups). Free testosterone levels also significantly increased within the active group and between groups, from 0.3±0.2 to 1.9±1.1 ng/dL in the testosterone group and 0.3±0.2 to 0.4±0.2 ng/dL in the placebo group (both p<0.001). Morning serum cortisol decreased within the testosterone group (p<0.05) and did not change in the placebo group; there was no significant difference between groups. Serum estradiol decreased in the testosterone versus placebo group (p=0.036). Estrone levels were not affected by testosterone or placebo administration.
Depression Symptom Severity
MADRS scores decreased in both groups [testosterone group: 26.8±6.3 baseline to 15.3±9.6 at 8 weeks vs. placebo: 26.3±5.4 baseline to 14.4±9.3 at 8 weeks], with no statistical difference between the groups (p=0.91) (Table 3, Figure 1). Remission status (MADRS score of ≤10) was achieved at study end by 36% in the testosterone group and 44% in the placebo group (p=0.52). Categorical response (improvement in MADRS ≥50%) was experienced by 47% vs. 49% (p=1.00) of the testosterone vs. placebo groups, respectively. Response was not moderated by baseline free testosterone level or menopausal status (i.e., there was no interaction between treatment effect and baseline free testosterone level or menopausal status). Additionally, Clinician Global Impressions-Severity Score (CGI-S) ratings of “much improved” or “very much improved” characterized 31% of the testosterone group vs. 41% of the placebo group (p=0.37). All other measures, including measures of fatigue and sexual function, improved from baseline to 8-weeks in both the testosterone and placebo groups, with no significant difference between the groups (Table 3). There was no effect of depression symptom severity or study site (MGH/Butler) in post-hoc analyses (data not shown).
Table 3.
Psychiatric assessments and hormone levels.
| Testosterone | Placebo | P-value* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 8 Weeks | Baseline | 8 Weeks | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Depression Symptom Severity | |||||||||
| MADRS Score | 26.8 | 6.3 | 15.3 | 9.6 | 26.3 | 5.4 | 14.4 | 9.3 | 0.91 |
| CGI-S Score | 4.3 | 0.6 | 3.1 | 1.4 | 4.3 | 0.6 | 2.8 | 1.2 | 0.78 |
| IDS-SR | 35.9 | 10.5 | 20.1 | 11.2 | 34.9 | 10.0 | 19.1 | 10.1 | 0.81 |
| Fatigue and Sleepiness | |||||||||
| BFI | 6.2 | 1.9 | 4.0 | 2.3 | 5.6 | 2.0 | 3.7 | 2.8 | 0.92 |
| ESS | 8.0 | 4.7 | 6.0 | 4.2 | 9.4 | 4.7 | 6.8 | 3.6 | 0.80 |
| FSS | 45.0 | 12.5 | 38.6 | 13.8 | 42.4 | 12.3 | 35.9 | 13.9 | 0.81 |
| Sexual Function | |||||||||
| DISF | 32.8 | 27.3 | 45.1 | 31.3 | 38.7 | 30.1 | 47.5 | 34.0 | 0.37 |
| Quality of Life | |||||||||
| SF-36 PCS | 66.7 | 20.3 | 72.6 | 19.6 | 66.7 | 20.6 | 74.3 | 18.3 | 0.43 |
| SF-36 MCS | 29.5 | 13 | 54.5 | 22.4 | 33.1 | 14.5 | 54.5 | 19.7 | 0.27 |
| Hormone Levels | |||||||||
| Total testosterone | 19 | 11 | 105 | 70 | 18 | 9 | 18 | 10 | p<0.0001 |
| Free testosterone | 0.3 | 0.2 | 1.9 | 1.1 | 0.3 | 0.2 | 0.4 | 0.2 | p<0.001 |
P -value for comparison of change in psychiatric assessment or hormone level from baseline to 8 weeks in subjects randomized to receive testosterone vs placebo. For SF-36, lower scores indicate worse quality of life. For DISF, lower scores indicate worse sexual function. For all other scales/questionnaires, higher scores indicate worse symptom severity. Abbreviations: MADRS: Montgomery-Asberg Depression Rating Scale; CGI-S: Clinician Global Impressions-Severity Scale; IDS-SR: Inventory of Depressive Symptomology-Self-Report; BFI: Brief Fatigue Inventory; ESS: Epworth Sleepiness Scale; FSS: Fatigue Severity Scale; DISF: Derogatis Interview for Sexual Function; SF-36 PCS: Short Form-36 Physical Component Score; SF-36 MCS: Short Form-36 Mental Component Score.
Figure 1.
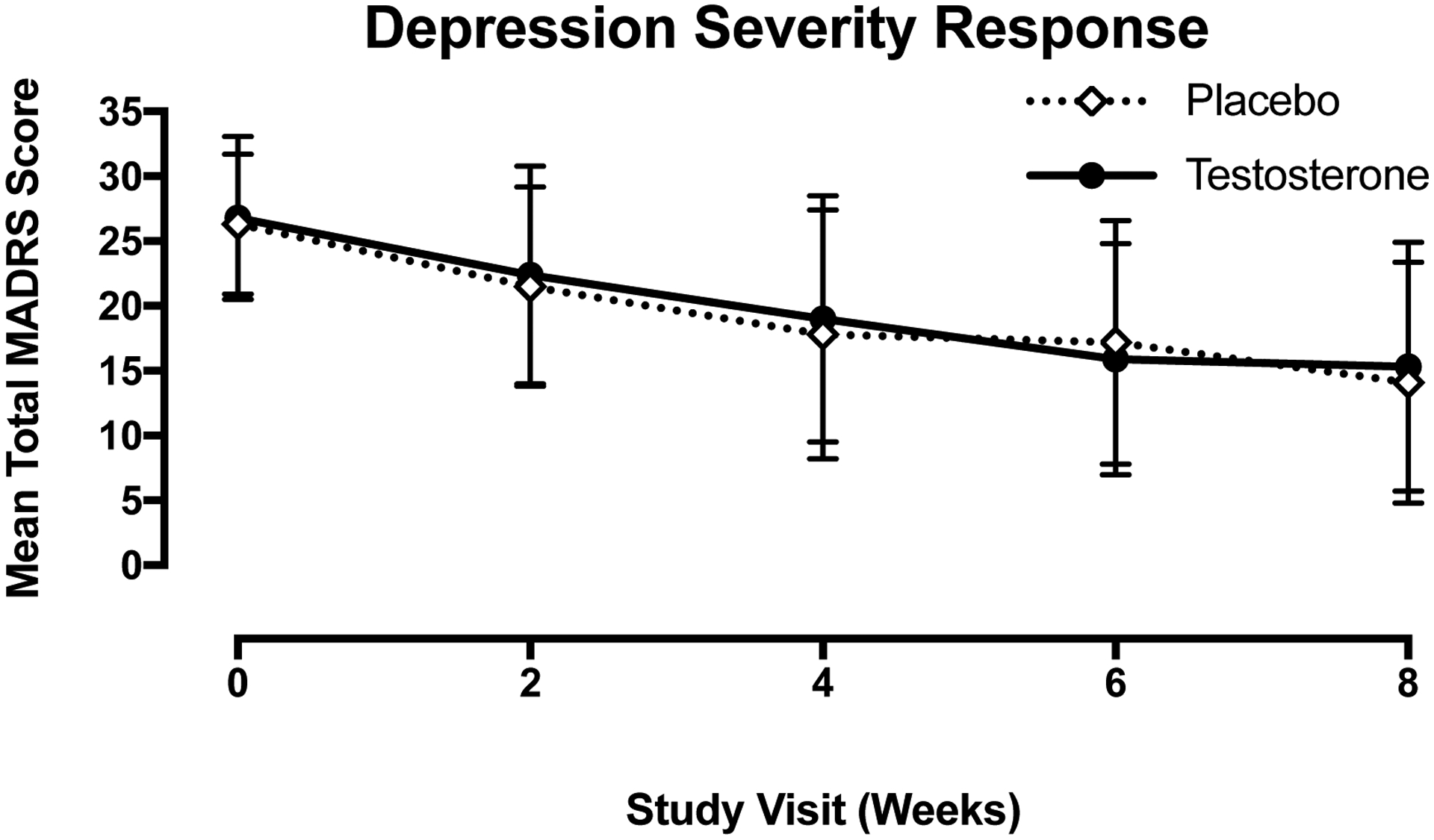
Depression severity over time represented by mean MADRS score (±SD) in the testosterone (solid black circle, solid black line) and placebo (open diamond, dotted line) groups by study visit. There was no significant difference between the testosterone and placebo groups at any visit.
Safety and Tolerability
Changes in total SAFTEE-SI scores, which measure treatment-emergent symptoms, did not differ between the groups at any time point. The following side effects were reported in >5% of subjects: acne, hot flashes and headache. All side effects are reported in Table 4. No reported side effect differed significantly between the testosterone and placebo groups and there were no serious adverse events.
Table 4.
Subject-reported adverse events.
| Testosterone | Placebo | P -value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Hot flashes | 2 | 4 | 6 | 12 | 0.16 |
| Acne | 7 | 14 | 3 | 6 | 0.32 |
| Headache | 6 | 12 | 5 | 10 | 1.00 |
| Increase in facial or body hair | 1 | 2 | 4 | 8 | 0.20 |
| Skin irritation at site of cream application | 1 | 2 | 3 | 6 | 0.36 |
| Delayed menses | 0 | 0 | 2 | 4 | 0.24 |
| Breast tenderness | 2 | 4 | 1 | 2 | 1.00 |
| Scalp hair loss | 1 | 2 | 0 | 0 | 1.00 |
fMRI Substudy Findings
Of post-treatment scan completers (n=18), eight were randomized to receive testosterone and 10 to placebo. Baseline free testosterone levels in all fMRI subjects (n=20) were inversely associated with baseline dorsal ACC activation (x=0, y=8, z=40, Ke=43, Z=2.64, p=0.004 uncorrected) and posterior cingulate cortex activation (x=6, y=−42, z=50, Ke=24, Z=2.65, p=0.004 uncorrected) in response to the emotional conflict task. Pre- to post-treatment change in ACC and PCC activation in response to the emotional conflict task (incongruent vs. congruent stimuli) was no different in the testosterone vs placebo group. Only these pre-specified ROI were analyzed.
Discussion
Augmentation with transdermal testosterone, administered at low doses designed to raise free testosterone levels to the high-normal female reference range, did not improve severity of depression, fatigue, or sexual dysfunction symptom severity to a greater degree than placebo. Additionally, fMRI data from a subset of trial participants showed no difference in activation of the dorsal and pregenual ACC following testosterone compared to placebo. We found that low-dose adjunctive testosterone had an excellent safety profile in this 8-week trial. The placebo response rate in this trial was high (49%), which might have accounted in part for the lack of observed treatment effect.
Major depression disproportionally affects women and inadequate treatment response in depression is highly prevalent. For example, the STAR*D multi-center trial demonstrated that approximately two-thirds of patients with MDD do not achieve remission after 8–12 weeks of adequate antidepressant therapy.29 Although there are effective augmentation therapies available, such as lithium and atypical antipsychotics, many patients do not respond to or are intolerant of these interventions. Even when adequate responses are obtained, these drugs may cause significant side effects which limit their long-term use.30,31,32,33,34,35,36 Previous trials and our pilot data supported the evaluation of transdermal testosterone in a properly-scaled randomized, prospective trials. Testosterone was an excellent candidate for study in women with antidepressant-resistant depression because of its potential antidepressant effects, ability to improve fatigue and sexual function, and favorable side effect profile.13,15,37–42 Nevertheless, in this rigorously designed, placebo-controlled study, we did not find a significant improvement in mood with testosterone compared with placebo, although, as noted above, the high placebo response rate might have accounted for the lack of significant differences. As these were antidepressant-resistant subjects, who by definition are a more challenging population to treat, the results of this study may not be generalizable to other depressed patients.
It should also be noted that response and remission rates that we observed for testosterone administration in this study were in line with what the literature typically suggests for antidepressants.43 However, the placebo response rate was also high and similar to that of testosterone. Placebo response rates in depression studies are often high,44 and it has been shown that studies with greater than a 40% placebo response rate are unlikely to demonstrate a statistically significant effect of the antidepressant.45 Additionally, studies demonstrate a higher placebo response rate when patients with a lower severity of depression are included,46,47 as was the case in this study. High placebo response rates may challenge underlying assumptions when designing trials using current methods, and several initiatives have focused on novel strategies to reduce placebo response in depression studies.48–51 Studies implementing these strategies may produce better quality data with greater separation rates between active treatment and placebo in cases where the drug is truly effective. Therefore, further studies employing these strategies may be warranted.
To our knowledge, this is the first randomized, placebo-controlled study of adjunctive low-dose testosterone in women with antidepressant-resistant MDD. Fooladi et al.52 randomized women (n=44) with treatment-emergent loss of libido who were on stable SSRI or SNRI therapy to low-dose testosterone or placebo to assess effects on libido and sexual function. They observed no improvements in their primary endpoint, reported level of libido, but did find an increase in frequency of sexual activity. Consistent with our findings in women with antidepressant-resistant MDD, they reported no group difference in change in depression severity as a secondary endpoint. However, women with severe depression (Beck Depression Inventory-II score > 28) were excluded from this study, resulting in recruitment of a sample with mild depression (mean baseline Beck Depression Inventory-II score of 8.0).52 Although these results may not be generalizable for a more severely ill or antidepressant-resistant population, they are consistent with the results of our study.
The anterior cingulate cortex (ACC) has been implicated in the pathophysiology of MDD.53,54 Our prior fluorodeoxyglucose-positron emission tomography (FDG-PET) study examined the effects of low-dose testosterone in women with anorexia nervosa and relative androgen deficiency, and found lower cerebral metabolism in women with anorexia nervosa than in controls, with increases in subgenual ACC activation following testosterone administration.55 Consistent with these prior findings, we report an inverse association between baseline free testosterone levels and both ACC and PCC activity (as measured by fMRI), suggesting that further study of the possible role of gonadal steroids, including androgens, in the etiopathology and/or as treatment targets in antidepressant-resistant depression is warranted. However, we did not find a difference in activation of the dorsal and pregenual ACC or PCC following testosterone compared to placebo in this study.
Limitations
Limitations of this study were those inherent in all blinded clinical trials of a disorder characterized by symptom heterogeneity and response vulnerable to placebo effect, as is MDD. It is possible that a more homogeneous population of postmenopausal women with lower levels of testosterone at baseline may allow detection of differences in testosterone versus placebo. Moreover, we cannot rule out Type II error in the context of a higher than expected placebo response.
Conclusions
This rigorously designed, double-blinded clinical trial did not find significant group differences between adjunctive low dose transdermal testosterone and placebo for antidepressant augmentation in women with treatment resistant MDD and had a high placebo response rate. Low-dose testosterone was well tolerated but failed to differentially impact overall depressive symptom severity, fatigue, or sexual dysfunction in women treated for 8 weeks with doses titrated to achieve blood levels near the upper end of the normal reference range. Additionally, testosterone did not result in greater activity in a brain region (ACC and PCC) implicated in MDD etiopathology compared to placebo. Based on our findings and the results of several other recent clinical trials, we conclude that the addition of low-dose testosterone to ongoing, ineffective antidepressant medications should not be recommended for women with MDD. These negative results are important given the number of women who use off-label male-branded and/or compounded testosterone. Further studies of adjunctive testosterone in antidepressant-resistant MDD using strategies designed to reduce placebo effects may be warranted.
Acknowledgements:
This work was supported by NIMH grant R34 MH099315 (Miller). Additionally, individual investigators were supported by NIH K23 DK113220 (Dichtel), K23 MH100623 (Brady), K23HD087464 (Fisher), K23AT008043 (Nyer), K23 DK097356-02 (Chang) and T32 DK007028 (Kimball), the Dupont-Warren Fellowship and Livingston Award through the Harvard Psychiatry Department (Cassano) and the National Alliance for Research on Schizophrenia & Depression (NARSAD) Young Investigator Award from the Brain and Behavior Research Foundation (Cassano). Lawley Pharmaceuticals provided study medication and identical placebo at no cost. The Foundation for Women’s Wellness provided funding for the fMRI substudy (Dichtel/Miller).
Disclosures:
Dr. Linda Carpenter and her research team have received research support from Janssen, NeoSync, Feelmore Labs, and Neuronetics. She has been a paid consultant for Magstim LTD and Janssen. Butler Hospital has received research equipment support from Neuronetics and Nexstim.
Dr. Paolo Cassano has received an unrestricted grant from Photothera Incorporated, drug donation from TEVA, travel reimbursement from Pharmacia-Upjohn, consultation fees from Janssen Research and Development and device donation from PhotoMedex. He has filed several patents related to the use of near-infrared light in psychiatry, received unrestricted funding from Litecure Incorporated, co-founded and consults for Niraxx Light Theraputics, and has received funding from Cerebral Sciences.
Dr. Christina Cusin has received research support from Janssen, Shenox, Otsuka and has participated on advisory boards for Janssen, Takeda, Boehringer and Alkermes.
Dr. Laura Dichtel has received study medication from Pfizer.
Dr. Maurizio Fava: For a list of lifetime disclosures of Dr. Maurizio Fava, please go to http://mghcme.org/faculty/faculty-detail/maurizio_fava
Dr. Karen K. Miller has received research support from Amgen, Pfizer and Ipsen, study medication from Lawley Pharmaceuticals, Marinus Pharmaceuticals, Pfizer, and Procter & Gamble and has equity in GE, Bristol-Myers Squibb, Becton Dickinson, Amgen and Boston Scientific.
Dr. David Mischoulon has received research support from Nordic Naturals. He has provided unpaid consulting for Pharmavite LLC and Gnosis USA, Inc. He has received honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy, Peerpoint, LLC, and from Blackmores. He has received royalties from Lippincott Williams & Wilkins for published book “Natural Medications for Psychiatric Disorders: Considering the Alternatives.”
This article was prepared while Benjamin G. Shapero was employed at the Massachusetts General Hospital / Harvard Medical School. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
All other authors report no relevant financial relationships with commercial interests.
Footnotes
Previous Presentation: none
References
- 1.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. [DOI] [PubMed] [Google Scholar]
- 2.Celotti F, Melcangi RC, Martini L. The 5 alpha-reductase in the brain: molecular aspects and relation to brain function. Front Neuroendocrinol. 1992;13(2):163–215. [PubMed] [Google Scholar]
- 3.Patchev VK, Schroeder J, Goetz F, Rohde W, Patchev AV. Neurotropic action of androgens: principles, mechanisms and novel targets. Exp Gerontol. 2004;39(11–12):1651–1660. [DOI] [PubMed] [Google Scholar]
- 4.Rubinow DR, Schmidt PJ. Androgens, brain, and behavior. Am J Psychiatry. 1996;153(8):974–984. [DOI] [PubMed] [Google Scholar]
- 5.Celotti F, Negri-Cesi P, Poletti A. Steroid metabolism in the mammalian brain: 5alpha-reduction and aromatization. Brain research bulletin. 1997;44(4):365–375. [DOI] [PubMed] [Google Scholar]
- 6.Hutchison JB. Gender-specific steroid metabolism in neural differentiation. Cellular and molecular neurobiology. 1997;17(6):603–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poletti A, Coscarella A, Negri-Cesi P, Colciago A, Celotti F, Martini L. 5 alpha-reductase isozymes in the central nervous system. Steroids. 1998;63(5–6):246–251. [DOI] [PubMed] [Google Scholar]
- 8.Poletti A, Martini L. Androgen-activating enzymes in the central nervous system. The Journal of steroid biochemistry and molecular biology. 1999;69(1–6):117–122. [DOI] [PubMed] [Google Scholar]
- 9.Steckelbroeck S, Heidrich DD, Stoffel-Wagner B, et al. Characterization of aromatase cytochrome P450 activity in the human temporal lobe. The Journal of clinical endocrinology and metabolism. 1999;84(8):2795–2801. [DOI] [PubMed] [Google Scholar]
- 10.Weber KS, Jacobson NA, Setchell KD, Lephart ED. Brain aromatase and 5alpha-reductase, regulatory behaviors and testosterone levels in adult rats on phytoestrogen diets. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY). 1999;221(2):131–135. [DOI] [PubMed] [Google Scholar]
- 11.Fink G, Sumner B, Rosie R, Wilson H, McQueen J. Androgen actions on central serotonin neurotransmission: relevance for mood, mental state and memory. Behavioural brain research. 1999;105(1):53–68. [DOI] [PubMed] [Google Scholar]
- 12.Pope HG Jr., Cohane GH, Kanayama G, Siegel AJ, Hudson JI. Testosterone Gel Supplementation for Men With Refractory Depression: A Randomized, Placebo-Controlled Trial. Am J Psychiatry. 2003;160(1):105–111. [DOI] [PubMed] [Google Scholar]
- 13.Shifren J, Braunstein G, Simon J, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. New Engl J Med. 2000;343:682–688. [DOI] [PubMed] [Google Scholar]
- 14.Miller KK BB, Beauregard C, Lipman JG, Jones J, Schoenfeld D, Sherman JC, Swearingen B, Loeffler J, Klibanski A. Effects of testosterone replacement in androgen-deficient women with hypopituitarism: a randomized, double-blind, placebo-controlled study. The Journal of clinical endocrinology and metabolism. 2006;91:1683–1690. [DOI] [PubMed] [Google Scholar]
- 15.Shifren JL DS, Moreau M, Waldbaum A, Bouchard C, DeRogatis L, Derzko C, Bearnson P, Kakos N, O’Neill S, Levine S, Wekselman K, Buch A, Rodenberg C, Kroll R. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 Study. Menopause. 2006;13(5):770–779. [DOI] [PubMed] [Google Scholar]
- 16.Miller KK, Perlis RH, Papakostas GI, et al. Low-dose transdermal testosterone augmentation therapy improves depression severity in women. CNS spectrums. 2009;14(12):688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Islam RM, Bell RJ, Green S, Page MJ, Davis SR. Safety and efficacy of testosterone for women: a systematic review and meta-analysis of randomised controlled trial data. The lancet Diabetes & endocrinology. 2019. [DOI] [PubMed] [Google Scholar]
- 18.Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology. 2003;28 Suppl 3:1–23. [DOI] [PubMed] [Google Scholar]
- 19.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders. 1996.
- 20.Chandler GM, Iosifescu DV, Pollack MH, Targum SD, Fava M. RESEARCH: Validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS neuroscience & therapeutics. 2010;16(5):322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawley Pharmaceuticals. AndroFemme® 1 Product Information. 2012.
- 22.Fava M, Mischoulon D, Iosifescu D, et al. A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study). Psychotherapy and psychosomatics. 2012;81(2):87–97. [DOI] [PubMed] [Google Scholar]
- 23.Fava M, Thase ME, DeBattista C. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry. 2005;66(1):85–93. [DOI] [PubMed] [Google Scholar]
- 24.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. [DOI] [PubMed] [Google Scholar]
- 25.Fournier JC, Chase HW, Greenberg T, et al. Neuroticism and Individual Differences in Neural Function in Unmedicated Major Depression: Findings from the EMBARC Study. Biological psychiatry Cognitive neuroscience and neuroimaging. 2017;2(2):138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. [DOI] [PubMed] [Google Scholar]
- 27.Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage. 2004;21(1):450–455. [DOI] [PubMed] [Google Scholar]
- 28.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. [DOI] [PubMed] [Google Scholar]
- 29.Trivedi MD, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006. 163:28–40. [DOI] [PubMed] [Google Scholar]
- 30.Trivedi MD, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. New Engl J Med. 2006;354:1243–1252. [DOI] [PubMed] [Google Scholar]
- 31.Papakostas G, Shelton RC, Smith J, Fava M. Augmentation of antidepressants with atypical antipsychotic medications for treatment-resistant major depressive disorder: a meta-analysis. J Clin Psychiatry. 2007;68:826–831. [DOI] [PubMed] [Google Scholar]
- 32.Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980–991. [DOI] [PubMed] [Google Scholar]
- 33.Shelton RC, Papakostas G. Augmentation of antidepressants with atypical antipsychotics for treatment-resistant major depressive disorder. Acta Psychiatr Scand. 2008;117:253–259. [DOI] [PubMed] [Google Scholar]
- 34.Thase ME, Nelson JC, Papakostas G, Gitlin MJ. Augmentation strategies in the treatment of major depressive disorder. CNS Spectr. 2007;12(12(Suppl 22)):1–18. [PubMed] [Google Scholar]
- 35.Berman RM, Marcus RN, Swanink R. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68:843–583. [DOI] [PubMed] [Google Scholar]
- 36.Marcus RN, McQuade RD, Carson WH, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder. J Clin Psychopharm. 2008;28:156–165. [DOI] [PubMed] [Google Scholar]
- 37.Miller K, Corcoran C, Armstrong C, et al. Transdermal testosterone administration in women with acquired immunodeficiency syndrome wasting: a pilot study. The Journal of clinical endocrinology and metabolism. 1998;83:2717–2725. [DOI] [PubMed] [Google Scholar]
- 38.Miller K, Grieco K, Klibanski A. Testosterone administration in women with anorexia nervosa. The Journal of clinical endocrinology and metabolism. 2004;90:1428–1433. [DOI] [PubMed] [Google Scholar]
- 39.Braunstein GD SD, Katz M, Shifren JL, Buster JE, Simon JA, Bachman G, Aguirre OA, Lucas JD, Rodenberg C, Buch A, Watts NB Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Arch Int Med. 2005;165:1571–1572. [DOI] [PubMed] [Google Scholar]
- 40.Buster JE KS, Aguirre O, Brown C, Breaux JG, Buch A, Rodenberg CA, Wekselman K, Casson P. Testosterone patch for low sexual desire in surgically menopausal women: a randomized trial. Obstet Gynecol. 2005;105(5):944–952. [DOI] [PubMed] [Google Scholar]
- 41.Simon JBG, Nachtigall L, Utian W, Katz M, Miller S, Waldbaum A, Bouchard C, Derzko C, Buch A, Rodenberg C, Lucas J, Davis S. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. The Journal of clinical endocrinology and metabolism. 2005;90:5226–5233. [DOI] [PubMed] [Google Scholar]
- 42.Davis SR, van der Mooren MJ, van Lunsen RH, et al. Efficacy and safety of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Menopause. 2006;13(3):387–396. [DOI] [PubMed] [Google Scholar]
- 43.Insel TR, Wang PS. The STAR*D trial: revealing the need for better treatments. Psychiatric services (Washington, DC). 2009;60(11):1466–1467. [DOI] [PubMed] [Google Scholar]
- 44.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. Jama. 2002;287(14):1840–1847. [DOI] [PubMed] [Google Scholar]
- 45.Iovieno N, Papakostas GI. Correlation between different levels of placebo response rate and clinical trial outcome in major depressive disorder: a meta-analysis. J Clin Psychiatry. 2012;73(10):1300–1306. [DOI] [PubMed] [Google Scholar]
- 46.Sonawalla SB, Rosenbaum JF. Placebo response in depression. Dialogues in clinical neuroscience. 2002;4(1):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rutherford BR, Roose SP. A model of placebo response in antidepressant clinical trials. Am J Psychiatry. 2013;170(7):723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desseilles M, Witte J, Chang TE, et al. Massachusetts General Hospital SAFER criteria for clinical trials and research. Harvard review of psychiatry. 2013;21(5):269–274. [DOI] [PubMed] [Google Scholar]
- 49.Freeman MP, Pooley J, Flynn MJ, et al. Guarding the Gate: Remote Structured Assessments to Enhance Enrollment Precision in Depression Trials. J Clin Psychopharmacol. 2017;37(2):176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fava M, Evins AE, Dorer DJ, Schoenfeld DA. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychotherapy and psychosomatics. 2003;72(3):115–127. [DOI] [PubMed] [Google Scholar]
- 51.Papakostas GI, Ostergaard SD, Iovieno N. The nature of placebo response in clinical studies of major depressive disorder. J Clin Psychiatry. 2015;76(4):456–466. [DOI] [PubMed] [Google Scholar]
- 52.Fooladi E, Bell RJ, Jane F, Robinson PJ, Kulkarni J, Davis SR. Testosterone improves antidepressant-emergent loss of libido in women: findings from a randomized, double-blind, placebo-controlled trial. J Sex Med. 2014;11(3):831–839. [DOI] [PubMed] [Google Scholar]
- 53.Zheng H, Li F, Bo Q, et al. The dynamic characteristics of the anterior cingulate cortex in resting-state fMRI of patients with depression. J Affect Disord. 2018;227:391–397. [DOI] [PubMed] [Google Scholar]
- 54.Matsuo K, Harada K, Fujita Y, et al. Distinctive Neuroanatomical Substrates for Depression in Bipolar Disorder versus Major Depressive Disorder. Cerebral cortex (New York, NY : 1991). 2017:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller KK, Deckersbach T, Rauch SL, et al. Testosterone administration attenuates regional brain hypometabolism in women with anorexia nervosa. Psychiatry research. 2004;132(3):197–207. [DOI] [PubMed] [Google Scholar]


