Abstract
The present study aimed to evaluate the role of early F-18 2-deoxy-2-[fluorine-18] fluoro-D-glucose positron emission tomography/computed tomography (FDG PET/CT) in non-small cell lung cancer patients undergoing immune checkpoint inhibitor (ICI) treatment.
Twenty-four non-small cell lung cancer patients who received nivolumab or pembrolizumab and underwent FDG PET/CT as an interim analysis after 2 or 3 cycles of ICI treatment were retrospectively enrolled. Tumor response was assessed using the PET Response Criteria in Solid Tumors 1.0 (PERCIST) and the European Organization for Research and Treatment of Cancer (EORTC) criteria after 2 or 3 cycles of ICI treatment (SCAN-1) and after an additional 2 cycles of ICI treatment (SCAN-2). The best overall response was determined by FDG PET/CT or chest CT at ≥ 3 months after therapy initiation, and the clinical benefit was investigated. progression-free survival was investigated, and its correlation with clinicopathologic and metabolic parameters was examined using a Cox multivariate proportional hazards model.
In the interim analysis, 4 patients achieved a complete metabolic response (CMR), 1 patient exhibited a partial metabolic response (PMR), and 14 patients had Progressive metabolic disease (PMD) according to the PERCIST and EORTC criteria. Four patients showed stable metabolic disease (SMD) according to the PERCIST criteria, and 2 patients showed different responses (i.e., PMR) according to the EORTC criteria. Patients with a CMR or PMR at SCAN-1 had a clinical benefit. Among the 4 patients with SMD at SCAN-1, only 1 experienced a clinical benefit regardless of the percent change in the peak standardized uptake value. Two patients with discordant response assessments between the PERCIST and EORTC criteria showed conflicting clinical benefits. Among the 14 patients with PMD, none experienced any clinical benefit. Only metabolic parameters were significant factors for predicting progression in the multivariate analysis (peak standardized uptake value and metabolic tumor volume, HRs of 1.18 and 1.00, respectively).
Based on early F-18 FDG PET/CT after ICI treatment, metabolic parameters could predict post-treatment progression. Responses after ICI treatment were correctly assessed in patients with a CMR, a PMR, and PMD, but patients with SMD required a meticulous follow-up because of varying clinical benefits.
Keywords: early response, immunotherapy, non-small cell lung cancer, prognosis, response assessment
1. Introduction
Recently, immune checkpoint inhibitors (ICIs) targeting programmed death-1 (PD)-1 and programmed death ligand-1 (PD-L1) have been approved for use in patients with previously treated non-small cell lung cancer (NSCLC) based on their objective responses.[1,2] NSCLC is the leading cause of cancer-related death worldwide, and its socioeconomic burden is severe.[3,4] A deeper understanding of cancer and immune biology led to the development of ICIs as a new category of anticancer therapeutics. However, due to the novel mechanism of action of these drugs, ICIs cause unusual response patterns, and biomarkers for response prediction have not been not fully established.[5] Unusual patterns of response, including pseudo-progression and hyper-progression, complicate precise assessments of the treatment response after ICI therapy.[6–9] Consensus guidelines after ICIs were developed, such as iRECIST, introduced a standard approach to treating solid tumors.[6] Major changes to the previous response evaluation criteria in solid tumors (RECIST) criteria resulted in the concept of “unconfirmed” response assessments and subsequent assessments. The response assessment timepoint in the CheckMate 017 and 517 studies was 9 weeks after nivolumab injection, but the optimal timepoint after ICI treatment to precisely assess the treatment response was unclear.
2-deoxy-2-[fluorine-18] fluoro-D-glucose positron emission tomography/computed tomography (FDG PET/CT) is widely used in the response assessment of NSCLC after treatment with various drugs and in different settings due to its prognostic value.[10–14] Furthermore, previous reports have indicated the superiority of FDG PET/CT in response assessments after chemotherapy for NSCLC. The European Organization for Research and Treatment of Cancer (EORTC) criteria and the PET Response Criteria in Solid Tumors 1.0 (PERCIST 1.0), which are based on FDG PET/CT, are more sensitive and accurate than the RECIST 1.1 criteria for early response assessments and even for evaluations of anatomical bone lesions.[15,16] Based on altered glucose metabolism and its relationship with the FDG uptake of the lesions, several reports have indicated that an association exists between PD-L1 expression and metabolic activity on FDG PET/CT.[17–19] Metabolic parameters evaluated from FDG PET/CT and PD-L1 expression and are also related to patient prognosis.[12,20]
In the present study, we aimed to evaluate the tumor shrinkage pattern after ICI treatment according to previous response assessments such as the PERCIST, RECIST 1.1, and EORTC criteria. Throughout this investigation, we attempted to suggest guidelines for interpreting early response assessments to predict the best overall response (BOR). Additionally, we investigated clinicopathologic and metabolic parameters for the prediction of progression after ICI treatment.
2. Materials and methods
2.1. Subjects
All of patients with recurrent NSCLC who underwent FDG PET/CT before and after 2 or 3 cycles of immunotherapy (SCAN-1) from June 2016 to December 2018 were retrospectively enrolled. The exclusion criteria were uncontrolled diabetes mellitus or other primary malignancies. All of the patients were treated with nivolumab (3 mg/kg intravenously) or pembrolizumab (2 mg/kg intravenously). The patients were observed for at least 6 months after immunotherapy, except for those who died or received hospice care. The study was approved by the institutional review board (NCC2018-0537).
2.2. F-18 FDG PET/CT examination and analysis
FDG PET/CT scans were performed in 1 PET/CT scanner (Discovery LS; GE Healthcare), and the same scanner was used for pre- and post-treatment PET/CT scans. All patients were fasted for at least 6 hours, and the blood glucose levels of the patients were less than 200 mg/dL. The patients rested before injection of FDG (5.5 MBq/kg), and PET/CT scanning was performed 60 minutes after injection. Non-contrast CT images and subsequent PET images were acquired from the skull base to the upper thigh in the supine position with the arms raised. PET image acquisition was performed for 4 minutes per bed in 3-dimensional acquisition mode using 7 to 10 beds. Using ordered-subset expectation maximization (2 iterations and 8 subsets), PET images were reconstructed onto a matrix (128 ∗ 128) with attenuation correction. Image review and analysis were performed using dedicated workstations and software (AW; GE Healthcare and OsiriX MD; Pixmeo). As PET parameters, the standardized uptake value (SUV) was evaluated using the following equation: (decay-corrected activity [kBq] per millilitre of tissue volume)/(injected 18F-FDG activity [kBq]/body mass [g]). A spherical volume of interest (VOI) was placed around the cancerous lesion, which was selected according to the PERCIST criteria (the single hottest tumor). The peak standardized uptake value (SUVpeak) (the highest uptake of a 1-cm3 sphere in the VOI) and maximum standardized uptake value (the highest pixel uptake) were obtained. As additional PET parameters, volumetric parameters were obtained and used for further analysis. The metabolic tumor volume (MTV), which was defined as the VOI with an isoactivity contour (a margin threshold SUV of 2.0), and the total lesion glycolysis, which was defined as the product of the MTV and the mean SUV of the VOI, were obtained.
2.3. PD-L1 expression by immunohistochemical analysis
PD-L1 expression was determined using an immunohistochemical method developed by Ventana Medical Systems. PD-L1 protein expression was assessed by 2 experienced pathologists. Positive PD-L1 expression was defined as a cytoplasmic circular membranous staining pattern. The intensity of PD-L1 staining was divided into 4 scores (0–3); Less than 1% of stained cells (0), greater than or equal to 1% and less than 10% (1), greater than or equal to 10% and less than 50% (2), and greater than or equal to 50% (3), according to the previous study.[21] Except for a score of 0, all scores were regarded as positive.
2.4. Response evaluation
Pre- and post-treatment PET/CT scans were evaluated using the PERCIST 1.0 and EORTC criteria. Post-treatment scans were performed after 2 or 3 cycles of ICI treatment (SCAN-1) and after an additional 2 cycles of ICI treatment (SCAN-2). To evaluate PET/CT scans with the EORTC criteria, we assumed that all FDG-avid lesions were target lesions, and the sum of these lesions was calculated. An FDG-avid lesion was characterized by increased uptake with a focal pattern compared to the background activity. The response according to post-treatment PET/CT scans was classified as a complete metabolic response (CMR), a partial metabolic response Partial metabolic response (PMR), stable metabolic disease (SMD) or Progressive metabolic disease (PMD). In cases of absent FDG PET/CT results at SCAN-2, the treatment response was evaluated using the RECIST 1.1 criteria. The BOR was assessed at least 3 months after immunotherapy using the RECIST 1.1 criteria, and patients exhibiting a clinical benefit were defined as having a CMR, PMR, complete response, or partial response (PR). In cases of SMD or stable disease (SD), SMD or SD needed to be sustained for more than 6 months. If a patient died during the follow-up, the BOR was considered programmed death (PD). progression-free survival (PFS) was defined as the time from initiation of chemotherapy to disease progression (including death and hospice care due to determination of a terminal state). PFS was evaluated according to clinicopathologic factors such as age, gender, PD-L1 expression, patient performance status, tumor stage, previous smoking history, the histological type of the tumor, and PET parameters.
2.5. Statistical analysis
All statistical analyses were performed using MedCalc software (version 17.5; Broekostraat, Mariakerke, Belgium). Quantitative variables are expressed as the mean ± SD or the median with the range. Inter-criteria agreement at SCAN-1 and SCAN-2 was assessed using kappa coefficients.[22] Univariate and multivariate Cox stepwise proportional hazards regression analysis were performed and significant clinicopathologic and PET parameters were derived. Two-tailed P values less than .05 were considered significant.
3. Results
3.1. Patient characteristics of those who underwent ICI and FDG PET/CT as a response assessment
A total of 24 patients were enrolled in the present study and underwent FDG PET/CT at SCAN-1. Fourteen patients underwent FDG PET/CT at SCAN-1 and SCAN-2. The characteristics of the patients are summarized in Table 1. The ages of the patients ranged from 29 to 88 years (median, 58 years). More patients received nivolumab versus pembrolizumab (N = 19 versus 5). Most patients underwent previous chemotherapy, but for 2 patients, ICI therapy was the first-line treatment.
Table 1.
Patient characteristics.
| Characteristic | Total (N = 24) |
| Age (yr) | 58 (29–88) |
| Gender | |
| Male | 19 |
| Female | 5 |
| Histopathology | |
| Adenocarcinoma | 19 |
| Squamous cell carcinoma | 4 |
| Adenosquamous carcinoma | 1 |
| Stage | |
| III (a/b) | ½ |
| IV | 21 |
| ICI drug | |
| Nivolumab | 18 |
| Pembrolizumab | 6 |
| Previous chemotherapy | |
| 0 | 2 |
| 1 | 9 |
| > 2 | 13 |
| PD-L1 expression | |
| Negative (0) | 8 |
| Positive (1/2/3) | 4/10/2 |
| PS (ECOG) | |
| 0/1/2/3/4 | 6/9/7/2 |
3.2. Treatment response evaluation after ICI using the EORTC and PERCIST criteria
At SCAN-1 and SCAN-2, response assessments were performed using the EORTC and PERCIST criteria (Table 2). Among the 24 patients, 5 patients were considered to exhibit a CMR at SCAN-1 according to both sets of criteria. The prognosis of these patients was fair; 4 patients did not exhibit progression, and the other patient exhibited progression approximately 1 year after therapy (56 weeks). One patient exhibited a PMR according to the post-treatment scan according to the 2 sets of criteria. After a PMR was identified on the post-treatment scan, the patient was considered to have a PR as the BOR. Therefore, patients with a CMR or a PMR at SCAN-1 showed a clinical benefit. In contrast, 14 patients exhibited PMD on the post-treatment scan and did not have a clinical benefit. Four patients exhibited PMD, and 1 patient exhibited SMD at SCAN-2. Eleven patients did not undergo exams because of death or the decision to receive hospice care due to determination of a terminal state. Four patients had SMD at SCAN-1 according to the PERCIST criteria. The response assessments using the PERCIST and EORTC criteria from baseline in the patient cohorts are shown in Figure 1.
Table 2.
Response assessments excluding brain lesions in a total of 24 patients.
| Post-treatment SCAN-1 (after #2 or #3 ICI) | Post-treatment SCAN-2 (after at least #4 or #5 ICI) | ||||||
| Patient no. | PERCIST | EORTC | PERCIST | EORTC | RECIST | Best overall response at ≥3 mo | Observation period (wk) |
| 1 | SMD | PMR | SMD | SMD | SD | PD | 18 |
| 2 | PMD | PMD | PMD | PMD | PD | PD | 15 |
| 3 | PMD | PMD | ∗ | ∗ | ∗ | PD | 4 |
| 4 | PMD | PMD | ∗ | ∗ | ∗ | PD | 18 |
| 5 | CMR | CMR | CMR | CMR | CR | CR | 56 |
| 6 | CMR | CMR | CMR | CMR | CR | CR | 46 |
| 7 | PMD | PMD | PMD | PMD | PD | PD | 25 |
| 8 | PMD | PMD | ∗ | ∗ | ∗ | PD | 8 |
| 9 | SMD | SMD | PMD | PMD | PD | PD | 17 |
| 10 | PMD | PMD | ∗ | ∗ | ∗ | PD | 7 |
| 11 | CMR | CMR | CMR | CMR | CR | CR | 67 |
| 12 | CMR | CMR | CMR | CMR | CR | CR | 68 |
| 13 | PMD | PMD | ∗ | ∗ | ∗ | PD | 10 |
| 14 | PMD | PMD | ∗ | ∗ | ∗ | PD | 12 |
| 15 | PMD | PMD | ∗ | ∗ | ∗ | PD | 10 |
| 16 | PMD | PMD | SMD | PMR | PR | PD | 21 |
| 17 | SMD | PMR | ∗∗ | ∗∗ | PR | PR | 43 |
| 18 | PMD | PMD | ∗ | ∗ | ∗ | PD | 6 |
| 19 | SMD | SMD | PMD | PMD | PD | PD | 18 |
| 20 | PMD | PMD | PMD | PMD | PD | PD | 13 |
| 21 | CMR | CMR | CMR | CMR | CR | CR | 56 |
| 22 | PMD | PMD | ∗ | ∗ | ∗ | PD | 9 |
| 23 | PMR | PMR | PMR | PMR | PR | PR | 28 |
| 24 | PMD | PMD | PMD | PMD | PD | PD | 19 |
Figure 1.
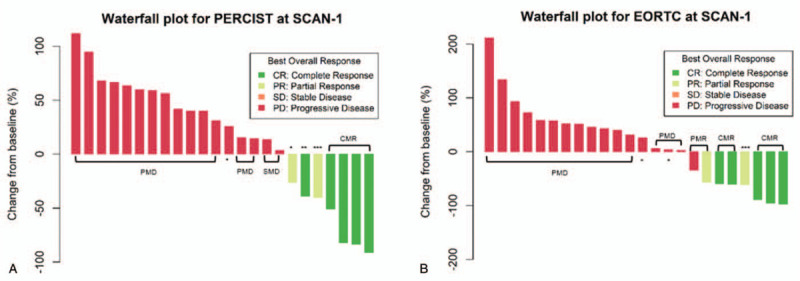
Waterfall plot of response by the (a) PET response criteria in solid tumors 1.0 and (b) European Organization for Research and Treatment of Cancer criteria. One asterisk (∗) is stable metabolic disease, 2 asterisks (∗∗) is complete metabolic response and 3 asterisks (∗∗∗) is partial metabolic response.
3.3. Comparisons of response evaluations at SCAN-1 and SCAN-2 and prognosis
Comparisons of tumor response assessments between the PERCIST and EORTC criteria at SCAN-1 and SCAN-2 demonstrated good inter-criteria agreement. An inter-criteria agreement test was performed, and kappa coefficient values were calculated at SCAN-1 and SCAN-2. At SCAN-1, the kappa value was 0.91, and at SCAN-2, the kappa value was 0.93 between the PERCIST and EORTC criteria.
Response assessments were not concordant in 2 patients who achieved SMD according to the PERCIST criteria and one who exhibited PMR according to the EORTC criteria. One patient achieved SMD at SCAN-2 but did not have any clinical benefit after 6 months of ICI treatment (Fig. 2). The other patient exhibited a PR at SCAN-2 and had a clinical benefit (Fig. 3). The other 2 patients who showed concordant response assessments exhibited progression at SCAN-2 and did not have a clinical benefit (PD as the BOR).
Figure 2.
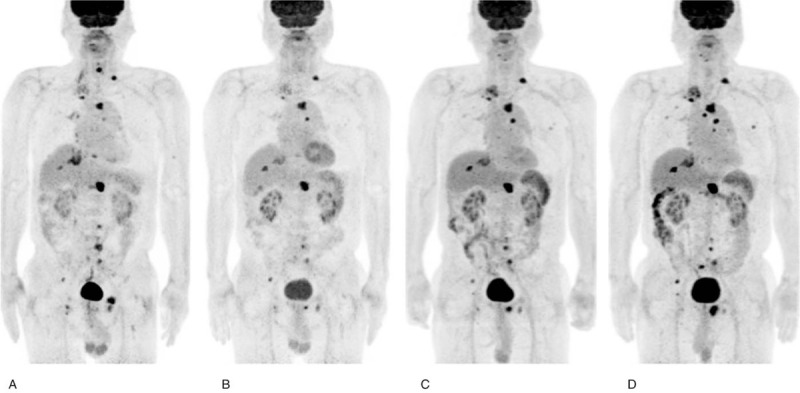
(a) Baseline 2-deoxy-2-[fluorine-18] fluoro-D-glucose positron emission tomography/computed tomography (b) after 2 rounds of nivolumab, (c) after 4 rounds of nivolumab, and (d) after 9 rounds of nivolumab treatment. This patient is an 82-year-old male treated with nivolumab. After 2 cycles of nivolumab therapy, the patient was diagnosed with stable metabolic disease according to the PET response criteria in solid tumors 1.0 criteria and exhibited a partial metabolic response according to the European Organization for Research and Treatment of Cancer criteria. During the follow-up period, the patient did not ultimately experience a clinical benefit.
Figure 3.
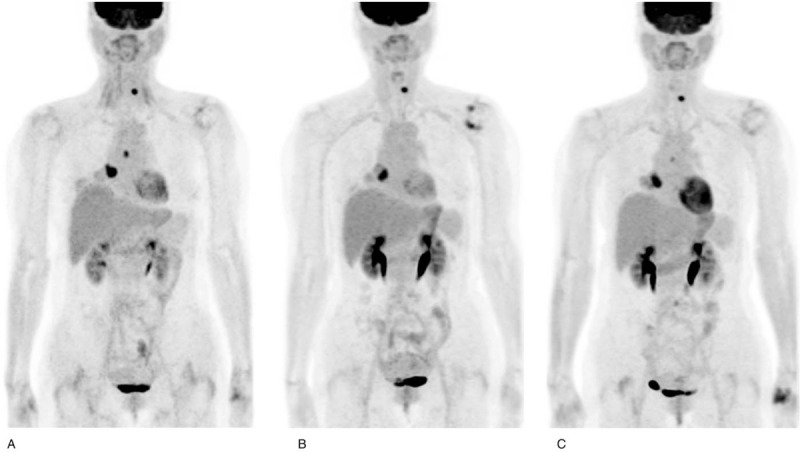
(a) Baseline 2-deoxy-2-[fluorine-18] fluoro-D-glucose positron emission tomography/computed tomography (b) after 3 rounds of pembrolizumab and (c) after 13 rounds of pembrolizumab treatment. This patient is a 53-year-old female treated with pembrolizumab. After 3 rounds of pembrolizumab, the patient was diagnosed with stable metabolic disease according to the PET response criteria in solid tumors 1.0 criteria and exhibited a partial metabolic response according to the European Organization for Research and Treatment of Cancer criteria. The patient did not undergo 2-deoxy-2-[fluorine-18] fluoro-D-glucose positron emission tomography/computed tomography after an additional 2 rounds of pembrolizumab but underwent chest CT. The patient showed a partial response on chest CT after 5 rounds of pembrolizumab therapy. During the follow-up period, the patient eventually experienced a clinical benefit.
3.4. Clinicopathologic and metabolic parameters for predicting progression after ICI treatment
During the follow-up of the patients after ICI therapy (median, 18 weeks; range, 4–68 weeks), progression was observed in 20 patients (83.3%). The median PFS was 10 weeks (range, 3–68 weeks). Among the clinicopathologic and metabolic parameters, only metabolic parameters (SUVpeak, maximum standardized uptake value, MTV, and total lesion glycolysis) were significant factors in the univariate analysis. However, in the multivariate analysis, the MTV and SUVpeak were the only significant factors that predicted progression (Table 3).
Table 3.
Multivariate logistic regression for prediction of progression after ICI treatment.
| Univariate analysis | Multivariate analysis | |||
| Hazard ratio | P value | Hazard ratio | P value | |
| Age | 1.02 (0.97–1.07) | .3425 | NS | |
| Gender (Female) | 1.11 (0.43–2.91) | .8261 | NS | |
| Pathology | 5.66 (0.62–51.66) | .3861 | NS | |
| PD-L1 expression | 0.45 (0.17–1.19) | .1133 | NS | |
| PS | 1.27 (0.77–2.08) | .3474 | NS | |
| Smoking history | 0.90 (0.34–2.34) | .8261 | NS | |
| Stage | 1.69 (0.22–13.29) | .6693 | NS | |
| SUVpeak | 1.18 (1.07–1.30) | .0002 | 1.18 (1.06–1.30) | .0017 |
| SUVmax | 1.10 (1.14–1.17) | .0014 | NS | |
| MTV | 1.00 (1.00–1.00) | .0060 | 1.00 (1.00–1.00) | .0142 |
| TLG | 1.00 (1.00–1.00) | .0045 | NS | |
3.5. Suggested optimal diagnostic flow for accurate response assessment after ICI
Based on the results from the response assessments, we suggest an optimal diagnostic flow for early FDG PET/CT after ICI therapy. After 2 or 3 rounds of ICI treatment, patients with a CMR or PMR on FDG PET/CT exhibit a clinical benefit, and patients with PMD do not exhibit a clinical benefit. However, patients with SMD, especially those with discordant response assessments between the PERCIST and EORTC criteria, require meticulous follow-up due to varying clinical benefits (Fig. 4).
Figure 4.
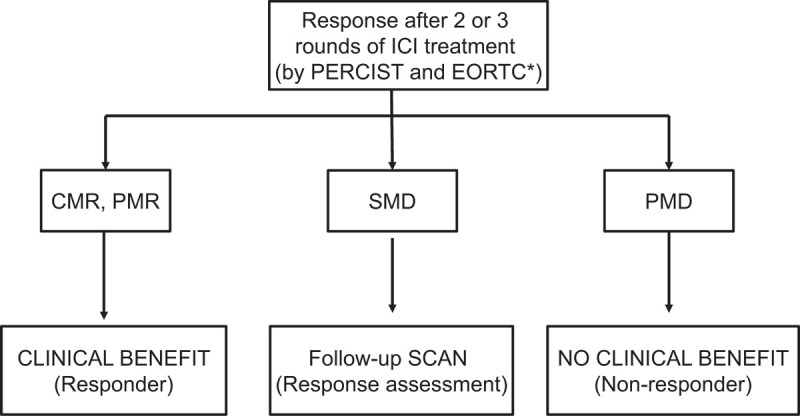
Recommendation for response assessment after immune checkpoint inhibitor treatment for non-small cell lung cancer. ∗ If discordance exists between the PET response criteria in solid tumors 1.0 and European Organization for Research and Treatment of Cancer criteria, meticulous follow-up is required.
4. Discussion
In the present study, we evaluated the value of early post-treatment scans to predict a clinical benefit after ICI treatment in patients with NSCLC. The clinical benefit was evaluated in each patient, and we evaluated whether early post-treatment scans could predict the clinical benefit. Additionally, we evaluated the prognostic value of PET parameters after ICI treatment.
ICIs are a new category of cancer therapeutics that have demonstrated striking advantages in cancer treatment in various human neoplasms. ICIs have demonstrated novel mechanisms compared to other therapies via reactivation of the immune system.[23] However, by reactivating the immune system, ICIs result in different patterns of response. Responses can be delayed and may be preceded by progression, which is known as pseudo-progression. Approximately 15% of melanoma patients experience pseudo-progression, but the presence of pseudo-progression is controversial.[5]
Kaira et al reported that metabolic parameters on an early post-treatment scan (1 month after nivolumab therapy) are predictive of prognosis after ICI treatment.[24] They analyzed 1 post-treatment scan, and the metabolic parameter predictive probability of a PR and PD was 100%. Four patients exhibited SD, one of whom was a responder, while the others were non-responders. In our study, all patients with a CMR or a PMR had a clinical benefit, and the patients with PMD had no clinical benefit, with a sensitivity of 100%. Based on these results, pseudo-progression in NSCLC was not apparent after ICI treatment. In the interpretation of post-treatment scans, the responses of patients considered to have a CMR, a PMR, or PMD were not debatable considering the clinical benefit to these patients. However, the results of the patients with SMD are questionable because these patients had different disease courses. In our study, some patients had a discrepancy in the response assessments between the PERCIST and EORTC criteria. Two patients showed SMD according to the PERCIST criteria but a PMR according to the EORTC criteria; however, the fates of these patients were different (Figs. 2 and 3). The difference between these 2 patients was apparent at SCAN-2, and the patients who experienced a clinical benefit showed a PR at SCAN-2. However, the patients who experienced no clinical benefit had SMD at SCAN-2, which led to PMD at 6 months after ICI treatment.
Cho et al suggested the PECRIT for response assessments after ICI treatment in melanoma patients.[25] According to the PECRIT, patients with a complete response, a PR or PD at 3 to 4 weeks after ICI treatment had a clinical benefit or no clinical benefit according to their assessments, with a sensitivity of 100%. However, patients with SD had different fates according to the percent change in the SUVpeak according to the PERCIST. Interestingly, patients with a greater than 15.5% change, who could be classified as having pseudo-progression, experienced a clinical benefit. Considering that melanoma patients experience an approximately 15% change, a positive percent change may reasonably reflect a clinical benefit, although this seems paradoxical. Of note, the authors explain this interesting phenomenon as result of an increased density of activated inflammatory cells around the tumor microenvironment. However, Kaira's group and our study demonstrated that only 1 patient who was a responder or had a clinical benefit exhibited SD or SMD at SCAN-1. Two of 4 patients with SMD at SCAN-1 exhibited difference response assessments between the EORTC and PERCIST criteria, and their prognoses differed (Figs. 2 and 3). Differences may exist in the ICI response and tumor shrinkage pattern in NSCLC compared to melanoma.
Kaira et al demonstrated that early post-treatment metabolic parameters are critical for predicting prognosis.[24] A high metabolic tumor burden is a biomarker that implies a poor prognosis after ICI treatment. In this study, we found that metabolic parameters, including the SUVpeak and MTV, were significant factors in the multivariate analysis for the prediction of progression, which is inconsistent with the findings of Cho et al and Grizzi et al who demonstrated that metabolic parameters reflect the tumor microenvironment, including immune infiltration, such as tumor infiltrating lymphocytes. In FDG-avid lesions, FDG is not a tumor-specific agent, and no definite method can distinguish inflammatory from malignant regions inside lesions. Controversies in the interpretation of metabolic parameters exist in follow-up assessments after ICI treatment.
The iRECIST primarily recommended follow-up radiologic evaluations in the CheckMate 017 and 517 studies in which response assessments occurred 9 weeks after ICI treatment. However, due to the high economic burden of ICIs, more precise recommendations are needed for the use of ICIs, and accurate, early response assessments are required.[26,27] Based on our study results, we recommend meticulous follow-up for patients with SMD via early post-treatment scans (Fig. 1), especially when discordant response assessments between the PERCIST and EORTC criteria occur. However, cautious interpretation is required in each step because of substantial immune-related adverse events related to ICI treatment and the presence of pseudo-progression.[28]
Apart from pseudo-progression, Anand et al revealed that secondary malignancy, especially T-cell related neoplasm was observed in the patient treated with ICI.[29] ICI related to “second hit” with loss of PD-1 suppression and could lead to T-cell malignancy. Therefore, patients with hyperprogression should be closely monitored considering secondary malignancy.
Limitations exist in the present study. The study was performed in a retrospective manner, and the patients who were selected to undergo scans at SCAN-1 and SCAN-2 may have reflected a selection bias. In general, however, frequent FDG PET/CT exams were performed in patients who showed questionable response assessment. Our results are more helpful for questionable patients. The small number of patients is also a limitation of the present study. We did not observe pseudo-progression, possibly due to the small number of patients enrolled in the present study. The response pattern varies after ICI treatment, and interpretation of the treatment response using functional imaging is more complex than interpretation of the treatment response to previous chemotherapy; thus, a large-scale prospective study is needed.
5. Conclusion
Early FDG PET/CT after ICI treatment accurately predicted the eventual clinical benefit of patients with a CMR, a PMR or PMD. Patients with SMD require meticulous follow-up after ICI treatment, including a post-treatment scan after 2 months. Additionally, a high metabolic burden after ICI treatment predicted progression and was related to a poor disease course.
Author contributions
Conceptualization: Tae-Sung Kim.
Data curation: Youngjoo Lee, Seok-ki Kim, Ji-Youn Han.
Formal analysis: Sohyun Park, Tae-Sung Kim.
Funding acquisition: Sohyun Park, Tae-Sung Kim.
Investigation: Sohyun Park, Tae-Sung Kim.
Methodology: Sohyun Park, Tae-Sung Kim, Seok-ki Kim.
Project administration: Tae-Sung Kim.
Resources: Sohyun Park, Tae-Sung Kim.
Software: Tae-Sung Kim.
Supervision: Youngjoo Lee, Seok-ki Kim, Ji-Youn Han.
Validation: Sohyun Park, Tae-Sung Kim, Seok-ki Kim.
Visualization: Sohyun Park, Tae-Sung Kim.
Writing – original draft: Sohyun Park, Tae-Sung Kim.
Writing – review & editing: Youngjoo Lee, Seok-ki Kim, Ji-Youn Han.
Footnotes
Abbreviations: BOR = best overall response, CMR = complete metabolic response, EORTC = European Organization for Research and Treatment of Cancer, FDG PET/CT = 2-deoxy-2-[fluorine-18] fluoro-D-glucose positron emission tomography/computed tomography, ICI = immune checkpoint inhibitor, MTV = metabolic tumor volume, NSCLC = non-small cell lung cancer, PD = progressive disease, PD-1 = programmed death-1, PD-L1 = programmed death ligand-1, PERCIST = PET Response Criteria in Solid Tumors 1.0, PFS = progression-free survival, PMD = progressive metabolic disease, PMR = Partial metabolic response, PR = partial response, RECIST = response evaluation criteria in solid tumors, SD = stable disease, SMD = stable metabolic disease, SUV = standardized uptake value, SUVpeak = peak standardized uptake value, VOI = volume of interest.
How to cite this article: Park S, Lee Y, Kim T-S, Kim S-k, Han J-Y. Response evaluation after immunotherapy in NSCLC: early response assessment using FDG PET/CT. Medicine. 2020;99:51(e23815).
This research was supported by National Cancer Center Research Grant (1610330 and 2010050) and Career Development Award (NCCCDA2018-06), National Cancer Center, Republic of Korea.
All authors declare that they do not have any conflict of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
ECOG = Eastern Cooperative Oncology Group, ICI = immune checkpoint inhibitor, PD-L1 = programmed death ligand-1, PS = performance status.
CMR = complete metabolic response, CR = complete response, EORTC = European Organization for Research and Treatment of Cancer, ICI = immune checkpoint inhibitor, PD = progressive disease, PERCIST = PET response criteria in solid tumors, PMD = progressive metabolic disease, PMR = partial metabolic response, PR = partial response, RECIST = response evaluation criteria in solid tumors, SD = stable disease, SMD = stable metabolic disease.
Among 24 patients, 10 patients did not perform FDG PET/CT on SCAN-2.
Among 24 patients, 1 patient perform chest CT instead of FDG PET/CT on SCAN-2.
MTV = metabolic tumor volume, PD-L1 = programmed death-ligand 1, PS = performance status, SUVmax = maximal standardized uptake value, SUVpeak = peak of the standardized uptake value, TLG = total lesion glycolysis.
References
- [1].Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–403. [DOI] [PubMed] [Google Scholar]
- [4].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [5].Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412–20. [DOI] [PubMed] [Google Scholar]
- [6].Seymour L, Bogaerts J, Perrone A, et al. IRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang GX, Kurra V, Gainor JF, et al. Immune checkpoint inhibitor cancer therapy: spectrum of imaging findings. Radiographics 2017;37:2132–44. [DOI] [PubMed] [Google Scholar]
- [8].Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920–8. [DOI] [PubMed] [Google Scholar]
- [9].Saada-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017;28:1605–11. [DOI] [PubMed] [Google Scholar]
- [10].de Jong EE, van Elmpt W, Leijenaar RT, et al. [18F]FDG PET/CT-based response assessment of stage IV non-small cell lung cancer treated with paclitaxel-carboplatin-bevacizumab with or without nitroglycerin patches. Eur J Nucl Med Mol Imaging 2017;44:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Higuchi M, Owada Y, Inoue T, et al. FDG-PET in the evaluation of response to nivolumab in recurrent non-small-cell lung cancer. World J Surg Oncol 2016;14:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sharma A, Mohan A, Bhalla AS, et al. Role of various semiquantitative parameters of 18F-FDG PET/CT studies for interim treatment response evaluation in non-small-cell lung cancer. Nucl Med Commun 2017;38:858–67. [DOI] [PubMed] [Google Scholar]
- [13].Usmanij EA, Natroshvili T, Timmer-Bonte JNH, et al. The predictive value of early in-treatment (18)F-FDG PET/CT response to chemotherapy in combination with bevacizumab in advanced nonsquamous non-small cell lung cancer. J Nucl Med 2017;58:1243–8. [DOI] [PubMed] [Google Scholar]
- [14].Yossi S, Krhili S, Muratet JP, et al. Early assessment of metabolic response by 18F-FDG PET during concomitant radiochemotherapy of non-small cell lung carcinoma is associated with survival: a retrospective single-center study. Clin Nucl Med 2015;40:e215–21. [DOI] [PubMed] [Google Scholar]
- [15].Shang J, Ling X, Zhang L, et al. Comparison of RECIST, EORTC criteria and PERCIST for evaluation of early response to chemotherapy in patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2016;43:1945–53. [DOI] [PubMed] [Google Scholar]
- [16].Costelloe CM, Chuang HH, Madewell JE, et al. Cancer response criteria and bone metastases: RECIST 1.1, MDA and PERCIST. J Cancer 2010;1:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Takada K, Toyokawa G, Okamoto T, et al. Metabolic characteristics of programmed cell death-ligand 1-expressing lung cancer on (18) F-fluorodeoxyglucose positron emission tomography/computed tomography. Cancer Med 2017;6:2552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Takada K, Toyokawa G, Tagawa T, et al. Association between PD-L1 expression and metabolic activity on (18)F-FDG PET/CT in patients with small-sized lung cancer. Anticancer Res 2017;37:7073–82. [DOI] [PubMed] [Google Scholar]
- [19].Lopci E, Toschi L, Grizzi F, et al. Correlation of metabolic information on FDG-PET with tissue expression of immune markers in patients with non-small cell lung cancer (NSCLC) who are candidates for upfront surgery. Eur J Nucl Med Mol Imaging 2016;43:1954–61. [DOI] [PubMed] [Google Scholar]
- [20].Kasahara N, Kaira K, Bao P, et al. Correlation of tumor-related immunity with 18F-FDG-PET in pulmonary squamous-cell carcinoma. Lung Cancer 2018;119:71–7. [DOI] [PubMed] [Google Scholar]
- [21].Kim HS, Lee JH, Nam SJ, et al. Association of PD-L1 expression with tumor-infiltrating immune cells and mutation burden in high-grade neuroendocrine carcinoma of the lung. J Thorac Oncol 2018;13:636–48. [DOI] [PubMed] [Google Scholar]
- [22].Psoter KJ, Roudsari BS, Dighe MK, et al. Biostatistics primer for the radiologist. AJR Am J Roentgenol 2014;202:W365–75. [DOI] [PubMed] [Google Scholar]
- [23].Lheureux S, Denoyelle C, Ohashi PS, et al. Molecularly targeted therapies in cancer: a guide for the nuclear medicine physician. Eur J Nucl Med Mol Imaging 2017;44: Suppl 1: 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kaira K, Higuchi T, Naruse I, et al. Metabolic activity by (18)F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging 2018;45:56–66. [DOI] [PubMed] [Google Scholar]
- [25].Cho SY, Lipson EJ, Im HJ, et al. Prediction of response to immune checkpoint inhibitor therapy using early-time-point (18)F-FDG PET/CT imaging in patients with advanced melanoma. J Nucl Med 2017;58:1421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Merino Almazan M, Duarte Perez JM, Marin Pozo JF, et al. A multicentre observational study of the effectiveness, safety and economic impact of nivolumab on non-small-cell lung cancer in real clinical practice. Int J Clin Pharm 2019;41:272–9. [DOI] [PubMed] [Google Scholar]
- [27].Aguiar PN, Jr, Perry LA, Penny-Dimri J, et al. The effect of PD-L1 testing on the cost-effectiveness and economic impact of immune checkpoint inhibitors for the second-line treatment of NSCLC. Ann Oncol 2017;28:2256–63. [DOI] [PubMed] [Google Scholar]
- [28].Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 2017;35:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Anand K, Ensor J, Pingali SR, et al. T-cell lymphoma secondary to checkpoint inhibitor therapy. J Immunother Cancer 2020;8: [DOI] [PMC free article] [PubMed] [Google Scholar]


