Abstract
Spontaneous intranodular hemorrhaging in benign partially cystic thyroid nodules was reported to cause neck swelling, difficulty swallowing, and other oppressive symptoms attributed to their growing progressively at high rates. In our study, the risk factors for hemorrhaging in these nodules were investigated.
We retrospectively analyzed benign partial cystic thyroid nodules from September 2017 to December 2019, and divided them into 2 groups according to the occurrence of intranodular hemorrhage. Age, gender, follow-up time nodules initial maximum diameter, blood supply, spongiform content, nodules solid components, and internal solid portion were compared between the 2 groups at the first ultrasound examination. Chi-Squared and multivariate analysis were performed to evaluate the association of hemorrhage with clinical and ultrasonographic characteristics. ROC analysis was performed to evaluate the utility of factors in predicting hemorrhage.
There were 59 occurrences of intranodular hemorrhage, which were associated with abundant blood supply, spongiform contents, and unsmooth margin of the internal solid portion. After multivariate analysis, abundant blood supply, and spongiform content were independent predictors for hemorrhage. In ROC analysis integrating these predictors, the sensitivity was 62.7% and specificity was 95.2% with the AUC 0.881.
Partially cystic thyroid nodules with abundant blood supply, non-smooth margin of the internal solid portion and a spongiform internal content were apt to spontaneous intranodular hemorrhaging, which can be recognized as soon as possible by ultrasound.
Keywords: partially cystic thyroid nodules, spontaneous intranodular hemorrhage, ultrasonography
1. Introduction
During the past decade, the number of detected euthyroid thyroid nodules (TNs) exponentially increased with the widespread use of ultrasonography (US).[1,2] Despite approximately 90% were benign TNs, the prevalence of spontaneous intranodular hemorrhage over half of adult populations,[3,4] and among the benign TNs, partially cystic thyroid nodules (PCTNs) were common. Nodules with solid components between 10 and 90% were classified as PCTNs.[5] The number of detected thyroid nodules exponentially increased with the widespread use of US. In diagnosis, evaluation, and management of TN, US is an essential noninvasive tool to help therapists make clinical decisions.[6] Studies have shown that echogenicity on US is a reliable standard to confirm diagnosis or determine therapeutic efficacy.[7]
Currently, the management of PCTNs is still problematic. On the one hand, some experts recommend following up these nodules because of their low risk of malignancy.[5] However, benign PCTNs may abruptly increase in size because of spontaneous intranodular hemorrhage and result in pain or palpable mass in the neck.[5,8] Even a small amount of spontaneous intranodular hemorrhage can compress the vital organs, and an emergency surgery would be demanded to relieve the suffering of patients.[9] On the other hand, others suggested percutaneous ethanol ablation of these nodules, but the management of asymptomatic nodules without spontaneous intranodular hemorrhage was over-treatment, which brought financial pressure and psychological burden to patients, and it may have an unsatisfactory therapeutic effect in the nodules with spontaneous intranodular hemorrhage due to increased volume and require further treatment to avoid recurrence.[10,11] So, for some PCTNs with spontaneous intranodular hemorrhage tendency, once hemorrhaging occurs, it is more difficult treatment and less effective therapeutic. In response to the above problems, our work proposes to follow up the nodules without spontaneous intranodular hemorrhage tendency, and therapeutic intervention the nodule with spontaneous intranodular hemorrhage tendency as soon as possible. Therefore, next we will investigate benign PCTNs features of spontaneous intranodular hemorrhage in order to accurately identify them and achieve a more reasonable therapy.
Unfortunately, no relevant studies have been conducted to report the diagnosis of PCTNs with spontaneous intranodular hemorrhage, which poses problems for our follow-up management of these nodules. Therefore, it is imperative to explore the risk factors of these nodules inclined to spontaneous intranodular hemorrhage, so that recommendations can be made for the conduct of strict and well-timed management. In our work, we denote ourselves to investigate the risk factors of spontaneous intranodular hemorrhage by US, so as to be helpieves the physical and psychological stress of patients, but also achieves accurate stratification for individualized therapy.
2. Patients and methods
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Affiliated Hospital of Jiangsu University (ethical review number:SWYXLL20190225-2).
A retrospective review of the records of all patients with benign PCTNs seen between September 2017 and December 2019 was performed. Study inclusion criteria included ultrasonography and FNA (Fine needle aspiration) confirmed benign nodules; nodule solid components accounts for 10% to 90%; a nodule measured at US ≥10 mm in 3 orthogonal dimensions; aged ≥18 years; follow-up was 6 to 24 months, and normal thyrotropin concentration. Exclusion criteria included history of neck irradiation; incomplete clinical data; abnormal blood pressure, blood glucose, platelets and coagulation; history of taking aspirin, warfarin, and other anticoagulants; the interval between the first and second ultrasound was more than 3 months and history of trauma.
2.1. US evaluation of nodules
PCTNs are nodules that have both solid and cystic components, which were categorized into predominantly cystic nodules (10% < solid component < 50%) and predominantly solid nodules (50% < solid component < 90%).[12,13] PTCNs with spontaneous intranodular hemorrhage showed a sudden enlarge in volume by at least 50% and an increase liquid dark area on US. Besides, intranodular hemorrhage appears as a reddish toothpaste-like muddy material in FNA, and cytopathological characteristics include numerous degenerated red blood cells.[14] Nodule volume was calculated by using the formula for a rotational ellipsoid (length × width × depth × π/6).[15] The growth of nodules was evaluated by the volume growth rate: [(last nodule volume - primary nodule volume)/ primary nodule volume],[5] and an increase ≥50% in volume defined as a significant growth.[5,16] Information obtained at the first US examination included gender, age, the size of nodules, blood supply, nodule internal content and internal solid portion. Compare the difference of the above information between hemorrhage group and non-hemorrhage group. US and US-guided FNA of TNs were performed by experienced radiologists.
Each nodule was evaluated at first examination for the following US findings: size (the longest diameter), nodule solid components (predominantly solid vs predominantly cystic), and a spongiform internal content (spongiform appearance was defined as aggregation of multiple microcystic components in more than 50% of the volume of an isoechoic nodule).[13] US examination of internal solid portion included margin (smooth vs non-smooth), echogenecity (hypoechoic vs isoechoic vs hyperechoic), and calcification (calcifications vs none).
The use of color Doppler imaging for characterization of TN vascularity is widely used currently, thereby performed Adler classification at the first US:[17] Grade 0 means no significant blood supply, which showed no obvious signal of blood flow in the nodules on the US blood flow imaging. Grade 1 is minimal vascularity, manifested by 1 or 2 dotted or short rod-shaped blood flow signals visible inside the nodules. Grade 2 is moderate vascularity manifested by a visualization of main vessel in the area and/or several small vessels. In our work, grade 0–1 was defined as scanty blood supply and grade 2 was abundant blood supply.
2.2. Statistical analysis
Continuous variables were presented as means ± standard deviation (SD). Comparison was performed using a two-sample Student t test for continuous variables and the χ2 test for categorical variables. A P < .05 was considered significant. Multivariate logistic regression analysis was used to assess the relationship between the predicting factors and the spontaneous intranodular hemorrhage. A risk score for each patient was constructed based on the identified risk factors, including proportion of cystic components, blood supply, and gender. Receiver operating characteristic (ROC) curve and area under the ROC curve (AUC) were used to estimate the predictive power. Statistical analyses were performed with SPSS for Windows version 22.0 (SPSS Inc., Chicago, Ill., USA).
3. Results
A total of 101 participants (Fig. 1), 59 nodules with spontaneous intranodular hemorrhage (mean age, 54.25 years ± 1.58 [standard deviation]; 35 women) and 42 nodules without spontaneous intranodular hemorrhage (mean age, 55.29 years ± 2.08 [standard deviation]; 28 women) were analyzed. The characteristics of patients and nodules are summarized in Table 1. The spontaneous intranodular hemorrhage can be characterized by an abundant blood supply (P < .001), non-smooth margin of the internal solid portion (P = .045) and a spongiform internal content (P<.001) (Figs. 2 and 3).
Figure 1.
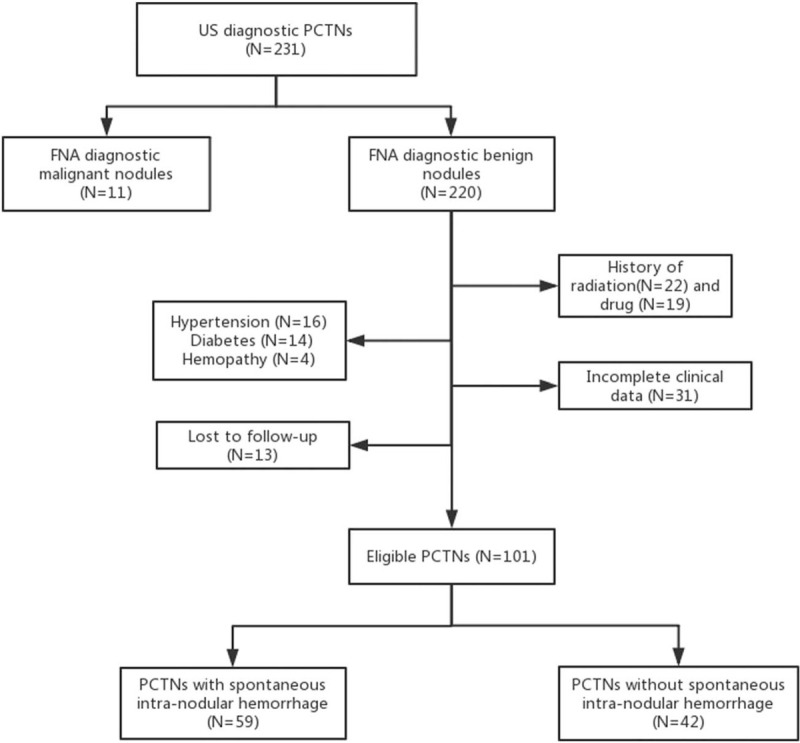
Flowchart of study participants included and excluded in the study.
Table 1.
Patient and thyroid nodule characteristics.
| Non-hemorrhage group (n = 42) | Hemorrhage group (n = 59) | P value | |
| Patient characteristics | |||
| Age (years)‡ | 55.29 ± 2.08 | 54.25 ± 1.58 | .321 |
| Gender | .453 | ||
| Male | 14 (33.3%) | 24 (40.7%) | |
| Female | 28 (66.7%) | 35 (59.3%) | |
| Fllow-up time (month)‡ | 16.17 ± 0.68 | 16.68 ± 0.53 | .475 |
| Nodule characteristics∗ | |||
| Nodule size (mm)‡ | 16.19 ± 0.67 | 16.66 ± 0.54 | .763 |
| Nodule blood supply type† | <.001 | ||
| scanty blood supply | 35 (83.3%) | 14 (23.7%) | |
| abundant blood supply | 7 (16.7%) | 45 (76.3%) | |
| Nodule solid components | .056 | ||
| Predominantly solid | 28 (66.7%) | 28 (47.5%) | |
| Predominantly cystic | 14 (33.3%) | 31 (52.5%) | |
| Spongiform content | <.001 | ||
| Yes | 8 (19.0%) | 36 (61.0%) | |
| No | 34 (81%) | 23 (39.0%) | |
| Internal solid portion | |||
| Margin | .045 | ||
| Smooth | 27 (64.3%) | 26 (44.1%) | |
| Non-smooth | 15 (45.7%) | 33 (55.9%) | |
| Echogenecity | .291 | ||
| Hypoechoic | 11 (26.2%) | 23 (39.0%) | |
| Isoechoic | 27 (64.3%) | 31 (52.5%) | |
| Hyperechoic | 6 (9.5%) | 5 (8.5%) | |
| Calcification | .364 | ||
| Yes | 6 (14.3%) | 4 (6.8%) | |
| No | 36 (85.7%) | 55 (93.2%) |
Figure 2.
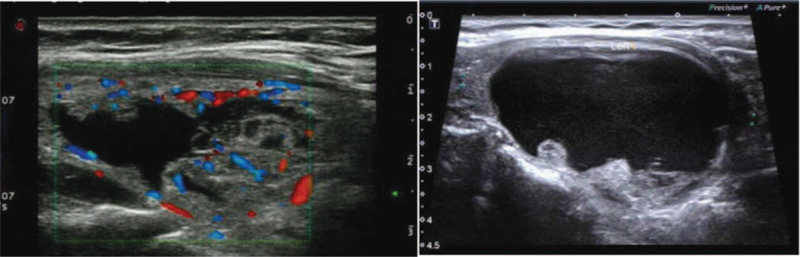
Longitudinal ultrasound image of PCTN spontaneous intranodular hemorrhage in 45-year old woman shows an abundant blood supply.
Figure 3.
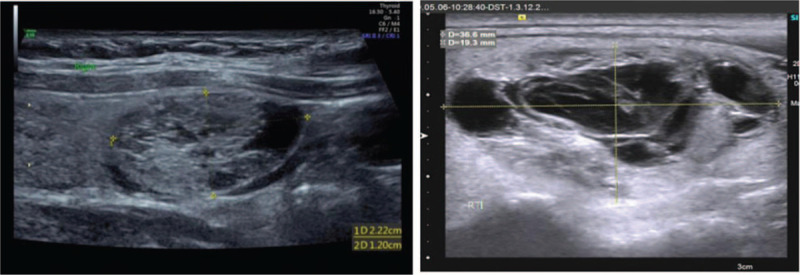
Longitudinal ultrasound image of PCTN spontaneous intranodular hemorrhage in 38-year old woman shows a spongy form.
There were no statistically significant differences found between 2 groups in patient age (P = .321), gender (P = .453), follow-up time (P = .457), maximum diameter of the initial nodules (P = .763) and nodule solid components (P = .056). In terms of internal solid portion, the echogenicity and calcification were no statistically significant differences in 2 groups (P = .291, P = .364).
We next performed multivariate logistic regression to examine the independent associations between US features and the risk of spontaneous intranodular hemorrhage. Abundant blood supply (OR: 17.072, 95% CI: 5.423–53.740) and spongiform content (OR: 6.860, 95% CI: 2.143–21.964) turned out to be independent risk factors for intra-nodular hemorrhage (Table 2).
Table 2.
Multivariate analyses of factors for predicting spontaneous intranodular hemorrhage in benign PCTNs.
| Nodule characteristics | β-Coefficient | OR | 95% Confidence interval | P value |
| Nodule blood supply | 2.837 | 17.072 | 5.423–53.740 | <.001 |
| Spongiform internal content | 1.926 | 6.860 | 2.143–21.964 | .001 |
| Non-smooth margin | 1.026 | 2.789 | 0.918–8.471 | .070 |
Finally, we computed a risk score for each patient based on the above-identified significant predictors and constructed a ROC curve using the risk score. The AUC was 0.881 with a cut-off value of 0.58, sensitivity of 62.7%, and specificity of 95.2% (Fig. 4).
Figure 4.
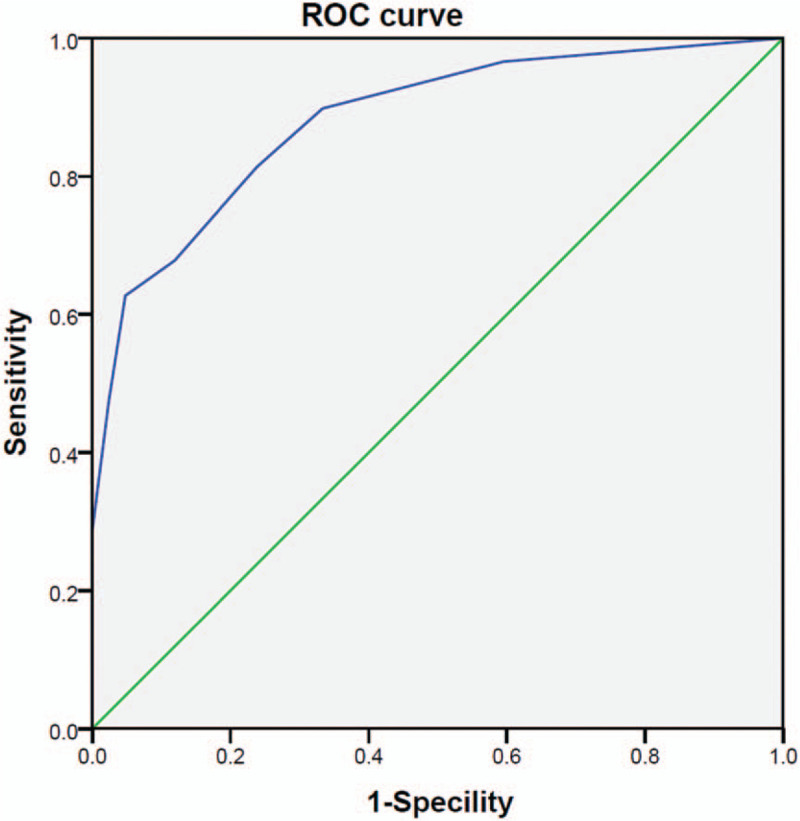
Receiver-operating characteristic (ROC) curve for the scoring system. The area under the ROC curve (AUC) was 0.881.
4. Discussion
US has the advantages of high safety, convenient operation, no radiation, and non-invasiveness in the diagnosis of TNs. Therefore, US has excellent clinical value and has become one of the most commonly used tools for diagnosing TNs. To the best of our knowledge, the focus of thyroid nodules has invariably been on the theranostic of malignant nodules, there is little information regarding the US findings of PCTNs, associated with spontaneous intranodular hemorrhage.[18–20]
Currently, medical scholars have put forwarded follow-up of benign PCTNs.[21,22] Novelly, we proposed to explore the risk factors relating to the spontaneous intranodular hemorrhage of benign PCTNs, and theat these nodules as soon as possible.
The mechanism of spontaneous intranodular hemorrhage of benign TNs is still not completely known, and several reports argue the oozing fluid bleeds out of the rupture of a weak vessel in the solid component.[23,24] Excitingly, our work sought to find abundant blood supply, nonsmooth margin of the solid portion and spongiform content were associated with spontaneous intranodular hemorrhage. Furthermore, abundant blood supply and spongiform content were independent factors for spontaneous intranodular hemorrhage.
Some reports have come up that the blood supply could provide rich nutrients for the growth of nodules affects.[25] Hence, abundant blood supplement are more prone to hemorrhage upon their spontaneous rupture, which is consistent with our findings of blood supply as 1 of the risk factors. In 2005, a classical “honeycomb” pattern was described as a heterogeneous nodule with multiple cystic areas separated by septations that indicate a hyperplastic benign nodule.[26] Then, Berna et al determined a soft appearance according to color map on USE (real-time ultrasound elastography) and low strain ratio values of dominantly solid areas,[27] revealing that dominantly solid areas of spongiform nodules were soft and low compactness. Therefore, it is logically explained that the loose tissue is more conducive to the occurrence of spontaneous intranodular hemorrhage.
Spontaneous intranodular hemorrhage is often resulted from the rupture of the blood vessel in solid content.[25] It is suspected that the non-smooth solid portion of the nodule is more vulnerable to the impact of the liquid in the nodule and hemorrhage. But, the nonsmooth solid portion was not sufficient to be an independent risk factor for spontaneous intranodular hemorrhage in our work. In addition, theoretically, the predominantly solid nodules are more prone to hemorrhage than the predominantly cystic nodules. Whereas, there was no significant statistical difference between them in our research (P = .056), which may be related to the small sample.
Despite our research achieved accepted results, there were still several limitations to be fully discussed. First, the sample of the research was small, a larger sample was needed. Second, whether spontaneous intranodular hemorrhage is related to thyroid function and autoimmune diseases was uncertain, because the existence of these diseases has been excluded in our work. Third, the time when growth occurred cannot be known precisely and, thus, may overestimate the stated time estimates for nodule growth. Fourth, there was variation in the evaluation of nodules intra and inter-radiologist. Fifth, the diagnosis of benign nodules were based on cytologic, rather than pathologic findings, because none of the nodules showed surgical indications. Therefore, the possibility of nodules malignancy cannot be completely ruled out, we try to consider obtaining pathologic findings in the later study. Finally, the application for estimating the nodules were only limited to the technique of color Doppler ultrasound and lack new technologies such as Ultrasound Elastography (USE) and Superb Microvascular Imaging (SMI), so that more detailed information could not be provided. Therefore, in the future, more larger, multicenter and longer follow-up investigations are necessary, accompanied with emerging techniques of USE and SMI.
5. Conclusion
In conclusion, benign partially cystic thyroid nodules with abundant blood supply and spongiform contents were prone to spontaneous intranodular hemorrhage. Besides, nodules with nonsmooth margin of the internal solid portion was more likely to have spontaneous intranodular hemorrhage, but it cannot be an independent risk factor. Thus, the thyroid nodule liable to spontaneous intranodular hemorrhage can be recognized as soon as possible, which laid the foundation for the development of individualized therapy.
Author contributions
Conceptualization: Baoding Chen.
Data curation: Zheng Zhang, Yanwei Chen, Mengyuan Shang.
Formal analysis: Haizhen Yang, Shuangshuang Zhao, Yanwei Chen.
Investigation: Haizhen Yang, Yanwei Chen.
Methodology: Haizhen Yang, Keke Wang.
Validation: Zheng Zhang, Baoding Chen.
Writing – original draft: Haizhen Yang.
Writing – review & editing: Haizhen Yang, Shuangshuang Zhao, Keke Wang.
Footnotes
Abbreviations: FNA = fine needle aspiration, PCTNs = partially cystic thyroid nodules, SMI = superb microvascular imaging, TNs = thyroid nodules, US = ultrasonography, USE = ultrasound elastography.
How to cite this article: Yang H, Zhao S, Zhang Z, Chen Y, Wang K, Shang M, Chen B. The associated factors for spontaneous intranodular hemorrhage of partially cystic thyroid nodules: a retrospective study of 101 thyroid nodules. Medicine. 2020;99:51(e23846).
This study was supported by the Zhenjiang Social Development Fund (SH2018035) and the Doctoral Start-up Fund of Affiliated Hospital of Jiangsu University (jdfyRC2017013). All the authors consent publish the article.
The authors report no conflicts of interest.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
At the time of initial ultrasound.
Blood supply type: a blood flow classification method was proposed by Adler.
Data are means ± standard deviations.
PCTNs = partially cystic thyroid nodules.
References
- [1].Cronan JJ. Thyroid nodules: is it time to turn off the US machines? Radiology 2008;247:602–4. [DOI] [PubMed] [Google Scholar]
- [2].Russ G, Royer B, Bigorgne C, et al. Prospective evaluation of thyroid imaging reporting and data system on 4550 nodules with and without elastography. Eur J Endocrinol 2013;168:649–55. [DOI] [PubMed] [Google Scholar]
- [3].Hegedüs L. The thyroid nodule. New Engl J Med 2004;351:1265–8. [DOI] [PubMed] [Google Scholar]
- [4].Oki T, Sugimoto T, Ogawa M, et al. Evaluation of follow-up examinations using ultrasonography for patients with thyroid nodules initially diagnosed as benign. Anticancer Res 2019;39:2061–7. [DOI] [PubMed] [Google Scholar]
- [5].Lim Hyun Kyung, Kim Dong Wook, Baek Jung Hwan, et al. Factors influencing the outcome from ultrasonography-guided fine-needle aspiration of benign thyroid cysts and partially cystic thyroid nodules: a multicenter study. Endocr Res 2018;43:65–72. [DOI] [PubMed] [Google Scholar]
- [6].Kangelaris GT, Kim TB, Orloff LA. Role of ultrasound in thyroid disorders. Ultrasound Clin 2012;7:197–210. [DOI] [PubMed] [Google Scholar]
- [7].Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol 2008;18:1244–50. [DOI] [PubMed] [Google Scholar]
- [8].Alexander EK, Hurwitz S, Heering JP, et al. Natural history of benign solid and cystic thyroid nodules. Ann Intern Med 2003;138:315–8. [DOI] [PubMed] [Google Scholar]
- [9].Covino Marcello, Princi Pietro, De Luca Giulio, et al. Spontaneous thyroid nodule hemorrhage in the emergency department. Endocr Pract 2020;26:192–6. [DOI] [PubMed] [Google Scholar]
- [10].Guglielmi R, Pacella CM, Bianchini A, et al. Percutaneous ethanol injection treatment in benign thyroid lesions: role and effificacy. Thyroid 2004;14:125–31. [DOI] [PubMed] [Google Scholar]
- [11].Suh CH, Baek JH, Ha EJ, et al. Ethanol ablation of predominantly cystic thyroid nodules: evaluation of recurrence rate and factors related to recurrence. Clin Radiol 2015;70:42–7. [DOI] [PubMed] [Google Scholar]
- [12].Lin Y, Li P, Shi Y-P, et al. Sequential treatment by polidocanol and radiofrequency ablation of large benign partially cystic thyroid nodules with solid components: Efficacy and safety. Diagn Interv Imaging 2019;undefined: undefined. [DOI] [PubMed] [Google Scholar]
- [13].Moon WJ, Baek JH, Jung SL, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol 2011;12:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ren Jie, Baek Jung Hwan, Chung Sae Rom, et al. Degenerating thyroid nodules: ultrasound diagnosis, clinical significance, and management. Korean J Radiol 2019;20:947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nakamura H, Hirokawa M, Ota H, et al. A 2015 is an increase in thyroid nodule volume a risk factor for malignancy? Thyroid 2015;25:804–11. [DOI] [PubMed] [Google Scholar]
- [17].Adler DD, Garson PI, Rubin J, et al. Doppler ultrasound color flow imaging in the study of breast cancer: preliminary findings. Ultrasound Med Biol1 1990;6:553–9. [DOI] [PubMed] [Google Scholar]
- [18].Kizilgul Muhammed, Shrestha Rupendra, Radulescu Angela, et al. Thyroid nodules over 4 cm do not have higher malignancy or benign cytology false-negative rates. Endocrine 2019;66:249–53. [DOI] [PubMed] [Google Scholar]
- [19].Kim M, Jeon MJ, Han M, et al. Tumor growth rate does not predict malignancy in surgically resected thyroid nodules classified as Bethesda category III with architectural atypia. Thyroid 2019;29:216–21. [DOI] [PubMed] [Google Scholar]
- [20].Cohen Oded, Zornitzki Taiba, Yarkoni Tom Raz, et al. Follow-up of large thyroid nodules without surgery: patient selection and long-term outcomes. Head Neck 2019;41:1696–702. [DOI] [PubMed] [Google Scholar]
- [21].Pemayun TGD. Current diagnosis and management of thyroid nodules. Acta medica Indonesiana 2016;48:247–57. [PubMed] [Google Scholar]
- [22].Shi You-Zhen, Jin Yan, Zheng Li, et al. Partially cystic thyroid nodules on ultrasound: the associated factors for malignancy. Clin Hemorheol Microcirc 2019;undefined: undefined. [DOI] [PubMed] [Google Scholar]
- [23].Rosen IB, Provias JP, Walfish PG. Pathologic nature of cystic thyroid nodules selected for surgery by needle aspiration biopsy. Surgery 1986;100:606–13. [PubMed] [Google Scholar]
- [24].Simeone JF, Daniels GH, Mueller PR, et al. 1982 High-resolution real-time sonography of the thyroid. Radiology 1982;145:431–5. [DOI] [PubMed] [Google Scholar]
- [25].Hofmann A, Gessl A, Girschele F, et al. Vascular endothelial growth factor in thyroid cyst fluids. Wiener Klinische Wochenschrift 2007;119:248–53. [DOI] [PubMed] [Google Scholar]
- [26].Reading CC, Charboneau JW, Hay ID, et al. Sonography of thyroid nodules: a “classic pattern” diagnostic approach. Ultrasound Q 2005;21:157–65. [DOI] [PubMed] [Google Scholar]
- [27].Aydoğan Berna İmge, Ceyhan Koray, Şahin Mustafa, et al. Are thyroid nodules with spongiform morphology always benign? Cytopathology 2019;30:46–50. [DOI] [PubMed] [Google Scholar]


