Abstract
PURPOSE:
Novel therapies are needed for the treatment of recurrent cervical cancer. The best chemotherapy regimen to date has a response rate of 48% with an overall survival of 17 months, with limited options for second-line chemotherapy. Immunotherapy can induce a strong immune response in cervical cancer due to retained viral antigens and is reviewed in this article.
RECENT FINDINGS:
Current clinical trials include treatment with Listeria that elicits an immune response against the E7 oncoprotein and active vaccines against the E7 oncoprotein. While the response rates to PD-1 inhibition alone have been modest, the landmark survival reported in these trials suggests the activity of these agents may not be measured by RECIST criteria. The KEYNOTE-158 trial has led to the approval of pembrolizumab in recurrent PD-L1 positive cervical cancer. Combinations of PD-1 and anti-CTLA-4 have shown promising and durable activity. There is active research with new combinations of checkpoint inhibitors, as well as combinations of these drugs with chemotherapy and radiation, and other novel approaches.
SUMMARY:
Immune therapy has broad activity in cervical cancer. Responses to immunotherapy can be dramatic and durable. Continued work to find the optimal combination and setting for immunotherapy is ongoing.
Keywords: Cervical cancer, Immunotherapy, Checkpoint inhibitors
INTRODUCTION
Chemotherapy options for recurrent cervical cancer are limited and are considered non-curative. As evaluated by the Gynecologic Oncology Group (GOG), the best regimen in recurrent or metastatic cervical cancer is the combination of cisplatin, paclitaxel, and bevacizumab with an overall response rate of 48% and a median survival of 17 months[1]. However, there is no consensus on the benefit of second line chemotherapy in recurrent cervical cancer due to the poor prognosis in this group of patients. Immunotherapy represents a new method to treat cancers dependent on the natural ability of the immune system to recognize and kill tumor cells directly. The original observation that a malignant tumor of the neck could respond dramatically after an infection first suggested the power of immunotherapy in the treatment of cancer [2]. Since that time therapies have been developed to increase the immune response to cancers and are now approved for the treatment of many cancers.
Almost all cervical cancer is a result of Human Papilloma Virus (HPV) infection[3]. After epithelial damage, HPV can infect the basal cell layer of keratinocytes [4, 5]. In most individuals this infection is cleared. However, high risk HPV types such as 16 and 18 are more likely to persist and integrate into the host genome such that there is excess expression of the oncoproteins E6 and E7. These oncoproteins interfere with natural immune response by down regulating critical pathways such as interferon production, the STING pathway, and inhibit the expression of HPV antigens by the class I MHC molecules[6]. HPV infection can also impair Langerhans cell function which is critical to the immune response. However, the retained viral antigens in cervical cancer makes immunotherapy an attractive treatment option since the antigens could be recognized as foreign. While checkpoint inhibition with blockade of the of PD-1 receptor has been the dominate class of drugs tested in cervical cancer, several other immunotherapy strategies are currently under development which are reviewed (Table 1).
TABLE 1.
Reported Trials of Immunotherapy in Cervical Cancer (Original table)
| Author/yr | Regimen | |||||||
|---|---|---|---|---|---|---|---|---|
| Prior Systemic Therapy | n | PD-L1 | ORR% (CI) (95%tile CI) | PFS months (95%tile CI) | OS months (95%tile CI) | |||
| ADXS11 | Basu [14] | ADXS11-001 | 0 | 56 | NA | 17.1% (8.1-32.7) | 6.1 (5.9-9.4) | 8.3 (5.8-10.5) |
| ADXS11-001 + cisplatin | 54 | NA | 14.7% (6.4-30.1) | 6.4 (4.2-8.9) | 8.8 (7.4-13.3) | |||
| GOG-0265 | Huh [20] | ADXS11-001 | 1-3 | 50 | NA | 3.7% (0.1-18.3) | 3.1 (2.8-3.7) | 6.2 (4.4-12.3) |
| KEYNOTE-028 | Frenel [22] | Pembrolizumab 10 mg/kg | 1-3 | 24 | + | 17% (5-37) | 2.0 (2.0-3.0) | 11.0 (4-15) |
| KEYNOTE-158 | Chung [23] | Pembrolizumab 200 mg | 0-5 | 98 | +/− | 12.2% (6.5-20.4) | 2.1 (2.0-2.1) | 9.4 (7.7-13.1) |
| 84 | + | 14.6% (7.8-24.2) | 2.1 (2.1-2.3) | 11.0 (9.1-14.1) | ||||
| GY002 | Santin [26] | Nivolumab 3 mg/kg | 1+ | 25 | +/− | 4.0% (0.4-22.9) | 3.5 (1.9-5.1) | 14.5 (8.3-26.8) |
| Ipilimumab | Lheureux [28] | Ipilimumab 10mg/kg | 1-3 | 42 | +/− | 2.7% (0.5-14.9) | 2.5 (2.1-3.2) | 8.5 (3.6-NR) |
| CheckMate 358 | Naumann [27] | Nivo Only | 1-2 | +/− | 26.3% (9.1-51.2) | 5.1mo (1.9-9.1) | 21.9 (15.1-NR) | |
| Naumann [41] | Ipi 1 + Nivo 3 | 0 | 19 | +/− | 31.6% (12.6-56.6) | 13.8 (2.1-NR) | NR (17.4-NR) | |
| 1-2 | 26 | +/− | 23.1% (9.0-43.6) | 3.6 (1.9-5.1) | 10.3 (7.9-15.2) | |||
| Ipi 3 + Nivo 1 | 0 | 24 | +/− | 45.8% (25.6-67.2) | 8.5 (3.7-NR) | NR (13.9-NR) | ||
| 1-2 | 22 | +/− | 36% (17.2-59.3) | 5.8 (3.5-17.2) | 25.4 (17.5-NR) |
NR- Not Reached
Ipi = Ipilimumab
Nivo = Nivolumab
Active Vaccines
Prophylactic HPV vaccines are currently approved and are highly effective in preventing HPV infections, but appear to be ineffective in clearing established HPV infection[7, 8]. Investigations of novel therapeutic vaccines targeting both cervical dysplasia and cervical cancer are ongoing. A preclinical trial utilizing Pseudomonas Exotoxin-HPV16 E70KDEL3 fusion protein or TVGV-1 combined with one of two different adjuvants showed promising results in a mouse model and was associated with longer survival and greater production of HPV16 E7 specific CD8+ T-cells.[9]. Results of the therapeutic vaccine pNGVL4a-CRT/E7(detox) have been reported in 32 women with CIN2/3 secondary to HPV16[10]. This vaccine consists of a DNA plasmid (pNGVL4a-A) that encodes calreticulin linked to a detox form of HPV type 16 E7 antigen. This vaccine was designed to increase cytotoxic T-cells in the hopes of clearing established HPV 16 infections. In this first-in-human clinical trial, 27 women were vaccinated and the resolution rate to CIN 1 or less was 30%. An increase in cytotoxic T-cells compared to matched controls was noted.
There is an interest regarding the role of combining vaccines with other immunotherapy approaches. One such novel strategy is the proposed VolATIL trial (NCT03946358) which combines the UCPVax vaccine with atezolizumab, a PD-L1 inhibitor, and has been developed for a group of 47 planned participants with HPV positive human cancers including cancers of the head and neck, anus and cervix. As designed, patients will receive both atezolizumab as well as the UCPVax for induction[11]. The primary endpoint is the objective response rate at 4 months with secondary endpoints to include OS, PFS, and health related QOL.
Listeria Based Treatments
Listeria monocytogenes is a gram-positive bacterium that stimulates cell mediated immunity[12, 13]. L monocytogenes has unique properties making this organism a reasonable choice for vector development including cytoplasmic replication and the lack of an endotoxin. Axalimogene filolisbac or ADXS11-001 was developed as a therapeutic agent via bioengineering and secretes a fusion protein that includes a truncated fragment of listeriolysin O (tLLO) which is fused to human HPV-16 E7[14]. Following administration, antigen presenting cells take up the agent by phagocytosis and the tLLO-HPV-16 E7 fusion protein activates the MHC class I pathway. In addition, following uptake via phagocytosis, antigens that are secreted by L monocytogenes can also be presented in the MHC class II pathway[15, 16]. ADXS11-001 may also alter the tumor microenvironment to facilitate T-cell infiltration and reduce immune suppression by immune regulatory cells[17, 18].
In a phase I trial, 15 patients with metastatic cervical cancer were treated with ADXS11-001 (Lm-LLO-E7). Treatment consisted of two vaccinations three weeks apart at one of three different dose levels (1 x 109 CFU/ml, 3.3 x 109 CFU/ml or 1 x 1010 CFU/ml) in conjunction with post treatment ampicillin[19]. Objectively, seven of 13 (54%) evaluable patients had stable disease and one patient had an unconfirmed partial response to therapy. A randomized phase 2 trial of ADXS11-001 was conducted in 25 centers within India[14]. This trial compared the dose of 1 x 109 CFU/ml either alone in combination with five weekly doses of cisplatin 40mg/m2 starting on day 29. The primary endpoint of the trial was OS. A total of 110 women were randomized in the study and median OS was similar for both groups of patients with a median OS of 8.3 months (95% CI, 5.9-10.5) for ADXS11-001 alone as compared to 8.8 months (95% CI, 7.4-13.3) for the combination. The landmark 12-month survival for this trial was 35% (38/109). The GOG also conducted (GOG-0265) a two stage, phase 2 trial of single agent ADXS11 in 50 patients[20]. The median OS was 6.2 months (95% CI, 4.4-12.3) and 12-month OS was 38% (range 12.0-40.1), which was found to be a 52% improvement from the expected survival. The treatment was well tolerated with anemia the only grade 3+ TRAE.
Pembrolizumab
Expression of PD-L1 by tumors allows the tumor to escape cell destruction by CD8+ killer T-cells. Significant expression of PD-L1 is more common in squamous cancer than in adenocarcinoma[21]. A meta-analysis has suggested that PD-L1 expression is associated with a worse prognosis independent of other risk factors such as stage, tumor size, depth of invasion, LVSI, or lymph node metastasis.
Pembrolizumab is a humanized antibody to PD-1. When bound to the PD-1 receptor it blocks the interaction of this receptor with the ligand (PD-L1). The KEYNOTE-028 included 24 patients with stage IVB or recurrent cervical cancer and were treated with the PD-1 antibody pembrolizumab at 10 mg/kg every two weeks for 24 months[22]. All patients had received prior systemic chemotherapy with 63% treated with two or more regimens and 42% had previously been treated with bevacizumab. There were 4 responses (4 PR) for a response rate of 16.7%. The PFS was 2 months and the median overall survival was 11 months.
The KEYNOTE-158 trial (NCT02628067) included 98 patients with recurrent or metastatic cancer[23]. The trial utilized 200 mg of IV pembrolizumab every 3 weeks for 24 months. Similar to the Keynote-028 trial, 65% patients had been treated with 2 or more prior lines of systemic chemotherapy. The overall RR was 12% (3 CR, 9 PR). However, 84% of patients were PD-L1 positive and all of the responses were limited to the PD-L1 positive group for a 14.6% RR in this cohort. The median PFS was 2.1 months with an overall survival of 9.4 months, but in the PD-L1 positive group the overall survival was 11 months. The landmark survival in the PD-L1 positive group was 80% at 6 months and 47% at 12 months. As with other trials of immunotherapy, responses tended to be durable. The median duration of response was not reached in the study (95%tile 4.1-18.6+) but 91% of responders were noted to have a duration of response greater than 6 months. Because of this trial and the high unmet need, the FDA granted accelerated approval of pembrolizumab in patients with previously treated recurrent cervical cancer who were PD-L1 positive with the companion diagnostic PD-L1 IHC 22C3 pharmDx assay with a CPS ≥ 1 (Dako North America, Carpinteria, CA)[24].
A phase III trial comparing the PD-1 inhibitor cemiplimab to investigator’s choice of chemotherapy is ongoing[25]. Preliminary results are not yet available and are expected to be mature in June of 2023.
Nivolumab
Similar to pembrolizumab, nivolumab is an antibody to PD-1. Nivolumab in cervical cancer was first evaluated in the phase II study NRG-GY002 (NCT022257528). Patients with metastatic or recurrent cervical cancer with one prior systemic chemotherapy were given nivolumab at 3 mg/kg every 2 weeks.[26]. The primary endpoint was PFS by RECIST 1.1. All patients had received one prior chemotherapy in the recurrent setting and PD-L1 was expressed in 77% of patients. Of the 25 patients evaluable for response, there was only 1 partial response noted for a RR of 4% (95% CI 0.4-22.9). Despite the minimal response rate, the median survival in this group was 14.5 months (95% CI 8.3-26.8). The survival was 78% at the 6 month landmark and 56% at the 12-month landmark. The grade 3+ treatment-related adverse event rate was 24%.
Checkmate 358 included a single agent nivolumab arm with nivolumab administered at 240 mg IV q 2 wk. All patients in this trial had received prior systemic chemotherapy with 58% receiving 2 or more prior therapies and 32% receiving prior bevacizumab. Patients were enrolled without respect to PD-L1 expression. The overall response rate was 26% (95% CI 9.1 – 52.2)[27]. In this trial 38% of patients were PD-L1 negative and 84% had two or more lines of therapy. Median PFS was 5.1 months and OS was 21.9 months. The landmark survival was 78% at 12 months and 50% at 24 months. Responses were seen in both PD-L1 positive and PD-L1 negative tumors suggesting this marker may not be necessary for PD-1 checkpoint inhibition in cervical cancer[27].
It is unclear why there is a discordance between GY002 and the single arm nivolumab arm of Checkmate 358. However, it should be noted that in GY002 there were 4 patients with an unconfirmed response. These patients were taken off study because of new lesions despite regression of target lesions and may have represented a significant response[26]. The median overall survival of 14.3 months for treatment in the recurrent second line metastatic setting in this trial does suggest activity that is not accurately measured by RECIST criteria.
CTLA4 antibodies
Blockade of the CTLA4 antigen increases antigen presentation to the immune system resulting in an expanded killer T-cell response to antigens. Anti-CTLA4 therapy is theoretically attractive in cervical cancer due to down regulation of antigen presentation by the viral oncoproteins[6]. A phase II trial of the anti-CTLA4 antibody ipilimumab 10 mg/kg every 3 weeks x 4 then every 12 weeks in 42 patients with recurrent squamous or adenocarcinoma has been reported[28]. The response rate in this trial was only 3% in the 34 evaluable patients with a PFS of 2.5 months (95% CI, 2.1-3.2) and an OS of 8.5 months (95% CI, 3.6-not reached).
Combination of Checkpoint Inhibitors and Radiation
The combination of immunotherapy and radiation is theoretically attractive as radiation may induce an immune response to the tumor due to tumor death[29]. There are multiple ongoing trials looking at adding checkpoint inhibitors either during radiation or following radiation to improve outcomes[30–40]. These trials are listed in Table 2.
TABLE 2.
Ongoing Trials of Immunotherapy in Cervical Cancer (Original table)
| NTC | Setting | Phase | Agents | n | Estimated Completion Date | |
|---|---|---|---|---|---|---|
| CHECKPOINT INHIBITORS | ||||||
| GOG 3016/ENGOT-cx9 | NTC03257267 | Second-line metastatic | Phase III | Cemiplimab Chemotherapy (Investigator’s choice) |
534 | June 2023 |
| CHECKPOINT INHIBITORS + RADIATION | ||||||
| NiCOL | NCT03298893 | Locally advanced untreated cervical cancer | Phase I | Cisplatin + WPRT + Nivolumab | 21 | May, 2022 |
| UVA-LACC-PD201 | NCT02635360 | Locally advanced untreated cervical cancer | Randomized phase I | Cisplatin + WPRT + Pembrolizumab Cisplatin + WPRT followed by Pembrolizumab |
88 | May, 2021 |
| GOG-9929 | NCT01711515 | Node (+) cervical cancer | Phase I | Cisplatin + WPRT + Ipiilimumab | 34 | June, 2017 |
| NRG GY017/NCI-2018-02791 | NCT03738228 | Locally advanced cervical cancer | Randomized Phase I | Cisplatin + WPRT + atezolizumab Atezolizumab followed by WPRT + cisplatin |
40 | November, 2021 |
| CALLA | NCT03830866 | Locally advanced untreated cervical cancer | Phase III | Cisplatin + WPRT + durvalumab cisplatin + WPRT + placebo |
714 | April, 2024 |
| ATEZOLACC | NCT03612791 | Locally advanced untreated cervical cancer | Randomized Phase II | Cisplatin + WPRT Cisplatin + WPRT + atzezolizumab |
190 | July, 2022 |
| KEYNOTE-826/ MK-3475-826 | NCT03635567 | First-line metastatic | Randomized Phase III | Paclitaxel + platinum ± bevacizumab followed by Pembrolizumab Paclitaxel + platinum ± bevacizumab followed by placebo |
600 | November, 2022 |
| BEATcc/GOG-3030/ENGOT Cx10/JGOG 1084/GEICO 68-C | NCT03556839 | Recurrent/Metastatic | Paclitaxel + cisplatin + bevacizumab followed by atezolizumab Paclitaxel + cisplatin + bevacizumab followed by placebo |
404 | July, 2023 | |
| MCC-19662/ML40521 | NCT03614949 | First-line metastatic | Phase II | Radiation + atezolizumab | 26 | December, 2022 |
| PD-1 and CTLA-4 COMBINATIONS | ||||||
| C-550-01 | NCT03495882 | Second-line metastatic | Phase I/II | AGEN0234 + AGEN1884 | 150 | March 2023 |
| RaPiDS/GOG-3028/C-750-01 | NCT03894215 | Second-line metastatic | Randomized Phase II | AGEN0234 ± AGEN 1884 | 200 | August, 2023 |
WPRT - whole pelvic radiation therapy
PD-1 and Chemotherapy Combinations
Taking a lead from other cancers where the addition of chemotherapy to checkpoint inhibition has improved outcomes, this approach is being evaluated in cervical cancer. Several trials of chemotherapy with PD-1 inhibitors are ongoing and listed in Table 2.
PD-1/ CTLA4 Checkpoint Inhibitor Combinations
The preliminary report on the combination arms of Checkmate 358 that included both nivolumab and ipilimumab at two different dosing schedules has been presented[41]. This combination of an anti-PD-1 and an anti-CTLA-4 agent demonstrated significant activity in treatment of metastatic cervical cancer for both first-line and second-line therapy in the metastatic setting. The first combination arm (Combo A, n=45) included nivolumab at 3 mg/kg every 2 weeks and ipilimumab at 1 mg/kg every 6 weeks for up to 2 years. The second combination (Combo B, n = 46) was nivolumab 1 mg/kg and ipilimumab 3 mg/kg every 3 weeks for 4 doses followed by nivolumab 240 mg every 2 weeks for up to 2 years. The study included patients with squamous cell cervical cancers regardless of PD-L1 expression. The overall response rate was 26.7% for Comb A and 41.3% for Combo B. When broken down by prior therapy, the Combo B arm demonstrated a 45.8% (95%CI: 25.6-67.2) response rate in patients without prior systemic therapy and a 36.4% (17.2-59.3) response rate in those previously treated with one or two prior lines of systemic chemotherapy. In patients on Combo B without prior systemic therapy, the PFS was 8.5 months with an OS that was not reached (95% CI: 13.9 - NR). In patients with prior therapy the PFS was 5.8 months with an OS of 25.4 months (95% CI: 17.5-NR). The 12 month OS in combo B was 78% without prior therapy and 85% with prior systemic chemotherapy. No new safety signals were noted in this trial. The treatment related grade 3/4 events were 29% in the Combo A and 37% in the Combo B arm. The discontinuation rate due to treatment related adverse events was 13% and 20% respectively.
Another combination of the PD-1 inhibitor AGEN2034 and the anti-CTLA 4 antibody AGEN1884 has been tested in combination in a phase I trial [42]. Two large phase II trials with this combination are ongoing and listed in Table 2[39, 40].
Other Checkpoint Inhibitors
Several other checkpoint inhibitors are currently under development. Targets of these antibodies include TIM-3, LAG-3, VISTA, and TIGIT[43]. Currently these agents are being tested as single agents or in combination with other checkpoint inhibitors. It is to be determined if they are more active than the currently available agents in cervical cancer.
Tumor Infiltrating Lymphocytes
It has been demonstrated that tumor infiltrating lymphocytes (TILs) are associated with a better clinical outcome in cervical cancer[44]. LN-145 is an ongoing clinical trial (NCT03108495) of a product derived from tumor infiltrating lymphocytes that are extracted and expanded in the laboratory. These TILs are then re-infused after marrow ablative chemotherapy. Complete and durable responses have been observed with this type of therapy[45]. In 18 evaluable patients with cervical cancer, responses were in 5 patients, including two with a complete response[46].
T-cells with genetically engineered T-cell receptors (TCR) that recognize HLA-A*02:01-restricted, HPV-16-oncoprotein have been developed. These T-cells have the ability to kill cells in vitro[47]. A phase I clinical trial (NCT02280811) using this therapy has shown a response in HPV16 positive anal cancer, demonstrating proof of principle[48].
CONCLUSIONS
There is tremendous interest in immunotherapy for cervical cancer with multiple ongoing trials. Although many of these approaches have demonstrated a low overall response rate, the prolonged response for some patients appears to be durable. Dramatic responses can seen with checkpoint combination checkpoint therapy as note in Figure 1. Given the limited options for patients with recurrent cervical cancer, it is likely that these agents will be the treatment of choice for first line metastatic disease or even as sensitizing agents with primary therapy to prevent recurrence. The optimal combination and regimen will need to be refined and balanced against the potential toxicity of these agents.
Figure 1.
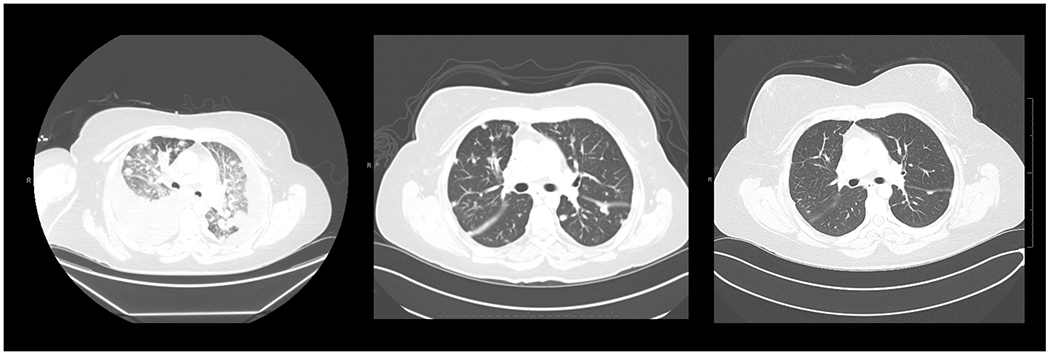
Dramatic response to therapy with the combination of ipilimumab and nivolumab on CHECKMATE 358. CT scans at baseline, 2 months, and 1 year of therapy. The patient remains without disease >12 months after completing two years of immunotherapy. Images © per the authors.
KEYPOINTS.
Immunotherapy is a promising treatment for cervical cancer
Active vaccination may also induce immune response and is being investigated
Checkpoint inhibitors are now approved in the treatment of recurrent cervical cancer and combination checkpoint inhibition have demonstrated responses that are better than current chemotherapy options
ACKNOWLEDGEMENTS
Assistance with this article: None
Financial Support: Dr. Leath was supported in part by the UG1 CA23330 and P50 CA098252
Relevant Conflict of Interest: Dr. Naumann reports institutional support of clinical research by Merck and Bristol-Myers-Squib. Consulting fees from Merck, Tesaro-GSK and is on the steering committee for GOG3028 (Sponsored by Agenus) without compensation.
Footnotes
Dr. Leath has no relevant conflicts of interest.
REFERRENCES
- [1].Tewari KS, Java JJ, Gatcliffe TA, et al. Chemotherapy-induced neutropenia as a biomarker of survival in advanced ovarian carcinoma: an exploratory study of the gynecologic oncology group. Gynecol Oncol 2014; 133: 439–45. [DOI] [PubMed] [Google Scholar]
- [2].Coley WB. The Treatment of Malignant Tumors by Repeated Innoculations of Erysipelas: with report of Ten Original Cases. Am J Med Sci 1893; 10: 487. [PubMed] [Google Scholar]
- [3].Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189: 12–9. [DOI] [PubMed] [Google Scholar]
- [4].Doorbar J, Egawa N, Griffin H, et al. Human papillomavirus molecular biology and disease association. Rev Med Virol 2015; 25 Suppl 1: 2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol 2010; 118: S12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].*Zhou C, Tuong ZK, Frazer IH. Papillomavirus Immune Evasion Strategies Target the Infected Cell and the Local Immune System. Front Oncol 2019; 9: 682. [DOI] [PMC free article] [PubMed] [Google Scholar]; A nice review of how HPV evades the immune system.
- [7].Hildesheim A, Herrero R, Wacholder S, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA 2007; 298: 743–53. [DOI] [PubMed] [Google Scholar]
- [8].Huh WK, Joura EA, Giuliano AR, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet 2017; 390: 2143–2159. [DOI] [PubMed] [Google Scholar]
- [9].*Da Silva DM, Skeate JG, Chavez-Juan E, et al. Therapeutic efficacy of a human papillomavirus type 16 E7 bacterial exotoxin fusion protein adjuvanted with CpG or GPI-0100 in a preclinical mouse model for HPV-associated disease. Vaccine 2019; 37: 2915–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]; A pre-clinical model for an active vaccine against the E7 HPV oncoprotien.
- [10].Alvarez RD, Huh WK, Bae S, et al. A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3). Gynecol Oncol 2016; 140: 245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].*NIH. Combination of UCPVax Vaccine and Atezolizumab for the Treatment of Human Papillomavirus Positive Cancers (VolATIL) (VolATIL).https://clinicaltrials.gov/ct2/show/NCT03946358. Accessed: 2/22/20; This is an ongoing trial of a novel combination of a vacine and a checkpoint inhibitor to boost immune response of the vacine.
- [12].Pan ZK, Ikonomidis G, Pardoll D, et al. Regression of established tumors in mice mediated by the oral administration of a recombinant Listeria monocytogenes vaccine. Cancer Res 1995; 55: 4776–9. [PubMed] [Google Scholar]
- [13].Wallecha A, Carroll KD, Maciag PC, et al. Multiple effector mechanisms induced by recombinant Listeria monocytogenes anticancer immunotherapeutics. Adv Appl Microbiol 2009; 66: 1–27. [DOI] [PubMed] [Google Scholar]
- [14].*Basu P, Mehta A, Jain M, et al. A Randomized Phase 2 Study of ADXS11–001 Listeria monocytogenes-Listeriolysin O Immunotherapy With or Without Cisplatin in Treatment of Advanced Cervical Cancer. Int J Gynecol Cancer 2018; 28: 764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]; A randomized trial of immune therapy compared to chemotherapy and immuntherapy showing that chemotherapy did not add to the immune response.
- [15].Ikonomidis G, Paterson Y, Kos FJ, et al. Delivery of a viral antigen to the class I processing and presentation pathway by Listeria monocytogenes. J Exp Med 1994; 180: 2209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol 2004; 4: 812–23. [DOI] [PubMed] [Google Scholar]
- [17].Campisi L, Soudja SM, Cazareth J, et al. Splenic CD8alpha(+) dendritic cells undergo rapid programming by cytosolic bacteria and inflammation to induce protective CD8(+) T-cell memory. Eur J Immunol 2011; 41: 1594–605. [DOI] [PubMed] [Google Scholar]
- [18].Wallecha A, French C, Petit R, et al. Lm-LLO-Based Immunotherapies and HPV-Associated Disease. J Oncol 2012; 2012: 542851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine 2009; 27: 3975–83. [DOI] [PubMed] [Google Scholar]
- [20].Huh W, Brady WE, Dizon DS, et al. A prospective phase II trial of the listeria-based human papillomavirus immunotherpay axalimogene filolisbac in second- and third-line metastatic cervical cancer: A NRG oncology group trial. Abstracts Presented for the 2017 Society of Gynecologic Oncology 48th Annual Meeting on Women’s Cancer 2017; 145: 220. [Google Scholar]
- [21].Heeren AM, Punt S, Bleeker MC, et al. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod Pathol 2016; 29: 753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].*Frenel JS, Le Tourneau C, O’Neil B, et al. Safety and Efficacy of Pembrolizumab in Advanced, Programmed Death Ligand 1-Positive Cervical Cancer: Results From the Phase Ib KEYNOTE-028 Trial. J Clin Oncol 2017; 35: 4035–4041. [DOI] [PubMed] [Google Scholar]; The first report of the activity of pembrolizumab in cervical cancer.
- [23].**Chung HC, Ros W, Delord JP, et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 2019; 37: 1470–1478. [DOI] [PubMed] [Google Scholar]; This study led to the approval of pembrolizumab in cervical cancer. The overall response rate was14.6% in women with PD-L1 postiive tumors with a median OS of 11 months as salvage therapy after failing systemic chemotherapy.
- [24].U.S. Food and Durg Administration. FDA approves pembrolizumab for advanced cervical cancer with disease progression during or after chemotherapy.https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-advanced-cervical-cancer-disease-progression-during-or-after-chemotherapy. Accessed: 2/15/20
- [25].Tewari KS, Vergote I, Oaknin A, et al. GOG 3016/ENGOT-cx9: An open-label, multi-national, randomized, phase 3 trial of cemiplimab, an anti-PD-1, versus investigator’s choice (IC) chemotherapy in ≥2 line recurrent or metastatic cervical cancer. Journal of Clinical Oncology 2018; 36: TPS5600–TPS5600. [Google Scholar]
- [26].**Santin AD, Deng W, Frumovitz M, et al. Phase II evaluation of nivolumab in the treatment of persistent or recurrent cervical cancer (NCT02257528/NRG-GY002). Gynecol Oncol 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]; This was the first trial of nivolumab in cervical cancer as GY-002. The response rate was low, but the OS was significant suggesting activity that was not measured by RECIST.
- [27].**Naumann RW, Hollebecque A, Meyer T, et al. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results From the Phase I/II CheckMate 358 Trial. J Clin Oncol 2019; 37: 2825–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report showed that nivolumab has a 26% response rate in patients with cervical cancer without regard to PD-L1 status. This was also the first report of checkpoint inhibitors in vulvar cancer.
- [28].Lheureux S, Butler MO, Clarke B, et al. Association of Ipilimumab With Safety and Antitumor Activity in Women With Metastatic or Recurrent Human Papillomavirus-Related Cervical Carcinoma. JAMA Oncol 2018; 4: e173776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].*Lee L, Matulonis U. Immunotherapy and radiation combinatorial trials in gynecologic cancer: A potential synergy? Gynecol Oncol 2019; 154: 236–245. [DOI] [PubMed] [Google Scholar]; This review highlights ongoing trials of checkpoint inhibitors and radiation in patients with cervical cancer.
- [30].*Shapira-Frommer R, Alexandre J, Monk B, et al. KEYNOTE-826: A phase 3, randomized, double-blind, placebo-controlled study of pembrolizumab plus chemotherapy for first-line treatment of persistent, recurrent, or metastatic cervical cancer. Journal of Clinical Oncology 2019; 37: TPS5595–TPS5595. [Google Scholar]; This is the confirmatory trial after accelerated approval of pembrolizumab.
- [31].NIH. Trial Assessing the Inhibitor of Programmed Cell Death Ligand 1 (PD-L1) Immune Checkpoint Atezolizumab (ATEZOLACC).https://clinicaltrials.gov/ct2/show/NCT03612791. Accessed: 2/22/20
- [32].*Monk BJ, Mayadev J, Nunes AT, et al. CALLA: Efficacy and safety of durvalumab with and following concurrent chemoradiotherapy (CCRT) versus CCRT alone in women with locally advanced cervical cancer: A phase III, randomized, double-blind, multicenter study. Journal of Clinical Oncology 2019; 37: TPS5597–TPS5597. [Google Scholar]; An ongoing phase III trial adding a checkpoint inhibitor to radiation and chemotherapy in the initial treatment of cervical cancer has the potential to improve response to initial therapy.
- [33].*Mayadev J, Zamarin D, Deng W, et al. Anti-PD-L1 (atezolizumab) as an immune primer and concurrently with extended-field chemoradiotherapy for node-positive locally advanced cervical cancer. Int J Gynecol Cancer 2019; 10.1136/ijgc-2019-001012. [DOI] [PMC free article] [PubMed] [Google Scholar]; This ongoing trial adds a checkpoint inhibitor to chemotherapy and radiation in the initial treatment of a high risk population.
- [34].Mayadev J, Brady WE, Lin YG, et al. A phase I study of sequential ipilimumab in the definitive treatment of node positive cervical cancer: GOG 9929. Journal of Clinical Oncology 2017; 35: 5526–5526. [Google Scholar]
- [35].*Grau JF, Farinas-Madrid L, Oaknin A. A randomized phase III trial of platinum chemotherapy plus paclitaxel with bevacizumab and atezolizumab versus platinum chemotherapy plus paclitaxel and bevacizumab in metastatic (stage IVB), persistent, or recurrent carcinoma of the cervix: the BEATcc study (ENGOT-Cx10/GEICO 68-C/JGOG1084/GOG-3030). Int J Gynecol Cancer 2020; 30: 139–143. [DOI] [PubMed] [Google Scholar]; This combination of a checkpoint inhibitor to chemotherapy in recurrent or metastatic cervical cancer.
- [36].Duska LR, Showalter TN, Petroni GR, et al. A randomized phase II study of chemoradiation and pembrolizumab for locally advanced cervical cancer. Journal of Clinical Oncology 2017; 35: TPS5601–TPS5601. [Google Scholar]; This ongoing randomized phase II study will look at concurrent versus sequential use of checkpoint inhibitors with chemtherapy and radiaiton in patients with locally advanced cervical cancer.
- [37].Ahmed KA, Kim Y, Apte SM, et al. Trial in progress: Phase II study of stereotactic body radiation therapy and atezolizumab in the management of recurrent, persistent, or metastatic cervical cancer. Journal of Clinical Oncology 2019; 37: TPS5596–TPS5596. [Google Scholar]; This ongoing trial will look at the abscobal effect of combining radiation with checkpoint inhibition due to the potential for radiation to produce an immune response to the cancer.
- [38].NIH. Nivolumab in Association With Radiotherapy and Cisplatin in Locally Advanced Cervical Cancers Followed by Adjuvant Nivolumab for up to 6 Months (NiCOL).https://clinicaltrials.gov/ct2/show/NCT03298893. Accessed: [Google Scholar]
- [39].NIH. Phase 2 Study of Anti-PD-1 Independently or in Combination With Anti-CTLA-4 in Second-Line Cervical Cancer.https://clinicaltrials.gov/ct2/show/NCT03894215. Accessed: 2/16/20
- [40].NIH. Subjects With Metastatic or Locally Advanced Solid Tumors, and Expansion Into Select Solid Tumors (Cervical).https://clinicaltrials.gov/ct2/show/NCT03495882?term=03495882&draw=2&rank=1. Accessed: 2/16/20 [Google Scholar]
- [41].**Naumann RW, Oaknin A, Meyer T, et al. LBA62 - Efficacy and safety of nivolumab (Nivo) + ipilimumab (Ipi) in patients (pts) with recurrent/metastatic (R/M) cervical cancer: Results from CheckMate 358. Abstract Book of the 44th ESMO Congress (ESMO 2019) 27 September – 1 October 2019, Barcelona, Spain 2019; 30: v898–v899. [Google Scholar]; This presentation at ESMO showed the dramatic response to the combination of ipilimumab and nivolumab in women with squamous cell cancer of the cervix that was better than existing chemotherapy regimens. The repsonse rate in second-line metastatic disease was 36% with an OS that was not reached (> 13.9 months).
- [42].Coward J, Lemech C, Meniawy T, et al. 1168PPhase I/II study of CTLA-4 inhibitor AGEN1884 + PD-1 Inhibitor AGEN2034 in patients with advanced/refractory solid tumors, with expansion into 2L cervical cancer and solid tumors. Annals of Oncology 2018; 29: [Google Scholar]
- [43].*Qin S, Xu L, Yi M, et al. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer 2019; 18: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nice review of novel checkpoint inhibitors currently under development.
- [44].Shah W, Yan X, Jing L, et al. A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4(+)FOXP3(+) regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell Mol Immunol 2011; 8: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stevanović S, Draper LM, Langhan MM, et al. Complete Regression of Metastatic Cervical Cancer After Treatment With Human Papillomavirus–Targeted Tumor-Infiltrating T Cells. Journal of Clinical Oncology 2015; 33: 1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].*Stevanovic S, Helman SR, Wunderlich JR, et al. A Phase II Study of Tumor-infiltrating Lymphocyte Therapy for Human Papillomavirus-associated Epithelial Cancers. Clin Cancer Res 2019; 25: 1486–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first report of treatment with TIL (Tumor Infiltrating Lymphocytes) for treatment of women with recurrent cervical cancer.
- [47].Draper LM, Kwong ML, Gros A, et al. Targeting of HPV-16+ Epithelial Cancer Cells by TCR Gene Engineered T Cells Directed against E6. Clin Cancer Res 2015; 21: 4431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hinrichs CS, Doran SL, Stevanovic S, et al. A phase I/II clinical trial of E6 T-cell receptor gene therapy for human papillomavirus (HPV)-associated epithelial cancers. Journal of Clinical Oncology 2017; 35: 3009–3009. [Google Scholar]


