Abstract
Chagas disease affects approximately 7 million people, causing disability and mortality in the most productive life stages of infected individuals. Considering the lifestyle of the world population, metabolic syndrome is a synergistic factor for an increased cardiovascular risk of patients with Chagas disease.
This study transversally evaluated the metabolic and immunological profiles of patients with indeterminate (IF) and cardiac (CF) forms of Chagas disease and their correlations with left ventricular dysfunction (LVD).
Clinical and electrical bioimpedance analysis, levels of cytokines (interferon [IFN]-γ, tumor necrosis factor [TNF]-α, interleukin [IL]-17, IL-10, and IL-33) and adipocytokines (adiponectin, leptin, and resistin), metabolic syndrome components, and brain natriuretic peptide (BNP) levels were assessed in 57 patients (13 IF and 44 CF) with a mean age of 61.63 ± 12.1 years. Chest x-ray, electrocardiogram, and echocardiogram were performed to classify the clinical forms.
The CF group had a higher number of individuals with metabolic syndrome components blood pressure altered, while more participants in the CF group with LVD had low high-density lipoprotein (HDL) levels. The IF group had more participants with a higher waist-to-hip ratio (WHR). No significant difference was observed between metabolic syndrome, cytokine and adipocytokine level, and clinical forms of the disease or in relation to LVD.
Individuals with the IF showed metabolic and immunological profiles compatible with increased disease control, whereas those with CF showed marked inflammatory immune response.
Keywords: adipokines, Chagas disease, cytokines, metabolic syndrome
1. Introduction
Chagas disease, endemic in 21 countries of Latin America, is responsible for approximately 10,000 deaths annually and disability and mortality in the most productive stages of life of affected individuals.[1,2]
The chronic phase of the disease includes an indeterminate form (IF) that is generally asymptomatic, in addition to forms involving other organs that are associated with specific manifestations; among these, the cardiac form (CF) is the most common, occurring in 20% to 40% of cases.[1,3] Chronic Chagas cardiomyopathy (CCC) is characterized by fibrosing myocarditis of variable and permanent intensity that causes progressive myocardial damage ranging from asymptomatic conditions to severe cases, with heart failure, arrhythmias, thromboembolism, and anginal manifestations. Immune response is crucial for disease control and is characterized by a balance between proinflammatory and antiinflammatory responses. Interferon (IFN)-γ and tumor necrosis factor (TNF)-α play roles directly related to the myocardial inflammatory process and more severe chronic heart damage, whereas interleukin (IL)-10 and other Th2 cytokines regulate Th1 response.[1,3,4,5–12]
Just as CCC significantly affects population survival, other comorbidities may have synergistic effects with this impairment. Given global lifestyles and the endocrine function of adipose tissue to produce adipocytokines that balance inflammatory responses, metabolic syndrome may be an aggravating factor for the increased cardiovascular risk of individuals with Chagas disease.[13–17]
This study quantified metabolic syndrome components and pro- (IFN-γ, TNF-α, IL-17, leptin, adiponectin, and resistin), and anti- (IL-10 and IL-33) inflammatory cytokines and assessed their correlations with left ventricular dysfunction (LVD) in patients with IF and CF of Chagas disease.
2. Materials and methods
2.1. Study cases
A total of 57 patients with Chagas disease who were regularly monitored at the Chagas Disease and Cardiology Outpatient Clinic of the University Hospital of the Federal University of Triangulo Mineiro (FUTM) participated in this study (spontaneous demand). The exclusion criteria were age <18 years; refusal to sign the informed consent form; advanced CF (American Heart Association functional classes III and IV) or the digestive form and other cardiomyopathies, possibly with LVD, including hypertensive, ischemic, diabetic, and restrictive etiologies; pregnant women; patients with liver, thyroid, autoimmune, or chronic obstructive pulmonary diseases, neoplasms; or chronic alcoholics. The study was approved by the FUTM Research Ethics Committee (number 2.780.184), and all individuals were informed about the study and signed the informed consent form.
2.2. Diagnostic criteria
The participants underwent anamnesis, physical examinations, and metabolic evaluation through anthropometric measurements (weight, height, waist circumference, and waist and hip), body mass index (BMI) calculations, and electrical bioimpedance analysis in those without metallic prostheses. Group categorization was performed based on the results of the following tests: electrocardiogram (ECG), echocardiogram, chest x-ray imaging, and serological testing for Trypanosoma cruzi (indirect immunofluorescence, indirect hemagglutination, enzyme-linked immunosorbent assay [ELISA]). T cruzi infection was confirmed by seropositivity in at least 2 of the 3 techniques used. Biochemical tests measuring blood glucose, total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, urea, creatinine, alanine aminotransferase, aspartate aminotransferase, total and indirect bilirubin, brain natriuretic peptide (BNP), thyroid-stimulating hormone (TSH), and free T4, ferritin, C-reactive protein, and hemogram levels were also conducted. Based on the biochemical results, the patients were tested for other diseases including diabetes, thyroid disorders, chronic alcoholism, autoimmune diseases, neoplasms, chronic obstructive pulmonary disease, and kidney and liver function aberrations. Chagas disease was classified according to 2nd Brazilian Consensus on Chagas Disease criteria. The participants were grouped into IF, CF, CF without LVD, and CF with LVD groups. LVD was defined as an ejection fraction <45% in transthoracic echocardiograms, according to the American Heart Association guidelines, or altered ventricular function even with an ejection fraction >45%.
2.3. Cytokine and adipocytokine levels
Cytokines (IFN-γ, TNF-α, IL-17, IL-10, and IL-33) and adipocytokines (adiponectin, leptin, resistin) present in serum samples from patients with the chronic form of Chagas disease were quantified by ELISAs using pairs of monoclonal antibodies according to the manufacturer's specifications. Briefly, the wells of high-affinity 96-well plates (Greiner Bio-One) were coated with cytokine-specific monoclonal antibodies overnight at 4 °C, followed by blocking with phosphate-buffered saline PBS containing 10% fetal calf serum (Sigma–Aldrich) for 4 hours at room temperature. Diluted serum from patients with Chagas disease and recombinant cytokines were added and the plates were incubated overnight at 4 °C, washed, and incubated with a biotinylated anti-cytokine monoclonal antibody at room temperature for 4 hours, followed by washing and incubation with horseradish peroxidase-conjugated streptavidin at room temperature for 4 hours. The reaction was developed with tetramethylbenzidine (Sigma-EUA) according to the manufacturer's specifications. Absorbance was determined by subtracting the absorbance at 570 nm from that at 450 nm using an Inspire microplate reader (Perkin-Elmer, Waltham, MA). The cytokine concentrations were measured by 5-parameter logistic (5PL) regression analysis of the absorbance values obtained for the recombinant cytokines, with the results expressed in picograms per milliliter.[18]
2.4. Statistical analysis
The results were analyzed in Graph Pad Prism version 5 (GraphPad Software, Inc.; GraphPad Software, San Diego, CA), IBM SPSS Statistics for Windows, version 20.0, and Statview 4.57 (SAS Institute, Inc., São Paulo, Brazil). The data were assessed for normality and homogeneity in variance by Shapiro-Wilks and Levene tests, respectively. Gaussian and homogenous distributions were analyzed by analysis of variance (ANOVA), whereas data with non-Gaussian distributions were analyzed using Kruskal–Wallis or Mann–Whitney U tests. Pearson chi-squared or Fisher exact tests were used to assess categorical variables. The correlations between the study variables were analyzed by Spearman rank correlation tests. P-values <.05 were considered significant when.
3. Results
3.1. General characteristics of the study population
The CF group had a significantly higher number of men (30:14, P = .0087) and mean age (63.7 ± 11.68 years; P = .0162). Among those without LVD, a proportionally higher number of individuals were physically inactive and more often ate fruits, vegetables, meat, carbohydrates, and dairy products.
3.2. Metabolic and immunological profiles by clinical form of Chagas disease
Overweight was the predominant profile in the IF group (6 participants, 46.1%), compared with 16 (36.3%) eutrophic individuals in the CF group. No significant differences were observed between the forms of Chagas disease and the median BMI values.
Significant differences were observed in waist-to-hip ratio (WHR) and the clinical form of the disease, and the main changes were observed in the CF group (Table 1).
Table 1.
Medians (minimum and maximum) of metabolic and immunological variables according to clinical form of Chagas disease.
| Variable | IF | CF without LVD | CF with LVD |
| Weight, kg | 67.0 (44.0–91.0) | 73.25 (42.2–90.4) | 64.2 (42–121) |
| BMI, kg/m2 | 25.7 (18.8–34.7) | 25.6 (16.7–40.7) | 24.7 (16–38.2) |
| WHR∗ | 0.9 (0.7–1.0) | 0.9 (0.8–1.9) | 0.9 (0.8–1.5) |
| Lean mass (%) | 27.2 (17.3–38.2) | 27.2 (11.1–42.5) | 29.2 (20.7–55.9) |
| Body fat (%) | 23.6 (16.9–40.9) | 30.6 (7.8–50.2) | 30.1 (3.0–36.3) |
| Adiponectin, ng/mL | 1599 (500.7–2750) | 1412 (297.9–3410) | 1584 (706.6–3039) |
| Leptin, ng/mL | 2.1 (0.0–22.65) | 4.8 (0.8–40.3) | 7.1 (0.15–51.2) |
| Rel A/L | 0.5 (0.02–22.2) | 0.25 (0.03–2.5) | 0.2 (0.04–16.6) |
| Resistin, ng/mL | 12.3 (1.93–42.9) | 8.9 (1.05–42.6) | 14.4 (2.4–49.1) |
| IFN-γ, pg/mL | 0.0 (0.0–1697) | 0.0 (0.0–121) | 0.0 (0.0–1013) |
| TNF-α, pg/mL | 0.0 (0.0–1191) | 0.0 (0.0–2278) | 0.0 (0.0–3122) |
| IL-10, pg/mL | 0.0 (0.0–1099) | 0.0 (0.0–641) | 0.0 (0.0–697) |
| IL-33, pg/mL | 8.0 (0–6832) | 1.5 (0.0–2196) | 1.0 (0.0–10969) |
| BNP, pg/mL | 55.5 (12–224) | 222.5 (43–2640) | 895 (17–10001) |
| LVEF (%) | 70 (56–84) | 64.5 (56–73) | 41.5 (14–70) |
| HDL, mg/dL∗ | 51 (33–77) | 52 (35–69) | 42 (25–74) |
| MBP | 86.6 (70–96.6) | 88.3 (70–100) | 84.9 (66.6–110) |
| Glycemia, mg/dL | 91.7 (70.7–109.7) | 91.4 (72.7–126.6) | 87.5 (66.9–226.3) |
| Triglyceride, mg/dL | 122 (70–294) | 120 (70–247) | 114.5 (45–491) |
Analysis of the presence of metabolic syndrome by the clinical form of the disease showed statistically similar distribution patterns, although with a proportionally higher number of patients without metabolic syndrome (Table 1). Regarding metabolic syndrome components, only altered blood pressure and antihypertensive medication use differed significantly, with higher percentages of individuals in the CF group (Table 1).
Serum levels of adipocytokines and proinflammatory and anti-inflammatory cytokines did not differ significantly according to the clinical forms of Chagas disease (Table 1). Only one patient in the IF group had detectable IL-17 levels.
3.3. Metabolic and immunological profiles according to LVD status
The serum HDL levels were lower in the CF group without LVD and no significant differences were observed for the other variables.
Among the CF patients with LVD, most individuals with indication for medication use according to American Heart Association guidelines showed optimal medication use.
3.4. Correlations between the study variables
The variables of metabolic and immunological profiles and complementary examinations were correlated according to the clinical form of the disease. The IF group showed positive correlations between HDL and IL-10 (P = .015; r = 0.651), between waist circumference and IFN-γ (P = .025; r = 0.604), and between mean blood pressure (MBP) and IFN-γ (P = .025; r = 0.607) and negative correlation between IL-10 and triglyceride level (P = .033; r = –0.593) (Fig. 1). The CF group without LVD showed positive correlations between glycemia and adiponectin level (P = .046; r = 0.508), between MBP and leptin level (P = .046; r = 0.508), and between BNP and leptin levels (P = .002; r = 0.716) and negative correlations between HDL and resistin level (P = .026; r = –0.598) and between triglyceride and IL-10 levels (P = .044; r = –0.513) (Fig. 2). The group of patients with CF and LVD showed positive correlations between glycemia and IL-33 level (P = .011; r = 0.471) and between LV ejection fraction and IFN-γ level (P = .0006; r = 0.625). Furthermore presented negative correlations between BNP level and BMI and between IFN-γ level land ejection fraction (P = .023; r = –0.436; P = .002; r = –0.561; P < .001; r = –0.726 respectively) (Fig. 3).
Figure 1.
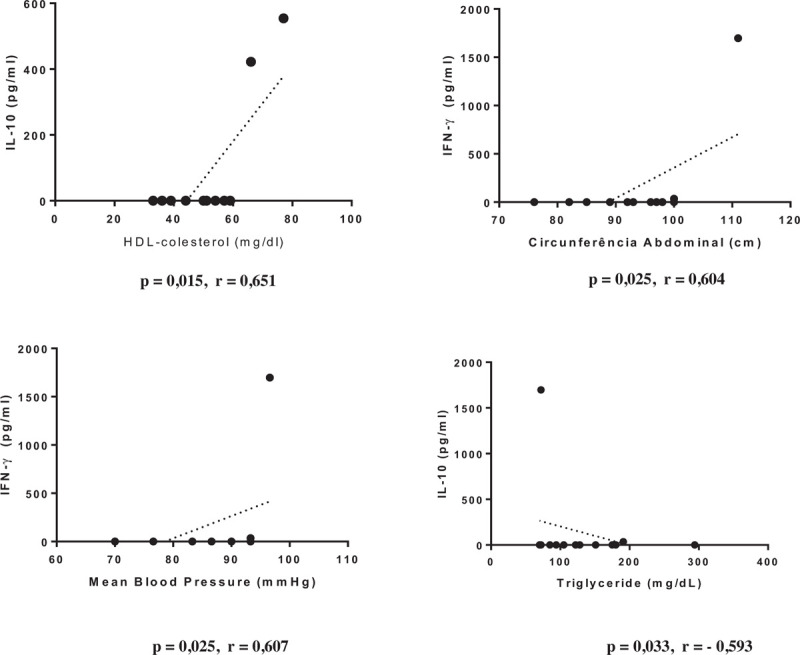
Graphical representation of Spearman correlations in the group of indeterminate form.
Figure 2.
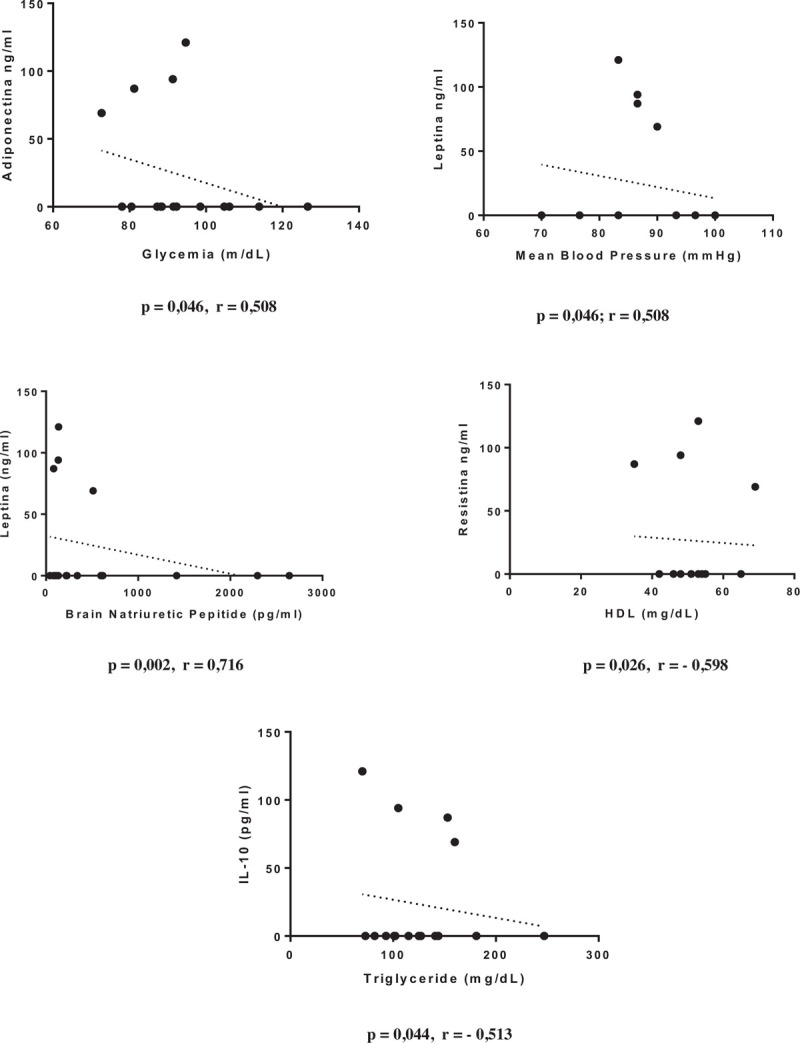
Graphical representation of Spearman correlations in the group of cardiac form without left ventricular dysfunction.
Figure 3.
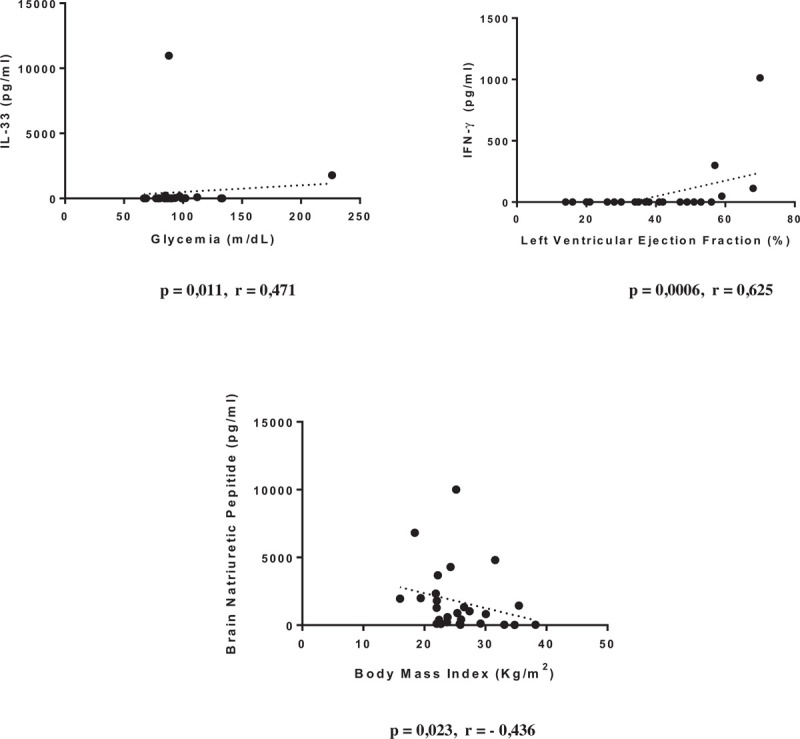
Graphical representation of Spearman correlations in the group of cardiac form with left ventricular dysfunction.
4. Discussion
The predominance of older men with CF is concordant with other reports in the literature.[1,19] While the results of this study do not support the hypothesis that patients with CF have higher levels of proinflammatory cytokines (TH1 pattern) than those in patients with IF, they corroborate the findings reported by Sousa.[19] Dutra et al[10] and Ferreira et al,[20] showed that proinflammatory cytokines prevail over IL-10 levels in patients with CF compared with individuals with IF. Navarro et al[21] concluded that, in addition to the presence of dyslipidemia, factors such as optimal medication use, changes in eating habits and physical activity, and regular outpatient follow-up also affect the natural disease progression and must be analyzed in patients with indeterminate Chagas disease.
While cytokine levels were not significantly associated with the presence of LVD, the directly proportional correlation between ejection fraction percentage and serum IFN-γ levels in patients with CF and LVD suggests the role of other factors in the control of the cardiac inflammatory process and the reduced impairment of cardiac function in these patients. Accordingly, considering BNP level an important marker of congestive heart failure, including in patients with Chagas disease,[22–26] the negative correlations between BNP levels and ejection fraction percentage, between BNP and IFN-γ, and between BNP and BMI in CF patients with LVD also support the role of other factors in disease progression.
Regarding metabolic syndrome, although the prevalence of overweight individuals in the IF group was consistent with that reported by Navarro et al,[21] we observed no association between body mass index and metabolic syndrome concerning the clinical progression of Chagas disease. A higher percentage of patients with CF had altered blood pressure components or antihypertensive use, most likely due to the pathophysiology of microcirculatory changes characteristic of the disease[3,5,8] and higher waist-to-hip ratios from lower physical activity levels, although they reported more balanced food intake.
No significant differences were observed for the presence of LVD, metabolic syndrome components, glycemia, triglyceride level, waist circumference, altered blood pressure, or antihypertensive use. These results differed from those reported by Santos et al,[27] who suggested an association between the degree of parasympathetic denervation, severity of heart disease, and exhaustion of glycemic homeostasis maintenance mechanisms. However, our findings regarding blood pressure were comparable to those by Gurgel et al,[28] who reported similar variations in blood pressure levels in patients with and without Chagas disease. The LVD group had a higher percentage of individuals with altered HDL levels, although they reported more frequent physical activity compared with that in the other groups. This observation is similar to those of Navarro et al,[21] who showed increased cardiovascular risk among patients with IF due to increased changes in HDL-cholesterol and triglyceride levels and waist circumference.
The levels of adipocytokines, which link obesity, peripheral insulin resistance, and inflammatory changes,[13–16] did not differ significantly in the clinical forms of Chagas disease and in the presence of LVD. The plasma levels were high, especially for adiponectin, in contrast to those reported in murine Chagas disease.[14] The higher median leptin levels observed in patients with LVD were likely due to the relationship between leptin and inflammatory process stimulation.[13,16,29] The positive correlations between BNP and leptin and between MBP and leptin in patients with CF but without LVD are consistent with those reported by Tilg and Moschen,[13] Francisco et al,[16] and Frühbeck et al,[29,30] who reported the relationship between inflammatory processes to and levels and, therefore, to cardiovascular risk.
This study had limitations. The sample comprised patients who were regularly monitored at the outpatient clinic and who are frequently advised about the importance of physical activity and adequate eating habits. A high percentage of individuals showed optimal use of the recommended medications for each clinical stage of the disease. Thus, these assessments must be performed in a larger number of individuals and should also assess markers of cardiac function to refine the findings and broaden the conclusions of the present study.
5. Conclusion
Most of the study participants had increased cardiovascular risk. Those with IF had more balanced metabolic and immunological measurements, consistent with greater disease control. In contrast, patients with CF showed correlations indicating marked inflammatory immune response, albeit with correlation patterns suggesting the effect of healthy lifestyle habits and optimal medication use for disease progression and control. More refined studies on cytokine and adipocytokine production and LV function are needed for further inferences.
Acknowledgments
The authors thank the immunology and clinical pathology laboratories and the Clinical Nutrition Unit of the University Hospital of the Federal University of Triângulo Mineiro (FUTM) for supporting this study.
Author contributions
Conceptualization: Ivonete Helena Rocha, Marcos Vinicius Silva, Virmondes Rodrigues, Daniel Ferreira Cunha, Dalmo Correia.
Data curation: Ivonete Helena Rocha, Ana Luisa Ferreira Marques, Giselle Vanessa Moraes, Djalma Alexandre Alves da Silva, Marcos Vinicius Silva, Virmondes Rodrigues, Daniel Ferreira Cunha, Dalmo Correia.
Formal analysis: Ivonete Helena Rocha, Giselle Vanessa Moraes, Djalma Alexandre Alves da Silva, Marcos Vinicius Silva, Virmondes Rodrigues, Daniel Ferreira Cunha, Dalmo Correia.
Funding acquisition: Ivonete Helena Rocha, Marcos Vinicius Silva, Virmondes Rodrigues, Dalmo Correia.
Investigation: Ivonete Helena Rocha, Marcos Vinicius Silva, Dalmo Correia.
Methodology: Ivonete Helena Rocha, Giselle Vanessa Moraes, Djalma Alexandre Alves da Silva, Marcos Vinicius Silva, Virmondes Rodrigues, Daniel Ferreira Cunha, Dalmo Correia.
Project administration: Ivonete Helena Rocha, Dalmo Correia.
Resources: Ivonete Helena Rocha, Dalmo Correia.
Software: Ivonete Helena Rocha.
Supervision: Ivonete Helena Rocha, Marcos Vinicius Silva, Virmondes Rodrigues, Daniel Ferreira Cunha, Dalmo Correia.
Validation: Ivonete Helena Rocha, Virmondes Rodrigues, Dalmo Correia.
Visualization: Ivonete Helena Rocha, Virmondes Rodrigues.
Writing – original draft: Ivonete Helena Rocha, Djalma Alexandre Alves da Silva, Dalmo Correia.
Writing – review & editing: Ivonete Helena Rocha, Marcos Vinicius Silva, Virmondes Rodrigues, Dalmo Correia.
Footnotes
Abbreviations: BMI = body mass index, BNP = brain natriuretic peptide, CCC = chronic Chagas cardiomyopathy, CF = cardiac form, ECG = electrocardiogram, HDL = high-density lipoprotein, IF = indeterminate form, IFN-γ = gamma interferon, IL-10 = interleukin 10, IL-17 = interleukin 17, IL-33 = interleukin 33, LVD = left ventricular dysfunction, MBP = mean blood pressure, TNF-α = tumor necrosis factor, WHR = waist-to-hip ratio.
How to cite this article: Rocha IH, Ferreira Marques AL, Moraes GV, Alves da Silva DA, Silva MV, Rodrigues V, Cunha DF, Correia D. Metabolic and immunological evaluation of patients with indeterminate and cardiac forms of Chagas disease. Medicine. 2020;99:51(e23773).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Source: own author.
BMI = body mass index, BNP = brain natriuretic peptide, CF = cardiac form, HDL = high-density lipoprotein, IF = indeterminate form, LVD = left ventricular dysfunction, LVEF = left ventricular ejection fraction, MBP = mean blood pressure, Rel A/L = adiponectin (μg/mL)/leptin (ng/mL), WHR = waist-to-hip ratio.
P < .05.
References
- [1].Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis 2001;1:92–100. [DOI] [PubMed] [Google Scholar]
- [2]. World Health Organization. Chagas disease (American trypanosomiasis); 2017. Available at: http://www.who.int/mediacentre/factsheets/fs340/en/. Accessed March 18, 2019. [Google Scholar]
- [3].Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet 2010;375:1388–402. [DOI] [PubMed] [Google Scholar]
- [4].Bozkurt B. Activation of cytokines as a mechanism of disease progression in heart failure. Ann Rheum Dis 2000;59:90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marin-Neto JA, Cunha-Neto E, Maciel BC, et al. Pathogenesis of chronic Chagas heart disease. Circulation 2007;115:1109–23. [DOI] [PubMed] [Google Scholar]
- [6].Dutra WO, Gollob KJ. Current concepts in immunoregulation and pathology of human Chagas disease. Curr Opin Infect Dis 2008;21:287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Boscardin SB, Torrecilhas AC, Manarin R, et al. Chagas’ disease: an update on immune mechanisms and therapeutic strategies. J Cell Mol Med 2010;14:1373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Maya Jd, Orellana M, Ferreira J, et al. Chagas disease: present status of pathogenic mechanisms and chemotherapy. Biol Res 2010;43:323–31. [PubMed] [Google Scholar]
- [9].Machado FS, Dutra WO, Esper L, et al. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin Immunopathol 2012;34:753–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dutra WO, Menezes CA, Magalhães LM, et al. Immunoregulatory networks in human Chagas disease. Parasite Immunol 2014;36:377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dias JC, Ramos AN, Jr, Gontijo ED, et al. Brazilian consensus on Chagas Disease, 2015. Epidemiol Serv Saude 2016;2:7–86. [DOI] [PubMed] [Google Scholar]
- [12].Simões MV, Romano MMD, Schmidt A, et al. Chagas disease cardiomyopathy. Int J Cardiovasc Sci 2018;31:173–89. [Google Scholar]
- [13].Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6:772–83. [DOI] [PubMed] [Google Scholar]
- [14].Nagajyothi F, Desruisseaux MS, Jelicks LA, et al. Perspectives on adipose tissue, chagas disease and implications for the metabolic syndrome. Interdiscip Perspect Infect Dis 2009;2009:824324.1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stofkova A. Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr Regul 2009;43:157–68. [PubMed] [Google Scholar]
- [16].Francisco V, Pino J, Gonzalez-Gay MA, et al. Adipokines and inflammation: is it a question of weight? Br J Pharmacol 2018;175:1569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. World Health Organization. Obesity and Overweight; 2018. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed April 9, 2018. [Google Scholar]
- [18].Llaguno M, Silva MV, Batista LR, et al. T-cell immunophenotyping and cytokine production analysis in patients with Chagas disease 4 years after Benznidazole treatment. Infect Immun 2019;87:e00103–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Sousa, RC. 2016. Correlation between cytokine profile and left ventricular function in chronic chagasic patients. Dissertation (Master in Tropical Medicine and Infectious Diseases) - Federal University of Triângulo Mineiro, Uberaba, 2016. [Google Scholar]
- [20].Ferreira RC, Ianni BM, Abel LCJ, et al. Increased plasma levels of tumor necrosis factor-alpha in asymptomatic/“indeterminate” and Chagas disease cardiomyopathy patients. Memórias do Instituto Oswaldo Cruz 2003;98:407–11. [DOI] [PubMed] [Google Scholar]
- [21].Navarro EC, Abreu MM, Tavares FC, et al. Indeterminate form of Chagas’ disease and metabolic syndrome: a dangerous combination. Am J Med Medical Sci 2013;3:68–73. [Google Scholar]
- [22].Fernandes F, Mady C. Qual o valor do BNP na prática clínica em pacientes com insuficiência cardíaca? Revista da Associação Médica Brasileira 2003;49:124. [Google Scholar]
- [23].Mayo DD, Colletti JE, Kuo DC. Brain natriuretic peptide (BNP) testing in the emergency department. J Emerg Med 2006;31:201–10. [DOI] [PubMed] [Google Scholar]
- [24].Barbosa MN, Nunes MC, Ribeiro Al, et al. N-terminal proBNP levels in patients with Chagas disease: a marker of systolic and diastolic dysfunction of the left ventricle. Eur J Echocardiogr 2007;8:204–12. [DOI] [PubMed] [Google Scholar]
- [25].Sherbuk JE, Okamoto EE, Marks MA, et al. Biomarkers and mortality in severe Chagas cardiomyopathy. Glob Heart 2015;10:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Curvo EOV, Ferreira RR, Madeira FS, et al. Correlation of transforming growth factor-β1 and tumour necrosis factor levels with left ventricular function in Chagas disease. Memórias do Instituto Oswaldo Cruz 2018;4:e170440.1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Santos VM, Cunha SFC, Teixeira VPA, et al. Frequency of diabetes mellitus and hyperglycemia in chagasic and non-chagasic women. Revista da Sociedade Brasileira de Medicina Tropical/Journal of the Brazilian Society of Tropical Medicine 1999;32:489–96. [DOI] [PubMed] [Google Scholar]
- [28].Gurgel CBM, Miguel Junior A, MendeS CR, et al. Frequency of arterial hypertension in chronic Chagas disease: a retrospective clinical study. Arq Bras Cardiol 2003;6:545–8. [DOI] [PubMed] [Google Scholar]
- [29].Frühbeck G, Catalán V, Rodríguez A, et al. Adiponectin-leptin ratio: a promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2017;7:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Frühbeck G, Catalán V, Rodríguez A, et al. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutrients 2019;2:454.1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


