Supplemental Digital Content is available in the text
Keywords: diagnosis, meta-analysis, microRNA-25, non-small cell lung cancer
Abstract
Objective:
Previous studies have shown that microRNA-25 (miR-25) plays a key role in the occurrence and development of non-small cell lung cancer (NSCLC). Many studies have shown that there is a significant increment of miR-25 in circulating blood of patients with NSCLC. The meta-analysis aims to explore diagnostic value of miR-25 in NSCLC in Chinese population.
Methods:
PubMed, Web of science, Excerpta Medica Database, China national knowledge infrastructure and China Wanfang database were searched to collect studies upon correlation between miR-25 and diagnosis of the patients with NSCLC until April 2020. Combined sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio and area under receiver operating characteristic curve were calculated by Stata 15.0 software. Literature assessment was conducted according to quality assessment of diagnostic accuracy studies, and documents with scores above or equal to 11 were included in this meta-analysis.
Results:
Six studies were included, including 480 cases with NSCLC and 451 healthy controls. The combined sensitivity (0.75, 95% confidence interval [CI]: 0.69∼0.80), specificity (0.81, 95% CI: 0.76∼0.86), positive likelihood ratio (4.04, 95% CI: 3.14∼5.20), negative likelihood ratio (0.31, 95% CI: 0.25∼0.37), diagnostic odds ratio (13.09, 95% CI: 9.37∼18.29) and area under curve (0.85, 95% CI: 0.82∼0.88) indicated that miR-25 had desirable diagnostic accuracy for NSCLC.
Conclusion:
MiR-25 can be applied in diagnosis of NSCLC and has potential of becoming a biomarker for detection of patients with early NSCLC in Chinese population.
1. Introduction
Globally, lung cancer remains the leading cause of cancer morbidity and mortality, with nearly 2.1 million new lung cancer cases and 1.8 million deaths in 2018, accounting for nearly 1/5 of total cancer deaths.[1] In male population, lung cancer is the leading cause of death in Eastern Europe, West Asia (especially the former Soviet Union), North Africa, and some countries in East Asia (China) and Southeast Asia (such as Myanmar, the Philippines and Indonesia). The region that has the highest incidence of lung cancer among men is Micronesia / Polynesia, East Asia (China, Japan and South Korea with an incidence of over 40 / 100000), and most part of Europe, particularly Eastern Europe (with an incidence of 77.4 / 100000 in males in Hungary). Among women, the regions with the highest incidence are North America, Northern and Western Europe and Australia / New Zealand, with Hungary at the top of the list.[1] Lung cancer is classified according to the type of disease, into small cell 1 with the English full name “small cell lung cancer,” abbreviated as small cell lung cancer, and non-small cell 1, with the English full name “non-small cell lung cancer,” abbreviated as non-small cell lung cancer (NSCLC). NSCLC makes up a substantial proportion of these 2 types of lung cancer, roughly more than 80% of them.[2] Improvement of living standards, accelerated pace of life, changes in eating habits and other aspects life, lead to yearly increasing incidence of lung cancer.[3,4]
Although with continuous progress of biological research, medical technology has been greatly improved, yet treatment of lung cancer has not been significantly improved. Prognosis of lung cancer is still not satisfactory, and 5-year survival rate is at a relatively low level.[5,6] To accurately evaluate the prognosis of patients with lung cancer, determination of individualized and effective treatment for different patients is 1 of the effective ways for improvement of survival rate of patients with lung cancer. Early detection, early diagnosis and early treatment are rather crucial for patients with lung cancer. Hence, the determination of effective biomarkers related to diagnosis is of great help for clinical application, which can guide treatment of patients, and improve survival status of patients. Therefore, it has drawn considerable attention from clinic.[7–9] Low-dose helical CT scanning is the main means to diagnose asymptomatic lung cancer at present, but it has some disadvantages, including tedious operation, high false positive rate and radiation damage to human body.[10] Non-invasive tumor markers can detect tumors early or predict progress of tumors,[11] which may become an effective tool for early diagnosis of lung cancer.
MicroRNA-25 (miRNA) is of great diagnostic value for many diseases, and early diagnosis and early treatment is of great significance.[12,13] Current main clinical tumor markers of lung cancer will facilitate diagnosis and pathological classification of lung cancer to some extent, but their sensitivity and specificity are relatively low. Circulating miRNA, which is the miRNA in serum or plasma, can stably exist in clinical samples. Abnormal expression of miRNA may be earlier than appearance of clinical symptoms,[14] which therefore may become a new tumor marker. However, not all miRNA abnormally expressed in cancer tissues can be detected in blood. Nad et al[15] compared expression of 334 types of miRNA in sera of patients with NSCLC and healthy people, and found that only 91 miRNA expressed abnormally, of which only 24 miRNA had the same changes in cancer tissues and serum, which was probably related to the source of serum. MiR-25 is not only highly expressed in tumor tissues of patients with NSCLC,[16,17] but also increases in serum or plasma samples, indicating that miR-25 is qualified to be a diagnostic marker of NSCLC. So far, a number of researchers have published studies on diagnostic value of miR-25 in NSCLC and raised concerns about effectiveness of miR-25 as a biomarker. Therefore, in this research, studies on the relationship between blood miR-25 levels and diagnostic value of patients with NSCLC were collected in Chinese population, and corresponding statistical model was used to calculate combined sensitivity, specificity, positive likelihood ratio, negative likelihood ratio (NLR) and diagnostic ratio, in order to evaluate diagnostic value of miR-25 in Chinese patients with NSCLC.
2. Materials and methods
2.1. Retrieval strategy
PubMed, Web of science, Excerpta Medica Database, China national knowledge infrastructure and China Wanfang database were searched to evaluate diagnostic value of miR-25 in NSCLC. The retrieval strategy was as follows:(microRNA-25 OR miRNA-25 OR microRNA25, OR miR-25 OR miRNA25 OR hsa-miR-25) AND (lung tumor OR NSCLC OR NSCLC OR lung neoplasms OR lung cancer). The retrieval time was from the establishment of the databases to April 2020. The language was limited to English and Chinese. A comprehensive database search was carried out independently by 2 researchers.
2.2. Literature selection criteria
2.2.1. Inclusion criteria:
The inclusion criteria were as follows:
-
(1)
The literatures were studies of miR-25 in NSCLC;
-
(2)
2)Human specimens must be used;
-
(3)
The relationship between miR-25 and diagnostic accuracy was studied;
-
(4)
The subjects were Chinese.
2.2.2. Exclusion criteria:
The exclusion criteria were as shown below:
-
(1)
The literatures studied the relationship between the expression of miR-25 and diagnostic accuracy, with a receiver operating characteristic curve (ROC), but without report of the specific values of sensitivity and specificity;
-
(2)
Letters, case reports, reviews, conference summaries, animal or laboratory studies were excluded;
-
(3)
Pathological subtype of NSCLC was conducted to calculate the relevant indicators of diagnostic accuracy;
-
(4)
The quality assessment of diagnostic accuracy studies (QUADAS) score was lower than 11.
2.3. Quality evaluation
The QUADAS was applied to assess diagnostic value of the study.[18] The QUADAS criteria included 14 evaluation items for systematic review of diagnostic accuracy studies, each of which was rated as yes (score 1), no (score-1) or unclear (score 0). If the QUADAS score was 11 or above, the quality of the study was defined as high-quality. Any differences between the 2 researchers were resolved through discussion by a third researcher.
2.4. Data extraction
The following information was extracted in this study:
-
(1)
first author, year of publication, tumor grade, detection method and cut-off value;
-
(2)
sensitivity, specificity, number of true positive cases, number of false positive cases, number of false negative cases, and number of true negative cases.
The table was designed according to the information extracted.
2.5. Statistical analysis
Stata 15.0 software was used to analyze the data. The inconsistency index (I2) and its P-value test were applied to evaluate the heterogeneity among studies. The bivariate mixed effect regression model was used to analyze the pooled diagnostic indicators.[19] Regarding the study of diagnostic value, the diagnostic threshold effect was evaluated by the ROC and the spearman correlation coefficient between sensitivity and specificity. The typical shoulder-arm-shaped representation in ROC space and the strong positive correlation between the logarithm of sensitivity and the logarithm of 1-specificity indicated the existence of threshold effect. In the bivariate mixed effect regression model, the pooled sensitivity, specificity, positive likelihood ratio (PLR), NLR, diagnostic odds ratio (DOR) and their forest maps were calculated using the corresponding 95% confidence interval (CI). The area under summary receiver operating characteristic (SROC) curve (AUC) was obtained. With AUC values ranging from 0.5 to 1.0, AUC values close to 0.5, it indicated poor diagnostic performance, and AUC values close to 1.0, it indicated good diagnostic performance. Deeks funnel diagram was applied to evaluate publication bias.
3. Results
3.1. Literature research and characteristic of studies
Initially, 436 articles were retrieved by keywords, of which the titles and abstracts were all reviewed, and 399 of which were excluded. Subsequently, the full text and data integrity of all the articles left were reviewed, 31 of which were excluded afterwards. Finally, 6 studies[13,20–24] that meet all the inclusion criteria were included in this study, including 480 patients with NSCLC and 451 healthy controls. The detailed screening flow diagram is shown in Fig. 1. The basic characteristics of the included literatures and the quality of the methods are shown in Table 1. The detailed evaluation of QUADAS is shown in supplementary table 1. All the QUADAS score included in this study was equal to or above 11.
Figure 1.
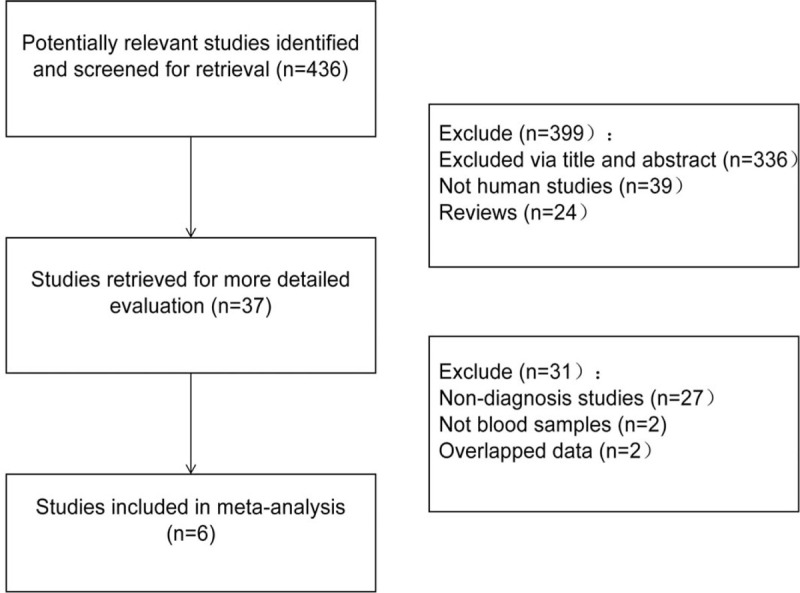
A flow diagram of the study selection process.
Table 1.
The basic characteristic and quality score of the studies included in this meta-analysis.
| First author | Year | Patients (control) | Country | Tumor stage | Sample | cut-off | Normalizer | Detection method | TP | FP | FN | TN | QUADAS |
| Wang P | 2015 | 94 (111) | China | IA–IIB | Serum | 0.551 | cel-miR-39 | qRT-PCR | 78 | 28 | 16 | 83 | 12 |
| Zhi SY | 2016 | 30 (30) | China | I–IV | Serum | 1.854 | U6 snRNA | qRT-PCR | 17 | 3 | 13 | 27 | 11 |
| Zhong JS | 2018 | 82 (82) | China | I–IV | Serum | 0.006 | Let-7d/g/i | qRT-PCR | 58 | 16 | 24 | 66 | 11 |
| Li SR | 2019 | 32 (20) | China | I–IV | Serum | NA | cel-miR-39 | qRT-PCR | 26 | 1 | 6 | 19 | 11 |
| Zhang YL | 2019 | 114 (80) | China | I–IV | Serum | 0.832 | cel-miR-39 | qRT-PCR | 85 | 20 | 29 | 60 | 12 |
| Li J | 2019 | 128 (128) | China | I–IV | Serum | 0.670 | U6 snRNA | qRT-PCR | 98 | 20 | 30 | 108 | 12 |
3.2. Meta-analysis
The results of diagnostic accuracy analysis showed that the heterogeneity was low in the studies of sensitivity (P = .07, I2 = 51.37%), specificity (P = .09, I2 = 47.27%), PLR (P = .26, I2 = 0.00%) and NLR (P = .11, I2 = 44.29%), while high heterogeneity was found in DOR (P = .00,I2 = 80.84%). There was no obvious threshold effect in the current meta-analysis, for the ROC curve was not a typical “shoulder-arm” pattern (Fig. 2). The Spearman correlation coefficient was 0.257(P = .623) between the logarithm of sensitivity and the logarithm of 1-specificity, so the difference was not statistically significant. In general, the results were the combined sensitivity (0.75,95%CI: 0.69∼0.80), specificity (0.81,95%CI: 0.76∼0.86), PLR (4.04,95%CI: 3.14∼5.20), NLR (0.31,95%CI: 0.25∼0.37), and DOR (13.09,95%CI: 9.37∼18.29). The forest plot of DOR is as shown in Fig. 3A; the forest plot of sensitivity and specificity is shown in Fig. 3B; and the forest plot of PLR and NLR is demonstrated in Fig. 3C. The SROC curve is shown in Fig. 2, with AUC (0.85,95%CI: 0.82∼0.88). The result of Fagan Nomogram suggested that the probability ratio of pre-test is 20%; the post-test probability of PLR is 50%, and the post-test probability of NLR is 7% (Fig. 3D), which indicates that miR-25 has an advanced diagnostic performance for NSCLC. The Deeks funnel plot in Fig. 4 illustrated a P value of .37, indicating that there was no publication bias.
Figure 2.
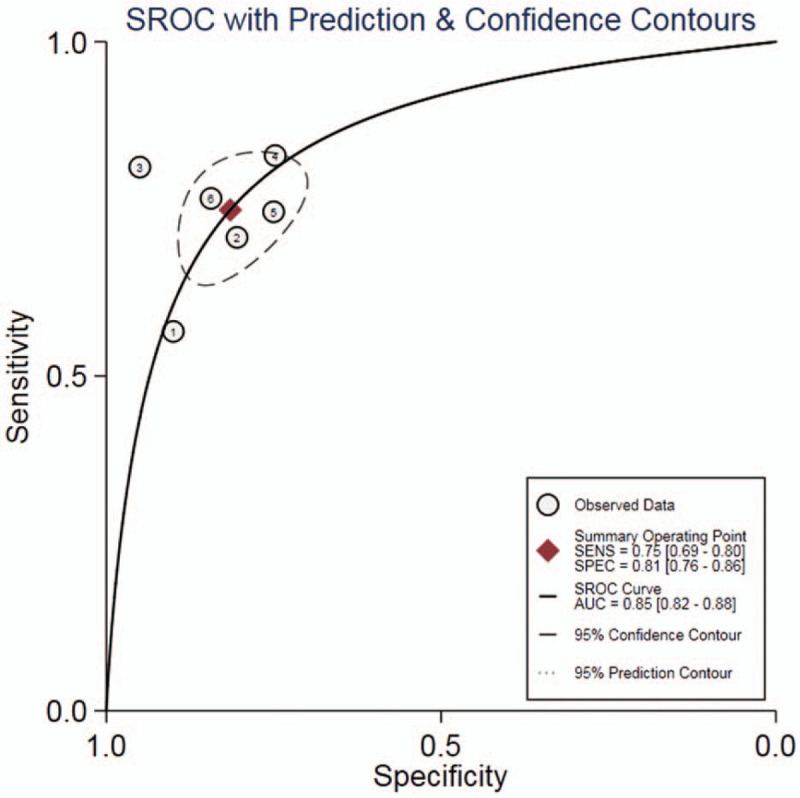
SROC curve for the accuracy of miR-25 in the diagnosis of NSCLC.
Figure 3.

Plot of miR-25 for the diagnosis of NSCLC (A:DOR;B:Sensitivity and specificity;C:PLR and NLR;D: Fagan's Nomogram).
Figure 4.
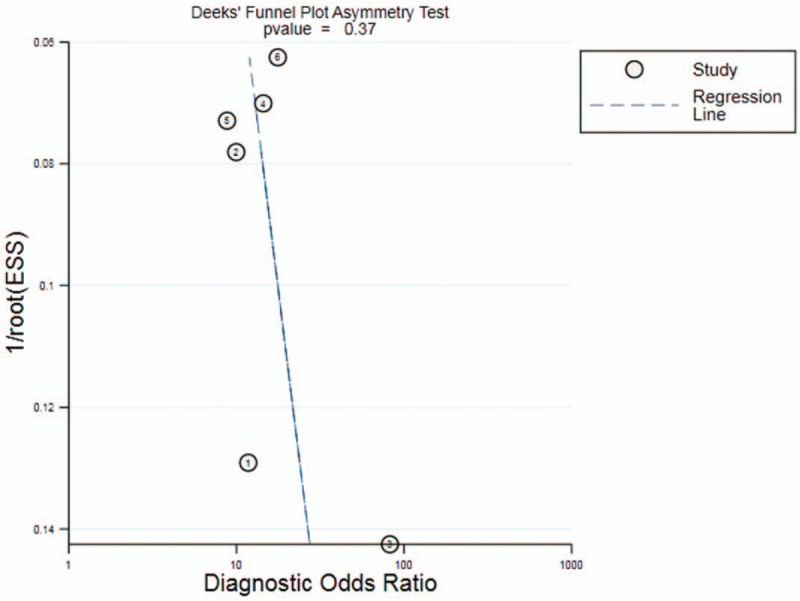
Funnel plot of miR-25 for the diagnosis of NSCLC.
3.3. Sensitivity analysis
The sensitivity analysis of diagnostic value is shown in Fig. 5. Through sensitivity analysis and outlier detection, a deviation study[13] which might affect the robustness of meta-analysis was determined. After excluding the study, there was no significant change in sensitivity (0.75 vs 0.77), specificity (0.81 vs 0.80), PLR (4.04 vs 3.80), NLR (0.31 vs 0.29), DOR (13.09 vs 13.00), and AUC (0.85 vs 0.83) between overall analysis with or without outlier. This showed that the meta-analysis of diagnostic value in this study was robust.
Figure 5.
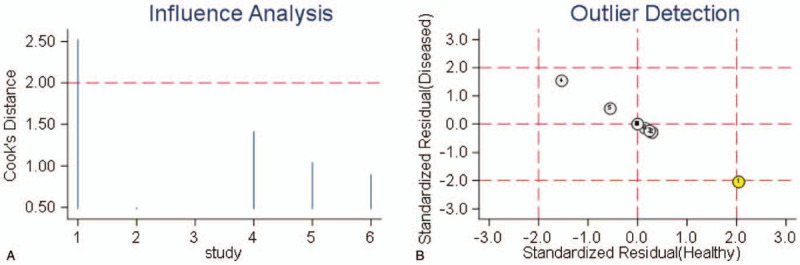
The results of sensitivity analysis.
4. Discussion
Mortality rate of lung cancer is very high in the world. Due to difficulty of early diagnosis and rapid metastasis of lung cancer, many patients had already developed blood or lymph node metastasis when they were diagnosed, which results in a significant reduction in survival rate of patients with lung cancer. Therefore, seeking biomarkers for early diagnosis of lung cancer is the key to improve the survival rate of patients with lung cancer. MiRNA is a kind of endogenous non-coding small RNAs composed of 18 to 24 nucleotides, which widely exists in eukaryotes. MiRNA can be completely or incompletely complementary to bases in the 3 ’untranslated region (3’UTR) of the target messenger RNA (mRNA), to induce the degradation of the target mRNA or inhibit its translation, and achieve the purpose of regulating the expression of the target gene at the post-transcriptional level.
More than 1000 mature miRNA have been discovered in human genes. Although the proportion of miRNA in human genome is only 1% to 3%, it regulates the expression of more than 30% of the protein-coded genes in human body.[25] Studies have shown that 50% of miRNA coding genes identified are located in tumor-related gene regions or fragile sites,[26] and there is a significant difference in expression of these microRNA between tumor cells and corresponding normal cells, which indicates that miRNA may play an critical role in occurrence and development of human tumors. The discovery of miRNA, especially serum miRNA, has opened up a new way for early diagnosis of cancer. Studies have found that not only can abnormal expression of miRNA be in lung cancer tissues, but also abnormal expression of miRNA in blood circulation, and miRNA can stably exist in serum .[27–29] Chen et al detected differential expression of serum miRNA of 400 patients with NSCLC and 220 healthy controls with Taqman probe quantitative RT- PCR. The study found that there was differential expression among 10 miRNAs (miR-20a, miR-24, miR-25, miR-145, miR-152, miR-199a-5p, miR-221, miR-222, miR-223, miR-320) in serum of NSCLC patients compared with the control groups. Then they evaluated diagnostic value of these serum miRNAs via risk score analysis, which suggested that this group of miRNAs could distinguish NSCLC patients from the control groups with high sensitivity and specificity.[30]
Foss et al. analyzed miRNA expression profiles in serum samples of 11 patients with early NSCLC and 11 healthy controls, and found that the expression levels of has-miR-1254 and has-miR-574–5p in serum of early NSCLC patients were significantly higher than those of the control samples. In verification group, the sensitivity and specificity were 73% and 71%, respectively.[31] Saitoh et al found that miR-375 can promote tumorigenesis by up-regulating the target gene FZD8 to activate the β-catenin-TCF signal pathway.[32] Zeng et al. found that the expression of miR-205 is up-regulated in lung cancer tissues and it can target suppression of SMAD4 to promote proliferation of lung cancer cells.[33] Yi et al.found that miR-375 is highly expressed in lung adenocarcinoma and small cell lung cancer, but low in lung squamous cell carcinoma, elucidating that miR-375 can promote growth of small cell lung cancer cell lines by directly down-regulating the target gene ITPKB.[34] In addition to typical miRNAs, miR-545[35] and miR-25[36–38] are also closely related to proliferation and apoptosis of lung cancer cells.
Six studies were included in the study, including 480 patients with NSCLC and 451 healthy controls. The results showed that the sensitivity, specificity, PLR, NLR, DOR and AUC were 0.75, 0.81, 4.04, 0.31,13.09, and 0.85, respectively. From the spearman correlation test of logarithm of sensitivity and logarithm of 1-specificity, as well as the shape of the SROC curve, a conclusion can be drawn that there was no significant threshold effect between the included studies. In terms of the sensitivity, specificity and the area under the SROC curve, miR-25 has diagnostic value for NSCLC. Combining + LR and-LR, + LR was less than 10 and-LR was greater than 0.1, thus the miR-25 in diagnosing NSCLC is still limited. The results of publication bias analysis showed that the publication bias of this meta-analysis was well controlled. The results of sensitivity analysis also confirmed the robustness of circulating miR-25 in diagnosis of NSCLC. In terms of sensitivity, specificity, PLR, NLR and diagnostic ratio, diagnostic value of circulating miR-25 in NSCLC is advanced. In practice, miR-25 can be combined with other biomarkers to improve its diagnostic accuracy in NSCLC. Wang et al[39] combined 5 markers, miR-483–5p, miR-193a-3p, miR-25, miR-214 and miR- 7, to diagnose NSCLC, and the area under the ROC curve was as high as 0.976 (95%CI:0.939∼1.000).
However, this study also has some limitations.
-
(1)
In terms of diagnostic accuracy, only 6 studies met the conditions of combined analysis, and the insufficient sample size may affect the overall estimation;
-
(2)
The population included in the study was relatively narrow, all of which were Chinese, so it may have some limitations for the generalization of the conclusion;
-
(3)
The study only focused on meta-analysis of diagnostic value of miR-25 in NSCLC, while no joint analysis was performed with other potential biomarkers.
In conclusion, miR-25 is a promising biomarker for the diagnosis of NSCLC in Chinese population, with advanced sensitivity and specificity. It provides a faster and less invasive assessment of NSCLC than other markers that require histopathological analysis. In addition, it is more valuable in finding circulatory prognostic markers than tissue markers in cancer patients. On the other hand, considering the effects of gender, age and race on large-scale studies, it is necessary to further evaluate the relationship between mir-25, NSCLC prognosis and chemotherapy sensitivity. In conclusion, our results proved the valuable diagnostic function of miR-25 in NSCLC, which may eventually contribute to a better understanding of the role of miR-25 in development of NSCLC and may enable it to become a biomarker in early diagnosis of NSCLC.
Author contributions
JG Chen: Critical revision of the manuscript; C Li, L Sun,HB Zhou: Substantial contribution to the conception and design of the work, manuscript drafting; Y Yang and Y Wang: Acquisition, analysis, and interpretation of the data; JG Chen, C Li and M She: Revising the manuscript critically, final approval of the version to be published. All authors have read and approved the final manuscript.
Conceptualization: Chang Li, Lin Sun, Hongbin Zhou, jianguo Chen.
Data curation: Chang Li, Lin Sun, Ying Yang, Yong Wang.
Formal analysis: Chang Li, Lin Sun, Hongbin Zhou, Ying Yang, Yong Wang.
Funding acquisition: Chang Li, jianguo Chen.
Investigation: Chang Li, Lin Sun, Hongbin Zhou, Ying Yang, Min She, jianguo Chen.
Methodology: Chang Li, Lin Sun, Hongbin Zhou, Yong Wang, jianguo Chen.
Project administration: Chang Li, Hongbin Zhou, jianguo Chen.
Resources: Chang Li, Ying Yang.
Software: Chang Li, Lin Sun, Ying Yang.
Supervision: Chang Li, Lin Sun, Min She, jianguo Chen.
Validation: Chang Li, Lin Sun, Yong Wang, Min She.
Visualization: Chang Li, Hongbin Zhou.
Writing – original draft: Chang Li, Lin Sun, Ying Yang, Yong Wang.
Writing – review & editing: Chang Li, Hongbin Zhou, Min She, jianguo Chen.
Supplementary Material
Footnotes
Abbreviations: AUC = area under curve, CI = confidence interval, DOR = diagnostic odds ratio, miR-25 = microRNA-25, NLR = negative likelihood ratio, NSCLC = non-small cell lung cancer, PLR = positive likelihood ratio, QUADAS = quality assessment of diagnostic accuracy studies, SROC = summary receiver operating characteristic curve.
How to cite this article: Li C, Sun L, Zhou H, Yang Y, Wang Y, She M, Chen J. Diagnostic value of microRNA-25 in patients with non-small cell lung cancer in Chinese population: a systematic review and meta-analysis. Medicine. 2020;99:51(e23425).
This work was supported by the Youth Innovation Medical Research Project of Chongzhou People's Hospital (Funding No.2019001).
Ethical approval was not needed because this is a meta-analysis.
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article [and its supplementary information files,].
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study. The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party. The datasets generated during and/or analyzed during the current study are publicly available.
FN = false negative, FP = false positive, qRT-PCR = Quantitative Real-time Polymerase Chain Reaction, QUADAS = Quality Assessment of Diagnostic Accuracy Studies, TN = true negative, TP = true positive.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Saad MI, Rose-John S, Jenkins BJ. ADAM17: an emerging therapeutic target for lung cancer. Cancers (Basel) 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barclay M, Lyratzopoulos G, Walter F, et al. Incidence of second and higher order smoking-related primary cancers following lung cancer: a population-based cohort study. Thorax 2019;74:466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Akhtar-Danesh N, Finley C. Temporal trends in the incidence and relative survival of non-small cell lung cancer in Canada: A population-based study. Lung Cancer 2015;90:8–14. [DOI] [PubMed] [Google Scholar]
- [5].Oskarsdottir G, Halldorsson H, Sigurdsson MI, et al. Lobectomy for non-small cell lung carcinoma: a nationwide study of short- and long-term survival. Acta Oncol 2017;56:936–42. [DOI] [PubMed] [Google Scholar]
- [6].Baine M, Verma V, Schonewolf C, et al. Histology significantly affects recurrence and survival following SBRT for early stage non-small cell lung cancer. Lung Cancer 2018;118:20–6. [DOI] [PubMed] [Google Scholar]
- [7].Abdel Ghafar MT, Gharib F, Abdel-Salam S, et al. Role of serum Metadherin mRNA expression in the diagnosis and prediction of survival in patients with colorectal cancer. Mol Biol Rep 2020;47:2509–19. [DOI] [PubMed] [Google Scholar]
- [8].El-Guindy D, Wasfy R, Abdel GM, et al. Oct4 expression in gastric carcinoma: association with tumor proliferation, angiogenesis and survival. J Egypt Natl Canc Inst 2019;31:3. [DOI] [PubMed] [Google Scholar]
- [9].Abdelghafar M, Allam A, Darwish S. Serum HOX transcript antisense RNA expression as a diagnostic marker for chronic myeloid leukemia 2019;44:91–7. [Google Scholar]
- [10].Mikhail O, Andrey V, Anna K, et al. PET-CT and Occupational Exposure in Oncological Patients. SciMedicine Journal 2020;2. [Google Scholar]
- [11].Ghafar M, Gharib F, Al-Ashmawy G, et al. Serum high-temperature-required protein A2: a potential biomarker for the diagnosis of breast cancer. Gene Reports 2020;20. [Google Scholar]
- [12].Khorasgani M, Nejad P, Bashi M, et al. Increased expression of miR-377-3p in patients with relapsing remitting multiple sclerosis. Med J 2019;1:48–54. [Google Scholar]
- [13].Wang P, Yang D, Zhang H, et al. Early detection of lung cancer in serum by a panel of microRNA biomarkers. Clin Lung Cancer 2015;16:313–9. [DOI] [PubMed] [Google Scholar]
- [14].Bianchi F, Nicassio F, Marzi M, et al. A serum circulating miRNA diagnostic test to identify asymptomatic high-risk individuals with early stage lung cancer. Embo Mol Med 2011;3:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nadal E, Truini A, Nakata A, et al. A novel serum 4-microRNA signature for lung cancer detection. Sci Rep-Uk 2015;5:12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xiang J, Hang J, Che J, et al. MiR-25 is up-regulated in non-small cell lung cancer and promotes cell proliferation and motility by targeting FBXW7. Int J Clin Exp Pathol 2015;8:9147–53. [PMC free article] [PubMed] [Google Scholar]
- [17].Yang T, Chen T, Li Y, et al. Downregulation of miR-25 modulates non-small cell lung cancer cells by targeting CDC42. Tumor Biol 2014;36:1903–11. [DOI] [PubMed] [Google Scholar]
- [18].Whiting P, Weswood M, Rutjes A, et al. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol 2006;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 2002;21:589–624. [DOI] [PubMed] [Google Scholar]
- [20].Zhi SY, Jiang C, Liu TZ, et al. Clinical diagnostic value of serum miR-25 expression in patients with lung cancer. Chin J Clinicians(Electronic Edition) 2016;10:2997–3001. [Google Scholar]
- [21].Zhong JS, Wang C, et al. Expression levels of serum miR-25 in the patients with non-small cell lung cancer and its clinical significance. hin J clin Lab sci 2018;36:86–9. [Google Scholar]
- [22].Li J, Yu M, Liu Z, et al. Clinical significance of serum miR-25 in non-small-cell lung cancer. Brit J Biomed Sci 2019;76:1–06. [DOI] [PubMed] [Google Scholar]
- [23].Zhang YL, Zhang ZL, Zhu XB, et al. Low plasma miR-25 expression is a favorite prognosis factor in non-small cell lung cancer. Eur Rev Med Pharmaco 2019;23:5251–9. [DOI] [PubMed] [Google Scholar]
- [24].Li SR, Liu Y, Wang ZM, et al. Diagnostic value of detecting serum miR-483-5p, miR-21, and miR-25 in patients with non-small-cell lung cancer. Shandong Med J 2019;59:19–22. [Google Scholar]
- [25].Lewis B, Burge C, Bartel D. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005;120:15–20. [DOI] [PubMed] [Google Scholar]
- [26].Calin G, Sevignani C, Dumitru C, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. P Natl Acad Sci Usa 2004;101:2999–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mitchell P, Parkin R, Kroh E, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008;105:10513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008;18:997–1006. [DOI] [PubMed] [Google Scholar]
- [29].Heegaard N, Schetter A, Welsh J, et al. Circulating micro-RNA expression profiles in early stage nonsmall cell lung cancer. Int J Cancer 2012;130:1378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen X, Hu Z, Wang W, et al. Identification of ten serum microRNAs from a genome-wide serum microRNA expression profile as novel noninvasive biomarkers for nonsmall cell lung cancer diagnosis. Int J Cancer 2012;130:1620–8. [DOI] [PubMed] [Google Scholar]
- [31].Foss K, Sima C, Ugolini D, et al. miR-1254 and miR-574-5p: serum-based microRNA biomarkers for early-stage non-small cell lung cancer. J Thorac Oncol 2011;6:482–8. [DOI] [PubMed] [Google Scholar]
- [32].Saitoh T, Hirai M, Katoh M. Molecular cloning and characterization of human Frizzled-8 gene on chromosome 10p11.2. Int J Oncol 2001;18:991–6. [DOI] [PubMed] [Google Scholar]
- [33].Zeng Y, Zhu J, Shen D, et al. MicroRNA-205 targets SMAD4 in non-small cell lung cancer and promotes lung cancer cell growth in vitro and in vivo. Oncotarget 2017;8:30817–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jin Y, Liu Y, Zhang J, et al. The Expression of miR-375 Is Associated with Carcinogenesis in Three Subtypes of Lung Cancer. Plos One 2015;10:e144187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cui J, Pan G, He Q, et al. MicroRNA-545 targets ZEB2 to inhibit the development of non-small cell lung cancer by inactivating Wnt/β-catenin pathway. Oncol Lett 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lo SF, Forcato M, Sacconi A, et al. MCM7 and its hosted miR-25, 93 and 106b cluster elicit YAP/TAZ oncogenic activity in lung cancer. Carcinogenesis 2017;38:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Caiazza C, Mallardo M. The roles of miR-25 and its targeted genes in development of human cancer. Microrna 2016;5:113–9. [DOI] [PubMed] [Google Scholar]
- [38].Chen Z, Wu Y, Meng Q, et al. Elevated microRNA-25 inhibits cell apoptosis in lung cancer by targeting RGS3. In Vitro Cell Dev Biol Anim 2016;52:62–7. [DOI] [PubMed] [Google Scholar]
- [39].Wang C, Ding M, Xia M, et al. A five-miRNA panel identified from a multicentric case-control study serves as a novel diagnostic tool for ethnically diverse non-small-cell lung cancer patients. EBioMedicine 2015;2:1377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


