Abstract
Here we report the development of an 18F-labeled, activity-based small molecule probe targeting the cancer-associated serine hydrolase NCEH1. We undertook a focused medicinal chemistry campaign to simultaneously preserve potent and specific NCEH1 labeling in live cells and animals, while permitting facile 18F radionuclide incorporation required for PET imaging. The resulting molecule, [18F]JW199, labels active NCEH1 in live cells at nM concentrations and greater than 1,000-fold selectivity relative to other serine hydrolases. [18F]JW199 displays rapid, NCEH1-dependent accumulation in mouse tissues. Finally, we demonstrate that [18F]JW199 labels aggressive cancer tumor cells in vivo, which uncovered localized NCEH1 activity at the leading edge of triple-negative breast cancer tumors, suggesting roles for NCEH1 in tumor aggressiveness and metastasis.
Keywords: Covalent activity-based probes, Positron emission tomography (PET), Radiotracer, NCEH1
Graphical Abstract

A Covalent PET probe: An 18F-labeled, activity-based small molecule probe, [18F]JW199, potently and selectively targets NCEH1 in live cells and displays rapid, NCEH1-dependent accumulation in mouse tissues and tumor xenografts.
Activity-based probes and profiling methods allow for interrogation of the active proteome in a wide range of biological contexts.[1–3] In general, chemical probes are applied to cell lysates or intact cells to quantify active enzymes by gel, mass spectrometry, or activity-dependent proximity ligation.[4–6] To detect and visualize specific enzyme in live cells, we and others have developed activity-based fluorescent probes targeting proteases, metabolic hydrolases, and kinases in mammalian and prokaryotic cells.[7–10] Most fluorescent probes, however, lack the pharmacologic and optical properties necessary for in vivo imaging applications. Instead, radiolabeled small molecules have been deployed for whole-body imaging of global molecular and physiologic processes.[11–12] For example, the radiotracer Fluorodeoxyglucose (18F) (FDG) is widely used for positron emission tomographic (PET) imaging of glucose-avid tumor cells in vivo.[12] Radiotracers that detect nutrient uptake, disease-associated protein conformation and abundance (e.g., Aβ aggregates in Alzheimer’s disease), cell surface markers, metals and other molecular features have been developed with the intention of clinical deployment.[13–16] Despite the longstanding use of PET in the clinic, there remains a need to identify new molecular targets and specific chemical probes for diagnostic imaging in disease. Toward this goal, here we tested an emerging paradigm in radiotracer development by combining the selective targeting capacity of covalent activity-based probes[17] with the imaging power of PET to create an activity-based radiotracer targeting the neutral cholesterol ester hydrolase 1 (NCEH1, also known as AADACL1 or KIAA1363). We demonstrate potent and specific target engagement in cells and in vivo, enabling direct visualization of active NCEH1 in the microenvironment of aggressive triple-negative breast cancer xenograft tumors.
Elevation of NCEH1 mRNA, protein abundance, and activity has been observed in a range of aggressive human cancer cell lines and primary tumors.[18–20] NCEH1 regulates levels of neutral ether lipids and cholesterol esters, but it remains unclear how these metabolites are involved in aggressive cancer cell phenotypes.[18, 21] The consistent upregulation of NCEH1 activity in multiple aggressive cancer cells suggests: 1) it is involved in or associated with general processes of tumor progression; 2) its activity may serve as a marker for malignant potential. We were therefore interested in visualizing this enzyme in cells, tissues and live animals. Potent, selective, and activity-dependent covalent inhibitors of NCEH1 have been reported, including a fluorescent probe capable of labeling active NCEH1 in cell culture.[22] None of these scaffolds permit in vivo imaging, however, which would be necessary for to study this enzyme in the tumor microenvironment and for potential diagnostic applications.
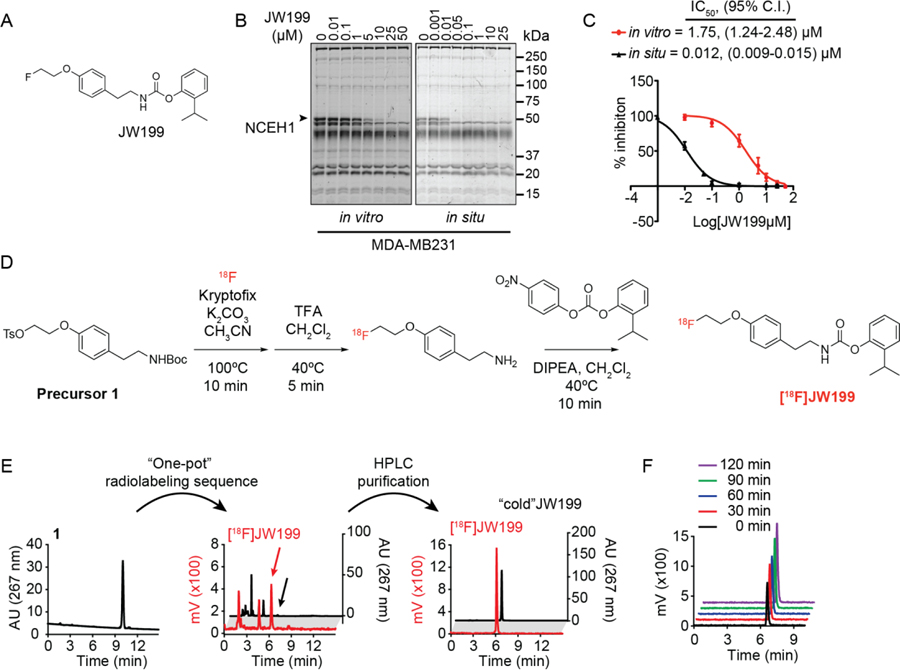
Taking into account previous NCEH1-dependent structure activity relationships and potential for facile radiosynthetic transformations, we synthesized and screened a focused series of carbamate-based small molecules. We designed compounds that might retain solubility, in vivo activity, NCEH1-target potency and specificity, and a latent synthon for radionuclide incorporation (Fig S1). For the latter attribute, we planned for 18F-compatible synthons in the aromatic/hydrophobic carbamoylating portion of the inhibitor. Three lead compounds displayed good selectivity for NCEH1, but two molecules (JW245 and JW291) were much less potent against NCEH1 compared to JW199 (Scheme S1; Fig 1A; Fig. S1). Family-wide profiling of serine hydrolases in MDA-MB231 and PC3 cell lines confirmed potent inhibition (low nM IC50) of NCEH1 in live cells by JW199 and ~1,000-fold selective over other detected serine hydrolase targets (Fig 1B, C and Fig S2). Though comparably selective, JW199 exhibited much higher IC50 values in lysate, underscoring the importance of live cell profiling to fully ascertain compound targeting landscapes. Kinetic profiling in live cells demonstrated rapid labeling of NCEH1, with target saturation within ~20 minutes (t1/2 = 10.37 min, Fig S3). Taken together, these properties prompted further development of a radiosynthetic route to generate 18F-labeled JW199 for in vivo PET imaging.
Figure 1.
JW199 is a potent and specific inhibitor of NCEH1. A. Chemical structure of JW199. B. Gel-based profiling of active serine hydrolases in breast MDA-MB231 cells (membrane fraction) treated with JW199 in vitro (left) and in situ (right). Bands mark active serine hydrolases labeled fluorophosphonate-rhodamine. C. Quantitation of JW199-dependent NCEH1 inhibition in MDA-MB231 cells in vitro (red) and in situ (black). Data shown represent the mean and 95% confidence interval (95% C.I.) from n = 3 biological replicates. D. Radiosynthesis of [18F]JW199. E. Absorbance (black) and crude radioactivity (red) chromatograms of pure TsO-JW199 starting material (1, left), 18F-labeled, crude JW199 (middle), and purified [18F]JW199 (right). Arrowheads indicate peaks corresponding to the [18F]JW199 radiosynthetic product. F. Stability of [18F]JW199 in phosphate buffered saline containing 10% ethanol over time, as measured by radioactive HPLC.
Radiosynthetic strategies generally install 18F in the final step of synthesis due to its short half-life of 109.8 minutes. We therefore considered strategies amenable to installation of a suitable electrophilic group at the 2-phenoxyethyl position in the elaborated carbamate structure of JW199, or installation of 18F on an earlier precursor followed by rapid construction of the molecule. Iterative rounds of synthesis ultimately identified a route that took the latter approach: incorporating 18F into JW199 using an N-Boc-phenoxyethyltosyl precursor 1 (Fig 1D and Scheme S1) to directly fluorinate the inhibitor scaffold via nucleophilic substitution facilitated by a tosylate leaving group.[23] This was accomplished by automated radiolabeling of precursor 1 with aqueous [18F]F− (4,750 ± 50 mCi, n=15) and K2CO3 in acetonitrile, followed by rapid, quantitative removal of the Boc protecting group using trifluoroacetic acid (TFA). The resulting 18F-labelled compound (2) was reacted with an activated carbonate, yielding [18F]JW199 (Fig 1D–E). [18F]JW199 was purified via semi-preparative HPLC, and the identity of the new radiotracer was confirmed via HPLC and co-injection with a cold (i.e., non-radiolabeled) JW199 standard (Fig 1E). Purified [18F]JW199 was chemically stable in physiologic buffers, consistent with the non-labile C-F bond (Fig 1F). Notably, our automated, one-pot and multistep synthetic route facilitates [18F]JW199 radiosynthesis (35 min) and purification (30 min) in total <65 minutes (Fig 1D, E). This route results in consistent radiochemical yields (4±0.5%, n=15), specific radioactivity (500–510 GBq/μmol) and purity (99±1%, n=15) that are comparable to radiosyntheses for preclinical and clinical radiotracers.
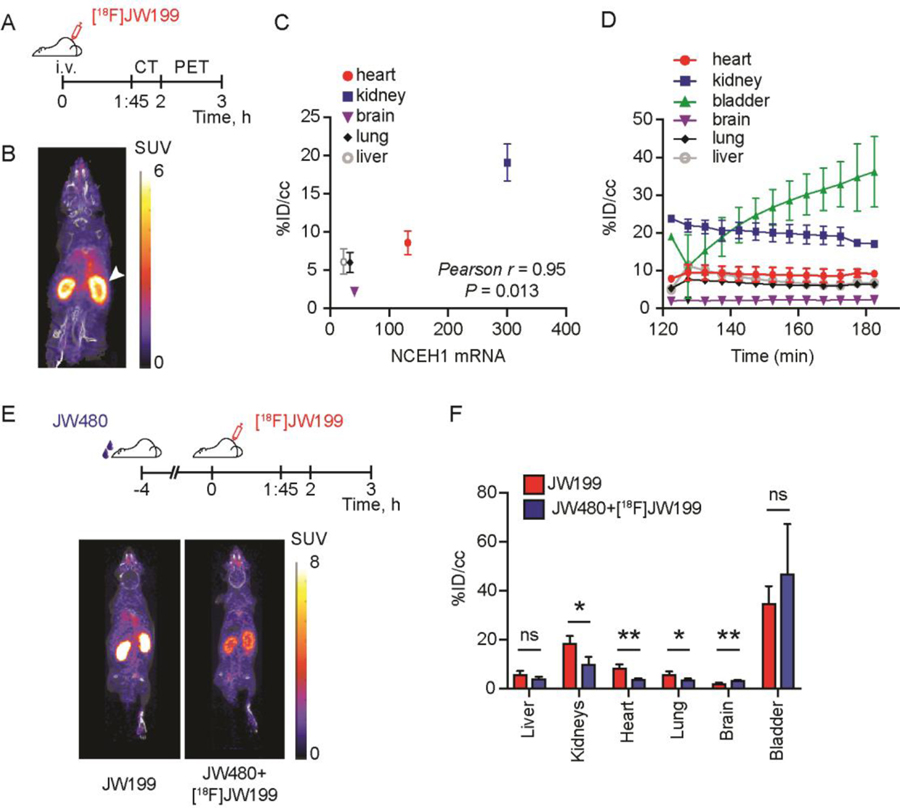
To test the utility of [18F]JW199 in visualizing active NCEH1 via PET imaging, we treated nude mice with [18F]JW199 (~100 µCi) intravenously (i.v.). We then conducted whole body X-ray computed tomography (CT) with subsequent PET imaging (Fig 2A) and assessed [18F]JW199 biodistribution. Significant signal accumulation was observed in tissues like kidney, heart and lung, which have relatively high Nceh1 expression in mice (Fig 2B, C; mRNA levels obtained from BioGPS.org). By contrast, relatively low uptake was observed in tissues with low Nceh1 mRNA levels such as adipose depots, liver, and bone. We also observed low PET signal in the brain despite relatively high levels of active NCEH1 in this tissue, consistent with low CNS penetration. Ex vivo quantification of whole tissue radioactivity corroborated the biodistribution pattern observed by PET imaging (Fig S4). Importantly, PET signal in target organs quickly reached and maintained constant levels during the entire imaging procedure (Fig 2D).
Figure 2.
NCEH1-dependent imaging with [18F]JW199. A. Treatment and imaging timeline. B. Whole-body PET-CT scan of wild-type mice following intravenous administration of [18F]JW199. Arrowhead marks radiosignal in the kidney. Image is representative of n = 3 mice. C. Correlation between Nceh1 mRNA abundance in mouse tissues (obtained from BioGPS.org) and [18F]JW199 accumulation, quantified as the percentage of initial dose per volume of bodyweight (%ID/cc). D. [18F]JW199 radiosignal kinetics in indicated organs following tracer injection. E. Timeline of JW480 competition experiment (top) and PET-CT images of two representative mice (bottom). F. Radiotracer signal quantification in tissues from mice in the [18F]JW199 (red) and competition (blue) experimental groups, 3 hours post injection of [18F]JW199. All images are representative of n = 3 mice per group and data quantitation represent mean ± standard deviation. ns, not significant, *p < 0.05, **p < 0.01, as determined by Student’s t-test.
To directly verify target-specific accumulation in vivo, we performed a competition experiment with a non-radioactive inhibitor of NCEH1, JW480 (Fig S5A)19. Pre-treatment of mice with JW480 followed by [18F]JW199 administration resulted in significantly diminished radiotracer signal in the heart, lung, and kidney (Fig 2E, F). Intriguingly, JW480 pre-treated mice also displayed slightly elevated radioactivity in urine and brain, suggesting that competition for enzymatic engagement may increase the likelihood of radiotracer accumulation in tissues with either low pharmacologic access (e.g., brain) or relative NCEH1 abundance. Gel-based profiling of serine hydrolase activity in these tissues confirmed specific and significant inhibition of NCEH1 in JW480 pre-treated mice (Fig S5B). Together, these data confirmed target labeling and retention in NCEH1-positive cells, which may provide differential imaging opportunities relative to reversible radiotracers.
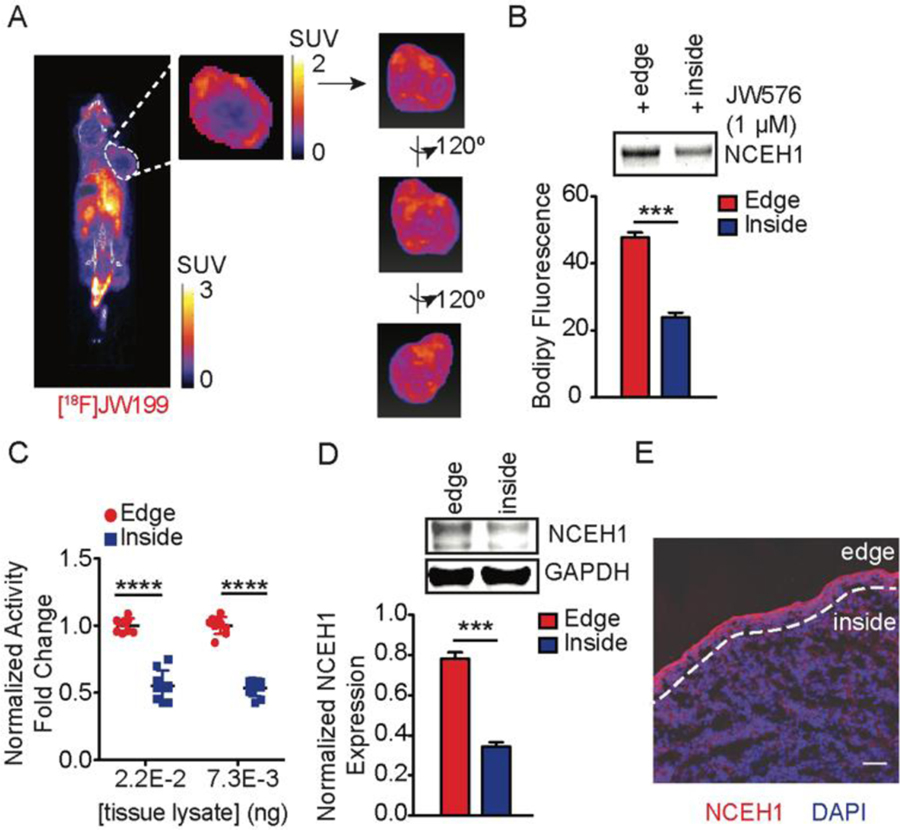
Since NCEH1 is elevated in many aggressive cancer cells, we reasoned that [18F]JW199 could allow for detection and tracking of malignant tumor cells in vivo. To test this, we established subcutaneous MDA-MB231 human breast cancer xenografts – a cell line with relatively high levels of active NCEH1 – in nude female mice. Once the tumors reached ~150–180 mm3, we i.v. administered 150 µCi of [18F]JW199, and performed dynamic PET-CT imaging. We observed a similar PET signal and biodistribution profile as in non-tumor bearing mice, as well as radiotracer accumulation in breast tumor xenograft cells (Fig 3A, Fig S6). Intriguingly, we observed marked heterogeneity within tumors, with the highest PET signal relegated to cells on the outer edge of the tumors (Fig 3A and Video S1). This distribution was independent of tumor size, and notably absent in other target tissues, suggesting that it was unique to the xenografts and/or tumor cells. To determine whether this pattern reproduced in other tumor types, we performed the same experiment mice bearing PC3 prostate cancer xenografts. [18F]JW199 treatment likewise resulted in enhanced labeling of the tumor boundary in PC3 xenografts (Fig S8A). Even with the heterogeneous signal distribution observed in tumors, ex vivo quantitation revealed that higher total radioactivity was present in bulk tumor tissue relative to surrounding muscle in both breast and prostate cancer xenografts, which collectively enabled direct visualization of tumor boundaries.
Figure 3.
[18F]JW199-mediated labelling of breast cancer tumor xenografts. A. Representative whole-body PET-CT scan of a MDA-MB231 tumor-bearing mouse following administration of [18F]JW199. Tumor boundary is delineated in the whole-body image and magnified in inset. Image is representative of n = 3 mice. B–C. NCEH1 activity profiling in the edge and core areas of MDA-MB231 tumor xenografts, as measured via gel-based profiling with JW576 (B) and the soluble activity-dependent proximity ligation (sADPL) profiling (C). D. Western blot of NCEH1 protein level in the edge (red) and core (blue) areas of tumor xenografts. E. Representative NCEH1 immunofluorescence of xenograft sections (5 µm thick). Blue channel is DAPI-stained nuclei, while red channel indicates anti-NCEH1. Division between the edge and inside areas are indicated with a dashed line. Scale bar = 5 µm. Data shown represent the mean ± standard deviation from n = 3 mice (C, E) and triplicate samples from n = 3 mice (D). ***p < 0.001, ****p < 0.0001 as determined by Student’s t-test.
To determine if the heterogeneous PET pattern observed in tumors was due to differential NCEH1 activity and/or expression throughout the tumor, we quantified NCEH1 protein abundance and activity in distinct tumor regions with several orthogonal methods. Gel-based measurements of active enzyme in microdissected tumor cells from the edge and core of xenografts were made using a specific fluorescent probe that labels active NCEH1, JW576[22], which confirmed significantly higher NCEH1 activity in cells isolated from the edge of both MDA-MB231 and PC3 xenografts (Fig 3B; Fig S7; Fig S8C). This difference in active NCEH1 was also confirmed using a more sensitive method, soluble activity-dependent proximity ligation (Fig 3C).[24–25] Likewise, Western blot revealed reduced NCEH1 abundance in the tumor core relative to the edge (Fig 3D). Finally, immunofluorescent imaging of whole xenograft tissue sections confirmed NCEH1 localization along the outer edges of the tumor, with comparatively little NCEH1 observed within the tumor, reinforcing the microenvironmental heterogeneity observed via PET imaging with [18F]JW199 (Fig 3E). Taken together, these results validate the utility of [18F]JW199 to detect active NCEH1, which displays spatially localized distribution in aggressive tumor xenografts.
Here we sought to develop a potent and selective PET radiotracer for an intracellular enzyme through covalent modification of an active site nucleophile. By balancing proteome-wide target engagement profiling, physicochemical properties, and demanding radiosynthetic processes, we developed a first-in-class activity-based PET probe targeting active NCEH1. Using [18F]JW199 in vivo, we discovered substantial intratumor heterogeneity in NCEH1 protein abundance and activity within breast and prostate cancer xenografts. These insights, which would not be possible with in vitro cellular models, raise intriguing hypotheses about the role of NCEH1 in tumor progression. For example, the increased NCEH1 abundance and activity at the growing edge of tumors could be related to its role in aggressive, pro-metastatic cellular processes. Spatially-restricted interaction with stromal cells or soluble factors could influence localized NCEH1 expression, and result in reciprocal effects on non-tumor cells. Finally, these data raise the question of whether this distribution pattern is consistent from primary to metastatic tumor lesions, as the environmental and mechanical forces are unique between these populations.
Finally, the recent resurgence of covalent small molecule chemical probes and therapeutics has established their unique capacity for increased potency, and potential specificity, relative to small molecules that bind protein targets reversibly. For example, recent studies have demonstrated that kinetic differentiation of targets can be leveraged for increased on-target in vivo activity of covalent probes[26]. Within this context, our data suggest that potent and specific covalent radiotracers may be able to differentiate between on- and off-target labelling through kinetic competition in vivo. This is supported by the rapid and stable signal observed in tissues with high NCEH1 activity, as well as the increased PET signal in tissues with either lower pharmacologic access and/or NCEH1 activity when NCEH1 is blocked in other tissues. We posit that tuning target potency and biodistribution properties with covalent imaging probes may allow for selectivity profiles that are unique from and potentially superior to traditional reversible tracers. Therefore, beyond the implications for detecting and studying NCEH1 activity, this work provides additional evidence supporting the development of covalent PET probes for precision imaging of molecular signatures and activities in animals.
Supplementary Material
Acknowledgements
We thank S. Ahmadiantehrani for text and figure editing and proofreading; E. Lengyel and M. Eckert for insightful discussions surrounding the manuscript. We are grateful for financial support of this work by the Mary Kay Foundation (to R.E.M.); this work was funded in part by the Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust (to R.E.M.); NIH 2T32DK007074-45 (supporting D.C.M.) and Shared Instrumentation Grant S10OD025265; and the University of Chicago Cancer Center Support Grant P30CA014599.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Nomura DK; Dix MM; Cravatt BF, Nat Rev Cancer 2010, 10, 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Moellering RE; Cravatt BF, Chem Biol 2012, 19, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sanman LE; Bogyo M, Annu Rev Biochem 2014, 83, 249–273. [DOI] [PubMed] [Google Scholar]
- [4].Liu Y; Patricelli MP; Cravatt BF, Proc Natl Acad Sci U S A 1999, 96, 14694–14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jessani N; Niessen S; Wei BQ; Nicolau M; Humphrey M; Ji Y; Han W; Noh DY; Yates JR 3rd; Jeffrey SS; Cravatt BF, Nat Methods 2005, 2, 691–697. [DOI] [PubMed] [Google Scholar]
- [6].Li G; Montgomery JE; Eckert MA; Chang JW; Tienda SM; Lengyel E; Moellering RE, Nat Commun 2017, 8, 1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Blum G; Mullins SR; Keren K; Fonovic M; Jedeszko C; Rice MJ; Sloane BF; Bogyo M, Nat Chem Biol 2005, 1, 203–209. [DOI] [PubMed] [Google Scholar]
- [8].Chang JW; Cognetta AB 3rd; Niphakis MJ; Cravatt BF, ACS Chem Biol 2013, 8, 1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shults MD; Imperiali B, J Am Chem Soc 2003, 125, 14248–9. [DOI] [PubMed] [Google Scholar]
- [10].Lentz CS; Sheldon JR; Crawford LA; Cooper R; Garland M; Amieva MR; Weerapana E; Skaar EP; Bogyo M, Nat Chem Biol 2018, 14, 609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Witney TH; James ML; Shen B; Chang E; Pohling C; Arksey N; Hoehne A; Shuhendler A; Park JH; Bodapati D; Weber J; Gowrishankar G; Rao J; Chin FT; Gambhir SS, Sci Transl Med 2015, 7, 310ra169. [DOI] [PubMed] [Google Scholar]
- [12].Kim W; Le TM; Wei L; Poddar S; Bazzy J; Wang X; Uong NT; Abt ER; Capri JR; Austin WR; Van Valkenburgh JS; Steele D; Gipson RM; Slavik R; Cabebe AE; Taechariyakul T; Yaghoubi SS; Lee JT; Sadeghi S; Lavie A; Faull KF; Witte ON; Donahue TR; Phelps ME; Herschman HR; Herrmann K; Czernin J; Radu CG, Proc Natl Acad Sci U S A 2016, 113, 4027–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rashidian M; Keliher EJ; Bilate AM; Duarte JN; Wojtkiewicz GR; Jacobsen JT; Cragnolini J; Swee LK; Victora GD; Weissleder R; Ploegh HL, Proc Natl Acad Sci U S A 2015, 112, 6146–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Marcus C; Mena E; Subramaniam RM, Clin Nucl Med 2014, 39, e413–22; quiz e423–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen Z; Mori W; Fu H; Schafroth MA; Hatori A; Shao T; Zhang G; Van RS; Zhang Y; Hu K; Fujinaga M; Wang L; Belov V; Ogasawara D; Giffenig P; Deng X; Rong J; Yu Q; Zhang X; Papisov MI; Shao Y; Collier TL; Ma JA; Cravatt BF; Josephson L; Zhang MR; Liang SH, J Med Chem 2019, 62, 8866–8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Muir RK; Zhao N; Wei J; Wang YH; Moroz A; Huang Y; Chen YC; Sriram R; Kurhanewicz J; Ruggero D; Renslo AR; Evans MJ, ACS Cent Sci 2019, 5, 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wilson AA; Garcia A; Parkes J; Houle S; Tong J; Vasdev N, Nucl Med Biol 2011, 38, 247–253. [DOI] [PubMed] [Google Scholar]
- [18].Chiang KP; Niessen S; Saghatelian A; Cravatt BF, Chem Biol 2006, 13, 1041–1050. [DOI] [PubMed] [Google Scholar]
- [19].Chang JW; Nomura DK; Cravatt BF, Chem Biol 2011, 18, 476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jessani N; Liu Y; Humphrey M; Cravatt BF, Proc Natl Acad Sci U S A 2002, 99, 10335–10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Okazaki H; Igarashi M; Nishi M; Sekiya M; Tajima M; Takase S; Takanashi M; Ohta K; Tamura Y; Okazaki S; Yahagi N; Ohashi K; Amemiya-Kudo M; Nakagawa Y; Nagai R; Kadowaki T; Osuga J; Ishibashi S, J Biol Chem 2008, 283, 33357–33364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chang JW; Moellering RE; Cravatt BF, Angew Chem Int Ed Engl 2012, 51, 966–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Betts HM; Milicevic Sephton S; Tong C; Awais RO; Hill PJ; Perkins AC; Aigbirhio FI, J Med Chem 2016, 59, 9422–9430. [DOI] [PubMed] [Google Scholar]
- [24].Li G; Moellering RE, Chembiochem 2019, 20, 1599–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li G; Eckert MA; Chang JW; Montgomery JE; Chryplewicz A; Lengyel E; Moellering RE, Proc Natl Acad Sci U S A 2019, 116, 21493–21500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lanning BR; Whitby LR; Dix MM; Douhan J; Gilbert AM; Hett EC; Johnson TO; Joslyn C; Kath JC; Niessen S; Roberts LR; Schnute ME; Wang C; Hulce JJ; Wei B; Whiteley LO; Hayward MM; Cravatt BF, Nat Chem Biol 2014, 10, 760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.