Abstract
With the increase in our older adult population, there is a need for dementia training for informal and formal dementia caregivers. The objective of this scoping study is to assess dementia knowledge instruments utilized in educational programs and interventions intended for formal and informal dementia caregivers. Scoping review methodology was used to search PubMed, PsycInfo, CINAHL and Web of Science with tailored database search terms. The search yielded 8101 results, with 35 studies meeting inclusion. Studies were conducted in eight countries, had varying study designs (randomized controlled trials [RCTs] = 9, non-RCTs = 6, one-group study design = 20) and utilized previously published (19) and author developed (16) instruments. Furthermore, the studies were internationally diverse, conducted in the United States (n = 18), Australia (n = 7), UK (n = 3), China (n = 2), Canada (n = 2), Taiwan (n = 1), Brazil (n = 1) and multi-country (n = 1). Only two studies focused on minority populations. While author-developed instruments may be more relevant and timesaving, studies should strive to validate instruments or use previously published instruments to help standardize findings across studies and understand better the effects of educational programs on caregiver knowledge.
Keywords: caregivers, dementia, evaluation, instruments, knowledge
Introduction
The United States will experience an unprecedented growth in its older population, one that will place pressure on both the informal and formal long-term care systems. The age structure of the population is also changing and as the proportion of older adults increases, the proportion of younger adults will remain stable.1 The AARP® estimates that the ratio of informal caregivers to dependent elders will decrease from about seven potential caregivers for every one 80-year-old (i.e., 7:1) in 2013 to 4:1 in 2030 and 3:1 in 2050.2 In terms of formal support systems, it is estimated that the United States currently only has 50% of the geriatricians needed to accommodate older adults.3 Moreover, only 6% of nurse practitioners have an adult-gerontology primary care focus,4 4% of social workers have certificates in gerontology5 and <1% of nurses, physician assistants and pharmacists identify as specializing in geriatrics.5 These three factors, i.e., an older population, a changing dependency ratio, and a lack of providers who specialize in geriatrics, will require significant changes to the US long-term care system.
In addition to the increasing demands on the long-term care system, there are unique and increasing care concerns among the growing number of individuals living with Alzheimer’s disease and related dementias (ADRD). The greatest risk factor for AD is age, and the percentage of older adults with AD will increase from 3% of all individuals aged 65–74 years to 17% of those aged 75–84 years and 32% of those aged ≥85 years.6 In 2017, the total number of caregivers who provided unpaid care for people with ADRD in the United States was 16 million.7 Lack of informal and formal dementia caregivers, along with the large financial cost of dementia care, is bound to lead to an increased caregiving burden. Evidence shows that informal dementia caregivers who sustain high levels of caregiving demands are more likely to report poor health, poor sleep and high levels of emotional stress.8,9
Currently, quality concerns about dementia care have been raised in the United States and other countries, including the inadequacy of workforce skills and knowledge to provide effective care.10,11 Gaps in dementia-related knowledge have been identified in family caregivers,12 the general public,13 physicians,14,15 and nurses or other healthcare staff.12,16 Caring for a person with dementia requires knowledge of the trajectory and appropriate care of the dementia. Having adequate knowledge about dementia is associated with lower levels of caregiver burden,17,18 caregiver depression19 and better quality of care for older adults with dementia.20 There is a need for continuing education and advanced training to increase knowledge about dementia for both informal and formal dementia caregivers to offer appropriate ongoing care.21,22 Two reviews found educational interventions for formal and informal caregivers were able to improve mental health and burden,23,24 while another study found that improving knowledge can improve care.25
Previous reviews of dementia knowledge training for caregivers indicate that in general, dementia education leads to improved caregiver outcomes.26–29 However, most of these reviews focused on training content such as attitudes and beliefs, confidence, perceived competence and self-efficacy. In addition, these reviews focused on intervention effectiveness and outcomes and did not focus on the instruments used to evaluate the programs. Burgio and colleagues recommended utilizing a standardized and recognized tool to help avoid biased results and to increase the methodological strength of studies.30 A previous systematic review of five validated instruments to evaluate dementia knowledge identified several limitations, including weaknesses in psychometric properties, being outdated and having limited scope.31 Thus, a better understanding of dementia knowledge instruments (both author constructed and previously published) is clearly needed. To expand upon previous reviews, the present study provides a scoping review of validated/previously published as well as author-constructed dementia knowledge assessment instruments, which are being utilized in educational programs, and interventions intended for informal and/or formal dementia caregivers.
Methods
Search strategy and selection criteria
We used scoping review methodology developed by Arksey and O’Malley,32 with modifications by Levac and colleagues.33 We searched PubMed, PsycInfo, CINAHL and Web of Science in April 2018. Query terms were: (dementia OR Alzheimer’s OR frontotemporal lobar degeneration OR Lewy body dementia OR HIV associated dementia) AND (caregivers OR health personnel OR family OR health facilities) AND (education OR health knowledge, attitudes, practice OR health promotion OR information dissemination OR program development OR programmed instruction as topic) AND (evaluation studies OR evaluation studies as topic OR program evaluation OR validation studies as topic OR educational measurement OR treatment outcome).
Studies were considered eligible if they met the following inclusion criteria: (i) used at least a pre- and post-test assessment of dementia knowledge in a dementia caregiver program(s) or intervention; (ii) published between June 1, 2007 and April 2018; and (iii) provided an interactive intervention, such as face-to-face or online modules, that educated formal or informal caregivers about dementia. The start date of June 1, 2007 was selected to reflect the work in the field associated with the first National Public Health Road Map to Maintaining Cognitive Health,34 which was developed to establish goals related to cognitive health, including dementia as well as caregiver burden. Excluded studies had the following characteristics: (i) participants younger than 18; (ii) a clinical focus; (iii) basic science; (iv) non-English articles; (v) case studies; (vi) abstracts; (vii) dissertations; (viii) review papers; (ix) used only pre- or post-test measures.
Data extraction and synthesis
Abstracts from all database searches were aggregated into EndNote® (Clarivate Analytics; Philadelphia, Pennsylvania) to combine results and eliminate duplicates. Next, the abstracts were uploaded to the Covidence systematic review online platform (Melbourne, Victoria, Australia) for management and review. Twelve reviewers assessed titles and abstracts for eligibility, with each article receiving two independent votes; conflicts were resolved by a 13th reviewer who was independent from the initial votes. The remaining articles from the initial review were divided up and distributed to seven team members to review for inclusion and to abstract data. This was conducted using an online abstraction tool adapted from previous research.35 The tool contained 26 questions, of which 15 were open-ended. Examples include characteristics of trainers and participants, program delivery methods, type of knowledge assessment tool used and the purpose of the study. Each full article was abstracted by one reviewer, with a quality check by another independent reviewer. In addition, reference lists within eligible articles were checked to identify other potential articles for inclusion. Once all articles deemed eligible were identified, the final data were converted and summarized from the online abstraction tool to Microsoft Excel for management.
Results
The search strategy yielded 8101 results. Of those, 1239 were eliminated due to duplication, while 6827 did not meet the inclusion criteria. The list was narrowed down to 35 for inclusion, all of which were found through the original search (see Fig. 1).
Figure 1.
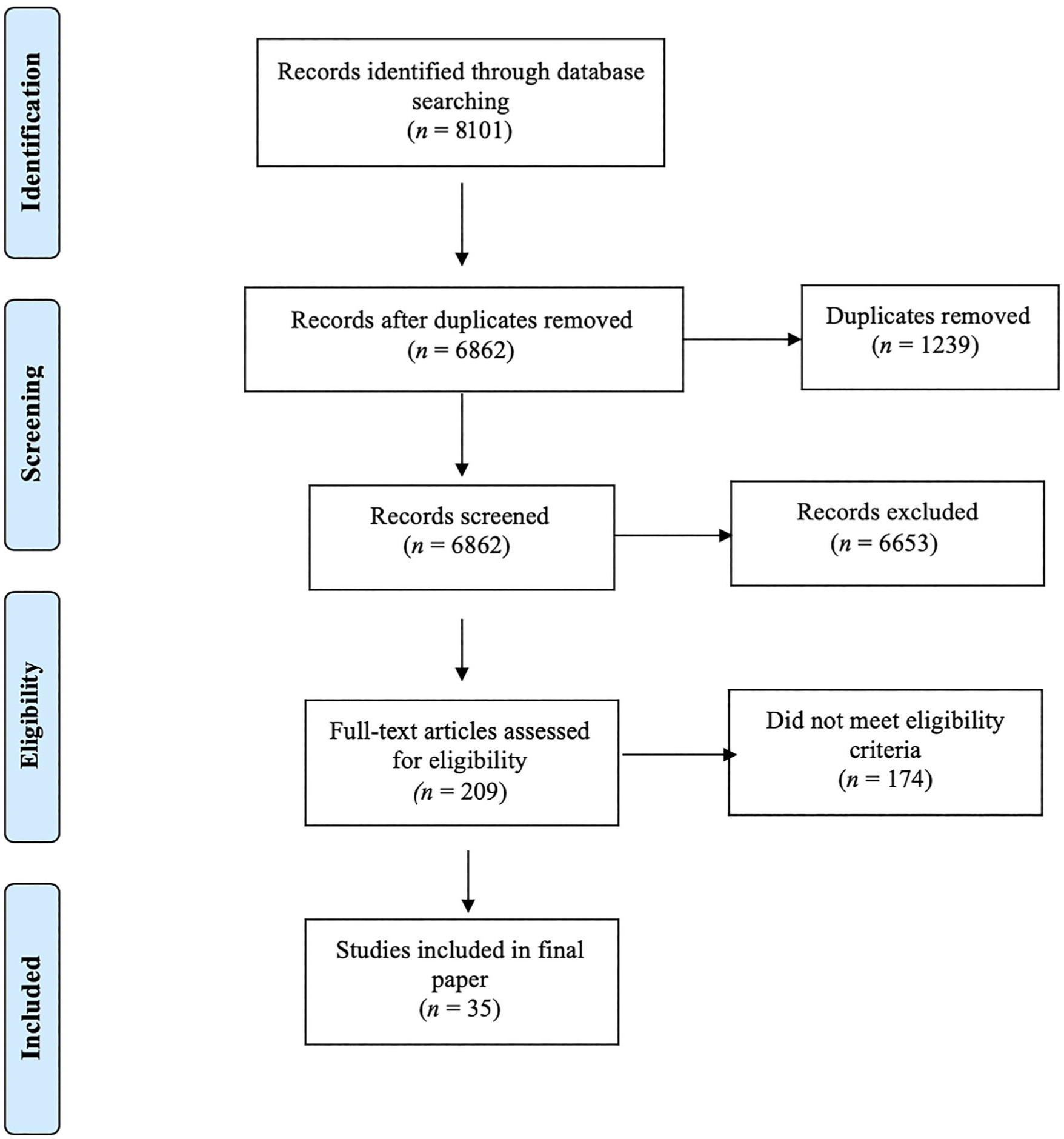
PRISMA diagram of scoping review process.
The results of the 35 articles (Table 1) showed that eight of the empirical articles included only informal caregivers, while 17 targeted formal caregivers (e.g., nurses, physicians, social workers, volunteers) and seven included students in health profession-related programs. An additional three studies were designed for both formal and informal caregivers. Moreover, the studies had various designs, including randomized controlled trials (n = 9), non-randomized controlled trials (n = 6) and one-group study designs (n = 20). According to our inclusion criteria, all studies had at least a pre- and post-test. The majority of the content was delivered face-to-face (n = 21), via the Internet (n = 8), or both (n = 4), whereas, two studies used DVDs to deliver content. Furthermore, the studies were internationally diverse, conducted in the United States (n = 18), Australia (n = 7), United Kingdom (n = 3), China (n = 2), Canada (n = 2), Taiwan (n = 1), Brazil (n = 1) and multi-country (n = 1). Only two studies focused on minority populations. Of the 30 studies that listed gender demographics, 28 had a sample >60% females, while only one had a greater proportion of males (52%).
Table 1.
Description of dementia focused educational programs and interventions for formal and informal caregivers
| References | Country | Disease focus | Type of caregiver and sample population | Study design | Delivery format | Content delivered | Program evaluation instrument |
|---|---|---|---|---|---|---|---|
| Gaugler et al. (2016)50 | United States | Dementia | Direct care workers: certified nurse assistants, personal care attendants, or similar healthcare providers (n = 40) Response rate = 50.0% | Single group pre-/post-test | Web-based | Communication with the patientBehavioral changes that accompany the disease | Author developed |
| DiZazzo-Miller et al. (2017)51 | United States | Dementia | Informal caregiver (n = 36; control group = 18, intervention group = 18) | RCT | Face-to-face | Communication with the patientManagement of diseaseEating, feeding and nutritionTransferring and toileting; dressing, bathing and grooming | The ADL Knowledge Test |
| Roberts & Silverio (2009)52 | United States | Dementia | Informal caregiver (n = 37) | Single group pre-/post-test | Face-to-face | Facts and figures about dementia/ADSigns and symptoms about dementia/ADDiagnosis and screeningTreatment of the diseaseCaregiving resourcesDirect contact with ADRD patients (in the study) | Author developed |
| Gaugler et al. (2015)53 | United States | Dementia | Informal caregiver (n = 41) Response rate = 33.3% | Single group pre-/post-test | Web-based | Communication with the patientBehavioral changes that accompany the diseaseManagement of disease | Dementia Care Knowledge |
| Karlin et al. (2017)54 | United States | Dementia | Care managers and registered nurses (n = 18) | Single group pre-/post-test | Face-to-face | Signs and symptoms about dementia/ADDisease progression Communication with the patient | Author developed |
| Grigsby et al. (2017)55 | United States | Dementia | Informal caregiver (n = 42) Response rate = 95.5% | Single group pre-/post-test | Face-to-face; YouTube Video | Signs and symptoms about dementia/AD | Author developed |
| Elvish et al. (2018)56 | United Kingdom | Dementia | Nurses, mental health educators, clinical educators or practice trainers (n = 476) Response rate = 92.1% | Single group pre-/post-test | Face-to-face | Facts and figures about dementia/ADSigns and symptoms about dementia/ADCommunication with the patientBehavioral changes that accompany the disease | Knowledge in Dementia (KIDE) scale |
| Chao et al. (2016)42 | Taiwan | Dementia | Nurses (n = 105)Response rate = 98.1% | Single group pre-, mid-, and post-test | Web-based | Signs and symptoms about dementia/ADCommunication with the patientBehavioral changes that accompany the diseaseDirect contact with ADRD patients (in the study) | The Communication Knowledge Scale-Chinese version (CKS-C) |
| DiZazzo-Miller et al. (2014)57 | United States | Dementia | Informal caregiver (n = 45) Response rate = 62.5% | Single group pre-, post-and follow-up test | Face-to-face | Communication with the patientBehavioral changes that accompany the diseaseManagement of disease | Author developed |
| Maharaj (2017)58 | United States | Dementia | Nursing students (n = 64; control group = 32, intervention group = 32) Response rate = 88.8% | RCT | Face-to-face | Facts and figures about dementia/ADSigns and symptoms about dementia/ADDirect contact with ADRD patients (in the study) | ADKS |
| Irvine et al. (2012)59 | United States | Dementia | Non-direct care workers (n = 57) Response rate = 83.8% | Single group with two pre-tests and one post-test | Web-based | Signs and symptoms about dementia/ADCommunication with the patientBehavioral changes that accompany the diseaseManagement of disease | Author developed |
| Wang et al. (2017)60 | China | Dementia | General practitioners and registered nurses (n = 170; control group = 85, intervention group = 85)Response rate = 93.4% | RCT | Face-to-face | Signs and symptoms about dementia/ADBehavioral changesManagement of diseasePerson-centered care | ADKS |
| Ducharme et al. (2015)61 | Canada | AD | Informal caregivers (n = 89; control group = 29, experimental group = 58, half randomized to booster and other half randomized to no booster) Response rate = 86.4% | RCT | Face-to-face | Communication, behavior, how to avert psychological distress and how to seek help from available formal services | Knowledge of Services Scale |
| Pleasant et al. (2017)62 | United States | Dementia | Informal and formal (paraprofessional nurse aides and licensed practical nurses or registered nurses) caregivers (n = 51) Response rate = 23.9% | Single group with pre-tests, post-test and follow-up test | Web-based | Facts and figures about dementia/ADSigns and symptoms about dementia/ADCommunication with the patientBehavioral changes that accompany the diseasePerson-centered care | Author developed |
| Kimzey et al. (2016)63 | United States | AD | Baccalaureate students (n = 94)Response rate = 94.0% | Non-RCT | One group web-based; one group face-to-face | Disease progression Communication with the patientBehavioral changes that accompany the diseaseCognitive assessmentPerson-centered care | ADKS |
| Hattink et al. (2015)41 | The Netherlands and United Kingdom | Dementia | Informal and formal (volunteer in dementia care or a professional caregiver) caregivers (n = 83)Response rate = 58.5% | RCT | Web-based | Facts and figures about dementia/ADSigns and symptoms about dementia/ADCauses of dementia/ADDisease progressionDiagnosis and screening Communication with the patientBehavioral changes that accompany the diseaseCaregiving resourcesManagement of disease | ADKS |
| Fenley et al. (2008)64 | United States | AD | Personal care worker (n = 61; English speaking = 37, Spanish speaking = 54, Mandarin/Cantonese speaking = 53)Response rate = 42.4% | Single group with pre-tests, post-test and follow-up test | Face-to-face | Knowledge of AD, care provision, communication and behavior management | Author developed |
| Perales et al. (2018)65 | United States | AD | Informal and formal (professionals) caregivers (n = 73; informal = 37, professional/formal = 40) Response rate = 94.8% | Single group with pre-test and post-test | Face-to-face | Facts and figures about dementia/ADSigns and symptoms about dementia/ADCauses of dementia/ADDiagnosis and screeningTreatment of the diseaseBrain health & AD resources | Author developed |
| Tannazzo et al. (2008)66 | United States | AD | Certified Nursing Assistants (n = 262) Response rate = 93.9% | Single group with pre-test, post-test and follow-up | Face-to-face | Signs and symptoms about dementia/ADBehavioral changes that accompany the diseaseManagement of diseasePutting the patient first in dementia care Environment Enhancing the bathing experienceActivities of daily livingMeals | Knowledge of Alzheimer Test |
| Crombie et al. (2008)67 | Australia | Dementia | Acute and residential aged care facilities staff (n = 62)Response rate = 96.9% | Single group pre-/post-test | Face-to-face | Signs and symptoms about dementia/ADDisease progressionTreatment of the diseaseBehavioral changes that accompany the diseaseManagement of disease | Author developed |
| Broughton et al. (2011)68 | Australia | Dementia | Nurses, nursing assistants, and recreational/activities officers (n = 52; control group = 15, intervention group = 37) Response rate = 76.5% | Non-RCT | DVD-based training | Communication with the patientBehavioral changes that accompany the diseaseCompensatory and communication strategies | Author developed |
| Bateman et al. (2016)69 | Australia | Dementia | Hospital volunteers (n = 16) Response rate = 88.8% | Non-RCT | Face-to-face | Facts and figures about dementia/ADCommunication with the patientBehavioral changes that accompany the diseaseDirect contact with ADRD patients (in the study)Assisting patients with eating and drinkingSafe walking and exercise | Alzheimer’s Disease Knowledge Test |
| Brody et al. (2016)43 | United States | Dementia | Registered nurses, physical therapists, and occupational therapists (n = 83)Response rate = 43.5% | Single group pre-/post-test | Web-based | Treatment of the diseaseBehavioral changes that accompany the diseaseAlso covered how to recognize and assess pain, depression and other neuropsychiatric symptoms; and educate primary informal caregivers on how to manage symptoms for the PLWD and how to perform clear and concise communication with other home healthcare clinicians and primary care providers (not with patients) | Author developed |
| Liddle et al. (2012)70 | Australia | Dementia | Informal caregivers (n = 29; control group = 16; intervention group = 13) Groups included dyads (i.e., caregiver and their patient) | RCT | DVD-based training | Communication with the patientBehavioral changes that accompany the diseaseCaregiver experience | Communication and Memory in Dementia (CMSD) knowledge test |
| Khan and Curtice (2011)71 | United Kingdom | Dementia | Staff members of care homes (numbers not provided) | Single group pre-/post-test | Face-to-face | Signs and symptoms about dementia/ADBehavioral changes that accompany the diseaseDirect contact with ADRD patients (in the study) Management of disease; pharmacological and non-pharmacological aspects of dementia management | Author developed |
| Wang et al. (2017)72 | China | Dementia | Nurses (n = 101; control group = 54, intervention group = 61)Response rate = 87.8% | RCT | Web-based, face-to-face and video chat | Facts and figures about dementia/ADSigns and symptoms about dementia/ADCauses of dementia/ADDiagnosis and screeningTreatment of the diseaseBehavioral changes that accompany the diseaseCaregiving resourcesManagement of disease | Chinese Alzheimer’s Disease Knowledge Scale (CADKS) |
| Lorio et al. (2017)73 | United States | Dementia | Doctor of physical therapy students (n = 31) Response rate = 96.8% | Single group pre-/post-test | Face-to-face | Facts and figures about dementia/ADSigns and symptoms about dementia/ADDisease progressionTreatment of the diseaseCommunication with the patientBehavioral changes that accompany the diseaseManagement of disease | Knowledge in Dementia Scale (KIDE) |
| Kouri et al. (2011)74 | Canada | AD | Informal caregiver (n = 50; control group = 25, intervention group = 25) | RCT | Face-to-face | Communication with the patientBehavioral changes that accompany the disease | Author developed |
| Gilmartin-Thomas and McNeil (2018)75 | Australia | Dementia | Medical and pharmacy students (n = 278; control group = 198, intervention group = 80) Response rate = 93.3% | Non-RCT | Face-to-face | Cognitive and perceptual difficulties faced by people with dementia | Dementia Attitudes Scale (DAS) |
| Hobday et al. (2017)76 | United States | Dementia | Hospital administrators and staff education (n = 25) | Single group pre-/post-test design | Web-based | Signs and symptoms about dementia/ADTreatment of the diseaseCommunication with the patientBehavioral changes that accompany the diseaseManagement of disease | Author developed |
| Fields et al. (2016)77 | United States | Dementia | Respite care providers (n = 23) | Single group pre-/post-test design | Face-to-face | Facts and figures about dementia/ADSigns and symptoms about dementia/ADCauses of dementia/ADTreatment of the diseaseCommunication with the patientBehavioral changes that accompany the diseaseCaregiving resourcesManagement of disease | Knowledge of Alzheimer’s disease/dementia (KAD) scale |
| Naughton et al. (2018)78 | United Kingdom | Dementia | Nursing students (n = 52; control group = 14, intervention group = 38) Response rate = 40% | RCT | Face-to-face | Communication, behavior | Dementia Knowledge 20 (DK-20) |
| Eccleston et al. (2015)79 | Australia | Dementia | Nursing students (n = 99; control group = 47, intervention group = 52) | Non-RCT | Face-to-face | Disease progression Dementia-associated care needs | Dementia Knowledge Assessment Tool Version 2 (DKAT 2) |
| Tomaz et al. (2015)80 | Brazil | Dementia | Physicians (n = 50; control group = 25, experimental group = 25)Response rate = 83.3% | Non-RCT | Face-to-face; web-based | Diagnosis and screeningKnowledge of and insight in dementia (general) | Author developed |
| Annear et al. (2016)81 | Australia | Dementia | Undergraduate medical, nursing, and paramedic students (n = 127) | Single group pre-/post-test design | Face-to-face | General dementia knowledge | Dementia Knowledge Assessment Tool Version 2 (DKAT 2) |
AD, Alzheimer’s disease; ADKS, Alzheimer’s Disease Knowledge Scale; ADRD, Alzheimer’s disease and related dementias; RCT, randomized controlled trial.
The knowledge domains assessed in these studies varied, but generally included information related to communication with patients with dementia, behavioral changes associated with dementias, management of disease, facts and figures related to dementias, and disease progression. To measure the change in knowledge from pre- to post-test, studies used either instruments that were previously published or a tool developed by the author. Specifically, 16 of the included studies used an author-developed instrument and 19 used previously published instruments. Of the 35 studies, 29 (82.9%) showed statistically significant improvement in knowledge from pre- to post-test. Furthermore, 15 (78.9%) of the 19 studies that used previously published instruments and 14 (87.5%) of the 16 studies that used author-constructed instruments found statistically significant improvement in knowledge from pre- to post-test. Thirteen published measures were identified. The Alzheimer’s Disease Knowledge Scale was used most frequently (n = 4 studies) followed by the Dementia Knowledge Assessment Tool Version 2 and Knowledge in Dementia Scale (n = 2 studies each). Eleven other published measures were used once each. Table 2 includes the psychometric properties and other characteristics of instruments used in these studies.
Table 2.
Psychometric properties and characteristics of previously published dementia knowledge instruments
| Previously published instrument | Type of caregiver intended for | Length and format of instrument | Domains covered | Reliability | Validity | Strengths | Limitations |
|---|---|---|---|---|---|---|---|
| ADKS82 | Formal and informal caregivers | 30-item, true/false scale | Dementia risk factors, assessment, diagnosis, symptoms, course, life impact, caregiving, treatment and management | Test-retest: Pearson correlation coefficient = 0.81; internal consistency reliability = 0.71 | Predictive validity: overall r = 0.50, dementia caregivers, r = 0.46, AD professionals, r = 0.39, older adults without cognitive impairment, r = 0.41 and undergraduates, r = 0.20; construct validity: difference between previous knowledge and experience; convergent validity: Pearson’s correlation coefficient = 0.65 | Quick and easy administration; can measure change in educational interventions and programs | Relatively low internal consistency; excludes some relevant domains; possible ceiling effects in more expert groups |
| Dementia Knowledge Assessment Tool Version 2 (DKAT2)83 | Formal and informal caregivers | 21-item, correct, incorrect, do not know | Disease etiology, course, prognosis, symptoms, psychosocial, management | Internal consistency: Cronbach’s alpha coefficient = 0.79 | Construct validity: difference between previous knowledge | Broad syndrome of dementia; provides indications of misunderstandings or where knowledge is lacking | Possible ceiling effects |
| Knowledge in Dementia (KIDE) scale84 | Formal caregivers | 16-item, agree/disagree scale | Communication, behavioral challenges, general dementia information | Internal consistency: Cronbach’s alpha = 0.72 | Adequate face validity and good content validity | Good internal consistency, face validity and content validity; ability to track changes in staff knowledge | Designed for only general hospital staff |
| The ADL Knowledge Test57 | Informal caregivers | 18-item, multiple-choice test | Communication and nutrition, bathing and dressing, and transfers and toileting | N/A | Content validity: intraclass correlation coefficient = 0.95 for communication and nutrition, 0.95 for toileting and transfers, and 0.83 for dressing and bathing | Great content validity for all three domains | Reliability not established; only validated for informal caregivers |
| Dementia Care Knowledge53 | Informal caregivers | 20-item, multiple-choice and true/false measure | Multiple knowledge domains regarding person-centered ADRD symptom management | Internal consistency: Cronbach’s alpha = 0.63 | Content validity established based on expert team recommendations | Iterative process to strengthen content validity; reduced ceiling effect | Reduced generalizability due to small sample size; only informal caregivers; online administration |
| The Communication Knowledge Scale-Chinese version (CKS-C)42 | Formal caregivers | 10-item, self-rated scale | Multiple domains related to communicating with people who have dementia | Internal consistency: Cronbach’s alpha = 0.94 | Content validity index = 0.92 | Ability to detect and track changes in communication knowledge | Criterion validity not determined; self-assessment may introduce bias |
| Knowledge of Services Scale85 | Informal caregivers | 7-item, Likert scale | Knowledge of available services | Internal consistency: Cronbach’s alpha = 0.92 | Validity supported by previous findings | Ability to detect and track changes in knowledge of dementia services | Construct validity not established |
| Knowledge of Alzheimer Test66 | Formal caregivers | 33-item, true/false assessment | AD pathology, signs and symptoms, and treatment and care of patients with AD | Internal consistency: Cronbach’s alpha = 0.90–0.96 | Evidence of validity supported | Good internal consistency | Older scale |
| Alzheimer’s Disease Knowledge Test86 | Formal and informal caregivers | 20-item, multiple-choice | First section: epidemiology, etiology, symptomatology, assessment. Second section: treatment, management, community support options | Internal consistency: Cronbach’s alpha = 0.71–0.92 | Demonstrates satisfactory validity | For formal and informal caregivers; able to detect overall and domain-specific knowledge levels; can be used for program evaluation | Older scale, may require revision and reevaluation of psychometric properties |
| Communication and Memory Support in Dementia (CMSD) knowledge test68 | Informal and formal caregivers | 17-item, brief responses | Communication and memory | N/A | N/A | Does not require information specific to the training materials | Reliability and validity not tested |
| Chinese Alzheimer’s Disease Knowledge Scale (CADKS)87 | Formal caregivers | 30-item, true/false scale | Risk factors, assessment and diagnosis, symptoms, disease progression, life impact, caregiving, treatment and management | Internal consistency: Cronbach’s alpha = 0.72; content Validity Index = 0.91 | Test-retest reliability = 0.71 | Similar validity and reliability to original ADKS | Only assesses overall knowledge; may not be generalizable |
| Dementia Attitudes Scale88 | Formal and informal caregivers | 20-item | Attitudes, cognitive and behavioral domains | Internal consistency: Cronbach’s alpha = 0.83 | Evidence of construct validity | Good reliability; replicability of factor structure across independent samples; convergent validity evidence; ease of administration | Possible ceiling effects; social desirability may influence results; self-report measures |
| Knowledge of AD/dementia (KAD) scale89 | Informal caregivers | 42-items, true/false, Likert and rating scales | Epidemiology and etiology of AD, perceived effectiveness of available treatments, beliefs regarding perceived threat of AD, how respondents learned about AD | Internal consistency: Cronbach’s alpha for Epidemiology/Etiology Disease Scale = 0.79 for whites, 0.76 for Hispanics, and 0.77 for Chinese; Cronbach’s alpha for AD treatment effectiveness = 0.95 for whites, 0.94, and 0.96 for whites, Hispanics, and Chinese for the healthy behaviors scale and 0.84 for whites, 0.87 for Hispanics, and 0.85 for Chinese for the medical behaviors scale; Cronbach’s alpha for perceived threat of AD = 0.57 for whites, 0.73 for Hispanics, and 0.70 for Chinese; Cronbach’s alpha for AD information sources = 0.39 for whites, 0.59 for Hispanics and 0.35 for Chinese | N/A | Tested in three ethnic groups (Hispanics, whites and Chinese); possibly reduces ceiling effects | Validity not tested; not tested with males |
| Dementia Knowledge 20 (DK-20)90 | Formal caregivers | 20-item, multiple-choice | Biopsychosocial knowledge and care-specific knowledge | Internal consistency: Cronbach’s alpha = 0.63; test-retest reliability: Pearson correlation = 0.73 | Evidence for face, content and construct validity | Possibly reduces ceiling effect; newer scale; may be used to identify gaps in knowledge, inform educational interventions | Designed for unqualified care staff, limits generalizability |
AD, Alzheimer’s disease; ADKS, Alzheimer’s Disease Knowledge Scale; ADRD, Alzheimer’s disease and related dementias.
Discussion
While previous studies have evaluated outcomes related to attitudes and beliefs, confidence, perceived competence, and self-efficacy or psychometric properties of dementia knowledge instruments, none has looked at the use of dementia knowledge instruments in evaluation assessments specifically for dementia caregiver educational programs. The major findings of the present review suggest there is a need for research on dementia knowledge among caregivers in at least two areas: first, development of programs focused on increasing knowledge among informal caregivers or facilitating transfer of knowledge between formal and informal caregivers; and second, development of knowledge assessment tools specific to informal caregivers or validation of existing tools among different types of caregivers. Furthermore, the development of dementia educational programs and interventions will only become more important as the population ages and more individuals are diagnosed with dementia. Thus, it is critical to evaluate the overall effectiveness of these programs and interventions. This review provides a comprehensive summary of previously published studies, the instruments used to evaluate the studies and psychometric properties of the instruments.
The majority of studies (n = 24) included in this review focused on dementia knowledge assessment for formal caregivers (e.g., nurses, occupational therapists, hospital administrators) or students in healthcare professions (e.g., nursing, medical or pharmacy students), while fewer (n = 8) focused specifically on informal caregiver education and assessment. Although formal caregivers and healthcare professionals serve central roles in the dementia caregiving continuum, the relative lack of educational programs or unique knowledge assessment tools for informal caregivers belies their significance and need. It is estimated that 83% of dementia-related caregiving is provided by informal caregivers,7 who experience most of the adverse financial and psychological effects of caregiving burden.8,9 In addition, previous research suggests that informal caregivers may receive little education or knowledge support from primary care physicians, despite considering physicians the most reliable, trustworthy sources for information about the caregiving process.36 Similarly, evidence suggests that lack of dementia knowledge among informal caregivers may act as a barrier to accessing formal community care,37 potentially leading to worse outcomes for both caregivers and their family members with dementia. Furthermore, previous evidence suggests increasing both formal and informal caregivers’ knowledge domains (e.g., behavior management, disease management/progression, communication) can lead to improved ability to care and better outcomes in care receivers. For example, a previous review found educational programs for formal caregivers that increased knowledge of challenging behavior allows staff to manage patients better,28 while other research found that improving knowledge and ability to communicate with the care receiver improves quality of life and well-being of people with dementia.38 Furthermore, our results demonstrate that educational programs are effective at increasing caregiver knowledge, with 82.9% of the studies showing significant improvement in knowledge as evaluated by pre- and post-assessments.
When deciding on an instrument to use in the evaluation of dementia educational programs and interventions, there is no “gold standard” instrument. Instead, the characteristics and goals of their program/intervention must be considered when choosing the appropriate method of evaluation. Specifically, investigators should consider the following factors during the instrument selection process: target population (formal caregivers, informal caregivers, or both); psychometric properties of the instrument(s); domains covered by the instrument and how these align with the program content; cultural appropriateness; and the readability level of the instrument and the literacy skills of the target population.
Informal and formal caregivers are likely to have very different education and training needs given their different roles in caregiving. Despite the limited number of education and training programs that were specifically focused on informal caregivers (e.g., family caregivers), the majority of these studies found that programs were successful at improving self-perceived competence and ability in providing care.39,40 Additionally, studies that focused on formal caregivers (e.g., nurse, physicians) found that education and training programs were successful at improving attitudes, communication, confidence and ability to provide care.41–43 However, there appears to be an underutilization of validated measures to assess knowledge programs for formal and informal dementia caregivers. While a previous review examined available measures to evaluate dementia knowledge within dementia education and training interventions, it was limited to the analysis of psychometric properties of the tools.31 These authors found that about half of the studies used previously validated instruments. While authors may choose to adapt instruments to save time, changing, eliminating or adding questions changes the construct and precision of these instruments.44 In addition, the content and level of dementia knowledge necessary for informal caregiving is distinct from those of healthcare professionals and may not be adequately captured by established assessment tools. Therefore, new, validated measures that are specific to caregiver type are needed to evaluate effectively if a program is providing the appropriate knowledge for both formal and informal caregivers.
Of further interest was the finding that the caregiver training programs reviewed were not tailored for specific minority groups. Culturally sensitive dementia interventions are important because different cultural groups and ethnicities attach different meanings to ADRD, which can influence caregiving practices. A recent systematic review described whether studies developed dementia caregiver interventions with cultural sensitivity as a key component of their study and program design.45 In their review, Napoles and colleagues found most of the studies that included ethnic minorities did not stratify their results by race/ethnicity, while even fewer studies tailored their programs for a particular culture or ethnic group(s). This is concerning as compared with non-Hispanic White dementia caregivers, racial/ethnic minorities may have different support needs and health effects.46 In addition, women, who typically adopt the role of the caregiver, have reported more depressive and anxiety symptoms than male caregivers.47 Therefore, to provide meaningful dementia training for racial/ethnic minorities and women, it is important to assess dementia knowledge and provide educational materials with reliable instruments that are grounded in culturally relevant interpretations of ADRD and caregiving,48 while interpreting and stratifying the results of ADRD and caregiving educational programs by race/ethnicity and sex.
Despite its strengths, this scoping review has limitations. First, comparing findings across studies must be done with caution, given the variations in how data were reported. Second, these findings cannot be generalized to other education programs or populations. Lastly, the search for manuscripts was systematic and comprehensive, but the exclusion of non-English manuscripts might limit the applicability of the findings to English-speaking parts of the world. Another important consideration when constructing and evaluating educational programs and interventions for dementia caregivers is the sociocultural context (e.g., primary language, literacy levels, health beliefs and health literacy) of intended program participants. To address some of these challenges, input from these participants is critical during the design, implementation and evaluation of the intervention programs, and to ensure content and format are culturally and literacy appropriate.49 Future studies should also be mindful of timing and readiness of the target population to engage in these educational programs.
In conclusion, dementia caregiver educational programs can improve skills and knowledge in the demanding task of providing effective care for the growing population of persons with dementia. Given the heterogeneity of these programs, it would be helpful to have studies that compare interventions by educational content or training methods and studies that focus on the development of culturally sensitive caregiver trainings and dementia knowledge assessments. In addition, we recommend that future work in this area involves more systematic application of knowledge instruments across dementia caregiver studies. Using validated instruments to measure knowledge accurately and reliably is important for understanding program effectiveness and improving intervention delivery for individuals caring for individuals with dementia.
Acknowledgements
We would like to thank Amy Edwards, Health Sciences Librarian at the University of South Carolina, for her role in helping to develop the search terms and initial review process. This research is the result of work conducted by the Centers for Disease Control and Prevention (CDC)-funded South Carolina Healthy Brain Research Network. The CDC Healthy Brain Research Network is a Prevention Research Centers program funded by the CDC Alzheimer’s Disease and Healthy Aging Program through cooperative agreement U48/DP005000-01S7. This work was also partially supported by the Alzheimer’s Foundation of America and the Arnold School of Public Health Graduate Scholar in Aging Award.
Footnotes
Disclosure statement
The authors declare no conflict of interest.
References
- 1.Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The Population 65 Years and Older in the United States: 2016. Washington, DC: US Department of Commerce, Economics and Statistics Administration, US Census Bureau, 2018. [Google Scholar]
- 2.Redfoot D, Feinberg L, Houser AN. The Aging of the Baby Boom and the Growing Care Gap: A Look at Future Declines in the Availability of Family Caregivers. Washington, DC: AARP Public Policy Institute, 2013. [Google Scholar]
- 3.Eldercare Workforce Alliance. Geriatrics Workforce Shortage: A Looming Crisis for our Families. Washington, D.C.: Eldercare Workforce Alliance, 2012. [Google Scholar]
- 4.American Association of Nurse Practitioners (AANP). American Association of Nurse Practitioners (AANP) 2018. [cited 30 Mar 2019]. Available from https://www.aanp.org/all-about-nps/np-fact-sheet.
- 5.Committee on the Future Healthcare Workforce for Older Americans, and Institute of Medicine. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: National Academies Press (US); 2008. [PubMed] [Google Scholar]
- 6.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013; 80: 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement 2018; 14: 367–429. [Google Scholar]
- 8.Mausbach BT, Chattillion EA, Roepke SK, Patterson TL, Grant I. A comparison of psychosocial outcomes in elderly Alzheimer caregivers and noncaregivers. Am J Geriatr Psychiatry 2013; 21: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson TJ, Lee SJ, Berg-Weger M, Grossberg GT. Caregiver health: health of caregivers of Alzheimer’s and other dementia patients. Curr Psychiatry Rep 2013; 15: 367. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee S Living well with dementia-development of the national dementia strategy for Engalnd. Int J Geriatr Psychiatry 2010; 25: 917–922. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services. National plan to address Alzheimer’s disease. Washington, DC: Office of the Assistant Secretary for Planning and Evaluation; 2016, 2016; 1–70. Available at: https://aspe.hhs.gov/national-plan-address-alzheimers-disease. [Google Scholar]
- 12.Robinson A, Eccleston C, Annear M et al. Who knows, who cares? Dementia knowledge among nurses, care workers, and family members of people living with dementia. J Palliat Care 2014; 30: 158–165. [PubMed] [Google Scholar]
- 13.Connell CM, Scott Roberts J, McLaughlin SJ. Public opinion about Alzheimer disease among blacks, hispanics, and whites: results from a national survey. Alzheimer Dis Assoc Disord 2007; 21: 232–240. [DOI] [PubMed] [Google Scholar]
- 14.Hansen EC, Hughes C, Routley G, Robinson AL. General practitioners’ experiences and understandings of diagnosing dementia: factors impacting on early diagnosis. Soc Sci Med 2008; 67: 1776–1783. [DOI] [PubMed] [Google Scholar]
- 15.Turner S, Iliffe S, Downs M et al. General practitioners’ knowledge, confidence and attitudes in the diagnosis and management of dementia. Age Ageing 2004; 33: 461–467. [DOI] [PubMed] [Google Scholar]
- 16.Smyth W, Fielding E, Beattie E et al. A survey-based study of knowledge of Alzheimer’s disease among health care staff. BMC Geriatr 2013; 13: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schindler M, Engel S, Rupprecht R. The impact of perceived knowledge of dementia on caregiver burden. GeroPsych 2012; 25: 127–134. [Google Scholar]
- 18.Scott CB, Clay OJ, Epps F, Cothran FA, Williams IC. Associations of knowledge of Alzheimer’s disease and memory loss and employment status with burden in African American and Caucasian family caregivers. Dementia 2018. 10.1177/1471301218788147. [DOI] [PubMed] [Google Scholar]
- 19.Blieszner R, Roberto KA. Care partner responses to the onset of mild cognitive impairment. Gerontologist 2010; 50: 11–22. [DOI] [PubMed] [Google Scholar]
- 20.Chater K, Hughes N. Strategies to deliver dementia training and education in the acute hospital setting. J Res Nurs 2013; 18: 578–593. [Google Scholar]
- 21.Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M. World Alzheimer report 2016: improving healthcare for people living with dementia: coverage, quality and costs now and in the future Alzheimer’s Disease International (ADI), London: 2016. [Cited 30 Mar 2019]. Available from: https://www.alz.co.uk/research/WorldAlzheimerReport2016.pdf [Google Scholar]
- 22.2015 White House Conference on Aging: final report. US Department of Health and Human Services Website 2015. [30 Mar 2019]. Available from: https://whitehouseconferenceonaging.gov/2015-whcoa-final-report.pdf.
- 23.Beinart N, Weinman J, Wade D, Brady R. Caregiver burden and psychoeducational interventions in Alzheimer’s disease: a review. Dement Geriatr Cogn Dis Extra 2012; 2: 638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore KJ, Lee CY, Sampson EL, Candy B. Do interventions that include education on dementia progression improve knowledge, mental health and burden of family carers? A systematic review. Dementia 2019; 20: 1471301219831530. [DOI] [PubMed] [Google Scholar]
- 25.Handley M, Bunn F, Goodman C. Dementia-friendly interventions to improve the care of people living with dementia admitted to hospitals: a realist review. BMJ Open 2017; 7: e015257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boots LMM, van Knippenberg RJM, Kempen GIJM, Verhey FRJ, de Vugt ME. A systematic review of internet-based supportive interventions for caregivers of patients with dementia. Int J Geriatr Psychiatry 2013; 29: 331–344. [DOI] [PubMed] [Google Scholar]
- 27.Surr CA, Gates C, Irving D et al. Effective dementia education and training for the health and social care workforce: a systematic review of the literature. Rev Educ Res 2017; 87: 966–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spector A, Revolta C, Orrell M. The impact of staff training on staff outcomes in dementia care: a systematic review. Int J Geriatr Psychiatry 2016; 31: 1172–1187. [DOI] [PubMed] [Google Scholar]
- 29.Brodaty H, Green A, Koschera A. Meta-analysis of psychosocial interventions for caregivers of people with dementia. J Am Geriatr Soc 2003; 51: 657–664. [DOI] [PubMed] [Google Scholar]
- 30.Burgio L, Lichstein KL, Nichols L et al. Judging outcomes in psychosocial interventions for dementia caregivers: the problem of treatment implementation. Gerontologist 2001; 41: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spector A, Orrell M, Schepers A, Shanahan N. A systematic review of “knowledge of dementia” outcome measures. Ageing Res Rev 2012; 11: 67–77. [DOI] [PubMed] [Google Scholar]
- 32.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005; 8: 19–32. [Google Scholar]
- 33.Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010; 5: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention, and Alzheimer’s Association. Healthy Brain Initiative: A National Public Health Road Map to Maintaining Cognitive Health. Chicago, IL: Alzheimer’s Association, 2007. [Google Scholar]
- 35.Friedman DB, Becofsky K, Anderson LA et al. Cognitive health and impairment: a review of the literature. Int Psychogeriatrics 2015; 27: 1263–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson K, Hahn H, Lee AJ, Madison CA, Atri A. In the information age, do dementia caregivers get the information they need? Semi-structured interviews to determine informal caregivers’ education needs, barriers, and preferences. BMC Geriatr 2016; 16: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bieber A, Nguyen N, Meyer G, Stephan A. Influences on the access to and use of formal community care by people with dementia and their informal caregivers: a scoping review. BMC Health Serv Res 2019; 19: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eggenberger E, Heimerl K, Bennett MI. Communication skills training in dementia care: a systematic review of effectiveness, training content, and didactic methods in different care settings. Int Psychogeriatr 2013; 25: 345–358. [DOI] [PubMed] [Google Scholar]
- 39.Chiu M, Wesson V, Sadavoy J. Improving caregiving competence, stress coping, and mental well-being in informal dementia carers. World J Psychiatry 2013; 3(3): 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huis In Het Veld JG, Verkaik R, Mistiaen P, Van Meijel B, Francke AL. The effectiveness of interventions in supporting self-management of informal caregivers of people with dementia; a systematic meta review. BMC Geriatr 2015; 15: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hattink B, Meiland F, Van Der Roest H, Kevern P, Abiuso F. Web-based STAR E-learning course increases empathy and understanding in dementia caregivers: results from a randomized controlled trial in The Netherlands and the United Kingdom. J Med Internet Res 2015; 17: e241 10.2196/jmir.4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chao H, Kaas M, Su Y, Lin M, Huang M, Wang J. Effects of the advanced innovative internet-based communication education program on promoting communication between nurses and patients with dementia. J Nurs Res 2016; 24: 163–172. [DOI] [PubMed] [Google Scholar]
- 43.Brody AA, Guan C, Cortes T, Galvin JE. Development and testing of the dementia symptom Management at Home (DSM-H) program: an interprofessional home health care intervention to improve the quality of life for persons with dementia and their caregivers. Geriatr Nurs 2016; 37: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juniper EF. Validated questionnaires should not be modified. Eur Respir J 2009; 34: 1015–1017. [DOI] [PubMed] [Google Scholar]
- 45.Napoles A, Chadiha L, Eversley R, Moreno-John G. Reviews: developing culturally sensitive dementia caregiver interventions are we there yet? Am J Alzheimers Dis Dementias 2010; 25: 389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinquart M, Sörensen S. Ethnic differences in stressors, resources, and psychological outcomes of family caregiving: a meta-analysis. Gerontologist 2005; 45: 90–106. [DOI] [PubMed] [Google Scholar]
- 47.Pinquart M, Sörensen S. Gender differences in caregiver stressors, social resources, and health: an updated meta-analysis. J Gerontol B 2006; 61: P33–P45. [DOI] [PubMed] [Google Scholar]
- 48.Sun F, Ong R, Burnette D. The influence of ethnicity and culture on dementia caregiving: a review of empirical studies on Chinese Americans. Am J Alzheimers Dis Other Demen 2012; 27: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morano CL, King MD. Lessons learned from implementing a psycho-educational intervention for African American dementia caregivers. Dementia 2010; 9: 558–568. [Google Scholar]
- 50.Gaugler JE, Hobday JV, Robbins JC, Barclay MP. Direct care worker training to respond to the behavior of individuals with dementia: the CARES® dementia-related behavior™ online program. Gerontol Geriatr Med 2016; 2 10.1177/2333721415626888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DiZazzo-Miller R, Winston K, Winkler SL, Donovan ML. Family caregiver training program (FCTP): a randomized controlled trial. Am J Occup Ther 2017; 71: 7105190010p1–7105190010p10. [DOI] [PubMed] [Google Scholar]
- 52.Roberts JS, Silverio E. Evaluation of an education and support program for early-stage Alzheimer’s disease. J Appl Gerontol 2009; 28: 419–435. [Google Scholar]
- 53.Gaugler JE, Hobday JV, Robbins JC, Barclay MP. CARES® dementia care for families: effects of online, psychoeducational training on knowledge of person-centered care and satisfaction. J Gerontol Nurs 2015; 41: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karlin BE, Young D, Dash K. Empowering the dementia care workforce to manage behavioral symptoms of dementia: development and training outcomes from the VOICE dementia care program. Gerontol Geriatr Educ 2017; 38: 375–391. [DOI] [PubMed] [Google Scholar]
- 55.Grigsby TJ, Unger JB, Molina GB et al. Evaluation of an audio-visual novela to improve beliefs, attitudes and knowledge toward dementia: a mixed-methods approach. Clin Gerontol 2017; 40: 130–138. [DOI] [PubMed] [Google Scholar]
- 56.Elvish R, Burrow S, Cawley R, Harney K, Keady J. Getting to know me: the second phase roll-out of a staff training programme for supporting people with dementia in general hospitals. Dementia 2018; 17: 96–109. [DOI] [PubMed] [Google Scholar]
- 57.DiZazzo-Miller R, Samuel PS, Barnas JM, Welker KM. Addressing everyday challenges: feasibility of a family caregiver training program for people with dementia. Am J Occup Ther 2014; 68: 2012–2020. [DOI] [PubMed] [Google Scholar]
- 58.Maharaj T Live-model simulation: improving nursing students’ attitudes and knowledge of Alzheimer’s disease. Clin Simul Nurs 2017; 13: 446–451. [Google Scholar]
- 59.Irvine AB, Beaty JA, Seeley JR, Bourgeois M. Use of a dementia training designed for nurse aides to train other staff. J Appl Gerontol 2012; 32: 936–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Xiao LD, Ullah S, He GP, De Bellis A. Evaluation of a nurse-led dementia education and knowledge translation programme in primary care: a cluster randomized controlled trial. Nurse Educ Today 2017; 49: 1–7. [DOI] [PubMed] [Google Scholar]
- 61.Ducharme F, Lachance L, Lévesque L et al. Maintaining the potential of a psycho-educational program: efficacy of a booster session after an intervention offered family caregivers at disclosure of a relative’s dementia diagnosis. Aging Ment Health 2015; 19: 207–216. [DOI] [PubMed] [Google Scholar]
- 62.Pleasant ML, Molinari V, Hobday JV, Fazio S, Cullen N, Hyer K. An evaluation of the CARES® dementia basics program among caregivers. Int Psychogeriatrics 2017; 29: 45–56. [DOI] [PubMed] [Google Scholar]
- 63.Kimzey M, Clinical A, Mastel-smith B, Alfred D. The impact of educational experiences on nursing students’ knowledge and attitudes toward people with Alzheimer’s disease: a mixed method study. Nurs Educ Today 2016; 46: 57–63. [DOI] [PubMed] [Google Scholar]
- 64.Fenley RC, Bober SJ, Powell ME, Berman J, Altman BN. Effect of Alzheimer’s training on multicultural personal care aides. Care Manag J 2008; 9: 4–10. [DOI] [PubMed] [Google Scholar]
- 65.Perales J, Moore WT, Fernandez C et al. Feasibility of an Alzheimer’s disease knowledge intervention in the Latino community. Ethn Health 2018; 18: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tannazzo T, Breuer L, Williams SM, Andreoli NA. A dementia training program to benefit certified nurse assistant satisfaction and nursing home resident outcomes. Alzheimer’s Care Today 2008; 9: 221–229. [Google Scholar]
- 67.Crombie A, Boyd J, Snell T. The ABC of managing behavioral and psychological symptoms of dementia. Geriaction 2008; 26: 14. [Google Scholar]
- 68.Broughton M, Smith ER, Baker R et al. Evaluation of a caregiver education program to support memory and communication in dementia: a controlled pre-test – posttest study with nursing home staff. Int J Nurs Stud 2011; 48: 1436–1444. [DOI] [PubMed] [Google Scholar]
- 69.Bateman C, Anderson K, Bird M, Hungerford C. Volunteers improving person-centred dementia and delirium care in a rural Australian hospital. Rural Remote Health 2016; 16: 3667. [PubMed] [Google Scholar]
- 70.Liddle J, Smith-Conway ER, Baker R et al. Memory and communication support strategies in dementia: effect of a training program for informal caregivers. Int Psychogeriatr 2012; 24: 1927–1942. [DOI] [PubMed] [Google Scholar]
- 71.Khan F, Curtice M. Non-pharmacological management of behavioral symptoms of dementia. Br J Community Nurs 2011; 16: 441–449. [DOI] [PubMed] [Google Scholar]
- 72.Wang F, Xiao LD, Wang K, Li M, Yang Y. Evaluation of a WeChat-based dementia-specific training program for nurses in primary care settings: a randomized controlled trial. Appl Nurs Res 2017; 38: 51–59. [DOI] [PubMed] [Google Scholar]
- 73.Lorio AK, Gore JB, Warthen L et al. Teaching dementia care to physical therapy doctoral students: a multimodal experiential learning approach. Gerontol Geriatr Educ 2017; 38: 313–324. [DOI] [PubMed] [Google Scholar]
- 74.Kouri KK, Ducharme FC, Giroux F. A psycho-educational intervention focused on communication for caregivers of a family member in the early stage of Alzheimer’s disease: results of an experimental study. Dementia 2011; 10: 435–453. [Google Scholar]
- 75.Gilmartin-Thomas JF, McNeil J. Qualitative evaluation of how a virtual dementia experience impacts medical and pharmacy students’ self-reported knowledge and attitudes towards people with dementia. Dementia 2018; 19: 205–220. 10.1177/1471301218770270. [DOI] [PubMed] [Google Scholar]
- 76.Hobday JV, Gaugler JE, Mittelman MS. Feasibility and utility of online dementia care training for hospital staff: the CARES ® dementia-friendly hospital™ program. Res Gerontol Nurs 2017; 10: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fields NL, Xu L, Richardson VE. The senior companion program plus: a culturally tailored psychoeducational training program (innovative practice). Dementia 2016; 19: 453–460. 10.1177/147130121668562. [DOI] [PubMed] [Google Scholar]
- 78.Naughton C, Beard C, Tzouvara V et al. A feasibility study of dementia communication training based on the VERA framework for pre-registration nurses: part II impact on student experience. Nurse Educ Today 2018; 63: 87–93. [DOI] [PubMed] [Google Scholar]
- 79.Eccleston CEA, Lea EJ, Mcinerney F, Crisp E, Marlow A, Robinson AL. An investigation of nursing students’ knowledge of dementia: a questionnaire study. Nurs Educ Today 2015; 35: 800–805. [DOI] [PubMed] [Google Scholar]
- 80.Tomaz JB, Mamede S, Filho JM, Roris Filho JDES, van der Molen HT. Effectiveness of an online problem-based learning curriculum for training family medical doctors in Brazil. Educ Health (Abingdon) 2015; 28: 187–193. [DOI] [PubMed] [Google Scholar]
- 81.Annear MJ, Goldberg LR, Lo A, Robinson A. Interprofessional curriculum development achieves results: initial evidence from a dementia-care protocol. J Interprof Care 2016; 30: 391–393. [DOI] [PubMed] [Google Scholar]
- 82.Carpenter BD, Balsis S, Otilingam PG, Hanson PK, Gatz M. The Alzheimer’s disease knowledge scale: development and psychometric properties. Gerontologist 2009; 49: 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Toye C, Lester L, Popescu A, McInerney F, Andrews S, Robinson AL. Dementia knowledge assessment tool version two: development of a tool to inform preparation for care planning and delivery in families and care staff. Dementia 2014; 13: 248–256. [DOI] [PubMed] [Google Scholar]
- 84.Elvish R, Burrow S, Cawley R et al. ‘Getting to know me’: the development and evaluation of a training programme for enhancing skills in the care of people with dementia in general hospital settings. Aging Ment Health 2014; 18: 481–488. [DOI] [PubMed] [Google Scholar]
- 85.Sörensen S, Pinquart M. Developing a measure of older adults’ preparation for future care needs. Int J Aging Hum Dev 2001; 53: 137–165. [DOI] [PubMed] [Google Scholar]
- 86.Dieckmann L, Zarit SH, Zarit JM, Gatz M. The Alzheimer’s disease knowledge test. Gerontologist 1988; 28: 402–408. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, Xiao LD, He GP. A comprehensive approach to psychometric assessment of instruments used in dementia educational interventions for health professionals: a cross-sectional study. Int J Nurs Stud 2015; 52: 568–577. [DOI] [PubMed] [Google Scholar]
- 88.O’Connor ML, McFadden SH. Development and psychometric validation of the dementia attitudes scale. Int J Alzheimers Dis 2010; 2010: 10. [Google Scholar]
- 89.Gray HL, Jimenez DE, Cucciare MA, Tong HQ, Gallagher-Thompson D. Ethnic differences in beliefs regarding Alzheimer disease among dementia family caregivers. Am J Geriatr Psychiatry 2009; 17: 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shanahan N, Orrell M, Schepers AK, Spector A. The development and evaluation of the DK-20: a knowledge of dementia measure. Int Psychogeriatr 2013; 25: 1899–1907. [DOI] [PubMed] [Google Scholar]


