Abstract
Purpose of review:
Sapovirus, a genus in the Caliciviridae family alongside norovirus, is increasingly recognized as an important cause of childhood diarrhea. Some challenges exist in our ability to better understand sapovirus infections, including the inability to grow sapovirus in cell culture, which has hindered diagnosis and studies of immunity. Another challenge is that individuals with sapovirus infection are commonly co-infected with other enteric pathogens, complicating our ability to attribute the diarrhea episode to a single pathogen.
Recent findings:
Development of molecular methods for sapovirus detection has increased our ability to measure disease prevalence. The prevalence of sapovirus varies between 1 to 17% of diarrhea episodes worldwide, with the highest burden in young children and older adults. Further, epidemiological studies have used novel approaches to account for the presence of co-infections with other enteric pathogens; one multi-site cohort study of children under two years of age found that sapovirus had the second highest attributable incidence among all diarrheal pathogens studied.
Summary:
Especially in settings where rotavirus vaccines have been introduced, efforts to reduce the overall burden of childhood diarrhea should focus on the reduction of sapovirus transmission and disease burden.
Keywords: Sapovirus, diarrhea, gastroenteritis, childhood, Caliciviridae
Introduction
Sapoviruses form a genus in the Caliciviridae family, a family which also includes noroviruses. The burden of sapovirus disease went underestimated for several decades after its discovery due to the lack of diagnostic tests for sapovirus detection other than electron microscopy. With the development and increasing use of molecular diagnostic tests, there is growing evidence of the importance of sapovirus to the global burden of childhood diarrhea. Most notably in countries where rotavirus vaccines have been introduced, sapovirus is among the leading contributors to diarrhea in young children.(1,2) Sapovirus has also been identified as an important cause of diarrhea in older adults, especially those living in long-term care facilities(3), and in individuals with immunocompromising conditions.(4,5) An extensive review of sapovirus was published by Oka, et al, in 2015.(6) This review includes a concise update of the literature on sapovirus since that review.
Text of review
SAPOVIRUS CLASSIFICATION
Sapoviruses were named after an outbreak of diarrhea in children in Sapporo, Japan; (7,8) similar viruses were observed in other countries (Manchester UK 1993 (GI.1), Parkville USA 1997 (GI.2), Bristol UK 1998 (GII.1) and Mex340 (GII.2)).(9–11) Nucleotide sequencing, capsid cloning, and virus-like particles (VLP) synthesis of these Sapporo-like caliciviruses showed genetic and antigenic differences from other Caliciviridae (12–14), and Sapovirus was recognized as a separate genus. (10,15–17) Sapoviruses have been detected in several mammals, including swine, sea lions, dogs, bats, and rats(6), but spillover of animal sapoviruses to humans has not been reported, suggesting host restriction. Overall, 19 sapovirus genogroups (GI - GXIX) have been described, with genogroups GI, GII, GIV and GV limited to humans. A total of 19 genetic variants within genogroups, denominated genotypes, have been described in humans (GI.1–7; GII.1–8; GIV.1 and GV.1–2).
DIAGNOSIS OF SAPOVIRUS
Reverse transcription (RT)-PCR is the most common method of sapovirus diagnosis. However, diagnosis of sapovirus is typically not performed due to the cost and lack of access to PCR in most of the world. Also, detection of sapovirus does not usually change clinical management. However, there are a growing number of multiplex clinical diagnostic tests that include sapovirus.(18) Another challenge is that primers used for sapovirus detection were designed from limited numbers of nucleotides sequences available in the GenBank, thus, current circulating strains might not be efficiently detected.(19) Several efforts have been made to increase the number of full genome sequences (20–22) and develop broadly reactive primers which may be more sensitive.(23) Sapovirus genotyping is often performed for epidemiological studies. Genotyping is more successful for samples with higher viral loads (cycle threshold < 30) (24); the association between viral loads and clinical symptoms remains to be explored.
Due to the limitations of current molecular methods, outbreaks caused by sapovirus may be underestimated. For instance, electron microscopy of stools and whole genome sequencing was required for the identification of sapovirus in a food-borne outbreak in Sweden, because the RT-PCR used for screening was unable to detect this new variant, GV.2.(25,26) The identification of sapovirus as a cause of foodborne or waterborne outbreaks is limited and requires an improvement in molecular methods used for screening.
BURDEN OF DISEASE
Sapoviruses are responsible for both sporadic cases and outbreaks of acute diarrhea. Sapovirus outbreaks have mainly been reported in high-income countries, including in day care centers,(27,28) schools,(27,29,30) a military institution,(31) hospitals,(32,33) and nursing homes.(3,27,34,35) The detection frequency of sapovirus is variable across geographic locations and age groups, but has been reported to be associated with 1% to 17% of diarrhea cases in different settings (Table 1).
Table 1.
Sapovirus detection proportion in diarrhea cases or sapovirus diarrhea incidence from studies that involved more than 300 participants during the period 2014–2020
| Publication Year | Setting | Country | Study Design | Time Frame | Number of Subjects | Age | Detection Proportion by PCR | Reference |
|---|---|---|---|---|---|---|---|---|
| 2014 | Inpatients | Thailand | Prospective, facility-based | January to December 2007, 2010 to 2011 | 567 | < 5 years | 1.2% | (45) |
| 2014 | Outpatients | China | Prospective, facility-based | January to December 2011 | 1110 | ≤60 years | 1.5% | (46) |
| 2014 | Inpatients or outpatients | China | Prospective, facility-based | January 2006 to December 2011 | 334 | < 5 years | 0.60% | (47) |
| 2014 | Community | Nicaragua | Prospective, community-based cohort | January 2010 to January 2011 | 826 | <5 years | 17%; Incidence: 19 per 100 child-years | (38) |
| 2014 | Inpatients, outpatients, and community | Nicaragua | Prospective, facility-based | September 2009 to October 2010 | 330 | ≤ 5 years | 17% | (48) |
| 2015 | Outpatients | Japan | Retrospective, facility-based | 2010 to 2012 | 751 | <15 years | 4.8% | (49) |
| 2015 | Inpatients and outpatients | China | Prospective, facility-based | July 2010 to December 2012 | 1128 | <14 years | 1.2% | (50) |
| 2015 | Outpatients | China | Prospective, facility-based | January 2010 to December 2011 | 436 | ≤5 years | 0.46% | (51) |
| 2015 | Outpatients | China | Prospective, facility-based | April 2011 to March 2013 | 3832 | All ages | 8.3% | (52) |
| 2016 | Inpatients | South Africa | Prospective, facility-based | 2009 to 2013 | 3103 | <5 years | 7.7% | (36) |
| 2016 | Inpatient and emergency department visits | Canada | Prospective, facility-based | February 2012 to May 2014 | 734 | 8 weeks to < 3 years | 6.4% | (53) |
| 2016 | Community | Peru | Prospective, community-based cohort | 2009 to 2010 | 300 diarrheal stools | <2 years | 12% | (54) |
| 2016 | Outpatients | USA | Prospective, facility-based | August 2012 to September 2013 | 1099 | All ages | 2%; Incidence: 1.6 per 100 person-years | (55) |
| 2016 | Outpatients | Japan | Retrospective, facility-based | September 2008 to August 2014 | 1871 | 2 weeks - 84 years | 3.6% | (56) |
| 2016 | Inpatients | China | Prospective, facility-based | May 2011 to January 2013 | 461 | <5 years | 6.5% | (57) |
| 2016 | Outpatients | Dutch | Prospective, day care-based | April 2013 to October 2014 | 981 children | <5 years | 3.8% | (58) |
| 2016 | Inpatients (children only) and outpatients | Brazil | Prospective, facility-based | January 2012 to December 2014 | 341 | All ages | 3.5% | (59) |
| 2016 | Inpatients and outpatients | Burkina Faso | Prospective, facility-based | November 2011 to September 2012 | 263 with diarrhea; 50 controls | <5 years | 10% | (60) |
| 2017 | Inpatients | Thailand | Prospective, facility-based | January 2012 to December 2014 | 889 | <5 years | 0.7% | (61) |
| 2017 | Not stated | Japan | Retrospective, facility-based | September 2009 to August 2015 | 948 | <15 years | 7.5% | (62) |
| 2017 | Inpatients and outpatients | Burkina Faso | Prospective, facility-based | November 2011 to September 2012 | 443 | All ages (263 <5 years) | 8.8% | (63) |
| 2017 | Inpatients and outpatients | USA | Prospective, facility-based | October 2010 to September 2012 | 1089 | <18 years | 5.9% | (64) |
| 2018 | Inpatients | Russia | Retrospective, facility-based | January 2009 to January 2014 | 469 | <5 years old | 1.4% | (65) |
| 2018 | Community | Multicenter (Brazil, Bangladesh, India, Nepal, Pakistan, Peru, South Africa, Tanzania) | Prospective, community-based cohort | November 2009 to February 2012 | 1715 | <2 years old | Attributable incidence: 22.8 per 100 children-years | (1) |
| 2018 | Inpatients | Italy | Prospective, facility-based | November 2015 to October 2016 | 510 | 2 months to 15 years | 6.0% | (66) |
| 2019 | Inpatients | Thailand | Prospective, facility-based | January 2015 to February 2017 | 843 | 2 months to 15 years |
1.9% | (67) |
| 2019 | Outpatients | Ethiopia | Prospective, facility-based | November 2015 and April 2016 | 450 | <5 years | 10% | (68) |
| 2019 | Inpatients and emergency department visits | Finland | Prospective, facility-based | 2006 to 2014 | 1437 | <16 years | 1.4% before rotavirus vaccine introduction; 5.5% after introduction | (69) |
| 2019 | Outpatients | Spain | Prospective, facility-based | July 2010 to June 2011 | 2667 | All ages | 16% | (24) |
| 2019 | Outpatients | Spain | Retrospective, facility-based | January to April in 2016 and 2017 | 384 | ≤ 5years | 13% among samples testing negative for rotavirus, adenovirus, and enteropathogenic bacteria | (70) |
| 2019 | Inpatients and outpatients | Guatemala | Prospective, facility-based | January 2014 to December 2015 | 471 | <5 years | 7.4% | (71) |
| 2019 | Inpatients | Germany | Retrospective, facility-based | January 2010 to September 2018 | 5333 | All ages | 1.8% | (4) |
| 2019 | Inpatients | Germany | Prospective and retrospective, facility-based | 2008 to 2009 and 2010 to 2018 | 5897 | <5 years (2008 to 2009) and all ages (2010 to 2018) | 2.4% | (72) |
| 2019 | Inpatients and emergency department visits | USA | Prospective, facility-based | January to December 2012 | 330 cases | <2 years | 3.0% | (73) |
| 2019 | Outpatients | China | Prospective, facility-based | January 2010 to December 2014 | 4,548 | All ages | 1.1% | (74) |
| 2020 | Community and inpatients | China | Prospective, facility-based and community-based cohort | October 2011 to December 2013 | 10,056 | <5 years | 9.6%; Incidence: 12.4 and 11.7 per 1000 child-years in Zhengding and Sanjiang, respectively | (75) |
| 2020 | Inpatients | Thailand | Prospective, facility-based | 2010 to 2018 | 3057 | <15 years | 1.6% | (76) |
| 2020 | Inpatients and outpatients | USA | Prospective, facility-based | January 2012 to November 2015 | 3705 cases | 15 days to 17 years | 10% | (2) |
Sapovirus was detected in 10% of US children <18 years of age receiving care for diarrhea in both the inpatient and outpatient settings(2), 8% of South African children <5 years of age hospitalized for diarrhea,(36) and 12–19% of young children with diarrhea in Central American cohorts.(37,38) In general, studies limited to children, conducted in LMICs, focused on studying sapovirus, and performed in recent years show higher detection of sapovirus. It is possible that increased detection in recent years may be due to improvements in molecular detection, including the use of more specific primers(23), and the roll-out of rotavirus vaccines, which increases the proportion of remaining diarrhea cases in which sapovirus is detected. Finally, most but not all studies that include healthy controls show lower sapovirus detection in stools from asymptomatic as compared to symptomatic individuals.(1,2,37–41)
One factor that complicates our understanding of the burden of sapovirus disease is the common finding of co-infections with multiple enteric pathogens, especially in LMIC settings.(1,42,43) The multi-site Malnutrition and Enteric Disease (MAL-ED) study addressed this problem by estimating the attributable burden of each enteric pathogen by accounting for detection in healthy control children. Using this approach, MAL-ED reported sapovirus to have the third highest attributable incidence for diarrhea among children under 12 months of age, and the second highest attributable incidence among children between 12 and 24 months of age.(1) Further, in the three MAL-ED research sites where rotavirus vaccines had been introduced, the attributable incidence of sapovirus had a higher point estimate than rotavirus. However, unlike several other enteric pathogens, sapovirus diarrhea was not associated with delays in linear growth in children.(44)
BREATH AND DISTRIBUTION OF SAPOVIRUS GENOTYPES
Understanding genotype distribution provides insights into patterns of transmission, appropriate diagnosis of circulating strains, immunity in populations, and future vaccine development. GI.1 remains the most common genotype across age groups and recruitment settings, followed by GII.1, GII.2 and GI.1.(24,72) In surveillance studies of children with sapovirus diarrhea, the frequency of genotype GI.1 can range from 10% to 63%, with GI.2 and GII.1 the second most commonly reported genotypes. A hospital-based study showed fluctuation of genotypes between sampling years and geographic areas.(77) Varela and coworkers observed that the dominant genotypes, GI.1, GI.2 and GII.2, were more frequently detected in the first year of life but also in older adults.(24) It is possible that infection from these common sapovirus genotypes early in life may induce natural protection that wanes later in life. Another interesting trend is that studies reporting high frequencies of GI.1 usually report low frequencies of GI.2 and GII.1; and likewise, studies with high frequencies of GI.2 and GII.1 report lower frequencies of GI.1(60,62,78) (Fig. 1). GII.3, GII.4 and GII.5 have been reported at high frequencies in some studies and have been described as emerging genotypes.(48,61,79) In asymptomatic subjects, the distribution of genotypes is similar to that observed in symptomatic subjects with GI.1 ranging from 18% to 75%, followed by GI.2, GII.1 and GII.2.(73,78,81,82) The most common genogroups observed in outbreaks are GI and GIV.(83)Finally, unlike norovirus, which evolves to cause periodic global outbreaks from genetic variants, sapovirus showed limited intra-genotype diversity over a forty year period.(80)
Figure 1.
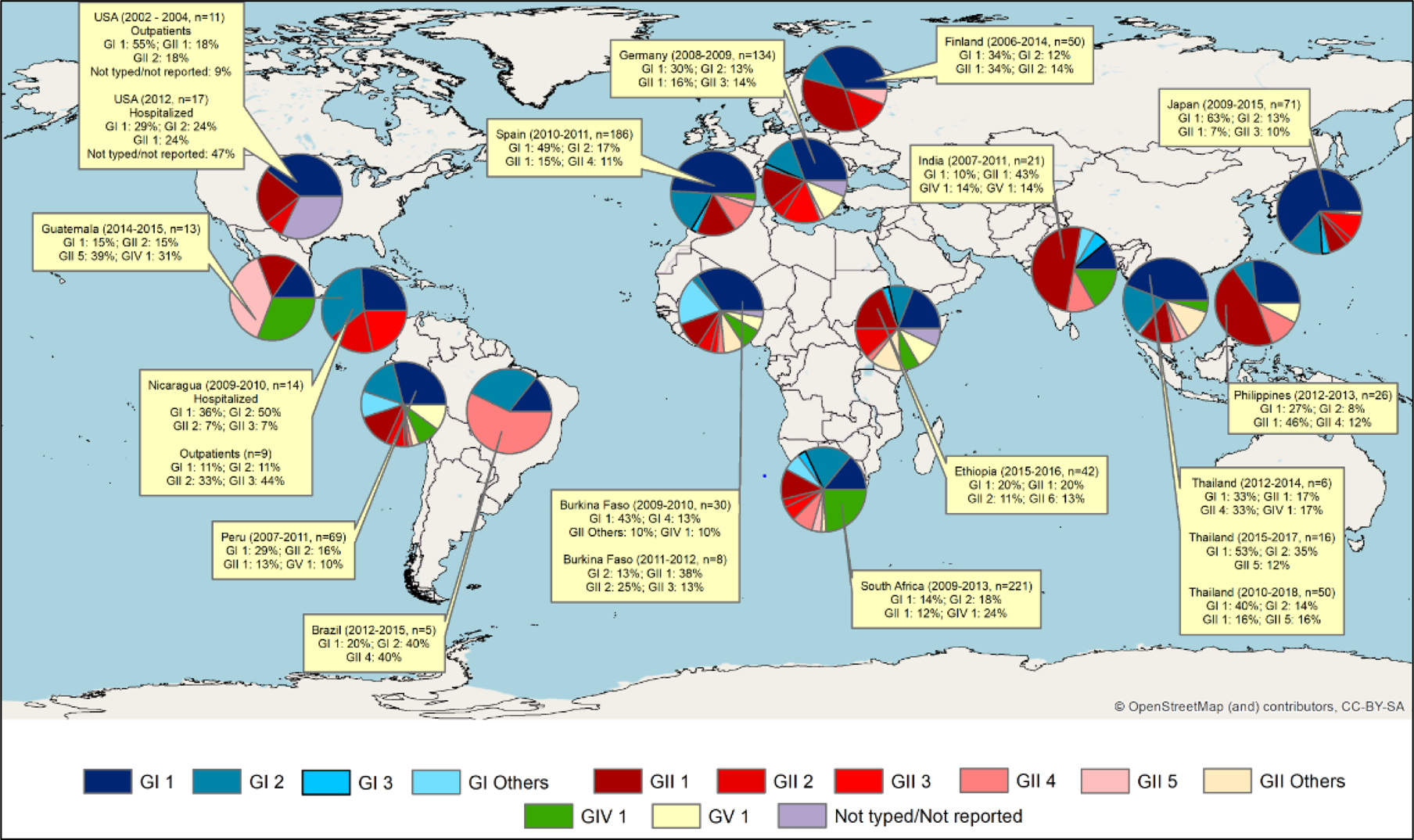
Sapovirus genotypes reported in surveillance studies lasting at least one year and using RT-PCR for sapovirus detection in diarrheic stools in the last decade.
In these studies, genotypes were assigned according to phylogenetic analysis of partial capsid gene, with some exceptions that used near full genomes. ArcGis 10.7 was used to display sapovirus distribution by country and study. The basic map was provided by OpenStreetMap.
Sapovirus genotype diversity may have implications for the development of broadly protective sapovirus vaccines. However, more studies are needed to understand the relationship between genotypes and antigenic types, as is it not currently known whether the immune response to natural sapovirus infections are genotype- or genogroup-specific or cross-reactive. One important challenge for these studies is the absence of cell culture systems for antigen production and for viral neutralization studies which could inform the appropriate vaccine design.
CHALLENGES TO STUDYING SAPOVIRUS
Human sapovirus cannot be adapted and propagated in the most efficient cell culture systems currently available and no animal model is available.(84) Such limitations preclude efforts to better understand the replication cycle and pathogenesis, and deter vaccine development and the search for agents that limit viral replication. Moreover, studies to understand the breath of the immune response are also challenging. An important advance was the transformation of cloned capsid genes from sapovirus into insect cells, which then assembles into VLPs providing opportunities to study antigenicity among genogroups and genotypes; however, expression levels are low, making it difficult to use these approaches in large studies. (85–87) The lack of efficient cell culture also limits the production of reagents (antigens and monoclonal antibodies) for rapid diagnostic tests. The observation that human norovirus, another difficult to culture calicivirus, was able to replicate in stem cell-derived human enteroids (88), suggests that sapovirus propagation may be possible in this system.
TRANSMISSION AND RISK FACTORS FOR SAPOVIRUS DIARRHEA
Sapovirus is thought to spread person-to-person,(89) through wastewater intrusion of drinking water sources,(90) and in contaminated food.(26,91,92) Household crowding(36) and having recent contact with individuals with gastrointestinal symptoms have been identified as risk factors for sapovirus diarrhea.(89) Among food-borne transmissions, shellfish are a recognized source of sapovirus infections.(93–95)
Sapovirus was reported to shed in the stool for a median of 18.5 days in children, with no difference in shedding duration between symptomatic and asymptomatic infections.(82) Children infected with sapovirus can shed approximately 1 million genome copies of sapovirus per gram of stool (range: 2 × 103 and 7 × 1011).(24,96,97) A study of children in Danish day cares found that 21% of all children shed sapovirus, suggesting that day cares may act as year-round reservoirs for sapovirus transmission.(39)
Age is a clear risk factor for sapovirus diarrhea: children under age 5 are at highest risk, followed by older adults.(98) Sapovirus caused 13% of gastroenteritis outbreaks in nursing homes studied in England.(3) A study of outpatients with diarrhea in Spain found that sapovirus detection followed a U-shaped curve: highest in children aged 0–2 years, decreasing in older children and young adults, and then increasing in adults > 60 years of age.(24) Among children, the incidence is greatest in the second year of life,(36) once maternal immunity wanes and the child is increasingly exposed to contamination of food or the environment. A birth cohort study in Peru fround the incidence of sapovirus infection in the first and second years of life was 4.3 and 11.1 per 100 child-months, respectively. By age 2 years, 82% of children had experienced at least one sapovirus infection, and 64% had experienced at least one sapovirus diarrhea episode.(82) Unlike norovirus infection, sapovirus infection does not appear to be associated with host histo-blood group antigen expression.(101,102) Finally, it is not known if contact with animals is a risk factor for sapovirus; the zoonotic potential of sapovirus needs further investigation.(99,100)
Sapovirus diarrhea can occur throughout the year, however, it was most frequently found in June and July in Japan.(62) In Spain, detection peaked during autumn and early spring,(24) while in the Netherlands, sapovirus was most commonly detected in the winter and spring.(58) In Nicaragua, sapovirus was most frequently detected early during the rainy season, and in a Peruvian birth cohort, sapovirus risk increased following La Niña-associated flooding.(101)
CLINICAL SYMPTOMS
Clinical symptoms of sapovirus infection are similar to other intestinal viruses. Common symptoms of sapovirus infection include diarrhea, nausea, vomiting, and abdominal pain which usually resolve within one week.(6,40,102) A birth cohort in Peru reported that 40% of children with sapovirus diarrhea experienced vomiting and 10% experienced fever.(40) As compared to norovirus episodes, sapovirus episodes had less vomiting,(102,103) and as compared to rotavirus episodes, sapovirus episodes had less fever.(103)
While earlier studies suggested that sapovirus resulted in milder symptoms as compared to diarrhea from other causes(103,104), more recent studies show that sapovirus is similar in severity to other etiologies(37,105) and is also detected in hospitalized children,(48,64,69,105) ranging from 15%(48) of children hospitalized for diarrhea in Nicaragua to 4% in a US-based study.(64) A hospital-based study in South Africa identified sapovirus in 11% of child deaths due to diarrhea.(36) Finally, while primarily associated with acute diarrhea, sapovirus has also been detected in cases of chronic diarrhea, particularly in individuals with immunocompromising conditions.(4,5)
DEVELOPMENT OF IMMUNITY
Humoral immunity to sapovirus has been demonstrated in acute and convalescent samples using purified viruses from infected stool samples.(7) Further, seroprevalence studies conducted in the years after sapovirus had been identified showed rising seroprevalence with age, reaching a high level in older children that continues into adulthood.(106) As the intestinal mucosa is the location of infection, it is presumed that IgA antibodies are important to immune protection and that innate immune activation is mediated through the interferon pathway.(107) Chronic sapovirus infections in individuals with immune deficiencies or those taking immunosuppressant medications show that clearance of infections is dependent on intact host immune responses.(4,108) There is little known about the breath of immunity following a sapovirus infection. Studies using hyperimmune sera show strong reactivity against VLPs of homologous sapoviruses, but little cross reactivity between genogroups, suggesting that human sapovirus genogroups are antigenically distinct.(17)
Observational field studies show that sapovirus diarrhea is more common in young children as compared to older children and adults, suggesting that early life infections provide immunity.(102,109) A Peruvian birth cohort found that repeat sapovirus infections do occur, but repeat infections with same genotype are rare: over two years of surveillance; 59/82 children who experienced a sapovirus infection went on to have a repeat sapovirus infection, however only three repeat infections were of the same genotype.(82) Taken as a whole, these data suggest that first sapovirus infections provide immunity against homologous infections, but little protection against infections of different genogroups. Further, the low incidence of symptomatic sapovirus episodes in the first months of life suggests early protection from maternal immunity.
A recent study by Rogawski, et al,(110) examined the protective efficacy of first enteric infections on subsequent infections in MAL-ED; they found that a first sapovirus infection provided modest protection against subsequent infections [adjusted Hazard Ratio of 0.78, 95% confidence interval (0.69, 0.88)]. Now that more tools to study sapovirus immunity are becoming available, research is needed to understand the bredth of immunity following infection and to identify protective epitopes. This knowledge would improve our understanding of the development of natural immunity and guide future vaccine development.
TREATMENTS
There are no specific therapeutics agents or vaccines for sapovirus diarrhea. Currently, the World Health Organization recommends treating diarrhea with oral rehydration solution, usual nutrition or continued breastfeeding, and zinc supplementation (in LMIC).(111) Anti-motility agents, such as loperamide, should be avoided in children <18 years, however, while not FDA-approved, the Infectious Diseases Society of America includes the anti-nausea medicine, ondansetron, as an option for individuals >4 years of age with diarrhea to support oral rehydration.(112) Rapid intravenous hydration should be provided to individuals with severe dehydration resulting from diarrhea.(111)
PREVENTION OF SAPOVIRUS INFECTION
While there is little known specifically about the environmental decontamination of sapoviruses, measures used to control norovirus are also likely effective against sapovirus, such as frequent hand washing, proper disposal of fecal- or vomit-soiled materials, limiting contact with infected individuals, and environmental disinfection with chlorine-containing cleaning agents.(113,114) As for norovirus, the safe handling of food would likely decrease food-borne transmission of sapovirus. While improving water quantity and quality is likely to contribute to decrease sapovirus transmission, the high burden of sapovirus in both high- and low-income settings argues against the potential that improvements in water sources alone will eliminate sapovirus burden. There is little known about the potential for breastfeeding to protect against sapovirus diarrhea in infants and no licensed vaccines against sapoviruses are available.
Conclusion
With increasing molecular detection, sapovirus is now recognized as an important cause of diarrhea, primarily in young children. As there are no vaccines currently available against sapovirus, prevention includes hygiene, safe food handling, and improved water sources. More research is needed to better understand transmission patterns and risk factors for sapovirus infection to guide prevention efforts. Treatment of sapovirus diarrhea episodes is similar to diarrhea due to other causes and includes the use of oral rehydration. Limited immunological and epidemiological data suggest that children develop natural immunity to sapovirus, providing optimism for the potential success of future sapovirus vaccines.
Key points.
With the increasing use of molecular methods of detection, sapovirus is now recognized as an important cause of diarrhea in children and older adults worldwide.
The severity of illness of sapovirus infections is similar to that of other intestinal viruses; sapovirus can also cause chronic diarrhea, particularly in individuals with immunocompromising conditions.
Observational studies suggest that sapovirus infections during early childhood confer immunity and repeat infections with the same sapovirus genotype are rare.
Sapovirus can spread person-to-person, and through contaminated drinking water and food; day care centers may serve as year-round reservoirs of sapovirus transmission.
Acknowledgements
S.B.D. is supported by NIH awards K24AI141744 and R01AI127845. F.G. is supported by a research capacity-building award from the NIH: D43TW010923. F.B. is supported by NIH award R01AI127845. We appreciate the technical support provided by Christian Toval for the figure preparation.
This work was supported by funding from the National Institutes of Health (NIH).
Footnotes
Conflicts of interest:
There are no conflicts of interest.
References
- 1.Platts-Mills JA, Liu J, Rogawski ET, Kabir F, Lertsethtakarn P, Siguas M, et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Heal. 2018;6(12):e1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Halasa N, Piya B, Stewart LS, Rahman H, Payne DC, Woron A, et al. The Changing Landscape of Pediatric Viral Enteropathogens in the Post-Rotavirus Vaccine Era. Clin Infect Dis. 2020. February; [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent large study of children <18 years old with gastroenteritis conducted in the hospital, ED, and outpatient settings in the US that detected sapovirus in 10% of stools from children with diarrhea vs. only 1% in control stools.
- 3**.Inns T, Wilson D, Manley P, Harris JP, O’Brien SJ, Vivancos R. What proportion of care home outbreaks are caused by norovirus? An analysis of viral causes of gastroenteritis outbreaks in care homes, North East England, 2016–2018. BMC Infect Dis. 2019. December;20(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of gastroenteritis outbreaks in nursing homes in England detected sapovirus as the cause of 13% of outbreaks.
- 4.Pietsch C, Liebert UG. Intrahost viral evolution during chronic sapovirus infections. J Clin Virol. 2019. April;113:1–7. [DOI] [PubMed] [Google Scholar]
- 5.Daniel-Wayman S, Fahle G, Palmore T, Green KY, Prevots DR. Norovirus, astrovirus, and sapovirus among immunocompromised patients at a tertiary care research hospital. Diagn Microbiol Infect Dis. 2018. October;92(2):143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oka T, Wang Q, Katayama K, Saifb LJ. Comprehensive review of human sapoviruses. Clin Microbiol Rev. 2015;28(1):32–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiba S, Sakuma Y, Kogasaka R, Akihara M, Horino K, Nakao T, et al. An outbreak of gastroenteritis associated with calicivirus in an infant home. J Med Virol. 1979;4(4):249–54. [DOI] [PubMed] [Google Scholar]
- 8.Kogasaka R, Nakamura S, Chiba S, Sakuma Y, Terashima H, Yokoyama T, et al. The 33- to 39-nm virus-like particles, tentatively designed as Sapporo agent, associated with an outbreak of acute gastroenteritis. J Med Virol. 1981;8(3):187–93. [DOI] [PubMed] [Google Scholar]
- 9.Noel JS, Liu BL, Humphrey CD, Rodriguez EM, Lambden PR, Clarke IN, et al. Parkville virus: a novel genetic variant of human calicivirus in the Sapporo virus clade, associated with an outbreak of gastroenteritis in adults. J Med Virol. 1997. June;52(2):173–8. [PubMed] [Google Scholar]
- 10.Nakata S, Honma S, Numata KK, Kogawa K, Ukae S, Morita Y, et al. Members of the family caliciviridae (Norwalk virus and Sapporo virus) are the most prevalent cause of gastroenteritis outbreaks among infants in Japan. J Infect Dis. 2000. June;181(6):2029–32. [DOI] [PubMed] [Google Scholar]
- 11.Robinson S, Clarke IN, Vipond IB, Caul EO, Lambden PR. Epidemiology of human Sapporo-like caliciviruses in the South West of England: molecular characterisation of a genetically distinct isolate. J Med Virol. 2002. June;67(2):282–8. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Cubitt WD, Berke T, Zhong W, Dai X, Nakata S, et al. Sapporo-like human caliciviruses are genetically and antigenically diverse. Arch Virol. 1997;142(9):1813–27. [DOI] [PubMed] [Google Scholar]
- 13.Liu BL, Clarke IN, Caul EO, Lambden PR. Human enteric caliciviruses have a unique genome structure and are distinct from the Norwalk-like viruses. Arch Virol. 1995;140(8):1345–56. [DOI] [PubMed] [Google Scholar]
- 14.Farkas T, Zhong WM, Jing Y, Huang PW, Espinosa SM, Martinez N, et al. Genetic diversity among sapoviruses. Arch Virol. 2004. July;149(7):1309–23. [DOI] [PubMed] [Google Scholar]
- 15.Numata K, Hardy ME, Nakata S, Chiba S, Estes MK. Molecular characterization of morphologically typical human calicivirus Sapporo. Arch Virol. 1997;142(8):1537–52. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi K, Tatsumi M, Kinoshita-Numata K, Tsugawa T, Nakata S, Tsutsumi H. Full sequence analysis of the original Sapporo virus. Microbiol Immunol. 2011. September;55(9):657–60. [DOI] [PubMed] [Google Scholar]
- 17.Hansman GS, Oka T, Sakon N, Takeda N. Antigenic diversity of human sapoviruses. Emerg Infect Dis. 2007. October;13(10):1519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman K, Mistry H, Tsertsvadze A, Royle P, McCarthy N, Taylor-Phillips S, et al. Multiplex tests to identify gastrointestinal bacteria, viruses and parasites in people with suspected infectious gastroenteritis: a systematic review and economic analysis. Health Technol Assess. 2017. April;21(23):1–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oka T, Katayama K, Hansman GS, Kageyama T, Ogawa S, Wu F-T, et al. Detection of human sapovirus by real-time reverse transcription-polymerase chain reaction. J Med Virol. 2006. October;78(10):1347–53. [DOI] [PubMed] [Google Scholar]
- 20.Diez-Valcarce M, Castro CJ, Marine RL, Halasa N, Mayta H, Saito M, et al. Genetic diversity of human sapovirus across the Americas. J Clin Virol. 2018. July;104:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diez-Valcarce M, Montmayeur A, Tatusov R, Vinje J. Near-Complete Human Sapovirus Genome Sequences from Kenya. Microbiol Resour Announc. 2019. February;8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oka T, Iritani N, Okada M, Ogawa T, Iizuka S, Tatsumi C, et al. First Complete Genome Sequences of Human Sapovirus Strains Classified as GI.3, GI.4, GI.6, GI.7, and GII.7. Genome Announc. 2018. March;6(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oka T, Iritani N, Yamamoto SP, Mori K, Ogawa T, Tatsumi C, et al. Broadly reactive real-time reverse transcription-polymerase chain reaction assay for the detection of human sapovirus genotypes. J Med Virol. 2019. March;91(3):370–7. [DOI] [PubMed] [Google Scholar]
- 24**.Varela MF, Rivadulla E, Lema A, Romalde JL. Human Sapovirus among Outpatients with Acute Gastroenteritis in Spain: A One-Year Study. Viruses. 2019. February;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]; This large, year-long study of outpatients with acute gastroenteritis in Spain found sapovirus to be the second most commonly identified enteric pathogen after norovirus. Also, they found that detection rate had a U-shaped curve, highest in young children, low in older children and young adults, and rising again in older adults. This suggests that infections early in life result in immunity, that may decline with aging.
- 25.Hallstrom B, Lagerqvist N, Lind-Karlberg M, Helgesson S, Follin P, Hergens M-P, et al. Complete Genome Sequence of a Sapporo Virus GV.2 Variant from a 2016 Outbreak of Gastroenteritis in Sweden. Genome Announc. 2017. February;5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hergens M-P, Nederby Ohd J, Alm E, Askling HH, Helgesson S, Insulander M, et al. Investigation of a food-borne outbreak of gastroenteritis in a school canteen revealed a variant of sapovirus genogroup V not detected by standard PCR, Sollentuna, Sweden, 2016. Euro Surveill Bull Eur sur les Mal Transm = Eur Commun Dis Bull. 2017. June;22(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iritani N, Yamamoto SP, Abe N, Kubo H, Oka T, Kaida A. Epidemics of GI.2 sapovirus in gastroenteritis outbreaks during 2012–2013 in Osaka City, Japan. J Med Virol. 2016. July;88(7):1187–93. [DOI] [PubMed] [Google Scholar]
- 28.Lyman WH, Walsh JF, Kotch JB, Weber DJ, Gunn E, Vinje J. Prospective study of etiologic agents of acute gastroenteritis outbreaks in child care centers. J Pediatr. 2009. February;154(2):253–7. [DOI] [PubMed] [Google Scholar]
- 29.Wang Md J, Li PhD Y, Kong Md X, Li PhD H, Zhang Ba Q, Jin PhD M, et al. Two gastroenteritis outbreaks caused by sapovirus in Shenzhen, China. J Med Virol. 2018. November;90(11):1695–702. [DOI] [PubMed] [Google Scholar]
- 30.Hassan-Rios E, Torres P, Munoz E, Matos C, Hall AJ, Gregoricus N, et al. Sapovirus gastroenteritis in preschool center, Puerto Rico, 2011 Vol. 19, Emerging infectious diseases. United States; 2013. p. 174–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neo FJX, Loh JJP, Ting P, Yeo WX, Gao CQH, Lee VJM, et al. Outbreak of caliciviruses in the Singapore military, 2015. BMC Infect Dis. 2017. November;17(1):719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pang XL, Lee BE, Tyrrell GJ, Preiksaitis JK. Epidemiology and genotype analysis of sapovirus associated with gastroenteritis outbreaks in Alberta, Canada: 2004–2007. J Infect Dis. 2009. February;199(4):547–51. [DOI] [PubMed] [Google Scholar]
- 33.Johansson PJH, Bergentoft K, Larsson PA, Magnusson G, Widell A, Thorhagen M, et al. A nosocomial sapovirus-associated outbreak of gastroenteritis in adults. Scand J Infect Dis. 2005;37(3):200–4. [DOI] [PubMed] [Google Scholar]
- 34.Nidaira M, Taira K, Kato T, Arakaki E, Kyan H, Takara T, et al. Phylogenetic analysis of sapovirus detected from an outbreak of acute gastroenteritis on Ishigaki Island (Okinawa Prefecture, Japan) in 2012. Jpn J Infect Dis. 2014;67(2):141–3. [DOI] [PubMed] [Google Scholar]
- 35.Lee LE, Cebelinski EA, Fuller C, Keene WE, Smith K, Vinje J, et al. Sapovirus outbreaks in long-term care facilities, Oregon and Minnesota, USA, 2002–2009. Emerg Infect Dis. 2012. May;18(5):873–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Page N, Groome MJ, Murray T, Nadan S, Netshikweta R, Keddy KH, et al. Sapovirus prevalence in children less than five years of age hospitalised for diarrhoeal disease in South Africa, 2009–2013. J Clin Virol. 2016;78:82–8. [DOI] [PubMed] [Google Scholar]
- 37.Gaensbauer JT, Lamb M, Calvimontes DM, Asturias EJ, Kamidani S, Contreras-Roldan IL, et al. Identification of Enteropathogens by Multiplex PCR among Rural and Urban Guatemalan Children with Acute Diarrhea. Am J Trop Med Hyg. 2019. September;101(3):534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becker-Dreps S, Bucardo F, Vilchez S, Zambrana LE, Liu L, Weber DJ, et al. Etiology of childhood diarrhea after rotavirus vaccine introduction: a prospective, population-based study in Nicaragua. Pediatr Infect Dis J. 2014. November;33(11):1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Hebbelstrup Jensen B, Jokelainen P, Nielsen ACY, Franck KT, Rejkjaer Holm D, Schonning K, et al. Children Attending Day Care Centers are a Year-round Reservoir of Gastrointestinal Viruses. Sci Rep. 2019. March;9(1):3286. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examined fecal shedding of enteric viruses in children under age 6 attending day care. They found that the prevalence of sapovirus shedding was 21%. The authors state that this high prevalence of shedding suggest that day care centers may be a reservoir for year-round transmission of these enteric viruses.
- 40.Liu X, Jahuira H, Gilman RH, Alva A, Cabrera L, Okamoto M, et al. Etiological role and repeated infections of sapovirus among children aged less than 2 years in a cohort study in a peri-urban community of Peru. J Clin Microbiol. 2016;54(6):1598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sala MR, Broner S, Moreno A, Arias C, Godoy P, Minguell S, et al. Cases of acute gastroenteritis due to calicivirus in outbreaks: clinical differences by age and aetiological agent. Clin Microbiol Infect. 2014. August;20(8):793–8. [DOI] [PubMed] [Google Scholar]
- 42.Lekana-Douki SE, Kombila-Koumavor C, Nkoghe D, Drosten C, Drexler JF, Leroy EM. Molecular epidemiology of enteric viruses and genotyping of rotavirus A, adenovirus and astrovirus among children under 5 years old in Gabon. Int J Infect Dis. 2015. May;34:90–5. [DOI] [PubMed] [Google Scholar]
- 43.Okitsu S, Khamrin P, Takanashi S, Thongprachum A, Hoque SA, Takeuchi H, et al. Molecular detection of enteric viruses in the stool samples of children without diarrhea in Bangladesh. Infect Genet Evol. 2020. January;77:104055. [DOI] [PubMed] [Google Scholar]
- 44.Rogawski ET, Liu J, Platts-Mills JA, Kabir F, Lertsethtakarn P, Siguas M, et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Heal. 2018. December;6(12):e1319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaimongkol N, Khamrin P, Malasao R, Thongprachum A, Kongsricharoern T, Ukarapol N, et al. Molecular characterization of norovirus variants and genetic diversity of noroviruses and sapoviruses in Thailand. J Med Virol. 2014. July;86(7):1210–8. [DOI] [PubMed] [Google Scholar]
- 46.Wu W, Yang H, Zhang H, Xian H-X, Yao X-J, Zhao D-J, et al. Surveillance of pathogens causing gastroenteritis and characterization of norovirus and sapovirus strains in Shenzhen, China, during 2011. Arch Virol. 2014. August;159(8):1995–2002. [DOI] [PubMed] [Google Scholar]
- 47.Lu L, Zhong H, Xu M, Su L, Cao L, Dong N, et al. Molecular epidemiology of human calicivirus infections in children with acute diarrhea in Shanghai: a retrospective comparison between inpatients and outpatients treated between 2006 and 2011. Arch Virol. 2014. July;159(7):1613–21. [DOI] [PubMed] [Google Scholar]
- 48.Bucardo F, Reyes Y, Svensson L, Nordgren J. Predominance of norovirus and sapovirus in nicaragua after implementation of universal rotavirus vaccination. PLoS One. 2014;9(5):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thongprachum A, Takanashi S, Kalesaran AFC, Okitsu S, Mizuguchi M, Hayakawa S, et al. Four-year study of viruses that cause diarrhea in Japanese pediatric outpatients. J Med Virol. 2015. July;87(7):1141–8. [DOI] [PubMed] [Google Scholar]
- 50.Zhang D-M, Ma M-M, Wen W-T, Zhu X, Xu L, He Z-J, et al. Clinical epidemiology and molecular profiling of human bocavirus in faecal samples from children with diarrhoea in Guangzhou, China. Epidemiol Infect. 2015. August;143(11):2315–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu L, Jia R, Zhong H, Xu M, Su L, Cao L, et al. Molecular characterization and multiple infections of rotavirus, norovirus, sapovirus, astrovirus and adenovirus in outpatients with sporadic gastroenteritis in Shanghai, China, 2010–2011. Arch Virol. 2015. May;160(5):1229–38. [DOI] [PubMed] [Google Scholar]
- 52.Gao Z, Li X, Yan H, Li W, Jia L, Hu L, et al. Human calicivirus occurrence among outpatients with diarrhea in Beijing, China, between April 2011 and March 2013. J Med Virol. 2015. December;87(12):2040–7. [DOI] [PubMed] [Google Scholar]
- 53.Doll MK, Gagneur A, Tapiero B, Charest H, Gonzales M, Buckeridge DL, et al. Temporal Changes in Pediatric Gastroenteritis after Rotavirus Vaccination in Quebec. Pediatr Infect Dis J. 2016. May;35(5):555–60. [DOI] [PubMed] [Google Scholar]
- 54.Liu X, Jahuira H, Gilman RH, Alva A, Cabrera L, Okamoto M, et al. Etiological Role and Repeated Infections of Sapovirus among Children Aged Less than 2 Years in a Cohort Study in a Peri-urban Community of Peru. J Clin Microbiol. 2016. June;54(6):1598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grytdal SP, DeBess E, Lee LE, Blythe D, Ryan P, Biggs C, et al. Incidence of Norovirus and Other Viral Pathogens That Cause Acute Gastroenteritis (AGE) among Kaiser Permanente Member Populations in the United States, 2012–2013. PLoS One. 2016;11(4):e0148395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura N, Kobayashi S, Minagawa H, Matsushita T, Sugiura W, Iwatani Y. Molecular epidemiology of enteric viruses in patients with acute gastroenteritis in Aichi prefecture, Japan, 2008/09–2013/14. J Med Virol. 2016. July;88(7):1180–6. [DOI] [PubMed] [Google Scholar]
- 57.Li LL, Liu N, Humphries EM, Yu JM, Li S, Lindsay BR, et al. Aetiology of diarrhoeal disease and evaluation of viral-bacterial coinfection in children under 5 years old in China: a matched case-control study. Clin Microbiol Infect. 2016. April;22(4):381.e9–381.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heusinkveld M, Mughini-Gras L, Pijnacker R, Vennema H, Scholts R, van Huisstede-Vlaanderen KW, et al. Potential causative agents of acute gastroenteritis in households with preschool children: prevalence, risk factors, clinical relevance and household transmission. Eur J Clin Microbiol Infect Dis. 2016. October;35(10):1691–700. [DOI] [PubMed] [Google Scholar]
- 59.Fioretti JM, Rocha MS, Fumian TM, Ginuino A, da Silva TP, de Assis MR, et al. Occurrence of human sapoviruses in wastewater and stool samples in Rio De Janeiro, Brazil. J Appl Microbiol. 2016. September;121(3):855–62. [DOI] [PubMed] [Google Scholar]
- 60.Ouedraogo N, Kaplon J, Bonkoungou IJO, Traore AS, Pothier P, Barro N, et al. Prevalence and Genetic Diversity of Enteric Viruses in Children with Diarrhea in Ouagadougou, Burkina Faso. PLoS One. 2016;11(4):e0153652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khamrin P, Kumthip K, Supadej K, Thongprachum A, Okitsu S, Hayakawa S, et al. Noroviruses and sapoviruses associated with acute gastroenteritis in pediatric patients in Thailand: increased detection of recombinant norovirus GII.P16/GII.13 strains. Arch Virol. 2017. November;162(11):3371–80. [DOI] [PubMed] [Google Scholar]
- 62.Yoneda M, Nakano M, Sugimoto D, Inada M, Fujitani M, Kitahori Y. Epidemiological Characteristics of Sapovirus and Human Astrovirus Detected among Children in Nara Prefecture, Japan, during the 2009/2010–2014/2015 Seasons. Jpn J Infect Dis. 2017. January;70(1):87–91. [DOI] [PubMed] [Google Scholar]
- 63.Ouedraogo N, Ngangas SMT, Bonkoungou IJO, Tiendrebeogo AB, Traore KA, Sanou I, et al. Temporal distribution of gastroenteritis viruses in Ouagadougou, Burkina Faso: seasonality of rotavirus. BMC Public Health. 2017. March;17(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stockmann C, Pavia AT, Graham B, Vaughn M, Crisp R, Poritz MA, et al. Detection of 23 Gastrointestinal Pathogens Among Children Who Present With Diarrhea. J Pediatric Infect Dis Soc. 2017. September;6(3):231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kiseleva V, Faizuloev E, Meskina E, Marova A, Oksanich A, Samartseva T, et al. Molecular-Genetic Characterization of Human Rotavirus A Strains Circulating in Moscow, Russia (2009–2014). Virol Sin. 2018. August;33(4):304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biscaro V, Piccinelli G, Gargiulo F, Ianiro G, Caruso A, Caccuri F, et al. Detection and molecular characterization of enteric viruses in children with acute gastroenteritis in Northern Italy. Infect Genet Evol. 2018. June;60:35–41. [DOI] [PubMed] [Google Scholar]
- 67.Supadej K, Khamrin P, Kumthip K, Malasao R, Chaimongkol N, Saito M, et al. Distribution of norovirus and sapovirus genotypes with emergence of NoV GII.P16/GII.2 recombinant strains in Chiang Mai, Thailand. J Med Virol. 2019. February;91(2):215–24. [DOI] [PubMed] [Google Scholar]
- 68.Gelaw A, Pietsch C, Mann P, Liebert UG. Molecular detection and characterisation of sapoviruses and noroviruses in outpatient children with diarrhoea in Northwest Ethiopia. Epidemiol Infect. 2019. January;147:e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pitkanen O, Vesikari T, Hemming-Harlo M. The role of the sapovirus infection increased in gastroenteritis after national immunisation was introduced. Acta Paediatr. 2019. July;108(7):1338–44. [DOI] [PubMed] [Google Scholar]
- 70.Vu D-L, Sabria A, Aregall N, Michl K, Rodriguez Garrido V, Goterris L, et al. Novel Human Astroviruses: Prevalence and Association with Common Enteric Viruses in Undiagnosed Gastroenteritis Cases in Spain. Viruses. 2019. June;11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diez-Valcarce M, Lopez MR, Lopez B, Morales O, Sagastume M, Cadena L, et al. Prevalence and genetic diversity of viral gastroenteritis viruses in children younger than 5 years of age in Guatemala, 2014–2015. J Clin Virol. 2019. May;114:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mann P, Pietsch C, Liebert UG. Genetic Diversity of Sapoviruses among Inpatients in Germany, 2008–2018. Viruses. 2019. August;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hassan F, Kanwar N, Harrison CJ, Halasa NB, Chappell JD, Englund JA, et al. Viral Etiology of Acute Gastroenteritis in <2-Year-Old US Children in the Post-Rotavirus Vaccine Era. J Pediatric Infect Dis Soc. 2019. November;8(5):414–21. [DOI] [PubMed] [Google Scholar]
- 74.Chen C, Wang L-P, Yu J-X, Chen X, Wang R-N, Yang X-Z, et al. Prevalence of Enteropathogens in Outpatients with Acute Diarrhea from Urban and Rural Areas, Southeast China, 2010–2014. Am J Trop Med Hyg. 2019. August;101(2):310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J-X, Zhou H-L, Mo Z-J, Wang S-M, Hao Z-Y, Li Y, et al. Burden of viral gastroenteritis in children living in rural China: Population-based surveillance. Int J Infect Dis. 2020. January;90:151–60. [DOI] [PubMed] [Google Scholar]
- 76.Kumthip K, Khamrin P, Ushijima H, Chen L, Li S, Maneekarn N. Genetic recombination and diversity of sapovirus in pediatric patients with acute gastroenteritis in Thailand, 2010–2018. PeerJ 2020;8:e8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murray TY, Nadan S, Page NA, Taylor MB. Diverse sapovirus genotypes identified in children hospitalised with gastroenteritis in selected regions of South Africa. J Clin Virol. 2016. March;76:24–9. [DOI] [PubMed] [Google Scholar]
- 78.Lasure N, Gopalkrishna V. Epidemiological profile and genetic diversity of sapoviruses (SaVs) identified in children suffering from acute gastroenteritis in Pune, Maharashtra, Western India, 2007–2011. Epidemiol Infect. 2017. January;145(1):106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Portal TM, Reymao TKA, Quindere Neto GA, Fiuza MKDC, Teixeira DM, Lima ICG, et al. Detection and genotyping of enteric viruses in hospitalized children with acute gastroenteritis in Belem, Brazil: Occurrence of adenovirus viremia by species F, types 40/41. J Med Virol. 2019. March;91(3):378–84. [DOI] [PubMed] [Google Scholar]
- 80.Tohma K, Kulka M, Coughlan S, Green KY, Parra GI. Genomic Analyses of Human Sapoviruses Detected over a 40-Year Period Reveal Disparate Patterns of Evolution among Genotypes and Genome Regions. Viruses. 2020. May;12(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grant LR, O’Brien KL, Weatherholtz RC, Reid R, Goklish N, Santosham M, et al. Norovirus and Sapovirus Epidemiology and Strain Characteristics among Navajo and Apache Infants. PLoS One. 2017;12(1):e0169491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sanchez GJ, Mayta H, Pajuelo MJ, Neira K, Xiaofang L, Cabrera L, et al. Epidemiology of Sapovirus Infections in a Birth Cohort in Peru. Clin Infect Dis. 2018. June;66(12):1858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu Y, Guo XH, Yan HQ, Gao ZY, Li WH, Liu BW, et al. [Systematic review on the characteristics of acute gastroenteritis outbreaks caused by sapovirus]. Zhonghua Liu Xing Bing Xue Za Zhi. 2019. Jan;40(1):93–8. [DOI] [PubMed] [Google Scholar]
- 84.Oka T, Stoltzfus GT, Zhu C, Jung K, Wang Q, Saif LJ. Attempts to grow human noroviruses, a sapovirus, and a bovine norovirus in vitro. PLoS One. 2018;13(2):e0178157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oka T, Yamamoto M, Miyashita K, Ogawa S, Katayama K, Wakita T, et al. Self-assembly of sapovirus recombinant virus-like particles from polyprotein in mammalian cells. Microbiol Immunol. 2009. January;53(1):49–52. [DOI] [PubMed] [Google Scholar]
- 86.Oka T, Hansman GS, Katayama K, Ogawa S, Nagata N, Miyamura T, et al. Expression of sapovirus virus-like particles in mammalian cells. Arch Virol. 2006. February;151(2):399–404. [DOI] [PubMed] [Google Scholar]
- 87.Hansman GS, Oka T, Katayama K, Takeda N. Enhancement of sapovirus recombinant capsid protein expression in insect cells. FEBS Lett. 2006. July;580(17):4047–50. [DOI] [PubMed] [Google Scholar]
- 88.Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, et al. Replication of human noroviruses in stem cell-derived human enteroids. Science. 2016. September;353(6306):1387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Wit MAS, Koopmans MPG, van Duynhoven YTHP. Risk factors for norovirus, Sapporo-like virus, and group A rotavirus gastroenteritis. Emerg Infect Dis. 2003. December;9(12):1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kauppinen A, Pitkanen T, Al-Hello H, Maunula L, Hokajarvi A-M, Rimhanen-Finne R, et al. Two Drinking Water Outbreaks Caused by Wastewater Intrusion Including Sapovirus in Finland. Int J Environ Res Public Health. 2019. November;16(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oka T, Doan YH, Haga K, Mori K, Ogawa T, Yamazaki A. Genetic Characterization of Rare Genotype GII.5 Sapovirus Strain Detected from a Suspected Food-Borne Gastroenteritis Outbreak among Adults in Japan in 2010. Jpn J Infect Dis. 2017. March;70(2):223–4. [DOI] [PubMed] [Google Scholar]
- 92.Kobayashi S, Fujiwara N, Yasui Y, Yamashita T, Hiramatsu R, Minagawa H. A foodborne outbreak of sapovirus linked to catered box lunches in Japan. Arch Virol. 2012. October;157(10):1995–7. [DOI] [PubMed] [Google Scholar]
- 93.Varela MF, Polo D, Romalde JL. Prevalence and Genetic Diversity of Human Sapoviruses in Shellfish from Commercial Production Areas in Galicia, Spain. Appl Environ Microbiol. 2016. February;82(4):1167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iritani N, Kaida A, Abe N, Kubo H, Sekiguchi J-I, Yamamoto SP, et al. Detection and genetic characterization of human enteric viruses in oyster-associated gastroenteritis outbreaks between 2001 and 2012 in Osaka City, Japan. J Med Virol. 2014. December;86(12):2019–25. [DOI] [PubMed] [Google Scholar]
- 95.Nakagawa-Okamoto R, Arita-Nishida T, Toda S, Kato H, Iwata H, Akiyama M, et al. Detection of multiple sapovirus genotypes and genogroups in oyster-associated outbreaks. Jpn J Infect Dis. 2009. January;62(1):63–6. [PubMed] [Google Scholar]
- 96.Silva TN, Dabilla N, Fiaccadori FS, Cardoso D das D de P, de Sousa TT, Almeida TNV, et al. Sapovirus in Rectal and Nasopharyngeal Swab Samples of Children with Symptoms of Acute Gastroenteritis. Pediatr Infect Dis J. 2018. April;37(4):e115–6. [DOI] [PubMed] [Google Scholar]
- 97.Bergallo M, Galliano I, Montanari P, Brusin MR, Finotti S, Paderi G, et al. Development of a quantitative real-time PCR assay for sapovirus in children under 5-years-old in Regina Margherita Hospital of Turin, Italy. Can J Microbiol. 2017. April;63(4):296–302. [DOI] [PubMed] [Google Scholar]
- 98.Leblanc D, Inglis GD, Boras VF, Brassard J, Houde A. The prevalence of enteric RNA viruses in stools from diarrheic and non-diarrheic people in southwestern Alberta, Canada. Arch Virol. 2017. January;162(1):117–28. [DOI] [PubMed] [Google Scholar]
- 99.Bank-Wolf BR, Konig M, Thiel H-J. Zoonotic aspects of infections with noroviruses and sapoviruses. Vet Microbiol. 2010. January;140(3–4):204–12. [DOI] [PubMed] [Google Scholar]
- 100.Sisay Z, Djikeng A, Berhe N, Belay G, Abegaz WE, Wang QH, et al. First detection and molecular characterization of sapoviruses and noroviruses with zoonotic potential in swine in Ethiopia. Arch Virol. 2016. October;161(10):2739–47. [DOI] [PubMed] [Google Scholar]
- 101*.Colston J, Paredes Olortegui M, Zaitchik B, Penataro Yori P, Kang G, Ahmed T, et al. Pathogen-Specific Impacts of the 2011–2012 La Nina-Associated Floods on Enteric Infections in the MAL-ED Peru Cohort: A Comparative Interrupted Time Series Analysis. Int J Environ Res Public Health. 2020. January;17(2). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examined the effect of flooding on sapovirus risk; sapovirus risk increased during the later period of the flood, suggesting environmental sources of infection are important.
- 102.Sala MR, Broner S, Moreno A, Arias C, Godoy P, Minguell S, et al. Cases of acute gastroenteritis due to calicivirus in outbreaks: Clinical differences by age and aetiological agent. Clin Microbiol Infect. 2014;20(8):793–8. [DOI] [PubMed] [Google Scholar]
- 103.Sakai Y, Nakata S, Honma S, Tatsumi M, Numata-Kinoshita K, Chiba S. Clinical severity of Norwalk virus and Sapporo virus gastroenteritis in children in Hokkaido, Japan. Pediatr Infect Dis J. 2001;20(9):849–53. [DOI] [PubMed] [Google Scholar]
- 104.Pang XL, Honma S, Nakata S, Vesikari T. Human caliciviruses in acute gastroenteritis of young children in the community. J Infect Dis. 2000. May;181 Suppl:S288–94. [DOI] [PubMed] [Google Scholar]
- 105.Praharaj I, Platts-Mills JA, Taneja S, Antony K, Yuhas K, Flores J, et al. Diarrheal Etiology and Impact of Coinfections on Rotavirus Vaccine Efficacy Estimates in a Clinical Trial of a Monovalent Human-Bovine (116E) Oral Rotavirus Vaccine, Rotavac, India. Clin Infect Dis. 2019. July;69(2):243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cubitt WD, McSwiggan DA. Seroepidemiological survey of the prevalence of antibodies to a strain of human calicivirus. J Med Virol. 1987. April;21(4):361–8. [DOI] [PubMed] [Google Scholar]
- 107.Penaflor-Tellez Y, Trujillo-Uscanga A, Escobar-Almazan JA, Gutierrez-Escolano AL. Immune Response Modulation by Caliciviruses. Front Immunol. 2019;10:2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roos-Weil D, Ambert-Balay K, Lanternier F, Mamzer-Bruneel M-F, Nochy D, Pothier P, et al. Impact of norovirus/sapovirus-related diarrhea in renal transplant recipients hospitalized for diarrhea. Transplantation. 2011. July;92(1):61–9. [DOI] [PubMed] [Google Scholar]
- 109.Rockx B, De Wit M, Vennema H, Vinjé J, De Bruin E, Van Duynhoven Y, et al. Natural history of human calicivirus infection: a prospective cohort study. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2002. August;35(3):246–53. [DOI] [PubMed] [Google Scholar]
- 110**.Rogawski McQuade ET, Liu J, Kang G, Kosek MN, Lima AAM, Bessong PO, et al. Protection from natural immunity against enteric infections and etiology-specific diarrhea in a longitudinal birth cohort. J Infect Dis. 2020. January; [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used MAL-ED data to examine the protection conferred by a prior enteric infection on future diarrhea episodes from that pathogen. For sapovirus, they found a modest reduction in the hazard of sapovirus diarrhea after a prior sapovirus infection [Hazard ratio= 0.82 (95% confidence interval, 0.66, 1.02)].
- 111.World Health Organization. The Treatment of Diarrhoea: A manual for physicians and other senior health workers [Internet]. 2005. Available from: https://apps.who.int/iris/bitstream/handle/10665/43209/9241593180.pdf;jsessionid=EE6B73F245E532D0A525258DD385391B?sequence=1
- 112.Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, et al. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clin Infect Dis. 2017. November;65(12):1963–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Costantini V, Morantz EK, Browne H, Ettayebi K, Zeng X-L, Atmar RL, et al. Human Norovirus Replication in Human Intestinal Enteroids as Model to Evaluate Virus Inactivation. Emerg Infect Dis. 2018. August;24(8):1453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Updated norovirus outbreak management and disease prevention guidelines. MMWR Recomm reports Morb Mortal Wkly report Recomm reports. 2011. March;60(RR-3):1–18. [PubMed] [Google Scholar]


