Abstract
Purpose
The COVID-19 pandemic has been widely reported to present stress to medical systems globally and to disrupt the lives of patients and health care practitioners (HCPs). Given that spontaneous reporting heavily relies on both HCPs and patients, an understandable question is whether the stress of the pandemic has diminished spontaneous reporting. Herein, the hypothesis that the COVID-19 pandemic has negatively affected the spontaneous reporting of adverse drug events was assessed.
Methods
Spontaneous-report counts from 119 weeks (January 1, 2018, to April 12, 2020) were identified using Pfizer's safety database and were analyzed. Autoregressive integrated moving-average models were fitted to aggregated and disaggregated time series (TSs). Model residuals were charted on individual-value and moving-range charts and exponentially weighted moving-average charts for the identification of statistically unexpected changes associated with the pandemic.
Findings
Overall, the reporting of serious adverse events showed no unexpected decline. Total global reporting declined, driven by HCP reporting (of both serious and nonserious events), starting after week 8 of 2020 and exceeding model expectations by week 15 of 2020, suggesting the pandemic as an assignable cause. However, reporting remained within longer-term historical ranges. The TS from Japan was the only national TS that showed a significant decline, and an unusual periodicity related to national holidays. A few countries, notably Taiwan, showed unexpected statistical increases in reporting associated with the pandemic, commencing as early as week 3 of 2020. In the literature, the reporting of adverse drug events was stable. Ancillary findings included prevalent year-end/beginning reporting minima, with more reports from HCPs than from consumers.
Implications
Using data from a large-scale and diverse safety database from a pharmaceutical company, a significant global decline in total reporting was detected, driven by HCPs, not consumers, and reports of nonserious events, consistent with the pandemic as an assignable cause, but the reporting remained within long-term ranges, suggesting relative durability. Importantly, the analyses found no unexpected decline in overall reporting of serious events. Future avenues of research include the use of data from large-scale, publicly available spontaneous reporting systems for assessing the generalizability of the present findings and whether they correlate with impaired signal detection, as well as a follow-up analysis of whether the effects on spontaneous reporting abate after the pandemic.
Key words: adverse drug events, coronavirus, COVID-19, pandemic, spontaneous reporting, time series
Introduction
The unprecedented COVID-19 pandemic has reportedly presented a huge stress to medical systems across the globe.1 , 2 Health care practitioners (HCPs) and institutions have been faced with large caseloads and serious and unexpected clinical phenotypes. Patients' lives have also been disrupted in significant ways.
Spontaneous reporting, a foundation for drug-safety monitoring, relies on the time and efforts of HCPs and patients for providing crucial information on the tolerability of drugs in clinical practice. Spontaneous reporting may be influenced by numerous factors, including the attitudes of HCPs; constraints on reportes' time; clinical recognition/suspicion, fear of litigation, publicity/notoriety bias; and secular trends related to the life-cycle of a product (eg, Weber curve). An important question is whether a disruptive strain of a pandemic infection diminishes spontaneous reporting by HCPs, patients, or both.
Serial data from long time periods, such as time series (TSs) of spontaneous reporting frequencies, cannot be fully understood if the time sequence is ignored. The observations at any point in a TS may reflect memory/carryover effects from previous time points (autocorrelation), trends, seasonality, other periodic effects, random variability, and systematic disturbances. Ignoring such structure, such as comparing the number of reports in a given week or month from one year with the number of counts in the same week or month from another year, can lead to erroneous conclusions.3
Herein, weekly frequencies of global and national spontaneous reporting across a TS were analyzed for the potential impact of the COVID-19 pandemic on spontaneous reporting.
Materials and Methods
To investigate the potential impact of the COVID-19 pandemic on spontaneous-reporting frequencies, the basic strategy was to apply TS regression to model the long-term temporal evolution of spontaneous-report counts. Then, statistical process control (SPC) charts were applied to assess whether significant deviations from well-fitting models were temporally related to the pandemic. As it might be expected that the effects of a pandemic on spontaneous reporting may vary as a function of multiple variables, total global spontaneous reports were analyzed, as was the source of reports (HCP vs consumer vs literature), reports of serious versus nonserious events, and reports from a selected set of countries with the highest numbers of confirmed COVID-19 cases.
Therefore, there were 3 elements of the methodology: (1) the use of a safety database for obtaining adverse-events report counts, (2) the use of a global database of confirmed numbers of COVID-19 cases, and (3) the use of statistical TS analysis.
Data Collection
Pfizer Safety Database
The analyses were performed on data from Pfizer's in-house safety database (New York, New York). This database consists of >3 million initial reports (from the past 10 years) originating from 208 countries, inclusive of a portfolio of >900 drugs; it is a large-scale, pharmacologically, clinically, and geographically diverse database.
Weekly global and national spontaneous-report frequencies were extracted from 119 weeks (January 1, 2018, to April 12, 2020) inclusive of a substantial time period after the World Health Organization declared on January 30, 2020, that COVID-19 was a public health emergency of international concern, and on March 11, 2020, that it was a global pandemic.4 , 5 This time span captured the latest available data and exceeded the frequently recommended minimum sample size recommendation for TS analysis of 50, and preferably 100, time points, but not overly so, which could have degraded the ability to approximate the true process with a model.6 , 7
In addition to TS for global spontaneous adverse-events report counts, the corresponding component TS were analyzed for events reported by HCPs versus consumers, serious versus nonserious events, and reports from the literature, plus weekly spontaneous-report counts from the top 12 countries ranked by confirmed COVID-19 cases as of May 8, 2020, on the John Hopkins COVID-19 Resource Center World Map (see next section).8 The robustness of the analysis was increased by the inclusion of 2 additional countries about which there was curiosity because they may be regarded as having pharmacovigilance systems with unique features of interest to this exercise: Taiwan and Japan. Taiwan implemented an early, intensive, and effective response to the COVID-19 pandemic,9 while Japan contributes a substantial number of spontaneous reports and has specialized features for intensive monitoring of the effects of new drugs.10 TS of literature reports were also examined as a comparator that was hypothesized to be resilient to the pandemic, although possibly showing delayed effects.
Johns Hopkins Coronavirus Resource Center
The Johns Hopkins Resource Center is a leading centralized source of data and information on COVID-19 infection. It collects, collates, tracks, and displays/plots data on global and national confirmed COVID-19 infections and deaths, on an open-source platform. The website was interrogated on May 8, 2020, for the selection of the countries with the top 12 numbers of confirmed COVID-19 cases, for the TS analysis.8
Statistical Analysis
The analysis involved an autoregressive integrated moving average (ARIMA) model/Box–Jenkins protocol supplemented with special-causes SPC charts of the model residuals11 (Minitab statistical analysis software version 18; Minitab LLC, State College, Pennsylvania). This combined ARIMA and SPC approach has been used in detection of epidemics,12 , 13 and ARIMA modeling with outlier detection has been implemented for high-throughput, hypothesis-free signal detection in pharmacovigilance.14 Recently it has also been used to forecast COVID-19 cases.15 ARIMA incorporates model terms for the aforementioned effects of trends, autocorrelation, and seasonality, thus maximally exploiting historical information embedded in the time sequence.
ARIMA is a form of regression, specifically regression of TS data—that is, a model that estimates or predicts the value of a variable of interest (eg, spontaneous-report counts) over time. In general, a regression model estimates or predicts the value of an outcome variable as a weighted combination of predictor variables plus random error. In an ARIMA model, the predictor variables can include previous values of the outcome (ie, the autoregressive term representing correlations with one or more previous time points), an underlying trend in the TS (ie, integrated term), and carryover effects of previous estimation errors (the moving-average term). In summary, in an ARIMA model, the value observed at any time point is modeled with a weighted sum of previous values, underlying trends, and/or previous estimation errors.
Sometimes the best model is purely autoregressive, other times purely moving average, and sometimes the best model is mixed (i.e., both autoregressive and moving-average terms and even an integration term). A specific ARIMA model is represented by 3 letters—pdq—in that order, corresponding to the AR, I, and MA order, respectively. The order refers to how far ahead in time the AR and MA effects reach. So, in an ARIMA model that is autoregressive of order 2, the estimated value at a given time point is a weighted sum of the values of the 2 preceding time points, without underlying trend or moving averages, and would be labeled ARIMA (2,0,0). The order of the I term denotes the number of times the difference is taken between consecutive values to eliminate trend.
SPC charts, of which there are many varieties for use with different types of data, were originally developed for manufacturing-quality monitoring. Common features of these graphs are a variable plotted over time, a line of central tendency, and so-called “control limits,” which are statistically derived lines above and below the line of central tendency. The latter define threshold values of the monitored variable that substantially deviate from the central, expected value, indicating a significant nonrandom change in the process that generated the data.
The protocol consisted of 4 steps for assessing whether observed changes in counts of spontaneous reports were consistent with the inherent nature of the entire TS (eg, common causes) versus a change of a magnitude sufficient to be considered unlikely without an external shock or disruption (ie, an assignable external cause, contributing to the change):
-
1.
Visual inspection of TS plots/run charts with testing for clustering indicative of autocorrelation, and trends;
-
2.
Initial model selection, starting with examination of autocorrelation function and partial autocorrelation function of the original TS to determine an initial ARIMA model;
-
3.
Iterative model selection and evaluation based on the following criteria, supporting model fit: approximate normality of residuals (Q–Q plot); no obvious structure on residuals versus fits plots; no significant residual correlations in the autocorrelation function, partial autocorrelation function, or Box–Ljung statistics; statistically significant parameter estimates; parameter parsimony; and minimum mean square error. An additional model-checking option (used only in the example in the Appendix 2 for didactic purposes; see the online version at https://doi.org/10.1016/j.clinthera.2020.12.008) is overfitting of the model with an additional higher-order term for assessing its statistical significance. Due to theoretical issues with the Box–Ljung statistic, small P values of the statistic at large lags is less important as an isolated finding. If more than one equally satisfactory model was identified, the minimum mean square error was decisive; and
-
4.
Generation of SPC charts (individual value–moving range [I-MR] and exponentially weighted moving-average [EWMA] charts of the model residuals, so-called “special-causes charts”).11
Two special-causes charts were used because they complement each other—I-MR charts are more suited for detecting sudden shifts, while EWMA charts are more suited for detecting more gradual drifts.16 In addition, because the EWMA calculates a moving average of current and past observations, it is robust to deviation from normality and thus useful for plotting individual values.16 The smoothing parameter (λ) for the EWMA was set at 0.2, given its use in supplementing the I-MR chart/detecting small shifts.16
If a finding from the aggregated global TS was inconsistent across component TS (eg, HCP vs consumer, serious vs nonserious), the role of increased TS noisiness/volatility decreasing the signal/noise ratio was evaluated using a comparison of the respective TS consecutive disparity index (CDI), a measure of TS volatility used in quantitative ecology.17 Unlike conventional measures (eg, the coefficient of variation), the CDI takes into account the time-ordering of the observations. To assess whether differences in CDI between 2 TSs were statistically significant, paired t tests were performed on the summed consecutive differences in the formula for the CDI of each TS.
Unexpected changes were considered potentially pandemic related if observed from week 2 of 2020, or a later “time period of interest” (TPI), to avoid prevalent periodic year-end/beginning reporting effects.
Appendix 2 (see the online version at https://doi.org/10.1016/j.clinthera.2020.12.008) provides a detailed, step-by-step worked example of the TS-analysis methodology used herein.
Results
Overall Counts of Spontaneous Reports and Confirmed COVID-19 Cases
The top 12 countries, ranked by the number of confirmed COVID-19 cases as of May 8, 2020, were: the United States, Spain, Italy, the United Kingdom, Russia, France, Germany, Brazil, Turkey, Iran, China, and Canada.8 These countries collectively accounted for 71.67% (501,960 of 700,362) of spontaneous reports in the time period studied. With Taiwan and Japan included, the percentage rose to 85.1%. Reporting from Iran was extremely sparse, and therefore those data were excluded from further national analysis.
There was a total of 700,362 spontaneous reports in the time period studied. The median (range) number of spontaneous reports per week was 5857 (3248–8798). The corresponding number submitted by HCPs was 419,361 (median weekly count [range], 3481 [1320–5945]).
The Table displays the median (range) weekly worldwide counts from the TPI (weeks 2–15 of 2020) and from the corresponding time intervals from the previous 2 years. The minimum weekly total global report count during the TPI was 73% of the median weekly count for the entire TS (4269 of 5857). The corresponding figures for HCP versus consumer, and serious versus nonserious, reports in the TS were 60% (2097 of 3481) versus 93% (2154 of 2323), and 65% (966 of 1494) versus 71% (3134 of 4389), respectively.
Table.
Spontaneous reports of adverse events in weeks 2–15 of 2018, 2019, and 2020. Data are given as median (range) total weekly reports.
| Parameter | 2018 | 2019 | 2020 |
|---|---|---|---|
| Total | 5893 (5323–7949) | 6457 (5561–8128) | 5475 (4269–6061) |
| Reporter | |||
| HCP | 3666 (3242–5631) | 4029 (3404–5945) | 3051 (2097–3457) |
| Consumer | 2218 (2081–2661) | 2294 (2157–2954) | 2386 (2154–2604) |
| Seriousness | |||
| Serious | 1532 (1201–1929) | 1760 (1232–2675) | 1398 (966–1576) |
| Nonserious | 4489 (3906–6020) | 4701 (4217–6134) | 4115 (3134–4500) |
HCP = health care practitioner.
ARIMA Time Series Modeling
An adequate model was fitted for 25 of 38 TSs. Most models were pure low-order AR or MA models, with a few countries requiring mixed AR and MA models. Five TSs were fitted with seasonal models. Detailed results from the fitted models are provided in Supplemental Table I in Appendix 1 (see the online version at https://doi.org/10.1016/j.clinthera.2020.12.008).
Global
According to the combined ARIMA/SPC analysis, total spontaneous reporting of serious cases did not show unexpected declines. Total spontaneous reporting, total nonserious-event spontaneous reporting, and total reporting by HCPs showed unexpected declines either by ARIMA/SPC (when a good-fitting model was obtained) or by visual inspection (see Supplemental Table I in Appendix 1 in the online version at https://doi.org/10.1016/j.clinthera.2020.12.008). The relative stability of the serious-event reporting was partly related to opposing changes in HCP versus consumers reports—that is, an increase in consumer reports coincident with a decline in HCP reports.
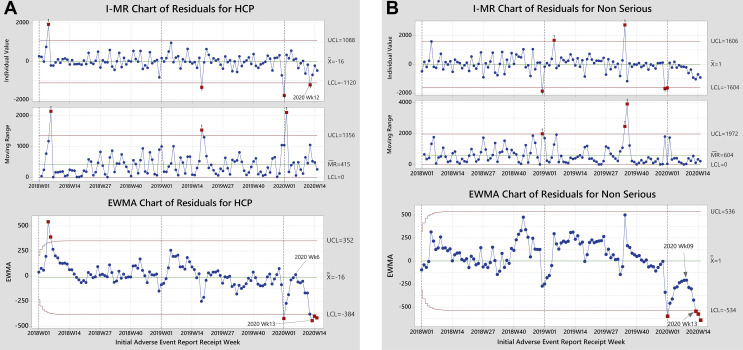
Figure 1A shows the charts on HCP reporting. The downward drift of overall and HCP spontaneous reporting exceeded the control limits of the special-causes charts by weeks 15 and 12 respectively, of 2020, and commenced earlier with HCP reporting (after week 6 vs after week 8, respectively). The decline in overall reporting, commencing after the World Health Organization declared a public health emergency of international concern, was detected on the EWMA special-causes chart, not on the I-MR chart (see Supplemental Table SI in Appendix 1 in the online version at https://doi.org/10.1016/j.clinthera.2020.12.008). A statistically signficant decline in HCP reporting was first detected on the I-MR chart.
Figure 1.
Individual value–moving range (I-MR) and exponentially weighted moving-average (EWMA) charts of the residuals of the time series models of spontaneous reports from health care practitioners (HCPs; A) and of nonserious events (B).
Figure 1B displays the corresponding charts for reports of nonserious events, which showed a decline in reporting commencing after week 9 and exceeding model limits on the EWMA chart by week 13 of 2020.
The differential TS behavior between HCPs and consumers was not explainable by differences in noise/volatility of the corresponding TS, as the CDI was numerically lower on the consumer TS versus on the HCP TS, but not significantly so (0.0936 vs 0.1556; P = 0.764). The possibility that the selectivity for reports of nonserious events reflected increased noisiness of the TS of serious events was discounted because the CDI for reports of serious events was numerically higher versus that of nonserious-event reports, but the difference was not statistically significant (0.133 vs 0.123; P = 0.989).
Data from literature reports were very stable, but with discrete year-end/beginning spikes due to the entry of cases listed in annual reports from the American Association of Poison Control Centers.
National
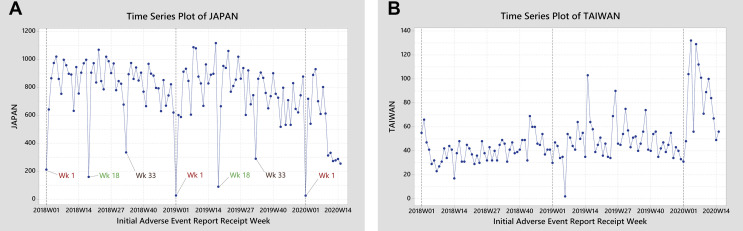
With exception of Japan (Figure 2 A), national TSs did not show unexpected declines (see Supplemental Table I in Appendix 1 in the online version at https://doi.org/10.1016/j.clinthera.2020.12.008). The Japan TS displayed a visually evident reporting decline during the TPI. It also displayed an intricate structure with the usual week-52 local minima superimposed on recurring local minima at weeks 18 and 33 (mid-May and early September, respectively). These periodic minima corresponded temporally to national holidays in Japan: Golden Week for the week-18 minima, Mountain Day and Obon for the week-33 minima.
Figure 2.
Time series of spontaneous reports from Japan (A) and Taiwan (B).
Interestingly, the Taiwan TS (both overall and from HCPs) showed a positive level shift in reporting starting in week 3 of 2020 (Figure 2B) that confounded ARIMA modeling but was nonetheless obvious on visual inspection. Notably, on January 20, Taiwan started implementing set of 124 action items including active periodic patient health checks,9 so these intensified interactions with patients possibly increased the ascertainment of all manner of health information, including suspected adverse drug reactions (ADRs).
The United Kingdom also showed a spike in reporting that exceeded special-causes control limits by week 11 of 2020. This spike reflected a change commencing by week 7 on the I-MR chart and week 8 on the EWMA chart. However, unlike the data from Taiwan, the increase in reporting was rather discrete, making it more difficult to rationalize as an effect of the pandemic.
Discussion
The present TS analysis of data from a large-scale, pharmacologically and geographically diverse safety database from a pharmaceutical company showed no unexpected (ie, inconsistent with the TS model) declines in overall spontaneous reporting of serious suspected ADRs, and no unexpected decline in consumer reports, in the TPI. This finding did not disprove a pandemic-related effect, but deviations from the models were not sufficient for rejecting the null hypothesis of no effect. Statistically unexpected declines in overall spontaneous reporting, reporting by HCPs (total, serious, and nonserious events), and nonserious-event reports were found. The decline in spontaneous reporting by HCPs manifested primarily as a downward drift rather than a large acute level shift, commencing after week 6 and reaching significance by week 13 of 2020, an onset somewhat earlier than that of overall reporting (as early as after week 8, and more clearly after week 11 of 2020). No signals of a decline in the reporting were found in the data from literature reports. Thus, selectivity of model deviations for HCPs over consumers is intuitively plausible, given the “front-line status” of HCPs in fighting the pandemic. When unexpected significant declines in spontaneous reporting were detected, they were more often detected by EMWA than I-MR charts, consistent with the superiority of EMWA charts in detecting more gradual downward drifts compared to I-MR charts, which are designed for detecting acute large declines. A more gradual decline seems to be the more intuitively plausible scenario of for a pandemic than a large, sudden change point. However, when both SPC charts demonstrated a significant decline, the I-MR chart tended to show an earlier decline.
The lack of unexplainable country-specific declines had several possible, not mutually exclusive explanations:
-
1.
Although “sample size” with regard to TSs typically refers to the number of included time periods, TS “noisiness” also affects power. The CDI for global reporting was in fact less than that for individual countries and the top 12 countries pooled;
-
2.
A contribution to global spontaneous reporting by countries other than those examined with more fragile or underdeveloped health care systems, including pharmacovigilance, and decreased availability of COVID-19 testing, that may have contributed disproportionately to global declines;
-
3.
Numbers of confirmed COVID-19 cases may have correlated imperfectly with health care system strain (eg, per-capita COVID-19 cases, COVID-19 case counts/physician or hospital bed ratios may have been better indicators);
-
4.
The data from the Johns Hopkins University Resource Center may have been a lagging indicator of relative disease activity. The ranking was rechecked on May 20, 2020, with observed relative ordinal stability, the only changes being the displacement of China and Canada by India and Peru in the top-ranked set.
-
5.
Some countries may have experienced delayed peaks in COVID-19 cases (eg, Eastern Europe/Russia)18;
-
6.
Overestimation of pandemic impacts.
Japan was the only country to demonstrate declines in overall and HCP reporting in the TPI. Of the few countries showing unexpected reporting increases, Taiwan had more sustained increases. Taiwan's intensive response to COVID-19, with 124 action items published on January 20, including periodic patient health checks,9 potentially increased the ascertainment of various health outcomes, including suspected ADRs. It would be interesting to determine whether the increased reporting was generalized, or drug, event, and/or drug–event combinations selective. Other interesting observations include highly prevalent year-end/beginning local minima, and more complex periodic patterns as in Japan.
The stability of the literature reporting TSs may have reflected the backlog of articles in press and in late-stage production, with delayed declines possible.
This analysis had limitations. ARIMA models are one methodologic choice for use in TS analysis, with advantages and disadvantages relative to others. Advantages include relative robustness to data fluctuations, and no required parameter selection. Disadvantages include the need for substantial amounts of data, especially for estimating seasonal effects; an inability to include effect modification; and sensitivity of results to model specification. While structured criteria for model fitting were followed, there is an element of trial and error, and some models fall between exemplars of good fit (eg, worked example in Appendix 2 in the online version at https://doi.org/10.1016/j.clinthera.2020.12.008) and poor fit, with subjective judgment sometimes needed. Similarly, the field of SPC charts is marked by vigorous controversy and debate on the robustness of these methods to theoretical assumptions, and optimum application.19 , 20
Levels of reporting, not signal-detection performance, were studied. But these are related, as signals may be detected from individual cases or by aggregate quantitative analysis. Decreased spontaneous reporting, if significant, could plausibly have hampered signal detection at the case level, although the decrease was within the range of the prepandemic TSs. The lack of an unexpected decline in reports of serious events is reassuring. However, altered ratios of different types of events (eg, serious vs nonserious) could positively or negatively affect disproportionality analysis in unpredictable and situation-dependent ways due to temporary changes in the distribution of reported drugs, events, and drug–event pairs, by COVID-19 disease–drug interactions/novel ADR phenotypes, and/or misattribution of clinical COVID-19 signs/symptoms to suspected ADRs. A dedicated online site for reporting suspected adverse reactions to medicines, future vaccines, and medical equipment related to COVID-19 treatment was launched by the UK's Medicines and Healthcare Products Regulatory Agency on May 4, 2020.21
Finally, while large-scale and diverse data from a pharmaceutical-company database were analyzed, it remains to be determined whether the results are generalizable to larger public databases containing data from numerous pharmaceutical companies.
Conclusions
In data from a large-scale and diverse pharmaceutical-company database, a significant global decline in total spontaneous reporting was detected, driven by HCPs, not consumers, and nonserious-event reports, consistent with the pandemic as an assignable cause, but the reporting remained within long-term ranges suggesting a relative durability. Importantly, the analyses showed no unexpected decline in overall serious-event reporting. Future avenues of research include assessing whether these effects impaired signal detection, performing the same analysis on data from a large-scale public spontaneous reporting system to assess the generalizability of the findings, and a follow-up analysis of whether the effects on spontaneous reporting abates following the pandemic.
Author Contributions
M. Hauben conceived and designed the study. Material preparation, data collection, and analysis were performed by both authors. The first draft of the manuscript was written by M. Hauben, and both authors commented on previous versions of the manuscript. Both authors read and approved the final manuscript.
Disclosures
No sources of funding were used for assisting in the preparation of this study. The authors are employees of, and hold stock options in, Pfizer Inc. The authors have indicated that they have no other conflicts of interest with regard to the content of this article.
Availability of Data
Spreadsheets containing case counts used for the analyses can be obtained by contacting the corresponding author.
Appendix 1.
Table 1 Time Series Analysis of Spontaneous Reporting
| World/Countries | Overall/Subsets | Visual Inspection of Original Time Series for Special Causesa | Best Fitting ARIMA Model (p,q,d) (P,Q,D)b | Adequate Fit? | Special Causes by Chart? |
|
|---|---|---|---|---|---|---|
| I-MR | EWMA | |||||
| World | Overall | ↓ Week 13 | (1,0,0) | Yes | No | ↓ Week 15 |
| HCP | ↓ Week 15 | (4,0,0) (0,0,1)52 | Yes | ↓ Week 12 | ↓ Week 13 | |
| Consumer | → | (1,0,0) | Yes | No | No | |
| Serious | → | (1,0,0) | Yes | No | No | |
| Non-serious | ↓ Week 13 | (0,0,1) | Yes | No | ↓ Week 13 | |
| HCP, Non-serious | ↓ Week 13 | (4.0,0) (0,0,1)52 | Yes | ↓ Week 12 | ↓ Week 13 | |
| HCP, Serious | ↓ Week 15 | (1,0,0) (0,0,1)52 | Yes | ↓ Week 12 | ↓ Week 12 | |
| Consumer, Non-serious | → | (1,0,0) | No | No | No | |
| Consumer, Serious | → | (1,0,0) | Yes | No | No | |
| Pooled Top Countries by Confirmed COVID-19 Cases | Overall | ↓ Week 13d | (0,0,0) | NA | NA | NA |
| HCP | ↓ Week 15 | (0,0,1) | Yes | No | ↓ Week 15 | |
| China | Overall | → | NAe | No | NA | NA |
| HCP | → | NAe | No | NA | NA | |
| Spain | Overall | → | (0,0,1) | Yes | No | No |
| HCP | → | (0,0,1) | Yes | No | No | |
| Russia | Overall | → | (1,1,1) | Yes | No | No |
| HCP | → | (1,1,1) | Yes | No | No | |
| France | Overall | →d | (0,0,0) | NA | Noc | Noc |
| HCP | →d | (0,0,0) | NA | NA | NA | |
| Brazil | Overall | ↓ Week 9 | (1,0,1) | Yes | No | No |
| HCP | ↓ Week 9d | (0,0,0) | NA | NA | NA | |
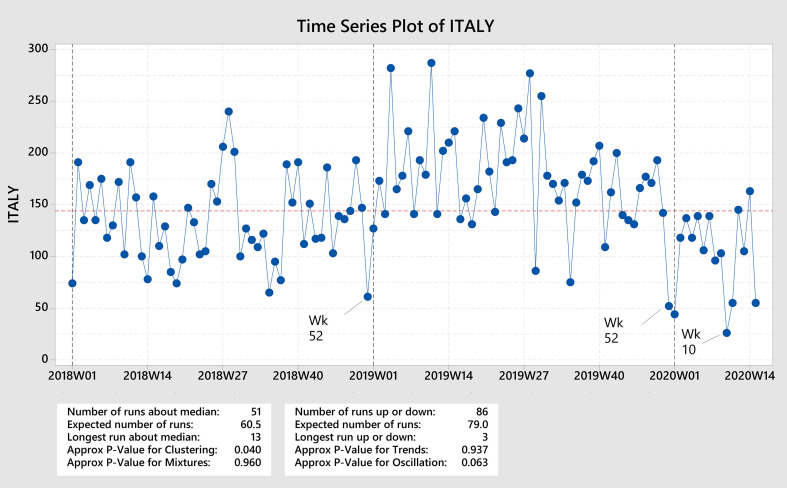
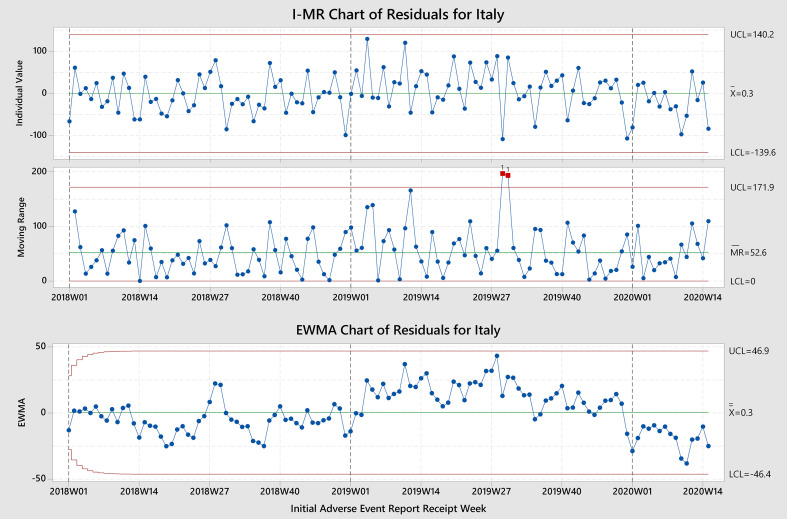
| Italy | Overall | ↓ Week 10 | (2,0,0) | Yes | No | No |
| HCP | ↓ Week 10 | (2,0,0) | Yes | No | No | |
| Japan | Overall | ↓ Week 15 | (1,0,0) (0,0,1)52 | No | No | ↓ Week 12 |
| HCP | ↓ Week 15 | (1,0,0) (0,0,1)52 | No | No | ↓ Week 12 | |
| United States | Overall | ↓ Week 13 | (0,0,1) | Yes | No | No |
| HCP | ↓ Week 13 | (1,1,2) | Yes | No | No | |
| Canada | Overall | ↑ Week 6 | (2,0,0) | Yes | No | No |
| HCP | ↑ Week 6 | (1,0,2) | No | No | No | |
| Taiwan | Overall | ↑ Week 4 | (4,0,0) | No | ↑ Weeks 3,4,6 | ↑ Weeks 4, 6-13 |
| HCP | ↑ Week 4 | (1,0,0) | No | ↑ Weeks 3,4,6 | ↑ Weeks 4, 6–8, 10-12 | |
| United Kingdom | Overall | ↑ Week 11 | (0,0,1) | Yes | ↑ Week 11 | No |
| HCP | ↑ Week 11 | (0,0,1) | Yes | ↑ Week 11 | ↑ Week 11 | |
| Germany | Overall | ↑ Week 11 | (0,0,1) | Yes | ↑ Week 10 | No |
| HCP | ↓ Week 14 | (1,0,0) | Yes | No | No | |
| Turkey | Overall | ↑ Week 11 | (2,0,0) | Yes | No | No |
| HCP | → | (1,0,0) | Yes | No | No | |
| Iran | Overall | NA | NAe | NA | NA | NA |
ARIMA = auto-regressive integrated moving average; EWMA = exponentially weighted moving average; I-MR= Individual value-moving range; ↓ = decreased reporting; ↑ = increased reporting; → = steady reporting.
Visually obvious local minimum during 1st quarter 2020 after week 1: (as assessed by Manfred Hauben).
Numbers in the first parenthesis define the non-seasonal model, numbers in second parenthesis, if present, define the seasonal mode with the subscript defining the temporal unit of observation, in this case weekly. (p/P = autoregressive; component; q/Q = integrated/trend component; d/D = moving average component).
Using first differenced series.
Based on original time series not residuals because of (0,0,0) ARIMA model.
No model achieved and even remotely acceptable fit.
Appendix 2.
Table 1 Final Estimates of Parameters
| Type | Coef | SE Coef | T-Value | P-Value |
|---|---|---|---|---|
| AR 1 | 0.2191 | 0.0908 | 2.41 | 0.017 |
| AR 2 | 0.2806 | 0.0908 | 3.09 | 0.003 |
| Constant | 73.69 | 4.33 | 17.03 | 0.000 |
| Mean | 147.30 | 8.65 |
Table 2 Modified Box-Pierce (Ljung–Box) Chi-Square Statistic
| Lag | 12 | 24 | 36 | 48 |
|---|---|---|---|---|
| Chi-Square | 10.77 | 36.57 | 48.61 | 75.34 |
| DF | 9 | 21 | 33 | 45 |
| P-Value | 0.292 | 0.019 | 0.039 | 0.003 |
References
- 1.Lombardi P., Petroni G. 2020. Virus Outbreak Pushes Italy's Health-Care System to the Brink [Wall Street Journal Online.https://www.wsj.com/articles/virus-outbreak-pushes-italys-healthcare-system-to-the-brink-11583968769 Available at: [Google Scholar]
- 2.Effects of COVID-19 on Global Healthcare Systems [IBISWorld online. https://www.ibisworld.com/industry-insider/coronavirus-insights/effects-of-covid-19-on-global-healthcare-systems Available at:
- 3.Nelson B.K. Statistical methodology: V. Time series analysis using autoregressive integrated moving average (ARIMA) models. Acad Emerg Med. 1998;5:739–744. doi: 10.1111/j.1553-2712.1998.tb02493.x. [DOI] [PubMed] [Google Scholar]
- 4.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Rolling updates on coronavirus Disease (COVID-19) [WHO website. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen Available at:
- 6.Beard E., Marsden J., Brown J., et al. Understanding and using time series analyses in addiction research. Addiction. 2019;114:1866–1884. doi: 10.1111/add.14643. [DOI] [PubMed] [Google Scholar]
- 7.Hyndman R.J., Athanasopoulos G. Forecasting: Principles and Practice. Springer; New York: 2018. Very long and very short time series. [Google Scholar]
- 8.COVID-19 dashboard by the center for Systems science and engineering (CSSE) at Johns Hopkins university [JHU website. https://coronavirus.jhu.edu/map.html Available at:
- 9.Wang C.J., Ng C.Y., Brook R.H. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. 2020;323:1341–1342. doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda S., Aoki K., Kawamata T., et al. Bias in spontaneous reporting of adverse drug reactions in Japan. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alwan L.C., Roberts H.V. Time-series modeling for statistical process control. J Bus Econ Stat. 1988;6:87–95. [Google Scholar]
- 12.Sharmin S., Rayhan I. Modelling of infectious diseases for providing signal of epidemics: a measles case study in Bangladesh. J Health Popul Nutr. 2011;29:567–573. doi: 10.3329/jhpn.v29i6.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steiner S.H., Grant K., Coory M., Kelly H.A. Detecting the start of an influenza outbreak using exponentially weighted moving average charts. BMC Med Inform Decis Mak. 2010;10:37. doi: 10.1186/1472-6947-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whalen E., Hauben M., Bate A. Time series disturbance detection for hypothesis-free signal detection in longitudinal observational databases. Drug Saf. 2018;41:565–577. doi: 10.1007/s40264-018-0640-8. [DOI] [PubMed] [Google Scholar]
- 15.Singh R.K., Rani M., Bhagavathula A.S., et al. Prediction of the COVID-19 pandemic for the top 15 affected countries: advanced autoregressive integrated moving average (ARIMA) model. JMIR Public Health Surveill. 2020;6 doi: 10.2196/19115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carson P.K., Yeh A.B. Exponentially weighted moving average (EWMA) control charts for monitoring an analytical process. Ind Eng Chem Res. 2008;47:405–411. [Google Scholar]
- 17.Fernández-Martínez M., Vicca S., Janssens I.A., Carnicer J., Martín-Vide J., Peñuelas J. The consecutive disparity index, D: a measure of temporal variability in ecological studies. Ecosphere. 2018;9 [Google Scholar]
- 18.WHO says 'Delayed epidemic' takes hold in eastern Europe as coronavirus cases in Russia rise [CNBC online. https://www.cnbc.com/2020/05/08/who-says-delayed-epidemic-takes-hold-in-eastern-europe-as-coronavirus-cases-in-russia-rise.html Available at:
- 19.Woodall W.H. Bridging the gap between theory and practice in basic statistical process monitoring. Qual Eng. 2017;29:2–15. [Google Scholar]
- 20.Woodall W.H. Controversies and contradictions in statistical process control. J Qual Technol. 2000;32:341–350. [Google Scholar]
- 21.Medicines and Healthcare Products Regulatory Agency Coronavirus: new website for reporting medicines side effects and equipment incidents [UK govt website. https://www.gov.uk/government/news/coronavirus-new-website-for-reporting-medicines-side-effects-and-equipment-incidents Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Spreadsheets containing case counts used for the analyses can be obtained by contacting the corresponding author.