Abstract
Many caregivers of patients with neurodegenerative disease experience physical and psychological strain, which is associated with negative health outcomes. Caregiver strain may be partly attributable to negative emotional responses (e.g., of resentment) to the behavioral, cognitive, and physical changes associated with patients’ disease. The philosopher Peter Strawson observed that in dealing with people who have neurological impairments, we often choose to suspend such emotional responses, adopting what he labeled the “objective attitude,” though this may come at the expense of our relationships with them. In this study, we assessed the mediating effect of caregivers’ adoption of the objective attitude on caregiver strain and relationship closeness in the setting of disease progression. Caregivers of patients with neurodegenerative disorders (n=215) completed the Clinical Dementia Rating, Relationship-Closeness scale, Caregiver Strain Index, and a novel questionnaire assessing the adoption of the objective attitude. A structural equation model assessing associations among these variables demonstrated good fit (X2 (88)=164.621, p<0.001; CFI=0.929, RMSEA=0.064.) and showed that adoption of the objective attitude mediated the association between disease progression and relationship closeness (total β= −0.233, 95% CI: −0.351, −0.113; indirect β= −0.483, 95% CI: −0.602, −0.364; direct β= 0.250, 95% CI: 0.117, 0.384), but did not mediate the association between disease progression and caregiver strain (total β= 0.323, 95% CI: 0.234, 0.412; indirect β= 0.089, 95% CI: −0.027, 0.206; direct β= 0.153, 95% CI: −0.043, 0.349). For future work, we propose longitudinal measurements of these constructs to test the directionality of associations and consideration of how models for caregiver support can draw upon interdisciplinary insights.
Keywords: dementia, relationships, caregiving, strain, attitudes
Introduction
In 2018, over sixteen million people in the United States self-identified as caregivers for someone with Alzheimer's disease or other related dementias (Alzheimer's Association, 2018). Many caregivers experience significant strain, which is likely attributable both to the burdens of caregiving and to the experience of observing a loved one with a progressive neuropsychiatric illness (Karg, 2018; Cao & Yang, 2020). Relatedly, progression and severity of neuropsychiatric disease have been linked to increased caregiver strain (Santos & De la Fuente-Fernandez, 2015; Oguh et al., 2013; Yang et al., 2019) and caregivers who report strain are at higher risk for a broad range of psychological and physical morbidities (Haley et al., 2010; Joling et al., 2011; Mausbach et al., 2010; Roepke et al., 2011).
Caregiver strain may also be due in part to caregivers’ negative emotional reactions (e.g., of resentment or indignation) when patients’ actions seem insufficiently attentive to the caregiver’s desires and needs (Shaji et al., 2009). The philosopher Peter Strawson has described such reactions as exemplifying “participant reactive attitudes,” which are central to ordinary human relationships. However, Strawson notes that these reactive attitudes can sometimes be inappropriate or counterproductive when directed at people who are neurologically impaired and/or display atypical patterns of thought or behavior (Strawson, 1962; Nelkin, 2019; Kennett et al., forthcoming). As a simple example, ordinarily in a marriage it would be natural for someone to feel resentment or anger that their spouse continually disrupts family outings by inappropriately approaching strangers at restaurants. However, if the spouse has dementia, then a caregiver might choose to suspend this normal reactive attitude on the grounds that the spouse is neurologically incapable of controlling their own behavior.
Strawson proposes that when we suspend our participant reactive attitudes, we adopt the “objective attitude (or range of attitudes)” toward people. This involves viewing another person as an object of “policy,” “treatment,” or “control,” rather than as a co-participant in a relationship (Strawson, 1962; Shabo, 2012a). Returning to our earlier example, the caregiver who has adopted an objective attitude toward the patient might then reschedule family outings, perhaps without the patient’s involvement or knowledge, for times and places in which the patient is less likely to encounter strangers; or might manage their embarrassment by distributing cards disclosing the patient’s diagnosis to people they meet. But as Strawson notes, while adopting the objective range of attitudes might have the benefit of mitigating the caregiver’s negative emotional reactions, this may come at a significant cost. Because participant reactive attitudes are central to human relationships, adopting an objective attitude toward patients could compromise the pre-existing relationship between the caregiver and care-recipient (Shabo, 2012). Previously, it has been shown that dyadic relationship closeness is inversely associated with disease progression and caregiver physical and psychological strain (Fauth et al, 2012; Norton et al., 2009; Vernon, 2019), highlighting the importance of such relationships in dementia care.
In this cross-sectional study involving caregivers of patients with neurodegenerative diseases, we assessed the mediating effect of caregivers’ adoption of the objective attitude on caregiver strain and relationship closeness in relation to the progression of a patient’s illness. We hypothesized first that disease progression is positively associated (total effect) with caregiver strain and the adoption of an objective attitude, and is negatively associated (total effect) with relationship closeness. We then hypothesized that the adoption of an objective attitude ameliorates the association between disease progression and caregiver strain, but at the cost of increasing the association between disease progression and loss of relationship closeness.
Method
Participants
Between 2013 and 2017, 215 caregiver/patient dyads were concurrently enrolled at the University of California, San Francisco (UCSF) Memory and Aging Center (MAC), a center for dementia care, research, and education. Patients underwent extensive neurological and psychological testing, assessment of cognitive and behavioral symptoms, informant report, and structural neuroimaging. This information was used by a multidisciplinary team of neurologists, neuropsychologists, and nurses to arrive at a consensus diagnosis. We included patients with a variety of neurodegenerative syndromes: behavioral variant frontotemporal dementia (bvFTD, n=65), Alzheimer’s disease (n=31), semantic variant primary progressive aphasia (svPPA, n=24), nonfluent variant primary progressive aphasia (nfvPPA, n=44), corticobasal syndrome (CBS, n=25), and progressive supranuclear palsy syndrome (PSPS, n=26) (Armstrong et al., 2011; Gorno-Tempini et al., 2011; McKhann et al., 2011; Litvan et al., 1996; Rascovsky et al., 2011). During patient visits, accompanying caregivers (co-enrolled in longitudinal research with patients) were asked to complete a series of questionnaires in either paper or electronic format. Caregivers were asked to specify their relationship to the patient (e.g., spouse, child) and whether they lived with the patient. For participants with multiple visits, we included only the initial evaluation. All participants or their legally authorized representatives gave written informed consent according to the Declaration of Helsinki, and the study was approved by the Committee on Human Research at UCSF.
Measures
Objective Attitude.
We developed a novel 18-item instrument to assess caregivers’ adoption of objective versus participant reactive attitudes toward patients with dementia. Instrument items were developed in collaboration with three university professors of philosophy. Eighteen questions assess an objective versus participant reactive attitude toward an individual patient (an additional eight questions, not utilized in the present study, assess an objective versus participant reactive attitude toward patients with neurodegenerative disease as a group). Each item was rated by the caregiver using a 6-level Likert scale (Strongly Disagree, Disagree, Slightly Disagree, Slightly Agree, Agree, Strongly Agree). Statements were framed in either a participant reactive (“My relative has as much of a say in important family decisions as other members of our family.”) or objective (“My relative can’t always tell the difference between right and wrong anymore.”) direction. Before analysis, responses to statements phrased in the participant reactive direction were reverse-coded so that they were in the objective direction. A full list of all statements can be found in Table 1.
Table 1.
Standardized loadings of questionnaire items on latent factor for adoption of objective attitude.
| Statement | λSTD |
|---|---|
| 1) When someone asks my relative a question, I usually let him/her try to answer the question instead of answering for him/her. | 0.23 |
| 2) My relative can' fully control the way he/she acts. | 0.68 |
| 3) My relative has the power to change himself/herself in order to live according to what he/she values. | 0.59 |
| 4) If my relative forgot my birthday, I wouldn’t feel hurt. | 0.09 |
| 5) I can' have a real conversation with my relative anymore. | 0.67 |
| 6) I wish there were a medication that would make my relative easier to control. | 0.37 |
| 7) I expect my relative to show appreciation when I do things for his/her sake. | 0.31 |
| 8) Sometimes I make up explanations for my relative because they are easier for him/her to understand than the truth. | 0.61 |
| 9) When my relative says something I know isn' true, I try to correct him/her. | 0.33 |
| 10) My relative can’t always tell the difference between right and wrong anymore. | 0.76 |
| 11) I can learn new things by talking with my relative about subjects that he/she knows well. | 0.72 |
| 12) When my relative does something thoughtful or considerate for me, I feel more surprised than grateful. | 0.62 |
| 13) While my relative may like or enjoy certain things, he/she no longer understands what is truly valuable or important. | 0.24 |
| 14) My relative has as much of a say in important family decisions as other members of our family. | 0.79 |
| 15) If my relative were to harm another person, he/she would deserve the same punishment as anyone else in his/her situation. | 0.42 |
| 16) My relative can behave appropriately when he/she really wants to. | 0.67 |
| 17) Sometimes I hide unpleasant facts from my relative to keep him/her from getting upset. | 0.37 |
| 18) When my relative doesn' do things that I ask, I sometimes think that he/she doesn’t care enough about my feelings. | −0.127 |
Caregiver Strain.
The Caregiver Strain Index (CSI) is a 13-item instrument that measures strain associated with caregiving in various domains: Employment, Financial, Physical, Social, and Time (e.g., “I feel completely overwhelmed”; “I find it is upsetting to find he/she has changed so much from his/her former self”) (Robinson, 1983). Items are dichotomously scored, and positive responses on this questionnaire are associated with more strain. A score of seven or more indicates the need for more support to the caregiver. This instrument has been validated in caregivers of disabled and hospitalized older adults and previously demonstrated an internal consistency (Cronbach’s Alpha) of 0.86. (Robinson, 1983).
Relationship Closeness.
Relationship closeness was measured using the Relationship Closeness scale (Noelker, 1996; Whitlatch et al., 2001). This instrument consists of 6 statements (e.g., “My relationship with my relative has always been close”; “My relative always understands what I value in life.”) scored on a 6-level Likert scaled (Strongly Disagree, Disagree, Slightly Disagree, Slightly Agree, Agree, Strongly Agree). Scores from this instrument were summed into an aggregate score with a higher score indicating greater relationship closeness. In a study involving a sample of familial caregivers, the scale has a reported Cronbach’s alpha of 0.90 (Whitlatch et al., 2001).
Disease Progression.
The Clinical Dementia Rating (CDR) is a global assessment instrument regularly used in clinical and research settings to stage the severity of neurodegenerative disease (Hughes et al., 1982). The instrument assesses six domains of cognitive functional performance relevant to patients with dementia: Memory, Orientation, Judgement & Problem Solving, Community Affairs, Home & Hobby. Each domain is rated on a 5-level scale of functioning as follows: 0, no impairment; 0.5, questionable impairment; 1, mild impairment; 2, moderate impairment; and 3, severe impairment (personal care is scored on a 4-level scale without a 0.5 rating available). This assessment was performed via a structured interview that was administered by a clinical nurse specialist to the caregiver. The Clinical Dementia Rating Sum of Boxes (CDR-SB) score, a sum of scores from each domain, was used to quantify patients’ disease progression. In a sample of 1,367 patients, the CDR-SB score was shown to have a Cohen’s kappa coefficient, a measure of inter-rater reliability for categorical items, that ranged from 0.86 to 0.94 (O’Bryant, 2008).
Statistical Analysis
Structural Equation Modeling can be divided into two components: a measurement model and a structural model. The first step in the development of our measurement model was an exploratory factor analysis to inform the number of latent factors. Following recommendations from Cattell (1966), analysis of the scree plot was in favor of a one-factor model. Next, we utilized a confirmatory factor analysis (CFA). CFA is an extension of factor analysis in which specific hypotheses about the structure of the factor loadings and inter-correlations are tested (Fox, 2010). We specified a CFA model in which responses on the Strawson questionnaire served as indicators of a latent variable. This latent variable represents the adoption of an objective attitude by the caregiver toward the care-recipient. We then specified a structural model that extends the measurement model by incorporating the latent objectifying variable into a path analysis. The structural model was utilized to assess the associations among a caregiver objective attitude, disease progression, caregiver strain, and relationship closeness. All models were adjusted for age, education, and gender of the patient. As many patients in our study sample had variants of frontotemporal dementia (bvFTD, svPPA, nfvPPA), we also conducted a sensitivity analysis focusing on these dyads.
To maintain consistency with previous publications, and because the Chi-Square statistic is sensitive to sample size and large correlations, several fit indices are reported. The Comparative Fit Index (CFI) estimates the fit of a specified model with a nested, baseline model. Larger values indicate a better fit, with values above 0.90 indicating adequate fit (20). The Root Mean Square Error of Approximation (RMSEA) is an approximate measure of fit to the data; better fit is indicated with a smaller RMSEA value, with 0.08 indicating adequate fit (Browne & Cudeck, 1992). All analyses were run in Mplus Version 8.0 (Asparouhov, Muthén, B. & Muthén, L, 2017).
Results
There were 215 questionnaires completed by caregivers who cared for a patient who met the diagnostic criteria for bvFTD, nfvPPA, svPPA, Alzheimer’s disease, PSPS, or CBS. Demographic characteristics of caregivers can be found in Table 2, while the characteristics of patients (i.e., care-recipients) can be found in Table 3. We assessed for normality in our data to inform which estimation procedure to specify for our structural equation models. Using the cutoff values established by Curran, West, and Finch (1996), all variables in our data exhibited univariate normality. Using the Doornik-Hansen test, however, our data did not meet the criteria for multivariate normality (X2 (48) = 509.321, p < 0.0001). Thus, our initial structural model was specified using Mplus’s MLR estimator, which estimates parameters using maximum likelihood with standard errors that are robust to non-normality. Finally, the bootstrapping feature of Mplus (1000 resamples; (Shrout & Bolger, 2002)) was used to obtain unbiased confidence intervals for the indirect (mediated) effects.
Table 2.
Characteristics of caregivers.
| Demographic Variable | Value |
|---|---|
| Caregiver Strain, mean (SD) | 6.5 (3.7) |
| Relationship to patient, n (%) | |
| Spouse | 139 (65 %) |
| Sibling | 32 (15 %) |
| Adult-Child | 35 (16.3%) |
| Other (e.g. friend, neighbor) | 9 (4%) |
| Lives with Patient, n (%) | |
| Yes | 136 (63%) |
| No | 79 (37%) |
Table 3.
Characteristics of patients with neurodegenerative disease.
| Demographic Variable | Value |
|---|---|
| Age (Std. deviation) | 66.38 (8.11) |
| Years of Education | 16.1 (4.51) |
| Clinical Dementia Rating, Sum of Boxes | 6.07 (3.27) |
| Male, % | 54.4 |
| Diagnosis: n (male) | |
| Alzheimer’s disease | 31 (15) |
| Behavioral variant frontotemporal dementia | 65 (41) |
| Nonfluent variant primary progressive aphasia | 44 (22) |
| Semantic variant primary progressive aphasia | 24 (11) |
| Corticobasal syndrome | 25 (14) |
| Progressive supranuclear palsy syndrome | 26 (14) |
| Race | |
| White | 184 |
| African-American | 3 |
| Hispanic or Latino | 3 |
| Asian | 8 |
| American Indian or Alaska Native | 2 |
| Other | 15 |
| Total | 215 |
Measurement Model
A one-factor CFA model of the 18 variables did not have adequate model fit: X2 (135) = 419.81, p <.001; comparative fit index (CFI) = 0.796, root-mean-square error of approximation (RMSEA) = 0.099. There were eight items with factor loadings below 0.4, and following recommendations from Fornell and Lacker (1981), these were dropped from the analysis. We proceeded with a one-factor CFA analysis with the remaining ten variables. After examination of modification indices, residual covariances were estimated between items 2 and 16 (Table 1) and between items 5 and 11 (Table 1) due to highly overlapping content. Estimates of this model suggested the model had good fit: X2 (114) = 1332.461, p < .001; CFI = 0.933, RMSEA = 0.067. To confirm the unidimensionality of our latent variable, we tested a two-factor CFA model, where one latent factor consisted of the ten remaining variables, and another latent factor consisted of the eight dropped variables. The two factor model had poor model fit (X2 (135) = 516.81, p < .001; comparative fit index (CFI) = 0.798; RMSEA = 0.099), each of the eight items on the second factor had low factor loadings, and there was high correlatability between the two latent factors. These findings support a latent variable with a single dimensionality.
Structural Model
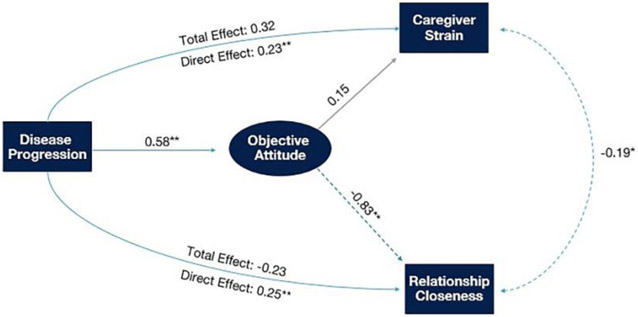
The total effect of disease progression on relationship closeness was negative (β = −0.233, 95% Confidence Interval, [CI]: −0.351, −0.113) as hypothesized. In a mediation model, disease progression was associated with greater adoption of the objective attitude (β = 0.584, 95% CI: 0.482, 0.687) which in turn was associated with decreased relationship closeness (β = −0.827, 95% CI: −0.951, −0.703). Adoption of the objective attitude significantly mediated the association between disease progression and relationship closeness (β = −0.483, 95% CI: −0.602, −0.364), as hypothesized. (While the total effect and indirect effect were both negative in sign in this model, the direct effect (β= 0.250, 95% CI: 0.117, 0.384) was positive in sign; such a change in sign may reflect hidden mediators or suppressors not included in the model.)
The total effect of disease progression on caregiver strain was positive (β = 0.323, 95% CI: 0.234, 0.412) as hypothesized. Contrary to our initial hypotheses and in contrast to the effect on relationship closeness, adoption of the objective attitude was not significantly associated with caregiver strain (β = 0.153, 95% CI: −0.043, 0.349), and did not mediate the relationship between disease progression and caregiver strain (β = 0.089, 95% CI: −0.027, 0.206).
Figure 1 displays direct, indirect, and total effects for these associations. All parameters for the mediation analysis are displayed in Table 4, with bootstrapped confidence intervals. Estimates of model fit suggested the model had good fit X2 (88) = 164.20, p < .001; CFI = 0.929, RMSEA = 0.064. A sensitivity analysis (X2 (88) = 157.90, p < .001; CFI = 0.926, RMSEA = 0.066) focusing on caregivers of patients with frontotemporal dementia (n=133) yielded effect sizes (and p-values) with similar magnitudes (Supplemental Table 1).
Figure 1.
An objective attitude is associated with decreased relationship closeness between caregiver and care-recipient while not protecting against caregiver strain. Because no significant effect was observed on mediation of disease progression and relationship closeness via caregiver strain, only the total and direct effects for mediation via an objective attitude are reported in that pathway. Note: Single-headed arrows represent postulated causal associations while double-headed arrows represent associations without presumed direction. Solid lines indicate positive associations while dashed lines indicate negative associations. Statistical significance (p < 0.05*, p < 0.005**) for a given path is indicated by a teal-colored arrow and an asterisk next to the standardized coefficient.
Table 4.
Direct, indirect, and total, effects of structural model (standardized regression coefficients and 95% CIs). Note: Direct and total effects were obtained through MLR estimation while 95% confidence intervals of indirect effects were obtained by bootstrapping. Disease progression was measured using the Clinical Dementia Rating Scale, caregiver strain was measured using the Caregiver Strain Index, relationship closeness was measured using the Relationship Closeness Scale, and Objective Attitude was measured using a novel questionnaire.
| Path | Standardized β (95% CI) | P-Value |
|---|---|---|
| Total effect (via objective attitude) of disease progression on relationship closeness | −0.233 (−0.351, −0.113) | <0.001 |
| Indirect effect (via objective attitude) of disease progression on relationship closeness | −0.483 (−0.602, −0.364) | <0.001 |
| Total effect (via objective attitude) of disease progression on caregiver strain | 0.323 (0.234, 0.412) | <0.001 |
| Indirect effect (via objective attitude) of disease progression on caregiver strain | 0.089 (−0.027, 0.206) | 0.131 |
| Direct effect of disease progression on relationship closeness | 0.250 (0.117, 0.384) | 0.013 |
| Direct effect of disease progression on objective attitudes | 0.584 (0.482, 0.687) | <0.001 |
| Direct effect of disease progression on caregiver strain | 0.231 (0.051, 0.411) | <0.001 |
| Direct effect of objective attitudes on relationship closeness | −0.827 (−0.951, 0-.703) | <0.001 |
| Direct effect of objective attitudes on caregiver strain | 0.153 (−0.043, 0.349) | 0.128 |
| Direct effect of caregiver strain with relationship closeness | −0.192 (−0.336, −0.049) | 0.011 |
Discussion
Peter Strawson characterized reactive attitudes—including negative emotional reactions such as resentment, hurt feelings, anger, and indignation—as central to human relationships, and in that way, as essential to shared human life. Strawson also suggested that in many cases of neuropsychiatric illness, these attitudes may need to be suspended in favor of the objective range of attitudes, in which we regard patients not as co-participants in a relationship but instead as objects of treatment or control (Strawson, 1962). In the present study, we consider the related empirical question of how such shifts in attitude are related to disease progression, caregiver well-being, and the closeness of the patient-caregiver relationship.
In formulating our study, we began with three hypotheses about the adoption of an objective attitude by caregivers. First, we expected that disease progression would lead to greater adoption of an objective attitude, as proposed in Strawson’s original conceptual analysis. Second, we hypothesized that the adoption of an objective attitude would diminish the association between disease progression and caregiver strain, as they (partially) protect caregivers from what Strawson termed “the strains of involvement”—i.e., emotional vulnerability to patients’ actions or omissions. Third, we hypothesized that such attitudes contribute to the association between disease progression and loss of relationship closeness.
Consistent with our first hypothesis, we found that caregivers of patients with more advanced disease reported greater adoption of an objective attitude and caregiver strain. With the onset of neurodegenerative disease, patients experience cognitive, behavioral, and physical changes that not only make caregiving more strenuous but also may result in the inability to sustain interpersonal relationships. Caregivers may be responding to this inability by suspending participant reactive attitudes (e.g., resentment, gratitude), and subsequently viewing the patient as an object, which must be controlled or attended to, as opposed to another co-participant in a relationship. Nonetheless, following Zhao et al. (2010), we remain cautious in our interpretation of this finding, given the limits of our theoretical framework (i.e., possible omitted mediators).
Contrary to our second hypothesis, the adoption of an objective attitude did not protect caregivers from increased caregiver strain with disease progression. This finding was unexpected, as, on a Strawsonian view, the participant reactive attitudes would make caregivers vulnerable to negative emotions such as hurt feelings, anger, and resentment toward the patient. However, as Strawson also emphasizes, the adoption of an objective attitude can come with a high cost to our meaningful relationships. Perhaps having to adopt an objective attitude toward someone to whom one was previously able to stand in a loving, personal relationship has its own deleterious consequences for caregiver strain because this is to lose the relationship one has valued. Alternatively, the association between disease progression and caregiver strain may simply be a more direct one, independent of the attitudes that a caregiver happens to adopt toward a patient.
Consistent with our third hypothesis, the adoption of an objective attitude mediated the association between disease progression and relationship closeness. This lends some empirical support to Strawson’s conceptual account of participant reactive attitudes as central to human relationships.
While our initial hypotheses were general hypotheses about dementia informed by Strawson’s account of human relationships, and were not specific to frontotemporal dementia, it should be noted that this study was conducted at a research center focused on frontotemporal dementia and that 62% of the data were from dyads with frontotemporal dementia. In a post-hoc sensitivity analysis restricted to these data, none of our findings were substantially changed. Still, the interpretation of our findings may be more secure in patients with frontotemporal dementia than in patients with other dementias.
Our study was an initial effort toward operationalizing Strawson’s conceptual insights about relationships in an empirical study of patients and caregivers. Given this, our research has several limitations. First, while our structural equation model includes theorized directions among constructs, data were collected cross-sectionally, and causal interpretations should be considered with caution. Relatedly, while the model specifies unidirectional and acyclic associations between constructs, this may be regarded as a more computationally-tractable simplification of more complex and (at least in many cases) bidirectional associations. Furthermore, while in some cases the underlying constructs included can be presumed to have unidirectional associations (e.g., we consider disease progression to be a mostly neurobiological process that in turn drives changes in caregiver attitudes, strain, and relationships), the instruments used to measure these constructs were all obtained from caregiver report, and these reports are likely to themselves be interrelated. Finally, for computational and sample-size considerations, we included patients with a variety of neurodegenerative conditions in a single model. It is likely that different forms of neurodegenerative disease pose different challenges for patient-caregiver relationships and caregiver attitudes that our research design is unable to distinguish.
In summary, disease progression in dementia is associated with caregivers’ adoption of an objective attitude toward patients. In our measurements, these attitudes do not protect against caregiver strain, but they do contribute to an association between disease progression and loss of relationship closeness. This work represents a first step toward applying philosophical insights about human relationships to empirical research on the patient-caregiver relationship. For future work, we propose longitudinal measurements of these constructs to test the directionality of these associations, and further consideration of how different models for caregiver support could draw upon insights from the humanities and social sciences.
Supplementary Material
Acknowledgements
The authors wish to thank Bennett Helm, Agnieszka Jaworska, Katherine Rankin, and Jennifer Beer for valuable interdisciplinary discussion of Strawson’s work and the design of the instrument; and our patients and caregivers for their generous participation in research.
Funding
This work was supported by the National Institutes of Health, National Institutes on Aging (grant numbers K23AG043553, K23AG061253, P01AG019724, R01AG022983, R01AG058817, and P50AG023501), the John Templeton Foundation (Love and Human Agency Project, Grant ID: 29246) and the Larry L. Hillblom Foundation (2018-A-025-FEL).
Abbreviations:
- bvFTD
behavioral variant frontotemporal dementia
- svPPA
semantic variant primary progressive aphasia
- nfvPPA
nonfluent variant primary progressive aphasia
- CBS
corticobasal syndrome
- PSPS
progressive supranuclear palsy syndrome
- CFA
Confirmatory Factor Analysis
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Alzheimer's Association. (2018). 2018 Alzheimer's disease facts and figures. Alzheimer's & Dementia, 14(3), 367–429. 10.1016/j.jalz.2018.02.001 [DOI] [Google Scholar]
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M & Josephs KA (2013). Criteria for the diagnosis of corticobasal degeneration. Neurology, 80(5), 496–503. 10.1212/WNL.0b013e31827f0fd1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asparouhov T, Muthén BO, Muthén LK (2017). Regression and mediation analysis using Mplus. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Browne MW, & Cudeck R (1992). Alternative ways of assessing model fit. Sociological Methods & Research, 21(2), 230–258. 10.1177/0049124192021002005 [DOI] [Google Scholar]
- Cattell RB (1966). The scree test for the number of factors. Multivariate behavioral research, 1(2), 245–276. 10.1207/s15327906mbr0102_10 [DOI] [PubMed] [Google Scholar]
- Cao Y, & Yang F (2020). Objective and subjective dementia caregiving burden: The moderating role of immanent justice reasoning and social support. International Journal of Environmental Research and Public Health, 17(2), 455. 10.3390/ijerph17020455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, West SG, & Finch JF (1996). The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychological Methods, 1(1), 16. 10.1037/1082-989X.1.1.16 [DOI] [Google Scholar]
- Fauth E, Hess K, Piercy K, Norton M, Corcoran C, Rabins P, Lyketsos C & Tschanz J (2012). Caregivers’ relationship closeness with the person with dementia predicts both positive and negative outcomes for caregivers’ physical health and psychological well-being. Aging & Mental Health, 16(6), 699–711. 10.1080/13607863.2012.678482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornell C, & Larcker DF (1981). Evaluating structural equation models with unobservable variables and measurement error. Journal of Marketing Research, 18(1), 39–50. 10.1177/002224378101800104 [DOI] [Google Scholar]
- Fox RJ (1983). Confirmatory factor analysis. Hoboken, NJ: John Wiley & Sons, Ltd. [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF & Manes F (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley WE, Roth DL, Howard G, & Safford MM (2010). Caregiving strain and estimated risk for stroke and coronary heart disease among spouse caregivers: differential effects by race and sex. Stroke, 41(2), 331–336. 10.1161/STROKEAHA.109.568279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger W, Coben LA, & Martin RL (1982). A new clinical scale for the staging of dementia. The British Journal of Psychiatry, 140(6), 566–572. 10.1192/bjp.140.6.566 [DOI] [PubMed] [Google Scholar]
- Joling KJ, van Hout HP, Schellevis FG, van der Horst HE, Scheltens P, Knol DL, & van Marwijk HW (2010). Incidence of depression and anxiety in the spouses of patients with dementia: a naturalistic cohort study of recorded morbidity with a 6-year follow-up. The American Journal of Geriatric Psychiatry, 18(2), 146–153. 10.1097/JGP.0b013e3181bf9f0f [DOI] [PubMed] [Google Scholar]
- Karg N, Graessel E, Randzio O, & Pendergrass A (2018). Dementia as a predictor of care-related quality of life in informal caregivers: a cross-sectional study to investigate differences in health-related outcomes between dementia and non-dementia caregivers. BMC Geriatrics, 18(1), 189. 10.1186/s12877-018-0885-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett Jeanette ; McConnell Doug & Snoek Anke (forthcoming). Reactive attitudes, relationships, and addiction. In Ahmed S & Pickard Hanna (eds.), Routledge Handbook of the Philosophy and Science of Addiction. London, UK: Routledge. [Google Scholar]
- Litvan I, Agid Y, Caine D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH & Hallett M (1996). Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology, 47(1), 1–9. 10.1212/WNL.47.1.1 [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Roepke SK, Ziegler MG, Milic M, von Känel R, Dimsdale JE, Mills PJ, Patterson TL, Allison MA, Ancoli-Israel S & Grant I (2010). Association between chronic caregiving stress and impaired endothelial function in the elderly. Journal of the American College of Cardiology, 55(23), 2599–2606. 10.1016/j.jacc.2009.11.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R & Mohs RC (2011). The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia, 7(3), 263–269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelkin DK (2019). Frontotemporal dementia and the reactive attitudes: two roles for the capacity to care?. Journal of Applied Philosophy, 36(5), 817–837. 10.1111/japp.12365 [DOI] [Google Scholar]
- Noelker LS. (1996). Promoting positive relationships between nursing assistants and the families of cognitively impaired nursing home residents. Final report to the Cleveland Foundation. Cleveland, OH. Benjamin Rose Institute. [Google Scholar]
- Norton MC, Piercy KW, Rabins PV, Green RC, Breitner JC, Østbye T, Corcoran C, Welsh-Bohmer KA, Lyketsos CG & Tschanz JT (2009). Caregiver–recipient closeness and symptom progression in Alzheimer’s disease. The Cache county dementia progression study. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 64(5), 560–568. 10.1093/geronb/gbp052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguh O, Kwasny M, Carter J, Stell B, & Simuni T (2013). Caregiver strain in Parkinson's disease: national Parkinson Foundation Quality Initiative study. Parkinsonism & Related Disorders, 19(11), 975–979. 10.1016/j.parkreldis.2013.06.015 [DOI] [PubMed] [Google Scholar]
- O’Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, Lupo PJ, Reisch JS & Doody R (2008). Staging dementia using clinical dementia rating scale sum of boxes scores: a Texas Alzheimer's research consortium study. Archives of Neurology, 65(8), 1091–1095. https://10.1001/archneur.65.8.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, Van Swieten JC, Seelaar H, Dopper EG, Onyike CU & Hillis AE (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(9), 2456–2477. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BC (1983). Validation of a caregiver strain index. Journal of Gerontology, 38(3), 344–348. 10.1093/geronj/38.3.344 [DOI] [PubMed] [Google Scholar]
- Roepke SK, Mausbach BT, Patterson TL, Von Känel R, Ancoli-Israel S, Harmell AL, Dimsdale JE, Aschbacher K, Mills PJ, Ziegler MG & Allison M (2011). Effects of Alzheimer caregiving on allostatic load. Journal of Health Psychology, 16(1), 58–69. 10.1177/1359105310369188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-García D, & De la Fuente-Fernández R (2015). Factors contributing to caregivers' stress and burden in Parkinson's disease. Acta Neurologica Scandinavica, 131(4), 203–210. 10.1111/ane.12305 [DOI] [PubMed] [Google Scholar]
- Shabo S (2012a). Incompatibilism and personal relationships: Another look at Strawson's objective attitude. Australasian Journal of Philosophy, 90(1), 131–147. 10.1080/00048402.2010.539621 [DOI] [Google Scholar]
- Shabo S (2012b). Where love and resentment meet: Strawson's intrapersonal defense of compatibilism. Philosophical Review, 121(1), 95–124. 10.1215/00318108-1426373 [DOI] [Google Scholar]
- Shaji KS, George RK, Prince MJ, & Jacob KS (2009). Behavioral symptoms and caregiver burden in dementia. Indian Journal of Psychiatry, 51(1), 45. 10.4103/0019-5545.44905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, & Bolger N (2002). Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological Methods, 7(4), 422. 10.1037/1082-989X.7.4.422 [DOI] [PubMed] [Google Scholar]
- Strawson P (1962). Freedom and resentment. Proceedings of the British Academy, 48(1), 1–25. 10.1073/pnas.48.1.1 [DOI] [Google Scholar]
- Vernon EK, Cooley B, Rozum W, Rattinger GB, Behrens S, Matyi J, Fauth E, Lyketsos CG & Tschanz JT (2019). Caregiver-care recipient relationship closeness is associated with neuropsychiatric symptoms in dementia. The American Journal of Geriatric Psychiatry, 27(4), 349–359. 10.1016/j.jagp.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlatch CJ, Schur D, Noelker LS, Ejaz FK, & Looman WJ (2001). The stress process of family caregiving in institutional settings. The Gerontologist, 41(4), 462–473. 10.1093/geront/41.4.462 [DOI] [PubMed] [Google Scholar]
- Yang F, Ran M, & Luo W (2019). Depression of persons with dementia and family caregiver burden: Finding positives in caregiving as a moderator. Geriatrics & Gerontology International, 19(5), 414–418. 10.1111/ggi.13632 [DOI] [PubMed] [Google Scholar]
- Zhao X, Lynch JG Jr, & Chen Q (2010). Reconsidering Baron and Kenny: Myths and truths about mediation analysis. Journal of Consumer Research, 37(2), 197–206. 10.1111/ggi.13632 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.