Abstract
The complete chloroplast genome sequences of vulnerable medicinal plant Saraca asoca (Roxb.) Willd. (Fabaceae) was sequenced. A total of 5,206,216,851 paired-end filtered reads of 151 bp were obtained. The plastome length (including LSC, SSC, IRa, and IRb) was 137,743 bp (GC content: 35.26%). A total of 126 coding genes which includes 97 CDS, 24 tRNA, and five rRNA genes were annotated. The phylogenetic analysis attempts to establish molecular signature in order to differentiate genuine sample of S. asoca from its adulterants easily.
Keywords: Chloroplast genome, vulnerable, medicinal plant, Saraca asoca, Fabaceae, adulterants, rbcL
Saraca asoca (Roxb.) Willd. (family Fabaceae, sub family Detarioideae), a rain-forest tree, native to Indian subcontinent, is one of the highly traded medicinal tree whose bark greatly valued for treatment of gynecological disorders (Singh et al. 2015). The over exploitation due to increasing commercial demand of the crude drug material mainly of its bark have resulted to vulnerable in wild (IUCN 2011); therefore, being extensively adulterated with Bauhinia variegata L., Mesua ferrea L., Polyalthia longifolia (Sonn.) Thwaites, Shorea robusta Gaertn., and Trema orientalis (L.) Blume in herbal raw drug trading (Hegde et al. 2018). An attempt to assess the extent of adulteration in the raw herbal trade of S. asoca using DNA barcoding was validated by NMR spectroscopic techniques (Urumarudappa et al. 2016). rbcL-ISSR based DNA barcodes (Hegde et al. 2018) are least user-friendly. The gradual development in NGS platforms and bioinformatics tools for the data analyses have revolutionized our understanding about comparative genomics, chloroplast biology, intracellular gene transfer, conservation biology, diversity, phylogeny, and also facilitated in the enhancement of the plant agronomic traits and to produce high-value agricultural products through genetic engineering.
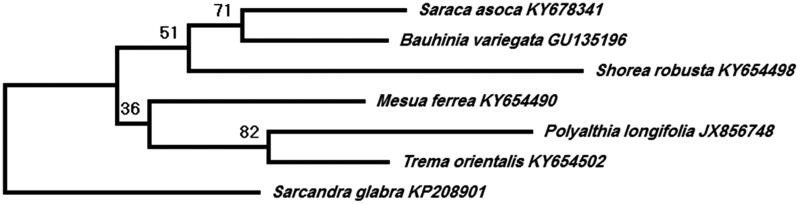
The chloroplast DNA was extracted from the leaves [Voucher: ‘MAA & TKPAN-116’ (BHAG)] collected from botanical garden (25°14′25″N 86°56′55″E), TMBU, Bhagalpur, India, and were sequenced at Illumina sequencing platform. A total of 5,206,216,851 paired-end filtered reads of 151 bp were obtained which were assembled using spades (Bankevich et al. 2012). A total of 126 coding genes including 97 protein-coding, 24 tRNA, and five rRNA genes were annotated using GeSeq (Tillich et al. 2017) and tRNAsacn-SE (Lowe and Eddy 1997). The total plastome length (Genbank accession number: MN866115) was 137,743 bp (GC content: 35.26%). The phylogenetic tree from the set of the GenBank accession number (see the phylogenetic tree) of rbcL[-the chloroplast gene proposed for plant DNA barcoding (CBOL 2009)] sequence of S. asoca and its adulterated with B. variegata, M. ferrea, P. longifolia, S. robusta, and T. orientalis using MEGA X (Kumar et al. 2018) revealed promising potential to be used as molecular authentication to discriminate genuine sample from adulterants (Figure 1).
Figure 1.
The maximum-likelihood (ML) tree based on rbcL gene from a total number of seven representative species of Rosids.
Funding Statement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project [RG-1439-84]. This study was supported by the KRIBB Initiative Program of the Republic of Korea. Help rendered by Sabiha Ali Ajmal during field trip at Bhagalpur, Bihar India for the collection of Plant material for the present study is thankfully acknowledged.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bankevich A, Nurk S, Antipov D, Gurevich A, Dvorkin M, Kulikov AS, Lesin V, Nikolenko S, Pham S, Prjibelski A, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19(5):455–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CBOL . 2009. Plant Wording Group. A DNA barcode for land plants. PNAS. 106:12794–12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde S, Archana S, Harsha VH, Sanjiva DK, Subarna R. 2018. Molecular identification of Saraca asoca from its substituents and adulterants. 3 Biotech. 8(3):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUCN . 2011. IUCN red list of threatened species. Version 2011.1. www.iucnredlist.org.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35(6):1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25(5):955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Anantha Krishna TH, Subban K, Gini C.K, Jinu MV, Chelliah J. 2015. Phytomedicinal importance of Saraca asoca (Ashoka): an exciting past, an emerging present and a promising future. Curr Sci. 109(10):1790–1801. [Google Scholar]
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq – versatile and accurate annotation of organelle genomes. Nuc Acids Res. 45(W1):W6–W11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urumarudappa SK, Gogna N, Newmaster SG, Venkatarangaiah K, Subramanyam R, Saroja SG, Gudasalamani R, Dorai K, Ramanan US. 2016. DNA barcoding and NMR spectroscopy-based assessment of species adulteration in the raw herbal trade of Saraca asoca (Roxb.) Willd, an important medicinal plant. Int J Legal Med. 130(6):1457–1470. [DOI] [PubMed] [Google Scholar]