Abstract
In this study, the complete mitogenome sequence of Ostrinia furnacalis was described. The assembled mitogenome is 15,241 bp in length with an extreme bias of high AT content (80.9%) (GenBank accession no. MN747041). The mitochondrial genome contains 13 protein-coding genes (PCGs), 22 transfer RNA (tRNA) genes, 2 ribosomal RNA (12S rRNA and 16S rRNA) genes, and a control region (D-loop region). The mitochondrial gene order was identical to that observed in most lepidopteran genomes, nine PCGs were located on the H-strand, others were located on the L-strand. 12 PCGs were initiated by typical ATN codons, except for COI with CGA instead. 21 tRNAs had the typical cloverleaf structure, while the DHU arm of the trnS1 gene did not form a stable stem-loop structure. The ‘ATAGT(A)’-like motif and a 19 bp poly-T stretch at the down-stream of the rrnS gene were observed in the A + T-rich region. The phylogenetic analysis showed that the relationship of O. furnacalis is very close to the three species in the subfamily Pyraustinae: O. nubilalis, O. penitalis and Loxostege sticticalis, and all the subfamilies of Spilomelinae, Pyraustinae, Crambinae and Nymphulinae within Crambidae formed monophyletic groups with the highest bootstrap value support.
Keywords: Ostrinia furnacalis, mitochondrial genome, phylogenetic analysis, Crambidae
The Asian Corn Borer (ACB), Ostrinia furnacalis, is one of the destructive lepidopteran pests of corn in Asian, and can cause significant loss in a corn field (Kojima et al. 2010). The moth larva destroys the fruit when it bores into the ear to feed on the silk and kernels, which is considered to be one of the aggravating factor for the epidemiology of Fusarium ear rot in maize (Folcher et al. 2009; Yang et al. 2014). Although partial mitochondrial genome sequences of O. furnacalis have been obtained (Coates et al. 2005), complete organelle genome information is still unclear. In the current study, the complete mitochondrial genome of O. furnacalis was reassembled and annotated, also the phylogenetic and taxonomic relationship with other species within Crambidae were discussed.
The larval of O. furnacalis selected for this study was collected from Jinan, Shandong Province, China (116°58.8′E, 36°58.7′N) in September 2017. Total genomic DNA was extracted using TIANamp Genomic DNA Kit (TIANGEN, Beijing, China) according to the manufacture’s instructions. The specimen (Voucher No. JNZQ012017) and isolated DNA were stored in the Institute of Plant Protection, Shandong Academy of Agricultural Sciences (Jinan, China). The complete mitochondrial genome of O. furnacalis was PCR amplified in overlapping fragments as described by Coates et al. (2005) and the fragments were assembled using MEGA6 (Tamura et al. 2013). The complete sequence was primarily annotated by MITOS WebServer (Bernt et al. 2013) and all the predicted tRNAs were confirmed using the tRNAscan-SE search server (Lowe and Chan 2016). Protein-coding genes (PCGs) and rRNA genes of O. furnacalis were annotated manually based on BLASTn results against published sequences of Loxostege sticticalis (GenBank: KR080490.1). The concatenated amino acid sequences of the 13 PCGs were used to reconstruct the phylogenetic relationships among the species within Crambidae using the Maximum Likelihood (ML) algorithm in MEGA6.0 software with the Jones–Taylor–Thornton (JTT) mode, considering 2000 replications with bootstrap analyses (Tamura et al. 2013).
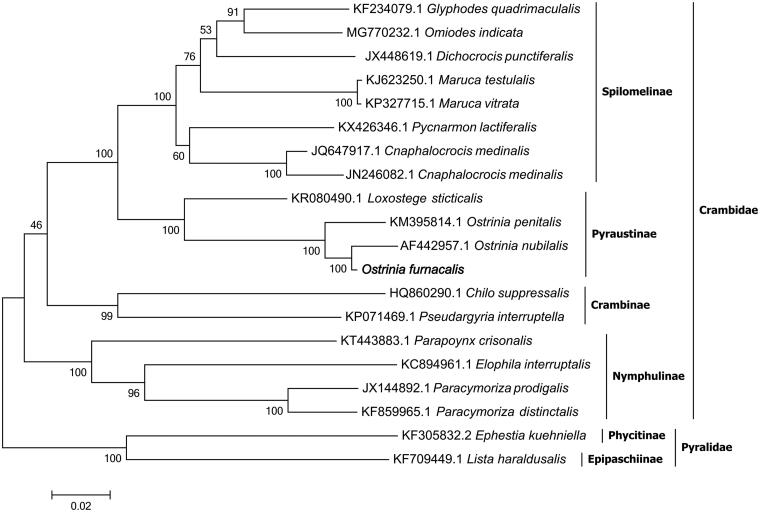
The complete mitochondrial genome of O. furnacalis is 15,241 bp in length and has a base composition of A (41.7%), T (39.2%), C (11.4%), G (7.7%), demonstrating an extreme bias of high AT content (80.9%) (GenBank accession no. MN747041). The mitochondrial genome contains a typically conserved structure among moth mitogenomes, encoding 13 protein-coding genes (PCGs), 22 transfer RNA (tRNA) genes, 2 ribosomal RNA (12S rRNA and 16S rRNA) genes, and a control region (D-loop region). The mitochondrial gene order was identical to that observed in most lepidopteran genomes, nine PCGs were located on the H-strand, others were located on the L-strand. 12 PCGs were initiated by typical ATN codons (ATA for ND2, COII and ATP8; ATG for ATP6, COIII, ND4, ND4L, CYTB and ND1; ATT for ND3, ND5 and ND6), except for COI with CGA instead. 21 tRNAs had the typical cloverleaf structure, while the DHU arm of the trnS1 gene did not form a stable stem-loop structure. The ‘ATAGT(A)’-like motif and a 19 bp poly-T stretch at the down-stream of the rrnS gene were observed in the A + T-rich region (Chai et al. 2012). The phylogenetic analysis showed that the relationship of O. furnacalis is very close to the three species in the subfamily Pyraustinae: O. nubilalis, O. penitalis and L. sticticalis, and all the subfamilies of Spilomelinae, Pyraustinae, Crambinae and Nymphulinae within Crambidae formed monophyletic groups with the highest bootstrap value support (Figure 1).
Figure 1.
Phylogenetic tree constructed for Crambidae in Lepidoptera, including O.furnacalis, using the concatenated amino acid sequences of 13 PCGs. GenBank accession numbers of each species were listed in the tree. Lista haraldusalis and Ephestia kuehniella from the family Pyralidae were used as the outgroups. The tree was constructed based on a complete protein sequence alignment by the ML method with bootstrapping analysis (2000 replicates).
Funding Statement
This study was supported by the National Key R&D Program of China [2017YFD0200400], the National Natural Science Foundation of China [31800349] and the Key R&D Program of Shandong Province [2018GNC111019], Agricultural Science and Technology Innovation Project of Shandong Academy of Agricultural Sciences [CXGC2019G01].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319. [DOI] [PubMed] [Google Scholar]
- Chai HN, Du YZ, Zhai BP. 2012. Characterization of the complete mitochondrial genomes of Cnaphalocrocis medinalis and Chilo suppressalis (Lepidoptera: Pyralidae). Int J Biol Sci. 8(4):561–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates BS, Sumerford DV, Hellmich RL, Lewis LC. 2005. Partial mitochondrial genome sequences of Ostrinia nubilalis and Ostrinia furnicalis. Int J Biol Sci. 1(1):13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folcher L, Jarry M, Weissenberger A, Gérault F, Eychenne N, Delos M, Regnault-Roger C. 2009. Comparative activity of agrochemical treatments on mycotoxin levels with regard to corn borers and Fusarium mycoflora in maize (Zea mays L.) fields. Crop Prot. 28(4):302–308. [Google Scholar]
- Kojima W, Fujii T, Suwa M, Miyazawa M, Ishikawa Y. 2010. Physiological adaptation of the 335 Asian corn borer Ostrinia furnacalis to chemical defenses of its host plant, maize. J Insect Physiol. 56(9):1349–1355. [DOI] [PubMed] [Google Scholar]
- Lowe TM, Chan PP. 2016. tRNAscan-SE On-line: search and contextual analysis of transfer RNA genes. Nucleic Acids Res. 44(W1):W54–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30(12):2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DB, Zhang LN, Yan XJ, Wang ZY, Yuan HZ. 2014. Effects of droplet distribution on insecticide toxicity to Asian corn borers (Ostrinia furnaealis) and spiders (Xysticus ephippiatus). J Integr Agric. 13:122–131. [Google Scholar]