Abstract
Rumex japonicus is a medicinal plant distributed in East Asia. Here, we report and characterize the complete plastid genome sequence of R. japonicus and size is 159,292 bp in length and contains the typical structure and gene content of other angiosperm plastomes, including two inverted repeat regions of 30,629 bp, a large single-copy region of 85,028 bp and a small single-copy region of 13,006 bp. There are 112 unique genes, including 78 protein-coding, 30 tRNAs and 4 rRNAs. We constructed a phylogenetic tree with 14 species and the phylogenetic topologies showed that R. japonicus was closely related to Rheum wittrockii.
Keywords: Rumex japonicus, chloroplast genome, medicinal plant, next-generation sequencing, Polygonaceae
A perennial herb plant, Rumex japonicus Houtt., belongs to the family Polygonaceae, is widely dispersed in the East Asia territories particularly, China, Japan and Korean Peninsula (Lee et al. 2016). It has been extensively used for the treatment of constipation, heat phlegm, jaundice, skin disease and uterine hemorrhage in traditional medicine due to presence of anthraquinones, oxanthrones, and flavonoid metabolites (Zee et al. 1998; Li et al. 2000; Zhou et al. 2005; Lee et al. 2006; Guo et al. 2011). Although, it has potential effects on antibacterial, anti-inflammatory, antioxidant and inhibitory activity against atopic dermatitis and skin disease (Zee et al. 1998; Elzaawely et al. 2005; Jang et al. 2005; Zhou et al. 2005; Lee et al. 2006; Guo et al. 2011; Xie and Yang 2014). Due to the unavailability of chloroplast (cp) information on this important medicinal plant, we sequenced and characterized the complete cp genome of Rumex japonicus Houtt. in the present study. The plant was collected from Dokdo island (geospatial coordinates: N37°14′20.2″, E131°52′10.6″) and the specimen stored at Yeungnam University Herbarium (YNUH), Republic of Korea (Specimen accession number: YNUH19D179). Total genomic DNA was extracted from young leaves using the Dneasy Plant Mini Kit (Qiagen) and whole-genome sequencing was performed using an Illumina HiSeq 2599 (Phyzen Ltd., South Korea).
The complete cp genome size of the plant, Rumex japonicus Houtt., is 159,292 bp with 37.5% of GC content which is similar to most of Polygonaceae cp genomes (GenBank accession number: MN720269). The genome has encoded two inverted repeat regions (IRa and IRb) of 30,629 bp, which is separated by one large single-copy region (LSC, 85,028 bp) and one small single-copy region (SSC, 13,006 bp). A total of 129 functional genes were identified, of which 112 were unique and included 78 protein-coding, 30 transfer RNA (tRNA) and 4 ribosomal RNA (rRNA) genes. Six protein-coding, seven tRNA and four rRNA genes were duplicated in the IR regions. This genome has encoded rpl23 gene as a pseudogene in both IR regions. Whereas, the two intact copies of the ycf1 gene were present in the IR region of R. japonicus cp genome. The similar patterns of pseudogene rpl23 and two copies of ycf1 were observed in other species of Polygonaceae cp genomes.
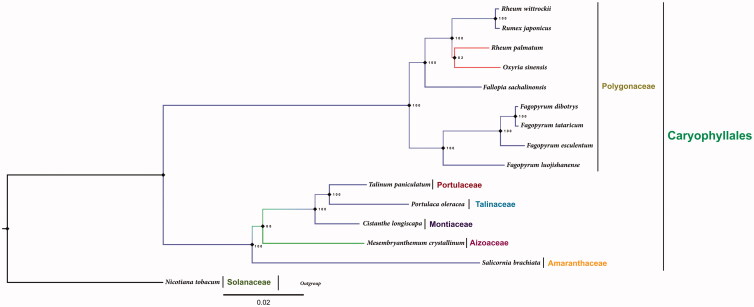
The maximum likelihood molecular phylogenetic tree was constructed using 76 protein-coding genes of 14 cp genome sequences (Figure 1). The molecular phylogenetic tree was divided into two clades, Polygonaceae species formed as one clade whereas other species of Caryophyllales formed as another clade. Interestingly, the phylogenetic topologies showed that the species R. japonicus is closely related to Rheum wittrockii with a maximum bootstrap value of 100%, whereas R. palmatum and Oxyria sinensis formed a sister clade to R. japonicus clade. This variation is due to presence of non-synonymous mutations in the protein-coding genes of clpP, matK, psbB, rpl2, rpoC1, rpoC2 and ycf2. The complete plastome sequence of R. japonicus will provide a useful resource for the conservative genetics of this species as well as for the phylogenetic studies for Polygonaceae.
Figure 1.
Maximum likelihood phylogenetic tree of 15 Caryophyllales species with 76 chloroplast protein-coding gene sequences. The tree was constructed by using the RAxML program and the GTR + G + I nucleotide model. The stability of each tree node was tested by bootstrap analysis with 1000 replicates. Nicotiana tabacum was set as the outgroup.
Funding Statement
This work was supported by the 2015 Yeungnam University Research Grant, Republic of Korea.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Elzaawely AA, Xuan TD, Tawata S. 2005. Antioxidant and antibacterial activities of Rumex japonicus Houtt. aerial parts. Biol Pharm Bull. 28(12):2225–2230. [DOI] [PubMed] [Google Scholar]
- Guo S, Feng B, Zhu R, Ma J, Wang W. 2011. Preparative isolation of three anthraquinones from Rumex japonicus by high-speed counter-current chromatography. Molecules. 16(2):1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang DS, Kim JM, Kim JH, Kim JS. 2005. 24-nor-Ursane type triterpenoids from the stems of Rumex japonicus. Chem Pharm Bull. 53(12):1594–1596. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim NH, Yang H, Bae SK, Heo Y, Choudhary I, Kwon YC, Byun JK, Yim HJ, Noh BS, et al. 2016. The hair growth-promoting effect of Rumex japonicus Houtt. Extract Evid Based Complement Alternat Med. 2016:1873746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-S, Kim S-K, Han J-B, Choi H-M, Park J-H, Kim E-C, Choi M-S, An H-J, Um J-Y, Kim H-M, et al. 2006. Inhibitory effects of Rumex japonicus Houtt. on the development of atopic dermatitis-like skin lesions in NC/Nga mice. Br J Dermatol. 155(1):33–38. [DOI] [PubMed] [Google Scholar]
- Li YP, Takamiyagi A, Ramzi ST, Nonaka S. 2000. Inhibitory effect of Rumex japonicus Houtt. on the porphyrin photooxidative reaction. J Dermatol. 27(12):761–768. [DOI] [PubMed] [Google Scholar]
- Xie QC, Yang YP. 2014. Anti-proliferative of physcion 8-O-β-glucopyranoside isolated from Rumex japonicus Houtt. on A549 cell lines via inducing apoptosis and cell cycle arrest. BMC Complement Altern Med. 14(1):377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee OP, Kim DK, Kwon HC, Lee KR. 1998. A new epoxynaphthoquinol from Rumex japonicus. Arch Pharm Res. 21(4):485–486. [DOI] [PubMed] [Google Scholar]
- Zhou X, Xuan L, Zhang S. 2005. Study on the chemical constituents from Rumex japonicus Houtt. Zhong Yao Cai. 28(2):104–105. [PubMed] [Google Scholar]