Abstract
Introduction
There is paucity of literature comparing outcomes of kidney transplant patients with COVID-19 to that of dialysis and waitlisted patients. This report describes our data, provides comparative analysis, together with a meta-analysis of published studies, and describes our protocols to restart the transplant program.
Methods
Data were analyzed on kidney transplant, dialysis, and waitlisted patients tested positive for SARS-CoV-2 (nasopharyngeal swab polymerase chain reaction [PCR] test) between March 1, 2020, and June 30, 2020, together with a meta-analysis of 16 studies.
Results
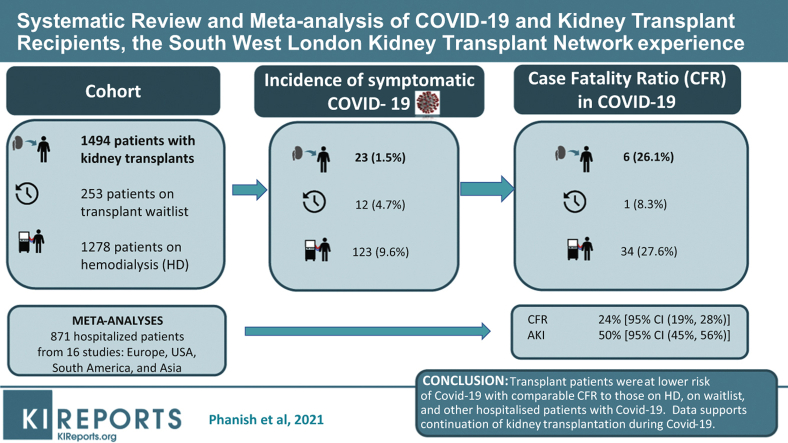
Twenty-three of 1494 kidney transplant patients tested positive for SARS-CoV-2 compared with 123 of 1278 hemodialysis patients (1.5% vs. 9.6%, P < 0.001) and 12 of 253 waitlisted patients (1.5% vs. 4.7%, P = 0.002). Nineteen patients required hospital admission, of whom 6 died and 13 developed AKI. The overall case fatality ratio was 26.1% compared with patients on hemodialysis (27.6%, P = 0.99) and waitlisted patients (8.3%, P = 0.38). Within our entire cohort, 0.4% of transplant patients died compared with 0.4% of waitlisted patients and 2.7% of hemodialysis patients. Patients who died were older (alive [median age 71 years] vs. dead [median age 59 years], P = 0.01).
In a meta-analysis of 16 studies, including ours, the pooled case fatality ratio was 24% (95% confidence interval [CI] 19%, 28%); AKI proportion in 10 studies was 50% (95% CI 45%, 56%), with some evidence against no heterogeneity between studies (P = 0.02).
Conclusions
From our cohort of transplant patients, a significantly lower proportion of patients contracted COVID-19 compared with waitlisted and dialysis patients. The case fatality ratio was comparable to that of the dialysis cohort and to a pooled case fatality ratio from a meta-analysis of 16 studies. The pooled AKI ratio in the meta-analysis was similar to our results.
Graphical abstract
SARS-CoV-2, the virus that causes COVID-19, continues to cause significant mortality and morbidity across the world as the pandemic evolves. As of July 27, 2020, a total of 300,111 people had tested positive for the virus in the United Kingdom and, of those tested positive, across all settings, 45,312 have died. The disease is primarily pulmonary, but involvement of other organs, including the kidneys and heart, during the course of illness is now well recognized. Kidney transplant recipients, because of their immunosuppressive burden and underlying comorbidities, are thought to be at higher risk of acquiring the infection as well as developing severe disease requiring hospitalization. We recently reported our initial experience of 7 renal transplant patients from 3 south London hospitals: 2 of 7 patients were managed at home and 1 patient died.1 All patients were managed with reduction of immunosuppression with no specific antiviral or anti-inflammatory therapies. In the same journal edition, Alberici et al. published their early experience of 20 kidney transplant patients admitted with SARS CoV-2 pneumonia in which they described a 25% mortality in spite of additional treatment with various drugs that included lopinavir/ritonavir, hydroxychloroquine, dexamethasone, and tocilizumab.2 Since these early reports, there have been several further reports of COVID-19 in kidney transplant patients describing overall case fatality ratios of 10% to 38% and 50% to 65% for patients requiring invasive ventilation.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19
We and others have advocated for immunosuppression reduction as a primary therapeutic strategy for hospitalized kidney transplant patients with COVID-19 pneumonia, with cessation of antiproliferative agents (mycophenolate mofetil/azathioprine) and continuation of calcineurin inhibitors either at the same or reduced dose depending on severity of disease along with continuation of corticosteroids.1, 2, 3,6
In this report, we describe 23 kidney transplant patients tested positive for SARS-CoV-2 from 2 tertiary care renal centers from South London Renal transplant Network, United Kingdom. This includes follow-up data on 5 patients described in our previous report.
The aim of this analysis was to further characterize SARS-CoV-2–infected transplant patients, describe their management and outcome, and compare the proportion of infections and case fatality ratios in transplant patients with waitlisted and the total cohort of dialysis patients. In addition, we have performed meta-analyses on 15 published studies on COVID-19 in kidney transplant patients in addition to ours to derive case fatality–acute kidney injury (AKI) ratios in hospitalized kidney transplant patients with COVID-19.
Methods
Data were collected on all kidney transplant recipients tested positive for SARS-CoV-2 between March 1, 2020, and June 30, 2020 (first wave of COVID-19 in the United Kingdom) and followed until October 15, 2020. The data collected included demographics, clinical and laboratory parameters, and outcomes. In addition, we collected data on dialysis patients that included all the patients on dialysis and those on the transplant waitlist. Data were collected as part of routine clinical processes and downloaded for the study from the electronic patient records. The study was approved by NHS Research Ethics Committee 20/SW/0077 and Heath Research Authority IRAS 283130.
Continuous variables were summarized by their means, medians, standard deviations, interquartile ranges (IQRs) and limits; categorical data were summarized as proportions. Two-sample independent tests tailored to the nature of the variables were used to test the null hypothesis of no difference between transplant patients and those on the waiting list. A 2×2 contingency table and Fisher exact tests were employed to assess the effect of dual vs. triple immunosuppression on the outcome of death.
Meta-analyses were performed to derive pooled proportions of deaths, AKI, and AKI stage 3 among positive patients using the available data from 15 published studies and our data. Using the keywords COVID-19, kidney transplant, mortality, and AKI for a PubMed search, we analyzed the outcomes for studies published between May 15, 2020, and October 20, 2020. Among 157 returns, we selected 15 studies that included at least 10 patients reporting mortality of hospitalized patients and/or AKI.2,3,5,8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Methods associated with analyses of proportions specific to binomial data allow computation of exact binomials and score test–based CIs. They also use appropriate methods for dealing with proportions close to or at the margins where the normal approximation procedures often break down, by use of the binomial distribution to model the within-study variability or by allowing Freeman-Tukey double arcsine transformation to stabilize the variances. (We used Metaprop command implemented in Stata, release 16 [StataCorp LLC, College Station, TX].)
Results
Clinical Characteristics
Twenty-three transplant patients tested positive for SARS-CoV-2 during the study period from a total cohort of 1494 kidney transplant recipients under follow-up in 2 renal centers (1.5% of total transplant cohort) (Table 1). Four were managed at home and 19 patients required hospitalization. The mean age was 62±9.2 years and the median age 62 years (IQR 55–69 years), compared with the median age of 51 years of the overall transplant cohort. There were 17 male and 6 female patients. Six (26%) patients were of black ethnicity, 9 white (39.1%), 4 South Asian (21.7%), 1 East Asian, 1 Hispanic, and 2 other. In comparison, the ethnicity of our entire transplant cohort as reported previously was black, 7.7%; white, 73.8%; and South Asian, 15.4%.7 Twenty-two patients had hypertension, 2 had a history of cancer, 8 had diabetes, and 1 had HIV. The 19 hospitalized patients had a mean age of 64.2 ± 8.7 years and a median age of 64 years (IQR 59–72 years). The median follow-up period was 183 days (range 169–199 days, IQR 173–192 days). The median transplant vintage (from transplant date to date of positive swab) was 1686 days (4.6 years; range 47–12,054 days, IQR 273–5326 days). Three patients (2 hospitalized, 1 managed at home) were within 3 months since receiving transplant (53 days, 56 days, and 47 days), 3 were between 3 and 12 months since their transplant, and the rest (17) had received their transplant >12 months ago. None of the patients who died had their transplant within the previous 6 months.
Table 1.
Patient Demographics, Comorbidities, Immunosuppressive Drugs, Hospital Management (Critical Care Admission, Type of Respiratory Support, and Renal Replacement Therapy)
| Variable / Summary Type or Category | All (N = 23) | Alive (n = 17; 73.9%) | Died (n = 6; 26.1%) | Two-Sample Independent Test (P Value) |
|---|---|---|---|---|
| Age, yr | ||||
| Mean (SD) | 62 (9.2) | 59.2 (8.2) | 70.5 (6.8) | 0.01 |
| Median (Q1-Q3) | 62 (55-69) | 59 (54-64) | 71 (68-76) | |
| Range | 45-78 | 45-73 | 59-78 | |
| Gender | ||||
| Female | 6 (26) | 5 (29) | 1 (17) | 0.99 |
| Male | 17 (74) | 12 (71) | 5 (83) | |
| Ethnicity | ||||
| Black | 6 (26.1) | 4 (23.5) | 2 (33.3) | 0.615 |
| East Asian | 1 (4.4) | 1 (5.9) | 0 (0) | |
| Other | 2 (8.7) | 1 (5.9) | 1 (16.7) | |
| South Asian | 5 (21.7) | 3 (17.7) | 2 (33.3) | |
| White | 9 (39.1) | 8 (47.1) | 1 (16.7) | |
| Immunosuppressive drugs | ||||
| 2 | 14 (61) | 12 (71) | 2 (33.3) | 0.162 |
| 3 | 9 (39) | 5 (29) | 4 (66.6) | |
| Transplant type | ||||
| DBD | 17 (73.9) | 12 (70.6) | 5 (83.3) | 0.99 |
| DCD | 4 (17.4) | 3 (17.7) | 1 (16.7) | |
| Living Donor | 2 (8.7) | 2 (11.8) | 0 (0) | |
| Tacrolimus, yes | 21 (91.3) | 15 (88.2) | 6 (100) | 0.99 |
| Cyclosporine, yes | 1 (4.4) | 1 (5.9) | 0 (0) | 0.99 |
| Azathioprine, yes | 2 (8.7) | 2 (11.8) | 0 (0) | 0.99 |
| MMF, yes | 13 (56.6) | 9 (52.9) | 4 (66.7) | 0.66 |
| Prednisolone, yes | 16 (69.6) | 11 (64.7) | 5 (83.3) | 0.621 |
| Cancer | ||||
| Yes | 2 (8.7) | 1 (5.9) | 1 (16.7) | 0.521 |
| Missing | 3 (13) | 3 (17.7) | 0 (0) | |
| Diabetes, yes | 8 (34.8) | 4 (23.5) | 4 (66.7) | 0.131 |
| Chronic lung disease | ||||
| Yes | 0 (0) | 0 (0) | 0 (0) | NA |
| Missing | 3 (13) | 3 (17.7) | 0 (0) | |
| Hypertension, yes | 21 (91.3) | 15 (88.2) | 6 (100) | 0.99 |
| Platelets | ||||
| Mean (SD) | 213 (50.1) | 221.1 (53) | 193.8 (39.9) | 0.30 |
| Median (Q1-Q3) | 206 (178-238.5) | 213.5 (186-251) | 193 (157-230) | |
| Range | 144-337 | 157-337 | 144-246 | |
| Missing | 3 (13) | 3 (17.6) | 0 (0) | |
| White blood cell count | ||||
| Mean (SD) | 6.6 (1.9) | 6.4 (1.9) | 7.1 (2.0) | 0.563 |
| Median (Q1-Q3) | 6.6 (5.3-7.4) | 6.5 (5.1-7.3) | 6.7 (6.4-9.1) | |
| Range | 3.2-10.3 | 3.2-10.3 | 3.9-9.5 | |
| Missing | 3 (13) | 3 (17.6) | 0 (0) | |
| Baseline lymphocyte count | ||||
| Mean (SD) | 1.5 (0.9) | 1.7 (0.9) | 1.2 (0.7) | 0.303 |
| Median (Q1-Q3) | 1.4 (1.1-1.9) | 1.6 (1.2-2) | 1.1 (0.5-1.7) | |
| Range | 0.3-4.2 | 0.3-4.2 | 0.4-2.3 | |
| Missing | 3 (13) | 3 (17.6) | 0 (0) | |
| Hemoglobin on admission | ||||
| Mean (SD) | 113.1 (20.6) | 115.6 (22.7) | 107.2 (14.5) | 0.283 |
| Median (Q1-Q3) | 116.5 (99-130) | 117.5 (101-131) | 105 (97-109) | |
| Range | 67-149 | 67-149 | 93-134 | |
| Missing | 3 (13) | 3 (17.6) | 0 (0) | |
| Lymphocyte nadir during admission | ||||
| Mean (SD) | 0.7 (0.9) | 0.8 (1) | 0.5 (0.4) | 0.334 |
| Median (Q1-Q3) | 0.4 (0.2-0.8) | 0.4 (0.2-0.8) | 0.25 (0.2-0.7) | |
| Range | 0.2-4.1 | 0.2-4.1 | 0.2-1.1 | |
| Missing | 3 (13) | 3 (17.6) | 0 (0) | |
| Highest ferritin during admission | ||||
| Mean (SD) | 1691.7 (1901.5) | 1598.1 (2004.4) | 1949 (1833.2) | 0.896 |
| Median (Q1-Q3) | 781 (544-2469) | 781 (549-1320) | 1506 (503-3395) | |
| Range | 462-6959 | 538-6959 | 462-4321 | |
| Missing | 8 (35) | 6 (35) | 2 (33) | |
| Highest CRP during admission | ||||
| Mean (SD) | 186.7 (106.1) | 161.1 (83.4) | 237.8 (135.3) | 0.223 |
| Median (Q1-Q3) | 178.5 (122-230) | 160.5 (114.5-212) | 217 (147-233) | |
| Range | 31-497 | 31-320 | 116-497 | |
| Missing | 5 (22) | 5 (29) | 0 (0) | |
| RRT during admission | ||||
| No | 15 (78.9) | 12 (85.7) | 3 (60) | 0.272 |
| Yes | 4 (21.1) | 2 (14.3) | 2 (40) | |
| ITU admission | ||||
| No | 10 (52.6) | 9 (69.2) | 1 (16.7) | 0.05 |
| Yes | 9 (47.4) | 4 (30.8) | 5 (83.3) | |
| On ACEi/ARB | ||||
| No | 9 (39.1) | 7 (41.2) | 2 (33.3) | 0.99 |
| Yes | 10 (43.5) | 7 (41.2) | 3 (50) | |
| Missing | 4 (17.4) | 3 (17.7) | 1 (16.7) | |
| Breathing support | ||||
| Nasal cannula/mask | 10 (52.6) | 9 (69.2) | 1 (16.7) | 0.045 |
| Invasive ventilation | 6 (31.6) | 3 (23.1) | 3 (50) | |
| NIV/HFNC | 3 (15.8) | 1 (7.7) | 2 (33.3) |
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CRP, C-reactive protein; DBD, deceased brain donor; DCD, deceased cardiac donor; HFNC, high-flow nasal cannula; ITU, intensive therapy unit; MMF, mycophenolate mofetil; NIV, noninvasive ventilation; Q1-Q3, quartile 1 to quartile 3; RRT, renal replacement therapy; SD, standard deviation.
Unless otherwise noted, values are n (%). Data for hospitalized patients are for 19 patients. Rest of the data are for 23 patients. Age, requirement of respiratory support, and intensive care unit admission were significantly different between the 2 groups (Alive vs. Died). P values in bold indicate significance.
All patients had received basiliximab induction. Fifteen patients, including 4 managed at home, were on dual maintenance immunosuppression, and 8 patients were on triple immunosuppression. Two of 19 hospitalized patients had received their transplant in February 2020.
Management
All hospitalized patients were managed with immunosuppression reduction, and antiproliferative agents (mycophenolate mofetil/azathioprine) were stopped on admission in all the patients (n=19). Tacrolimus dose was reduced in mild to moderate cases (n=11) and stopped in severe cases where there was progressive clinical and radiologic deterioration (n=8). Prednisolone dose was either unchanged (n=3) or increased (n=13) in all cases. Some of the patients were recruited into the RECOVERY trial (Randomised Evaluation of COVID-19 therapy, www.recoverytrial.net). As a part of this trial, 2 patients received hydroxychloroquine and 1 received dexamethasone. In addition, 2 patients received tocilizumab. Of the 4 patients managed at home, 1 patient had his mycophenolate mofetil dose reduced by 50% with increase back to the baseline dose after 2 weeks, and the remaining 3 patients were managed without any change to immunosuppression (Table 2).
Table 2.
Duration of Hospital Stay, Respiratory Support, AKI, RRT (Requirement and Type), and Outcome of Hospitalized Patients (n=19) and Nonhospitalized Patients (n=4)
| Patient | Hospital Stay (d) | Respiratory Support | Place of Management | AKI (Y/N), Stage | RRT Yes/No |
Comments and Outcome |
|---|---|---|---|---|---|---|
| 1 | 12 | Nasal cannula and mask | Renal ward | Y, St 3 | Y (Intermittent HD) | Discharged home, alive to date, graft failed, transplant nephrectomy—90 d postdischarge |
| 2 | 12 | Intubation and ventilation | Intensive (critical) care unit | Y, St 3 | Y (CVVHDF) | Died |
| 3 | 5 | Intubation and ventilation | Intensive (critical) care unit | Y, St 2 | N | Discharged home, alive to date with functioning graft, renal function back to baseline |
| 4 | 7 | Nasal cannula | Medical ward, palliative care | Y, St 3 | N | Died |
| 5 | 12 | HFNC, NIV, intubation and ventilation | Renal ward, respiratory ward, intensive (critical) care unit | Y, St 2 | N | Died |
| 6 | 5 | Nasal cannula and venturi mask | Renal ward | Y, St 3 | N | Discharged home, alive to date, poorly functioning graft |
| 7 | 8 | Nasal cannula | Renal ward | N | N | Discharged Home, alive to date, functioning graft |
| 8 | 7 | Nasal cannula and venturi mask | Renal ward | Y, St 2 | N | Discharged home, alive to date, functioning graft, renal function back to baseline |
| 9 | 80 | NIV, intubation, and ventilation (57 d of invasive ventilation) | Renal ward, intensive (critical) care unit | Y, St 3 | Y (CVVHDF and intermittent HD) | Discharged home, alive to date, graft failed |
| 10 | 13 | Nasal cannula | Renal ward | Y, St 2 | N | Discharged home, alive to date, functioning graft, renal function back to baseline |
| 11 | 4 | Nasal cannula | High-dependency unit | N | N | Discharged Home, Alive to date, functioning Graft |
| 12 | 5 | Nasal cannula | Renal ward | Y, St 1 | N | Discharged home, Alive to date, functioning Graft, renal function back to baseline |
| 13 | 5 | NIV | ITU | Y, St 1 | No | Died |
| 14 | 4 | Nasal cannula/venturi mask | High-dependency unit | N | N | Discharged home, alive to date, functioning graft |
| 15 | 5 | HFNC (refused intubation) | ITU | Y, St 2 | N | Died |
| 16 | 11 | NIV | ITU | N | N | Discharged home, alive to date, functioning graft |
| 17 | 23 | Intubated and ventilated | ITU | N | N | Discharged home, alive to date, functioning graft |
| 18 | 2 | Nasal cannula | Ward | N | N | Discharged home, alive to date, functioning graft |
| 19 | 21 | Intubated and ventilated | ITU | Y, St 3 | Y | Died |
| 20 | 0 | None | Home | N | N | Alive, no change in immunosuppression, no graft dysfunction |
| 21 | 0 | None | Home | N | N | Alive, MMF dose halved, No graft dysfunction |
| 22 | 0 | None | Home | N | N | Alive, no change in immunosuppression, no graft dysfunction |
| 23 | 0 | None | Home | N | N | Alive, no change in immunosuppression, no graft dysfunction |
AKI, acute kidney injury; CVVHDF, continuous venovenous hemodiafiltration; HD, hemodialysis; HFNC, high-flow nasal cannula; ITU, intensive therapy unit; N, no; NIV, noninvasive ventilation; RRT, renal replacement therapy; St, stage (of AKI); Y, yes.
All patients who had tacrolimus dose reduced had the dose progressively increased such that by 2 weeks postdischarge, the levels were in a therapeutic range (5-8 ng/ml). Mycophenolate mofetil was reintroduced around 2-3 weeks postdischarge provided patients were well with no fever or other symptoms of COVID-19 for at least 3 days and had a normal C-reactive protein level.
Patient demographics, laboratory parameters, and clinical outcomes are summarized in Table 1. Age, admission to intensive care unit, and type of respiratory support required were the only variables significantly different in patients who died compared with those discharged home. Patients who died were significantly older (59.2±8.2 vs. 70.5±6.8 years, P=0.01) and required more ventilatory support (P=0.04). There were no significant differences between the 2 groups (living vs. died) with regard to comorbidities, peak ferritin levels, C-reactive protein, baseline lymphocyte count, or lowest lymphocyte count during admission (Table 1).
Outcome of Infected Patients
Duration of hospital stay, respiratory support, AKI, renal replacement therapy (RRT), and outcome of hospitalized patients (n=19) are described in Table 2. Six of the 19 (31.57%) hospitalized patients died (Table 2). Of the total cohort of 1494 transplant patients, 6 patients died, which represents 0.4% of the total cohort.
Twelve of 14 (85.7%) patients on dual immunosuppression survived and 2 (14.3%) died. Of the 9 patients on triple immunosuppression, 5 (55.6%) survived and 4 (44.4%) died. Although nonsignificant (P=0.16), the relative risk of death on triple immunosuppression was 1.54 (95% CI 0.91, 3.28) and the odds ratio 4.8 (95% CI 0.71, 29.3) (Table 3). There was no difference in the proportion of patients on maintenance steroids between the 2 groups (survived vs. died) (Table 1).
Table 3.
The Effect of Baseline Dual vs. Triple Immunosuppression on Mortality (n=23)
| Immunosuppression (Whole Cohort) | Alive, n (%) | Died, n (%) | P | RR (95% CI) of Death | OR (95% CI) |
|---|---|---|---|---|---|
| Dual (n = 14) | 12 (85.7) | 2 (14.3) | |||
| Triple (n = 9) | 5 (55.6) | 4 (44.4) | 0.16 | 1.54 (0.91-3.28) | 4.8 (0.71-29.3) |
CI, confidence interval; OR, odds ratio; RR, relative risk.
RR of death in patients on triple immunosuppression was 1.54, but there was no statistical difference between the groups (Fisher exact test).
Among 6 patients who died, 1 patient was white, 2 black, 2 South Asian, and 1 other. The ethnicity was not significantly different between the 2 groups (survived and died), but this may be due to the small sample size. All patients who died had hypertension, and 4 had diabetes.
Respiratory support
Of 19 hospitalized patients, 3 were managed on high-flow nasal cannula or noninvasive ventilation, 6 (31%) were intubated and ventilated, and the remaining 10 were managed with oxygen delivered through nasal cannula or venturi mask (Tables 1 and 2). Of the 6 intubated and ventilated patients, 3 (50%) died. Of the 3 other patients who were discharged home, 2 had a functioning graft and 1 remained on dialysis. The patient who was discharged on dialysis had poor graft function before he had COVID-19 and was on hemodialysis preadmission. He was ventilated for a prolonged period of 57 days.
AKI
Thirteen of 23 patients (57%) developed AKI; 11 patients had stage 2 or 3 AKI (stage 2: 5 patients, stage 3: 6 patients) and 2 patients had stage 1 AKI. Four patients needed RRT (hemodialysis or continuous venovenous hemodiafiltration) (Tables 1 and 2). AKI resolved in all but 3 patients. These 3 patients in whom transplant kidney function failed to recover had poor baseline kidney function with a Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation-based estimated glomerular filtration rate of <20 ml/min per 1.73 m2. None of the patients underwent percutaneous kidney biopsy. Two patients continued on hemodialysis, and 1 was discharged with CKD stage 5 and commenced hemodialysis 5 months postdischarge. Of the 2 patients who have remained on hemodialysis postdischarge, 1 (patient 1 in Table 2) underwent transplant nephrectomy 3 months postdischarge as a result of severe rejection (clinical diagnosis). His nose and throat swabs for SARS-CoV-2 PCR both before and during this admission were negative. He made good recovery from surgery and is currently on outpatient hemodialysis. His histology of kidney postnephrectomy revealed severe vascular rejection, with widespread cortical infarctions and a thrombus in the main transplant artery.
At follow-up till October 15, 2020 (median follow-up of 183 days), all discharged patients and those managed at home (n=17) have remained well with no readmissions apart from the patient described above. Three patients have lost their graft function (all with baseline estimated glomerular filtration rate <20 ml/min per 1.73 m2).
Eight of 9 hospitalized patients discharged home (from 1 center) with baseline positive nasopharyngeal swab for SARS-CoV-2 PCR were reswabbed 3-4 weeks postdischarge. All of them had cleared the virus, as shown by negative nose and throat swab results.
Comparisons With Dialysis Cohort and Waitlisted Patients
Twenty-three from a cohort of 1494 kidney transplant patients were tested positive for SARS-CoV-2 compared with 123 of 1278 hemodialysis patients (1.5% vs. 9.6%, P < 0.001), 12 of 253 waitlisted patients (1.5% vs. 4.7%, P = 0.002), and 8 of 170 peritoneal dialysis patients (1.5% vs. 4.7%, P = 0.01) (Tables 4 and 5). The case fatality ratio was 26.1% for transplant patients, 8.3% for waitlisted patients, 27.6% for hemodialysis patients, and 75.0% for peritoneal dialysis patients. There was no statistically significant difference in case fatality ratio of transplant patients compared with waitlisted patients, hemodialysis patients, and peritoneal dialysis patients (Tables 4 and 5).
Table 4.
Proportions of Infections, Deaths in Transplant, Waitlisted, Hemodialysis, and Peritoneal Dialysis Patients
| Transplant Patients (1) | Patients on Transplant Waitlist (2) | Hemodialysis Patients (3) | Peritoneal Dialysis Patients (4) | |
|---|---|---|---|---|
| Total cohort (n) | 1494 | 253 | 1278 | 170 |
| COVID-19–positive (n) | 23 | 12 | 123 | 8 |
| Percentage of COVID-19–positive all patients in the cohort | 1.5 | 4.7 | 9.6 | 4.7 |
| Deaths (n) | 6 | 1 | 34 | 6 |
| Case fatality ratio (% of deaths among the COVID-19–positive) | 26.1 | 8.3 | 27.6 | 75.0 |
| Percentage of deaths (% of deaths among all patients in the cohort) | 0.4 | 0.4 | 2.7 | 3.5 |
Proportions of infections and case fatality ratio of transplant patients (1) compared to patients on transplant waitlist (2), hemodialysis (3), and peritoneal dialysis patients (4).
Table 5.
Comparisons of the proportions of Infections and Deaths in Transplant, Waitlisted, Hemodialysis, and Peritoneal Dialysis Patients
| Group Comparisons (as in Table 4)a |
||||||
|---|---|---|---|---|---|---|
| 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | |
| Proportion of COVID-19–positive among all | 0.002 | <0.001 | 0.01 | 0.011 | 0.999 | 0.033 |
| Case fatality ratio (% of deaths among the COVID-19–positive patients) | 0.380 | 0.999 | 0.032 | 0.185 | 0.004 | 0.010 |
There was a significant difference in infection risk in transplant patients compared to patients on waitlist and hemodialysis (1 vs. 2, P = 0.002, 1 vs. 3, P < 0.001). There was no significant difference in case fatality ratio between transplant patients and other groups.
Comparisons were performed between aggregated proportions. Only P values lower than 0.05/12 = 0.0042 are considered significant (shown in bold) due to Bonferroni correction for multiple comparisons.
Comparison groups: (1) transplant patients; (2) patients on transplant waitlist; (3) hemodialysis patients; and (4) peritoneal dialysis patients.
Meta-analyses
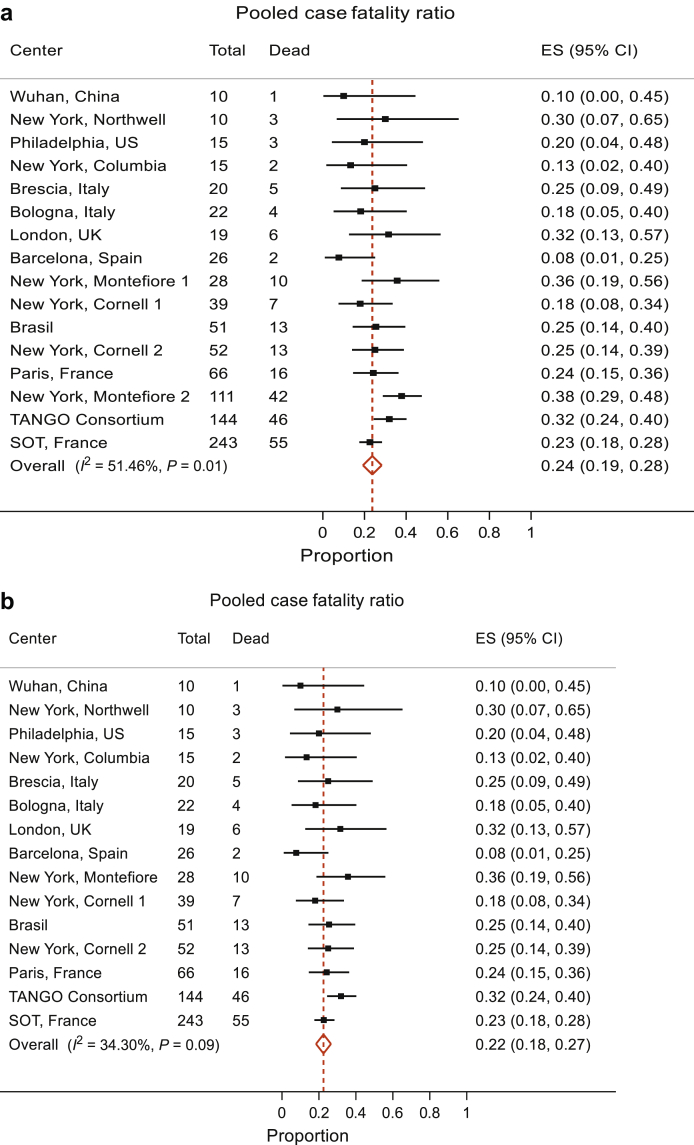
We performed meta-analyses of 15 published studies and our data to derive a pooled estimate of case fatality ratio (of hospitalized patients) and AKI in kidney transplant patients who tested positive for SARS-CoV-2, including a recent publication of TANGO international consortium.2,3,5,8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 The total number of hospitalized patients included in these studies were 871. The pooled case fatality ratio was 24% (95% CI 19%, 28%). The variability in the effect size attributable to between-study heterogeneity was moderate (I2 = 51.5%), consistent with some evidence against the null hypothesis stating no heterogeneity between studies (P = 0.01) (Figure 1a). The Montefiore 2 Study18—the third most influential in this analysis—exhibited a case fatality ratio of 38% (95% CI 29%, 48%), well above the pooled estimate of 24% (95% CI 19%, 28%). Excluding this study, the I2 drops to 34.3% with a P value of 0.09, indicating consistency with the magnitude of I2 and with the null hypothesis of not much heterogeneity between studies. The pooled case fatality ratio in this analysis was 22% (95% CI 18%, 27%), which was very close to our first analysis. Given the size and hence the precision of estimate in the Montefiore 2 Study (n=111), we opted to include all studies and provide evidence that the pooled case fatality ratio of hospitalized kidney transplant patients with COVID-19 is 24% (95% CI 19%, 28%).
Figure 1.
Meta-analyses of COVID-19 in transplant patients: case fatality ratio. (a) The pooled case fatality ratio was 24% (95% CI 19%, 28%). There was moderate heterogeneity between the studies (I2 = 51.5% [variation in effect size (ES) attributable to heterogeneity], heterogeneity χ2 = 30.90 [df = 15], P = 0.01). The New York Montefiore 2 Study—the third most influential in this analysis—exhibited a case fatality ratio of 38% (95% CI 29%, 48%), well above the pooled estimate of 24% (95% CI 19%, 28%). (b) We then analyzed 14 studies excluding this study, and with this analysis, the I2 drops to 34.3% with a P value = 0.09, consistent with the null hypothesis of not much heterogeneity between studies. The pooled case fatality ratio in this analysis was 22% (95% CI 18%, 27%).
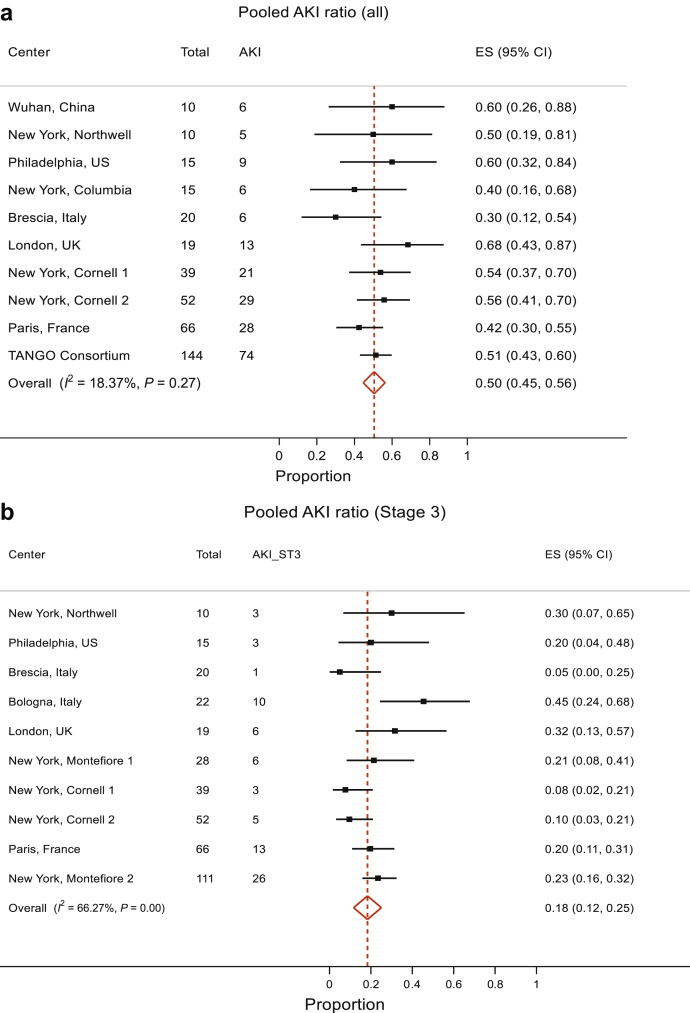
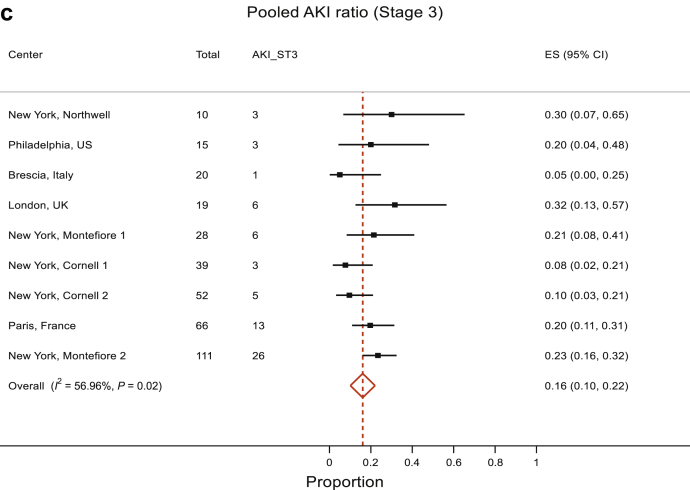
The analyses of AKI included 10 studies that reported AKI. The pooled proportion of AKI was 50% (95% CI 45%, 56%). There was no evidence to suggest heterogeneity between the studies, P = 0.27, and therefore, the data estimate that 50% (95% CI 45%, 56%) of kidney transplant patients with COVID-19 develop AKI (Figure 2a). We also separately analyzed the pooled proportion of severe AKI (stage 3 AKI or those requiring RRT). This analysis showed a pooled proportion of stage 3 AKI of 18% (95% CI 12%, 25%) (Figure 2b). However, there was evidence for presence of significant heterogeneity between studies (P < 0.001). Reanalysis after removal of the Bologna study showed that the high stage 3 AKI percentage of 45% yielded results with a pooled stage 3 / RRT–requiring AKI estimate of 16% (95% CI 10%, 22%), although the evidence against no heterogeneity between studies remained significant (P = 0.02) with I2 = 56.96% (Figure 2c).
Figure 2.
Meta-analyses of COVID-19 in transplant patients: (a) AKI, all stages. The pooled proportion of AKI was 50% (95% CI 45%, 56%). There was no significant heterogeneity between the studies, χ2 = 11.02 (df = 9), P = 0.27; I2 (variation in effect size [ES] attributable to heterogeneity) = 18.37%. Therefore, the pooled proportion of AKI is 50% (95% CI 45%, 56%). (b, c) AKI, stage 3 / RRT requirement. The pooled proportion of severe AKI (stage 3 / requiring RRT) was 18% (95% CI 12%, 25%) (b). However, there was a significant heterogeneity: I2 = 66.27%, P < 0.001. Reanalysis after removal of the Bologna study that showed a high stage 3 AKI percentage of 45% yielded results with pooled stage 3 / RRT-requiring AKI estimate of 16% (95% CI 10%, 22%) but the heterogeneity, although improved, remained significant, with I2 = 56.96%, P < 0.02 (c). Therefore, it appears from these analyses that the pooled proportion of severe AKI is 16% to 18%.
Discussion
In this report, we have described 23 kidney transplant recipients who tested positive for SARS-CoV-2 between March 1, 2020, and June 30, 2020 (this includes the entire period of the first surge of COVID-19 in London) with a median follow-up of 183 days. Nineteen were hospitalized and 4 managed at home, and 6 patients died (overall case fatality ratio of 26%, for hospitalized patients 31.6%). Six patients required intubation and ventilation, among whom 3 died (50% mortality in ventilated patients). Age and requirement of intensive care unit admission and respiratory support (noninvasive or invasive ventilation) were significantly different in patients who died compared with those who survived. In the meta-analysis of 16 available reports including ours, the pooled case fatality ratio for hospitalized transplant patients with COVID-19 was 24% (95% CI 19%, 28%), pooled proportion of AKI (all stages) was 50% (95% CI 45%, 56%) and that of AKI stage 3 / requiring RRT was 16% (95% CI 10%, 22%).
A small proportion of our overall transplant patient cohort got COVID-19 (1.5%) compared to 9.6% hemodialysis patients, 4.7% peritoneal dialysis patients, and 4.7% of waitlisted patients with an in-hospital case fatality ratio of 31.57%. A German multicenter study of 10,021 patients with COVID-19 admitted to 920 hospitals found an overall mortality of 22% and a 53% mortality in those requiring mechanical ventilation.20 A UK study of 20,133 patients admitted to 208 hospitals demonstrated an overall mortality of 26%21 compared to the pooled mortality of 24% in our meta-analyses. Our overall case fatality ratio of 26% is very similar to the 27% mortality observed in UK data in renal transplant recipients (NHSBT weekly COVID-19 reports). Transplant patients had case fatality ratios comparable to that of hemodialysis patients. The case fatality ratio of waitlisted patients was lower compared with transplant patients, but this difference was not statistically significant. Waitlisted patients tend to be younger and with fewer comorbidities compared to some of the older transplant patients, and this may largely explain this difference. Older age was associated with poor prognosis, with a median age of 71 years for transplant patients who died compared with 59 years for those who survived. This is consistent with the Spanish series by Perez-Saez et al., who reported a hazard ratio of death of 3.1 for patients older than 60 years.22 We observed 50% mortality of intubated patients, and this compares favorably with the 53% mortality among general medical patients with COVID-19 requiring invasive mechanical ventilation in the German study.20 Similarly, Rinaldi et al. found no difference in survival in transplant patients compared with the general population.17 All the patients from our cohort who were discharged home have survived to date.
It is likely that baseline immunosuppressive burden plays a role in the prognosis of COVID-19 as it does with other infections. Our units have a significant number of patients (approximately 60%-70%) on long-term dual immunosuppression.7 There was a trend toward higher risk of death in patients on triple immunosuppression, but this did not achieve statistical significance probably because of the small sample size. It needs to be seen in larger data sets if patients on triple immunosuppression are at higher risk of severe disease from SARS-CoV-2 compared with those on 2 drugs and if mycophenolate mofetil confers higher risk. We managed all the patients in line with NHS Blood and Transplant British Transplantation Society guidelines with immunosuppression reduction as the main strategy along with supportive medical care (https://bts.org.uk/information-resources/covid-19-information/) and recruitment into national clinical trials. Some of our patients were enrolled into the RECOVERY trial and received tocilizumab outside the trial on clinical grounds, but the numbers are too small to draw any conclusions on the effectiveness of these drugs. For current recommendations on the management of transplant patients with COVID-19 that include guidelines on the use of dexamethasone and remdesivir in transplant patients with COVID-19 pneumonia, the reader is referred to recent British Transplant Society (BTS)/UK renal association guidelines. (https://bts.org.uk/wp-content/uploads/2020/07/Clinical-management-of-transplants-and-immunosuppression-updated-9th-July.pdf). We would like to highlight here that some of the drugs used worldwide for COVID-19 such as azithromycin and lopinavir/ritonavir show significant interaction with tacrolimus, causing toxicity, and therefore, should be avoided where possible and, if used, tacrolimus levels should be monitored closely.
We observed a high percentage of AKI in these patients (68%) and 6 (31.5%) patients with stage 3 AKI. In comparison, the pooled proportion of AKI was 50% and that of stage 3 / RRT-requiring AKI was 16% to 18%. There was significant heterogeneity in studies reporting AKI as some reported all stages and some only reported patients requiring RRT. We analyzed these separately and included stage 3 / RRT requirement in 1 group as this indicates severe AKI. However, it must be noted that studies that reported patients requiring RRT only would have excluded patients with stage 3 AKI not needing RRT and, therefore, the true number of stage 3 AKI in these studies is likely to be higher than reported. Reassuringly, the AKI recovered in most cases. Two patients who remained dialysis dependent had poor baseline kidney function (estimated glomerular filtration rate <20 ml/min per 1.73 m2), and their kidney function deteriorated further during their hospital stay. It is possible that these patients developed rejection on immunosuppression reduction but we did not have transplant kidney biopsy results to prove this. On clinical grounds, we felt that treating COVID-19 pneumonia was the priority and, therefore, felt that the risk-benefit ratio did not favor doing a biopsy. During this period, we found an AKI risk of 26% in hospitalized patients with COVID-19 (i.e., 204 of 792 hospitalized patients in 3 hospitals from South London and Surrey developed AKI; personal communication). A recent publication on all hospitalized patients found an AKI in 36.6% of patients and 31.1% had stage 3 AKI23, very similar to the stage 3 AKI prevalence that we observed in our transplant cohort.
Following discharge from the hospital, patients were followed up in a dedicated COVID-19 outpatient setting for 4 weeks. In one of the 2 hospitals, we performed repeat SARS-CoV-2 nasal and throat swabs 3-4 weeks following discharge. All of the 8 patients tested had cleared the virus, with negative follow-up swab PCR results. The negative swab enabled us to deisolate these patients and return them to their normal outpatient pathway provided they have been free from symptoms for at least 3 days. The recently published New York study reported that 8 of 13 hospitalized patients retested were negative (median retest 29 days).9 Difference in management of immunosuppression may account for this difference; we stopped mycophenolate mofetil in all of our hospitalized patients whereas in this study 24 of 39 patients (61%) discontinued mycophenolate mofetil. It needs to be seen if continuation of mycophenolate mofetil in transplant patients admitted with COVID-19 is associated with more prolonged viral shedding.
During the early stages of the pandemic in the United Kingdom, between February 1, 2020, and March 23, 2020, we performed 19 transplants, among whom 3 patients tested positive for SARS-CoV-2. One patient had mild illness that was managed at home with regular outpatient reviews. Two of these 3 patients developed COVID-19 pneumonia requiring hospitalization. One of the hospitalized patients had delayed graft function and rejection requiring methylprednisolone infusions; he spent 60 days in the intensive care unit with 57 days of invasive ventilation. He was successfully discharged home stable on regular hemodialysis as his graft failed. A limitation of our study is that we did not systematically screen all transplant recipients or dialysis patients at regular intervals. The data presented include only symptomatic patients who reported to our centers and tested positive for SARS-CoV-2. Therefore, it is possible to underestimate the true prevalence of SARS-CoV-2 infection in our cohort. However, it does capture all symptomatic infections requiring hospitalization. It is worth noting that the studies analyzed showed a case fatality ratio of 22% to 38%, in spite of differences in management strategies, in particular with regard to the use of specific pharmacotherapy.2,3,5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19
Our transplant program was suspended on March 23, 2020, because of the increasing number of COVID-19 patients in South-west London and the unprecedented demand on critical care services. Based on our data analysis, out clinical experience of managing transplant patients with COVID-19, and the national guidelines, we reopened our transplant program in the latter part of June 2020. All the potential recipients received letters with specific counseling and consenting along the lines of advice given by the NHS Blood and Transplant (NHSBT) and Organ Donation and Transplantation UK (https://www.odt.nhs.uk/covid-19-advice-for-clinicians/re-opening-of-transplant-programmes/). These guidelines were used to develop pathways that include access to “green” theaters, with dedicated outpatient clinic areas for follow-up and plans for re- admission into non–COVID-19 wards if needed.
We have as of this writing (until October 15, 2020) performed 32 kidney transplants since our reopening. We have limited donor case selection to lower-risk donors (i.e., DBD [donation after brainstem death] donors age <60 years, DCD [donation after cardiac death] donors age <50 years, no significant AKI in the donor [to minimize the risk of delayed graft function and prolonged hospital stay], and no extended-criteria donors). The current national policy is to only offer organs from donors who did not die of COVID-19 and after obtaining negative SARS-CoV-2 PCR test (nasopharyngeal swab and endotracheal aspirate). We have reactivated lower-risk patients who are aged <65 years, body mass index <30 with low to medium cardiovascular risk, and not expected to require critical care admission during their inpatient stay. For living-donor kidney transplants, both the donor and the recipient are requested to “shield” for 14 days pretransplant with SARS-CoV-2 testing (nasal and throat swabs for PCR) at 14 and 2 days prior to surgery. We intend to expand donor and recipient acceptance criteria in a phased manner.
In conclusion, from our large cohort of transplant patients, a small proportion got COVID-19, with the proportion of infection significantly lower than that of waitlisted patients and those on dialysis. The overall case fatality ratio (26%) was comparable to that of the dialysis cohort and patients on waitlist. Thirty-one percent required intubation and ventilation, of whom 50% died. Within our entire cohort, a significantly lower proportion of transplant patients died of COVID-19 compared with hemodialysis and peritoneal dialysis patients. The case fatality ratio of hospitalized transplant patients with COVID-19 was 31.57%. The older age and severity of illness were associated with mortality. We observed a high proportion of AKI (68%), but the majority recovered. Meta-analysis of 16 studies including ours revealed pooled case fatality ratio of 24% for hospitalized patients, pooled AKI proportion of 50%, and pooled proportion of severe AKI of 16% to 18%. We have successfully restarted our transplant program with defined donor and recipient criteria to minimize the risk and optimize the outcomes.
Disclosures
DB has received research grants and speaker fees from AstraZeneca, Pfizer, and Vifor Pharma and a research grant from kidney research UK. All the other authors declared no competing interests.
Acknowledgments
We thank all the members of the transplant team involved in taking care of transplant patients and planning COVID-19–specific pathways.
Footnotes
The PRISMA Checklist.
Supplementary Material
The PRISMA Checklist.
References
- 1.Banerjee D., Popoola J., Shah S. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97:1076–1082. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberici F., Delbarba E., Manenti C. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akalin E., Azzi Y., Bartash R. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vistoli F., Furian L., Maggiore U. COVID-19 and kidney transplantation: an Italian Survey and Consensus. J Nephrol. 2020;33:667–680. doi: 10.1007/s40620-020-00755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair V., Jandovitz N., Hirsch J.S. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:1819–1825. doi: 10.1111/ajt.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kronbichler A., Gauckler P., Windpessl M. COVID-19: implications for immunosuppression in kidney disease and transplantation. Nat Rev Nephrol. 2020;16:365–367. doi: 10.1038/s41581-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phanish M.K., Hull R.P., Andrews P.A. Immunological risk stratification and tailored minimisation of immunosuppression in renal transplant recipients. BMC Nephrol. 2020;21:92. doi: 10.1186/s12882-020-01739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montagud-Marrahi E., Cofan F., Torregrosa J.V. Preliminary data on outcomes of SARS-CoV-2 infection in a Spanish single center cohort of kidney recipients. Am J Transplant. 2020;20:2958–2959. doi: 10.1111/ajt.15970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lubetzky M., Aull M.J., Craig-Schapiro R. Kidney allograft recipients, immunosuppression, and coronavirus disease-2019: a report of consecutive cases from a New York City transplant center. Nephrol Dial Transplant. 2020;35:1250–1261. doi: 10.1093/ndt/gfaa154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Columbia University Kidney Transplant Program Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31:1150–1156. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu L., Gong N., Liu B. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77:748–754. doi: 10.1016/j.eururo.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cravedi P., Suraj S.M., Azzi Y. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140–3148. doi: 10.1111/ajt.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias M., Pievani D., Randoux C. COVID-19 infection in kidney transplant recipients: disease incidence and clinical outcomes. J Am Soc Nephrol. 2020;31:2413–2423. doi: 10.1681/ASN.2020050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig-Schapiro R., Salinas T., Lubetzky M. COVID-19 outcomes in patients waitlisted for kidney transplantation and kidney transplant recipients [e-pub ahead of print] Am J Transplant. 2020 doi: 10.1111/ajt.16351. Accessed January 30, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierrotti L.C., Reusing Junior J.O., Freire M.P. COVID-19 among kidney-transplant recipients requiring hospitalization: preliminary data and outcomes from a single-center in Brazil [e-pub ahead of print] Transpl Int. 2020 doi: 10.1111/tri.13745. Accessed January 30, 2021. [DOI] [PubMed] [Google Scholar]
- 16.Katz-Greenberg G., Yadav A., Gupta M. Outcomes of COVID-19-positive kidney transplant recipients: A single-center experience. Clin Nephrol. 2020;94:318–321. doi: 10.5414/CN110311. [DOI] [PubMed] [Google Scholar]
- 17.Rinaldi M., Bartoletti M., Bussini L. COVID-19 in solid organ transplant recipients: no difference in survival compared to general population. Transpl Infect Dis. 2020 doi: 10.1111/tid.13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azzi Y., Parides M., Alani O. COVID-19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney Int. 2020;98:1559–1567. doi: 10.1016/j.kint.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caillard S., Anglicheau D., Matignon M. An initial report from the French SOT COVID Registry suggests high mortality due to Covid-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karagiannidis C., Mostert C., Hentschker C. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8:853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Docherty A.B., Harrison E.M., Green C.A. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Saez M.J., Blasco M., Redondo-Pachon D. Use of tocilizumab in kidney transplant recipients with COVID-19. Am J Transplant. 2020;20:3182–3190. doi: 10.1111/ajt.16192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.