Dear Editor,
In their article “Rapid Salivary Test suitable for a mass screening program to detect SARS-CoV-2: A diagnostic accuracy study”, Azzi and colleagues reported on the validity of rapid antigen tests (RATs) performed on salivary samples from 119 patients.1 Comparing the results of RATs to those of reverse transcription polymerase chain reaction (RT-PCR)-based tests for viral RNA, the sensitivity was high (0.93, 95% CI: 0.77–0.99), while the specificity was low (0.42, 95% CI: 0.32–0.53). In their study, no differences were observed between subgroups or between asymptomatic and symptomatic individuals.1 We agree with the authors that RATs could be a valuable tool in mass screening strategies, especially to control the pandemic during the reopening period.
We propose that a similar strategy be adapted to emergency departments (EDs), in which the rapid and accurate identification of SARS-CoV-2-infected patients is crucial, especially in a phase of reduced prevalence of infections in the population.2 In addition to the rapid identification of SARS-CoV-2-infected patients admitted with COVID-19-like symptoms, it is also crucial for the ED to accurately identify patients with other symptoms who may be asymptomatic carriers of SARS-CoV-2 and who, if not promptly identified, could spread the infection within the hospital.2, 3, 4 The RT-PCR test for viral RNA is the gold-standard diagnostic for SARS-CoV-2 infection, yet the results are often delayed and are thus not immediately available for every patient. This is not suitable for the ED timing. Due to their quick execution and the timeliness of their results, RATs could overcome the limitations of RT-PCR testing and improve the risk management of infection and transmission in the ED.2 , 5 Initial validation studies of RATs were conducted in small laboratory cohorts with a high prevalence of infection.2 , 5, 6, 7 Clinical data on RATs in the ED setting that include symptomatic and asymptomatic patients with a disease prevalence similar to the general population (<10%) are still not available.7 Thus, we would like to take the opportunity to report preliminary findings from our observational study on the implementation of RATs in the initial screening of patients arriving in the ED for either COVID-like symptoms or other health issues.
From 1 July to 10 November 2020, at the ED of the Merano Hospital (Italy, 70,000 annual admissions), a RAT using the STANDARD Q COVID-19 Ag (R-Ag) kit (SD BIOSENSOR, KR) followed by an RT-PCR test (samples obtained using two different swabs) were performed in all patients with symptoms suspicious for SARS-CoV-2 infection, with a temperature >37.3 °C, with any epidemiological risk criteria (e.g. reported contact with an infected person) and evaluated in the ED for other conditions not related to SARS-CoV-2 infection that required hospitalisation.
In this period, 3410 patients (991 patients with COVID-19-like symptoms and 2419 asymptomatic patients) required an ED evaluation and were tested with both a RAT and RT-PCR. A positive RT-PCR for SARS-CoV-2 was found in 6.5% of patients (223/3410). In SARS-CoV-2-positive patients, the RAT was positive in 85.6% of cases (179/223), while a false-positive RAT was found in 0.9% (30/3187) of patients with a negative RT-PCR. Overall, the sensitivity and specificity of the RAT were 80.3% (95% CI 74.9%–85.4%) and 99.1% (95% CI 98.6%–99.3%), respectively, and the accuracy was 97.8% (95% CI 97.3%–98.2%, Cohen's k = 0.8171, 95% CI 0.774–0.853, p<0.001).
RAT performance noticeably differed between patients with COVID-19-like symptoms and asymptomatic patients (Table 1 ). In symptomatic patients, the sensitivity of the RAT was 89.9% (95% CI 85.4%–94.4%), its specificity was 97.6% (95% CI 96.5%–98.5%) and its accuracy was 96.3% (95% CI 95.0%–97.3%; Cohen's k = 0.869, 95% CI 0.802–0.904, p<0.001). When performed on patients without COVID-19 symptoms, the RAT had a sensitivity of 50.0% (95% CI 36.0%–63.0%), a specificity of 99.6% (95% CI 99.1%–99.9%) and an accuracy of 98.5% (95% CI 97.9%–98.9%; Cohen's k = 0.586, 95% CI 0.464–0.691, p<0.001).
Table 1.
Two 2 × 2 contingency tables in which the performance of the rapid antigen test is compared with that of a reverse transcription polymerase chain reaction-based test. In the upper 2 × 2 table, only the cohort of symptomatic patients is reported. In the lower 2 × 2 table, the asymptomatic patients are reported.
| Only considering symptomatic patients for COVID-19 | |||
|---|---|---|---|
| Negative antigen test for COVID-19 | Positive antigen test for COVID-19 | ||
| Patients COVID-19 non-infected | 802 | 20 | |
| Patients COVID-19 infected | 17 | 152 | |
| Sensitivity | 89.94% (85.4–94.4) | ||
| Specificity | 97.57% (96.5–98.5) | ||
| Positive predictive value | 85.37% (90.5–80.0) | ||
| Negative predictive value | 97.92% (96.9–98.8) | ||
| Accuracy (correctly classified) | 96.27% (95.0–97.3) | ||
| Only considering asymptomatic patients for COVID-19 | |||
| Negative antigen test for COVID-19 | Positive antigen test for COVID-19 | ||
| Patients COVID-19 non-infected | 2355 | 10 | |
| Patients COVID-19 infected | 27 | 27 | |
| Sensitivity | 50.00% (36.0–63.0) | ||
| Specificity | 99.58% (99.1–99.9) | ||
| Positive predictive value | 72.97% (58.6–87.2) | ||
| Negative predictive value | 98.87% (98.3–99.2) | ||
| Accuracy (correctly classified) | 98.47% (97.9–98.9) | ||
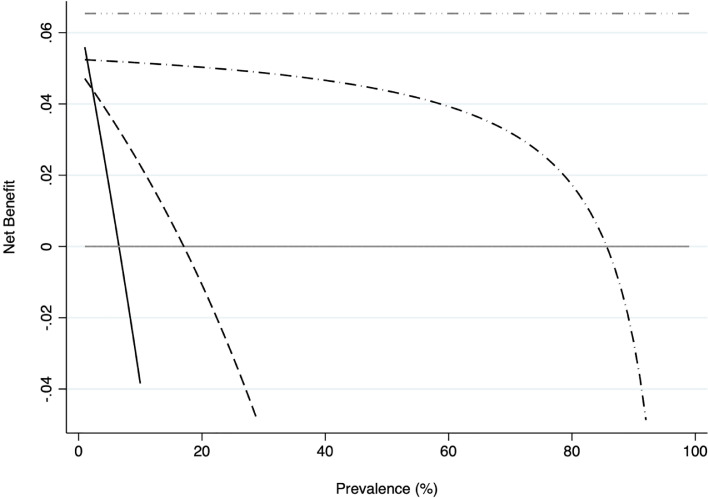
In addition to the assessment of the diagnostic accuracy of the RAT, a preliminary broader evaluation on the possible global clinical benefit derived from the use of RATs as an initial screening tool in the ED was performed via decision curve analysis (DCA). The DCA plot confirm that when the prevalence of infection in the general population is under 15% (similar to real data, in contrast to the validation laboratory cohorts), initial screening with RAT in the ED has a non-negligible net clinical benefit (Fig. 1 ).
Fig. 1.
Decision curve analysis and its distribution. Grey line: assume no patients have COVID-19. Black line: assume all patients have COVID-19. Grey dash-dotted line: a hypothetical perfect test. Black dashed line: the strategy of discovering COVID-19-infected patients only on the basis of their symptoms. Black dash-dotted line: the strategy of performing rapid antigen tests on patients in the ED.
Our preliminary findings on the diagnostic performance of RAT in clinical practice are in line with those previously observed in laboratory studies. In contrast to previous studies, in which the prevalence of COVID-19 in the study samples was high, the current study reports a prevalence that more closely reflects the true prevalence of infection in the general population.5, 6, 7 In symptomatic patients, the performance of the RAT seems to be high enough to propose its use as an initial screening test directly upon arrival in triage.7 In these patients, a positive RAT may accelerate the management of the infected patient, while in case of a negative test, the subsequent clinical management may depend on the degree of clinical suspicion. In asymptomatic patients, in whom RATs do not currently appear sufficient to properly identify the infected patients, a positive test may nonetheless identify patients who otherwise, without clinical suspicion, would not have been detected. However, a negative RAT in patients with low clinical suspicion cannot completely exclude the presence of SARS-CoV-2 infection.
Their immediate availability for each patient, the easy repeatability and the rapidity of the result are characteristics that make the RAT appealing for mass screening or for environments, such as the ED, in which rapid information is crucial. The introduction of a rapid screening tool for both symptomatic and asymptomatic patients upon arrival in the ED appears to improve the overall management of the infectious risk, with a net clinical benefit.
In conclusion, the use of a RAT implemented for infectious risk assessment directly in triage should be considered in EDs and could be an additional tool to address the challenge of containing the SARS-CoV-2 pandemic.
References
- 1.Azzi L., Baj A., Alberio T., Lualdi M., Veronesi G., Carcano G., Ageno W., Gambarini C., Maffioli L., Di Saverio S., Dalla Gasparina D., Genoni A.P., Premi E., Donati S., Azzolini C., Grandi A.M., Dentali F., Tangianu F., Sessa F., Maurino V., Tettamanti L., Siracusa C., Vigezzi A., Monti E., Iori V., Iovino D., Ietto G., Grossi P.A., Tagliabue A., Fasano M. Rapid salivary test suitable for a mass screening program to detect SARS-CoV-2: a diagnostic accuracy study. J Infect. Sep 2020;81(3):e75–e78. doi: 10.1016/j.jinf.2020.06.042. Epub 2020 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wee L.E., Fua T., Chua Y.Y., Ho A.F.W., Sim X.Y.J., Conceicao E.P., Venkatachalam I., Tan K.B., Tan B.H. Containing COVID-19 in the emergency department: the role of improved case detection and segregation of suspect cases. Acad Emerg Med. May 2020;27(5):379–387. doi: 10.1111/acem.13984. Epub 2020 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavezzo E., Franchin E., Ciavarella C., Cuomo-Dannenburg G., Barzon L., Del Vecchio C., Rossi L., Manganelli R., Loregian A., Navarin N., Abate D., Sciro M., Merigliano S., De Canale E., Vanuzzo M.C., Besutti V., Saluzzo F., Onelia F., Pacenti M., Parisi S.G., Carretta G., Donato D., Flor L., Cocchio S., Masi G., Sperduti A., Cattarino L., Salvador R., Nicoletti M., Caldart F., Castelli G., Nieddu E., Labella B., Fava L., Drigo M., Gaythrope K.A.M., Brazzale A.R., Toppo S., Trevisan M., Baldo V., Donnelly C.A., Ferguson N.M., Dorigatti I., Crisanti A. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo'. Nature. Aug 2020;584(7821):425–429. doi: 10.1038/s41586-020-2488-1. Epub 2020 Jun 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu X., Yang R. COVID-19 transmission through asymptomatic carriers is a challenge to containment. Influenza Other Respir Viruses. Jul 2020;14(4):474–475. doi: 10.1111/irv.12743. Epub 2020 Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porte L., Legarraga P., Vollrath V., Aguilera X., Munita J.M., Araos R., Pizarro G., Pablo V., Iruretagoyena M., Dittrich S., Weitzel T. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int J Infect Dis. Oct 2020;99:328–333. doi: 10.1016/j.ijid.2020.05.098. Epub 2020 Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirotsu Y., Maejima M., Shibusawa M., Nagakubo Y., Hosaka K., Amemiya K., Sueki H., Hayakawa M., Mochizuki H., Tsutsui T., Kakizaki Y., Miyashita Y., Yagi S., Kojima S., Omata M. Comparison of automated SARS-CoV-2 antigen test for COVID-19 infection with quantitative RT-PCR using 313 nasopharyngeal swabs, including from seven serially followed patients. Int J Infect Dis. Oct 2020;99:397–402. doi: 10.1016/j.ijid.2020.08.029. Published online 2020 Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinnes J., Deeks J.J., Adriano A., Berhane S., Davenport C., Dittrich S., Emperador D., Takwoingi Y., Cunningham J., Beese S., Dretzke J., Ferrante di Ruffano L., Harris I.M., Price M.J., Taylor-Philips S., Hooft L., Leeflang M.M., Spijker R. Van den Bruel A. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. Aug 26 2020;8 doi: 10.1002/14651858.CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]