Abstract
Purpose of Review:
To describe a collection of recent work published on thermal suitability for vector-borne diseases, in which mapping approaches illustrated the geographic shifts, and spatial approaches describe the demographic impact anticipated with a changing climate.
Recent Findings:
While climate change predictions of warming indicate an expansion in VBD suitability risk in some parts of the globe, while in others, optimal temperatures for transmission may be exceeded, as seen for malaria in Western Africa, resulting in declining risk. The thermal suitability of specific vector-pathogen pairs can have large impacts on geographic range of risk, and changes in human demography itself will intersect with this risk to create different vulnerability profiles over the coming century.
Summary:
Using a physiological approach to describe the thermal suitability of transmission for vector-borne diseases allows us to illustrate the future risk as mapped information. This in turn can be coupled with demographic projections to anticipate changing risk, and even changing vulnerability within that population change.
In recent years, the thermal biology of transmission has been described for a series of vector-borne diseases following an initial publication by Mordecai et al. (1), which described the optimal temperature for malaria transmission by Anopheles spp. mosquitoes. Since then, the thermal bounds of transmission have been systematically characterized for eleven mosquito-transmitted diseases of humans (2–7). These methods were extended to a vector-borne plant pathogen, citrus greening, transmitted by a psyllid (8), and a livestock disease, bluetongue, transmitted by midges (9). The methodology of assessing the individual thermal traits of coupled vector-parasite life histories establishes a temperature-dependent transmission curve, describing R0, the threshold of transmission, relative to its maximum. This provides a means to describe the temperature bounds of transmission, and to assess where thermal conditions are suitable to allow transmission to take place.
The equation for R0 in these studies is modified from MacDonald’s 1957 equations describing malaria transmission (10) as:
comprising mosquito biting rate (a), vector competence (b*c), vector density (m), vector survival (p), the parasite extrinsic incubation period, EIP (T), and r, the human recovery rate – all of which, except r, are temperature-sensitive parameters. All of these temperature sensitive traits have non-linear responses to temperature, and they must be measured from lab experimental data at constant temperatures to generate the curved responses used to parameterize the transmission equation. The resulting overall relationship for each trait is curvilinear with respect to temperature, which reveals an optimal temperature for transmission (see (1)).
Temperature is not the only factor that will determine, or constrain, transmission of vector- borne diseases on the landscape. For example, humidity, precipitation, availability of hosts, and the role of existing interventions will heavily influence the true transmission risk (11–13). This framing nonetheless presents a fundamental means to define areas on the landscape where temperature permits potential transmission. A key means to convey this information is to map the thermal transmission bounds to climate data products, demonstrating the implied geographic limits of these suitable conditions – i.e. where temperature allows for transmission. Using mean monthly temperature data at regional to global scales, this mapping approach appears in several descriptions of new thermal transmission curves, wherein lab-based measurements of life-history traits as functions of temperature were used in transmission equations, and mapped (6,8,9,14–18). These maps broadly illustrate the suitability of temperatures for transmission – in some cases, constrained by descriptions of sufficient moisture levels to support vector breeding (e.g. aridity bounds for malaria suitability mapping (15,16)).
Mapping approaches have also proven useful in communicating differential risk in emerging pathogens. The world’s attention was brought into focus on Aedes spp. transmitted diseases as Chikungunya swept through the Americas, starting in 2013(19). Zika leapt onto the world stage in the following two years with its terrifying syndrome of microcephaly in the children of infected mothers (20–25). While dengue had consistently been causing an estimated 390 million cases a year across the world prior to this (26), the appearance of low mortality impacts left it largely second chair to the far more lethal malaria (27,28). When Zika emerged, a multitude of studies estimating its potential spatial distribution followed suit – maps of Aedes suitability, generated with species distribution modeling (SDM) or ecological niche modeling (ENM) approaches (29–32), maps of Zika case suitability (33,34), and a comparison of models of Zika and dengue case data niche models, suggesting that Zika was somehow different than dengue, bioclimatically (35). Mordecai et al. published thermal suitability models for Aedes aegypti and Aedes albopictus transmission of flavivirus (lab strains of dengue), and validated these models with outbreak data for Chikungunya and Zika viruses to demonstrate the thermal suitability for transmission by these two vectors (4). Perhaps surprisingly, this revealed that optimal transmission by Ae. aegypti occurs at a higher temperature than by Ae. albopictus (Figure 1), underscoring the importance of the potential for different climate profiles of transmission for different vectors, even with the same human-disease causing pathogen.
Figure 1:
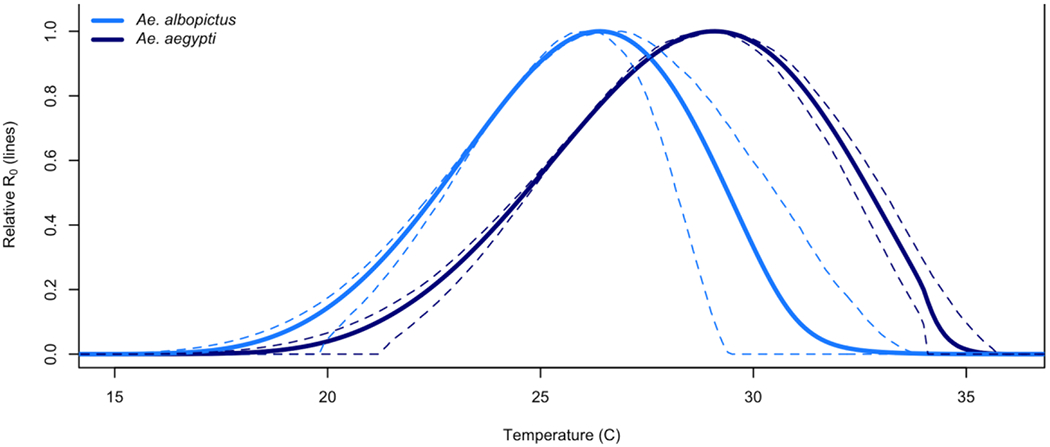
Temperature dependent transmissibility curves for Ae. aegypti (dark blue) and Ae. albopictus (light blue), with the 95% credible intervals shown as dashed lines, demonstrating the ‘hotter’ transmission suitability of Ae aegypti, and ‘cooler’ suitability for Ae albopictus. Adapted from Figure 2 in Mordecai et al. 2017 (4).
When we think of Aedes spp. transmitted diseases, we are no longer constrained by the natural occurrence of moisture on the landscape – mosquitoes like Ae. aegypti are anthropophilic, urban adapted, container-breeding mosquitoes (36). Therefore, they can exploit human altered environments and will take advantage of almost any type of water storage to oviposit, regardless of broader scale environmental conditions (37,38). Thus, while some studies can identify precipitation cues for Aedes aegypti occurrence at a household level (39), with sufficient social-ecological conditions to promote larval habitat (e.g. containers, abandoned tires, other water catchment in the domestic landscape), any signals of precipitation can be drowned out (40). In 2019, Ryan et al. described risk of disease transmission exposure in terms of the number of thermally suitable months for dengue transmission by Aedes aegypti and Aedes albopictus, geographically overlaying a spatial projection of the thermal suitability model of Mordecai et al. (4) on demographic projections of population density (17). In this way, the number of people at risk (PAR) across the globe was calculated under the assumptions that where there are people there is water storage (i.e. available mosquito habitat), and only thermal bounds would functionally limit range expansions and establishment by these two vectors (Figure 2).
Figure 2.
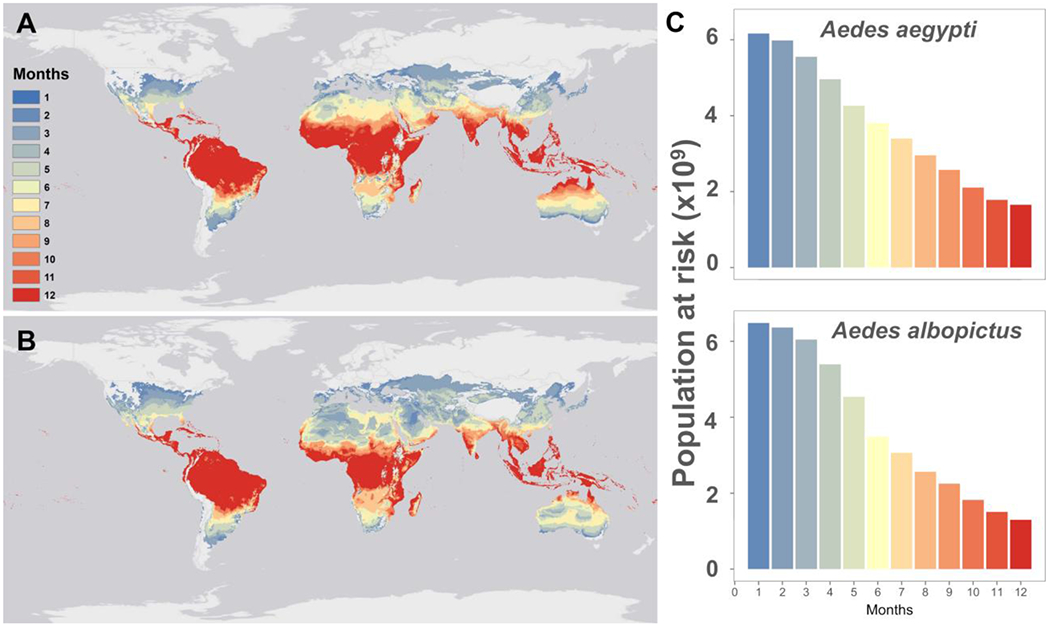
The differences in the thermal responses of two mosquito vectors results in different geographies of suitability for transmission – the cooler tolerant Ae. albopictus (b) has a much more temperate potential, while at baseline, current climate, Ae. aegypti (a) is constrained to warmer, more tropical areas, for much of the year (reproduced from Ryan et al. 2019 (17)).
Climate Change Induced Shifts in Geographic Risk
Understanding the potential impact that climate change will have on the vector-borne disease risk discussed here requires projecting transmission suitability under future scenarios of climate patterns and human mediation actions. This is conceptually straightforward, but rapidly becomes complicated by the myriad of increasingly sophisticated climate model projections available (41). In 2015, Ryan et al. used the future climate scenario framework presented in the 2007 Intergovernmental Panel on Climate Change (IPCC) 4th Assessment Report (AR) to map the physiological suitability of malaria transmission under climate change (42), specifically using the SRES (Special Report on Emissions Scenarios (43)) A1B emission scenarios, downscaled using the delta method (44). The A1B scenario falls in the center of projections of anthropogenic emissions and makes a general assumption of continuing globalization (45), balancing across fossil and non-fossil energy sources. This choice of projected future trajectory for climate change was chosen to present a balanced view of the impact of climate change, without illustrating extremes. We projected the shifting geography of the malaria season (i.e. the number of months suitable for transmission) across the continent of Africa using projected mean monthly temperatures for three endpoints, 2020, 2050, and 2080. As part of the study, the temperature-dependent spatial model was overlaid onto UN generated population density data estimates for 2015 to illustrate demographic risk, using the number of months suitable for transmission multiplied by log-transformed population density to illustrate a shifting gradient of risk over time. This revealed a transitioning zone of increased months of transmission suitability and high density population, where the highlighted zone of increased risk moves from West Africa across the center of the continent, and arrives over the Albertine Rift region of East Africa by 2080 (Figure 3)
Figure 3:
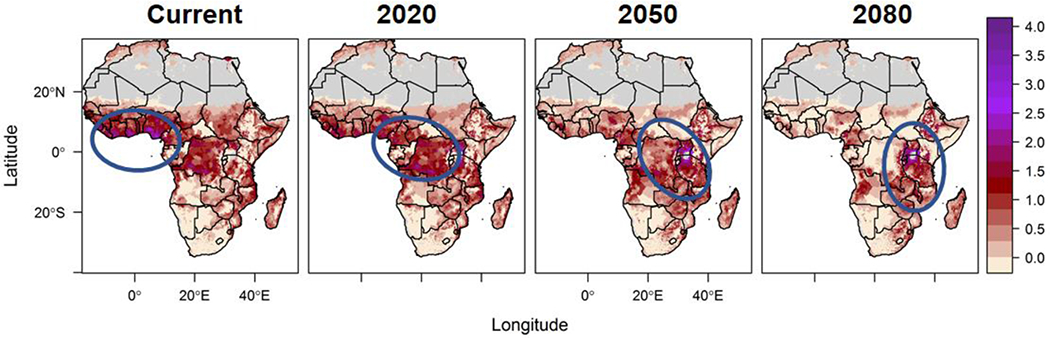
A hotspot of combined longer thermally suitable malaria transmission season and population density moves across the African continent under climate change scenarios (From Ryan et al. 2015 (15))
Since that publication, the framing of climate change scenarios and the projection models used have shifted with IPCC iterations, presenting the need to update study results that rely on these mapped projections. This is particularly vital when describing population risk, where mapped products are used to facilitate communication to decision makers, such as USAID’s President’s Malaria Initiative (PMI) (46). In 2020, Ryan et al. published a new study that used updated underlying climate models to describe regional shifts and population risk changes for malaria in Africa, using policy language that aligned with decision-making frameworks for intervention planning (16). Part of this effort was to engage in conversations about definitions and assumptions of transmission season length, given models of intervention for epidemic, seasonal, and endemic malaria, and these were used to frame the discussion in a series of reports (46–48).
While broad geographic and demographic shifts remain similar – a shifting zone of longer seasons moving across the continent from West Africa to East Africa – the updated paper reflects both a shift towards aligning with the language of funding decisions, as well as newer climate models. These papers, published only 5 years apart, reflect the changing descriptions of climate change upon which estimates of future VBD risk are based. Updating our projected risk estimates not only improve how multiple climate groups’ models can be incorporated into better understanding potential future climate dynamics, but also improve our own options and actions for mitigation. The Representative Concentration Pathways (RCPs), introduced with the AR5 IPCC assessment (49) describe futures in which the international community takes action of different magnitudes, at different time points, to mitigate climate change impacts. By linking the descriptions of malaria seasons to the intervention language – where seasonal and endemic risks had quantified lengths – and by using two pathways, RCP 4.5, a moderate trajectory, and RCP 8.5, the worst-case scenario, with ensembled climate models specifically created for the African continent (46), this paper cast the risk into decision-making frameworks. Figure 4, adapted from the study, exemplifies the differences between geographic risk (space on the landscape) and demographic risk (number of people impacted) involved in these projected shifts in suitability. The spatial risk decreases for both endemic (year-round) and seasonal (6-9 months) malaria transmission suitability (map panels), but the demographic risk is shifted dramatically (illustrated in right panels for endemic risk), as that suitability moves into high-density population areas.
Figure 4:
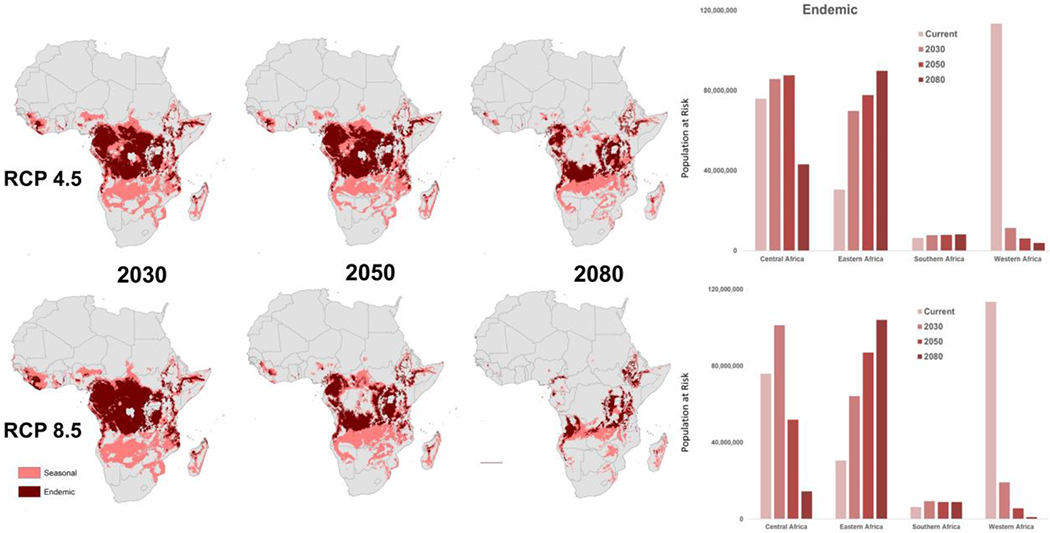
Shifting geographic zones of predicted seasonal (7-9 months) and endemic (10-12 months) suitability for transmission across three time horizons, under two Representative Concentration Pathways (RCP), and the corresponding PAR for endemic risk, for four African regions: Central, Eastern, Southern, and Western Africa (Adapted from Ryan et al. 2020 (16)).
Midtiple vectors, multiple pathogens, multiple futures
As stated previously, understanding risk of exposure to arboviral infections, particularly those spread by Ae. aegypti and Ae. albopictus, has become an urgent research priority in light of newly emerging pathogens. Like malaria, the potential geographic spread of emerging and re-emerging arboviruses is now of great interest to agencies tasked with communicating and preparing for risk (50). In order to project the future risk of Aedes spp. transmitted diseases with the new IPCC and RCP framework, in our 2019 paper, we were faced with the task of communicating futures for two vectors with different thermal profiles, under a variety of possible scenarios, and across different time horizons (17). Several variable factors contribute to IPCC climate change scenarios, including a multitude of mitigation strategy pathways, products from multiple circulation models from different climate research units, and choices of endpoints (e.g. the years 2050, 2080, and even 2100) When communicating geographic information, spatial scale can also become a complicating factor. While global scale maps are visually pleasing, they complicate the succinct communication of quantitative information. In the paper in 2019, we used a combination of synoptic boxplots to demonstrate the range of results from different GCMs, under two RCPs, at two future endpoints (2050 and 2080), in context of the number of people at risk due to thermal transmission suitability, either year-round (i.e. suitable transmission for 12 months) or 1 or more months. For the Aedes spp. transmission models, we used a different descriptor of seasonality than for the malaria work; in the context of emerging diseases, the question of “any” risk (i.e. a month or more) is important to know, and at the other extreme, describing areas of year-round suitability risk – potential endemic areas – is of interest. Given that intervention for these arboviral diseases, dengue, chikungunya, and Zika, is limited to vector control, with low availability of effective vaccines – unlike Yellow Fever – understanding the potential for emergence, or the need for continuous surveillance and vector control, guides the decision frameworks, and thus our model illustrations.
While we were developing spatial and demographic risk approaches in 2018, for the two Aedes spp. vectors, results from lab studies examining the vector-pathogen thermal responses of Aedes aegypti infected with Zika virus revealed that the optimal temperature for transmission was similar to that for dengue, as previously assumed. However, at lower temperatures, the two curves (dengue and Zika) differed (6). Zika appeared to have a higher minimum temperature bound on transmission suitability, and when mapped onto climate in the Americas, this difference in the thermal performance curve revealed a rather smaller potential geographic range of transmission risk compared to what was predicted for dengue in the same Aedes aegypti vector (6). This difference in important vector-pathogen coupled thermal responses has large geographic (and demographic) implications (51), suggesting that despite the commonality of the vector Aedes aegypti, more of the world is at risk of dengue transmission than Zika transmission. This highlights the importance of understanding the biological mechanisms fundamental to describing temperature dependent transmission suitability for vector-borne diseases. Tightly coupled vector-pathogen life histories will yield idiosyncratic transmission cycles, each with their own unique outcomes in terms of spatial and demographic risk. This specific contrast between dengue and Zika temperature-dependent transmission profiles must, however, be tempered with the caveat that these findings are based on infection experiments of mosquitoes in lab conditions with lab strains of the viruses, and both local adaptation of mosquitoes and evolution of viral strains will certainly occur in the ‘real world’(52). Future investigations will start to reveal how much deviation this can induce from our broad scale projections.
Climate Change and Demographic Shifts
One important aspect to keep in mind when thinking about global change is that we are prone to model single axes of change, assuming others remain constant. This is often for practical reasons, because to measure the impact of something we need a baseline to hold as a comparison. If we adjust both numerator and denominator together, it is harder to assess the impact and make that comparison. However, in order to understand demographic impact of future scenarios, we need to consider that the population is both changing (increasing overall), but also altering both compositionally (age structure, economics), and geographically (moving in response to climate change itself). The Shared Socioeconomic Pathways (SSP) projections (53) of demographic change are products of a series of modeled future population responses to climate change. The projections align with some of the SRES scenarios described by the RCP (Representative Concentration Pathway) framework in the IPCC (54), but are also informed by the Millennium Ecosystem Assessment (MEA) (55), UNEP’s Global Environment Outlook (GEO) scenarios (56), and others.
The SSPs present a range of five responses to climate change along a pair of axes representing the challenges of mitigation and adaptation, where responses are functions of how socioeconomic development will occur in terms of economic growth, environmental awareness, education, spatial patterns of urbanization, health equity, technological development, and economic inequalities (57,58). SSP1, on the lower end of both axes, is named “Sustainability” and emphasizes human well-being, achieving development goals, increasing sustainable consumption, reducing inequality, and concentrating urbanization. SSP3, placed high on both axes, is named “Regional Rivalry” and describes a world in which there is slowing global economic growth, increased inequality, regional conflict, continued fossil-fuel dependency, environmental degradation in some regions, and low technological advances to address mitigation or adaptation. SSPs 4 and 5, “Inequality”, and “Fossil-Fueled Development”, respectively, sit at opposing comers, with SSP 4 high on mitigation challenge (but adaptation in the form of a divided world between labor-intensive, low-tech economies, and a small but powerful high tech elite); and SSP 5 high on adaptation challenge, seeing continued global economic growth with high energy consumption, accelerated global development, local technological solutions to environmental issues, but overall de-prioritizing environmental concerns. The remaining SSP, SSP2, presents a ‘middle road’ scenario, to balance the four others. Although a given RCP might fit within the framing of several SSPs, not all combinations of RCP and SSP are plausible, given the role of mitigation (59,60).
Incorporating SSP scenarios into current modeling workflows can enhance our efforts to anticipate the future risk of vector-borne disease exposure, where we can plan for a wider breadth of possible scenarios that account for non-climatic changes. In a recent study, Rohat et al. (61) explored the combinations of projected demographic future risk of Aedes spp. transmitted diseases in the USA, first by overlaying the combinations of Ryan et al.’s projected future transmission models by RCP and year onto SSP1, SSP3 and SSP5. They then explored projected vulnerable population groups by age-sex-race/ethnicity (ASRE) cohorts (62) at the county level across the continental USA – those that are either more likely to get bitten, such as outdoor workers (projected from Bureau of Labor Statistics), children playing outside, or those more likely to suffer more adverse health effects if infected, such as the elderly. Additional considerations for vulnerability included being part of urban populations, as Aedes spp. mosquitoes are more likely to impact populations occupying urban and built environments. Under most combinations of scenarios in the study, exposure to transmission suitability in the USA is projected to increase, and the changes in exposure are driven largely by projected population change in vulnerable groups. In terms of climate change mitigation potential, Rohat et al. (61) found that changing emissions scenarios from RCP 8.5, under SSP3 (worst case climate scenario, ‘regional rivalry’ demographic trend – see above) to RCP 2.6, under SSP1 (best case climate scenario, ‘sustainability’ demographic trend – see above) would result in a difference of around 1.97 billion (3.16 vs. 1.19 billion) fewer people facing exposure risk to Aedes aegypti transmitted in the continental USA. This study underscores the importance of understanding the range of potential changes in future demography when assessing the impact of shifting geography of transmission risk under climate change.
The capacity for undertaking geospatial analyses of combined vulnerabilities is growing, as is our ability to explore more nuanced ways to describe potential climate impacts. This is due in no small part to the open data sharing data products for a range of spatial extents (e.g. regional to global) and projections of future scenarios. As recent work has suggested, we must now think not only of intersecting vulnerability, but also of overlapping risk. Carlson and Mendenhall (63) called attention to the potential for syndemics – co-occurring or overlapping epidemics – in the context of Zika emergence and spread, bringing the term into the spotlight for vector-borne diseases. This overview of temperature dependent, model derived projections of vector-borne diseases highlights the way in which vector-pathogen coupling proves to be an essential component of the geospatial suitability for Aedes spp. transmitted diseases. Within this framework we have illustrated overlapping areas of suitability for Zika and dengue, two diseases of major public health concern. However, it is important to note that there have been multiple waves of arboviral outbreaks in the Americas over the past decade (64–66). Whether these diseases will have multiplicative effects of risk and impact as syndemics in the future, or not, is complicated to assess at this point. In addition to considering overlaps, considering co-occurring shifts in suitability resulting in ‘swaps’ of risk is important. Mordecai et al. (67) (explored the changing suitability of the African landscape under climate change, in which many parts are becoming too hot for malaria transmission suitability while also becoming more suitable for Ae. aegypti transmission of dengue fever. In this study, both projected geospatial comparisons and empirical evidence support a changing febrile risk environment. This phenomenon is something which will also likely occur in other regions, or with other vector-pathogen systems. It was recently recognized that Anopheles stephensi, an urban, container-breeding malaria vector common in India, has become established in several African urban centers (68). With a ‘hotter’ transmission profile than the Anopheles gambiae complex (18), the expansion of An. stephensi is likely facilitated by longer periods of warmer climate, which in the context of ongoing global mobility, leads to increasing introductions of multiple potential vectors to new regions (69)
Using temperature-dependent models of transmission to describe future suitability, with available large-scale monthly mean temperature projections, captures a very broad outline of what we may expect to occur with regards to risk of vector-borne diseases. The role of variation in temperature at multiple scales – diurnal variation, seasonal anomalies, extreme events – remains to be explored, as do additional axes of bioclimatic limits on vector borne disease transmission (e.g. humidity, aridity, soil moisture capacity), and more nuanced descriptions of biologically meaningful features on the landscape (e.g. capturing larval habitat availability at large scales for mosquitoes). Given the range of variation in projected climate futures at such broad time horizons as those illustrated here, unpacking which uncertainty components or assumptions influence model outcomes is important for future work.
Reflections and Application
Synthesizing the important findings of this type of work for a journal article is a different endeavor than summarizing all the findings for a report to an organization, government agency, or surveillance unit. As has been asked of me by reporters: why do you (scientists) put the best stuff in supplementary information? In the 2019 Aedes spp. paper, we summarized our findings for policy (and journalists) further, to describe which regions, from the Global Burden of Disease regions (70) would see increases in novel exposures to risk, decreases, and by how many people. This allowed us to pinpoint parts of the globe that would move into greater exposure, and those that would move out of suitability – and for which vector. Just as there is a need for nuanced discussions on the impacts of biological limits, climate change, and human demographics on future risk of vector-borne disease transmission, so is there a need for clear communication and interpretation of these complex topics for agency partners and other stakeholders. Mapping remains a powerful tool when communicating the findings of complex models, providing the community of researchers an accessible means of presenting results and highlighting where, and when, populations are at risk.
Acknowledgements
SJR was funded by NSF EED DEB 1518681
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Mordecai EA, Paaijmans KP, Johnson LR, Balzer C, Ben-Horin T, de Moor E, et al. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecology Letters. 2013;16(1):22–30. [DOI] [PubMed] [Google Scholar]
- 2.Mordecai EA, Caldwell JM, Grossman MK, Lippi CA, Johnson LR, Neira M, et al. Thermal biology of mosquito-borne disease. Ecology letters. 2019;22(10):1690–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson LR, Ben-Horin T, Lafferty KD, McNally A, Mordecai EA, Paaijmans KP, et al. Understanding uncertainty in temperature effects on vector-borne disease: A Bayesian approach. Ecology. 2015;96( 1):203–13. [DOI] [PubMed] [Google Scholar]
- 4.Mordecai EA, Cohen JM, Evans MV, Gudapati P, Johnson LR, Lippi CA, et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLOS Neglected Tropical Diseases. 2017. April 27;11(4):e0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shocket M, Ryan S, Mordecai E. Temperature explains broad patterns of Ross River virus transmission. eLife. 2018;7:e37762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tesla B, Demakovsky LR, Mordecai EA, Ryan SJ, Bonds MH, Ngonghala CN, et al. Temperature drives Zika virus transmission: evidence from empirical and mathematical models. Proceedings of the Royal Society B: Biological Sciences. 2018;285:20180795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shocket MS, Verwillow AB, Numazu MG, Slamani H, Cohen JM, El Moustaid F, et al. Transmission of West Nile and other temperate mosquito-borne viruses peaks at intermediate environmental temperatures. bioRxiv. 2020. Jan 1;597898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor RA, Ryan SJ, Lippi CA, Hall DG, Narouei-Khandan HA, Rohr JR, et al. Predicting the fundamental thermal niche of crop pests and diseases in a changing world: A case study on citrus greening. Journal of Applied Ecology. 2019. August 1;56(8):2057–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Moustaid F, Thronton Z, Slamani H, Ryan SJ, Johnson LR. Understanding the effect of temperature on Bluetongue disease risk in livestock. bioRxiv. 2019;759860. [Google Scholar]
- 10.MacDonald G The Epidemiology and Control of Malaria. 1957;xv + 201 + xl + 11 pp. [Google Scholar]
- 11.Fouque F, Reeder JC. Impact of past and on-going changes on climate and weather on vector-borne diseases transmission: a look at the evidence. Infectious diseases of poverty. 2019;8( 1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parham PE, Waldock J, Christophides GK, Hemming D, Agusto F, Evans KJ, et al. Climate, environmental and socio-economic change: weighing up the balance in vector-borne disease transmission. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1665):20130551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson AL, Courtenay O, Kelly-Hope LA, Scott TW, Takken W, Torr SJ, et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS neglected tropical diseases. 2020;14(1):e0007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson LR, Lafferty KD, McNally A, Mordecai EA, Paaijmans KP, Pawar S, et al. Mapping the distribution of Malaria: Current Approaches and Future Directions In: Analyzing and Modeling Spatial and Temporal Dynamics of Infectious Diseases. John Wiley & Sons; 2014. [Google Scholar]
- 15.Ryan SJ, McNally A, Johnson LR, Mordecai EA, Ben-Horin T, Paaijmans K, et al. Mapping physiological suitability limits for malaria in Africa under climate change. Vector-Borne and Zoonotic Diseases. 2015;15(12):717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan SJ, Lippi CA, Zermoglio F. Shifting transmission risk for malaria in Africa with climate change: a framework for planning and intervention. Malaria Journal. 2020;19(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLOS Neglected Tropical Diseases. 2019;13(3):e0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miazgowicz K, Shocket M, Ryan S, Villena O, Hall R, Owen J, et al. Age influences the thermal suitability of Plasmodium falciparum transmission in the Asian malaria vector Anopheles stephensi. Proceedings of the Royal Society B. 2020;287(1931):20201093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer M, Staples JE. Chikungunya virus spreads in the Americas—Caribbean and South America, 2013–2014. MMWR Morbidity and mortality weekly report. 2014;63(22):500. [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson J, Sammon M, Garg M. Dengue, Zika and chikungunya: emerging arboviruses in the New World. Western Journal of Emergency Medicine. 2016;17(6):671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heymann DL, Hodgson A, Freedman DO, Staples JE, Althabe F, Baruah K, et al. Zika virus and microcephaly: why is this situation a PHEIC? The Lancet. 2016;387(10020):719–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly. New England Journal of Medicine. 2016;375(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, et al. Zika virus associated with microcephaly. New England Journal of Medicine. 2016;374(10):951–8. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues LC. Microcephaly and Zika virus infection. The Lancet. 2016. May;387(10033):2070–2. [DOI] [PubMed] [Google Scholar]
- 25.Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, et al. Possible association between Zika virus infection and microcephaly—Brazil, 2015. Morbidity and Mortality Weekly Report. 2016;65(3):59–62. [DOI] [PubMed] [Google Scholar]
- 26.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013. April 25;496(7446):504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. World Malaria Report 2019. [Internet], [cited 2020 May 31]. Available from: https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019
- 28.World Health Organization. Dengue and severe dengue. [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
- 29.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife. 2015. June 30;4:e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson TL, Haque U, Monaghan AJ, Eisen L, Hahn MB, Hayden MH, et al. Modeling the environmental suitability for Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in the contiguous United States. Journal of medical entomology. 2017;54(6):1605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamal M, Kenawy MA, Rady MH, Khaled AS, Samy AM. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PloS one. 2018;13(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alaniz AJ, Carvajal MA, Bacigalupo A, Cattan PE. Global spatial assessment of Aedes aegypti and Culex quinquefasciatus: a scenario of Zika virus exposure. Epidemiology & Infection. 2019;147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messina JP, Kraemer MU, Brady OJ, Pigott DM, Shearer FM, Weiss DJ, et al. Mapping global environmental suitability for Zika virus. Jit M, editor. eLife. 2016. April 19;5:e15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samy AM, Thomas SM, Wahed AAE, Cohoon KP, Peterson AT. Mapping the global geographic potential of Zika virus spread. Memorias do Instituto Oswaldo Cruz. 2016. September;111(9):559–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlson CJ, Dougherty ER, Getz W. An Ecological Assessment of the Pandemic Threat of Zika Virus. Johansson MA, editor. PLoS Neglected Tropical Diseases. 2016. August;10(8):e0004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Focks D, Sackett S, Bailey DL, Dame D. Observations on container-breeding mosquitoes in New Orleans, Louisiana, with an estimate of the population density of Aedes aegypti (L.). The American journal of tropical medicine and hygiene. 1981;30(6):1329–35. [DOI] [PubMed] [Google Scholar]
- 37.Pontes R, Freeman J, Oliveira-Lima JW, Hodgson JC, Spielman A. Vector densities that potentiate dengue outbreaks in a Brazilian city. The American journal of tropical medicine and hygiene. 2000;62(3):378–83. [DOI] [PubMed] [Google Scholar]
- 38.Hayden MH, Uejio CK, Walker K, Ramberg F, Moreno R, Rosales C, et al. Microclimate and human factors in the divergent ecology of Aedes aegypti along the Arizona, US/Sonora, MX border. EcoHealth. 2010;7(1):64–77. [DOI] [PubMed] [Google Scholar]
- 39.Martin JL, Stewart-Ibarra AM, Ayala EB, Mordecai EA, Sippy R, Heras F, et al. Household and climate factors influence Aedes aegypti risk in the arid city of Huaquillas, Ecuador. bioRxiv. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart Ibarra AM, Ryan SJ, Beltrán E, Mejía R, Silva M, Muñoz Á. Dengue Vector Dynamics (Aedes aegypti) Influenced by Climate and Social Factors in Ecuador: Implications for Targeted Control. PLoS ONE. 2013. November 12;8(11):e78263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hausfather Z, Drake HF, Abbott T, Schmidt GA. Evaluating the performance of past climate model projections. Geophysical Research Letters. 2020;47(1):e2019GL085378. [Google Scholar]
- 42.IPCC. IPCC 4 Assessment Report: the AR4 Synthesis Report [Internet] Valencia, Spain: Intergovernmental Panel on Climate Change; 2007. Nov. Report No.: IPCC AR4. Available from: http://www.ipcc.ch/pdf/assessment-report/ar4/syr/ar4_syr.pdf [Google Scholar]
- 43.Nakićenović N, Davidson O, Davis G, Grübler A, Kram T, La Rovere EL, et al. Intergovernmental Panel On Climate Change Species Report Emissions Scenarios: Summary For Policymakers. Geneva, Switzerland; 2000. (Intergovernmental Panel on Climate Change; ). [Google Scholar]
- 44.Ramirez J, Jarvis A. High resolution statistically downscaled future climate surfaces. International Center for Tropical Agriculture (CIAT). 2008;
- 45.Climate change 2007: The physical science basis. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change (IPCC). http://sswww.ipcc.ch/ipccreports/ar4-wg1.htm. Accessed 5 Feb 2015 Available from: http://sswww.ipcc.ch/ipccreports/ar4-wg1.htm
- 46.Zermoglio F, Ryan SJ, Swaim M. Shifting burdens: malaria risk in a hotter Africa. USAID; 2019. [Google Scholar]
- 47.Ryan SJ, Steynor A, Jack C, Wolski P, van Aardenne L, Lennard C, et al. Shifting Risks of Malaria in Southern Africa: a regional analysis. [Internet] United States Agency for International Development Adaptation Thought Ueadership and Assessments (USAID-ATLAS); 2020. p. 44 Available from: https://www.climatelinks.org/resources/shifting-risks-of-malaria-in-southern-africa [Google Scholar]
- 48.Ryan SJ, Zermoglio F Shifting Burdens: Malaria Risk Under Rising Temperatures in Botswana. [Internet] Washington D.C: United States Agency for International Development Adaptation Thought Ueadership and Assessments (USAID-ATLAS); 2020. p. 13 Available from: https://www.climatelinks.org/resources/shifting-burdens-malaria-risk-under-rising-temperatures-botswana [Google Scholar]
- 49.IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Barros VR, Field CB, Dokken DJ, Mastrandrea MD, Mach KJ, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, and White LL (eds.)]. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press; 2014. 688 p. [Google Scholar]
- 50.Espinal MA, Andrus JK, Jauregui B, Waterman SH, Morens DM, Santos JI, et al. Emerging and Reemerging Aedes-Transmitted Arbovirus Infections in the Region of the Americas: Implications for Health Policy. Am J Public Health. 2019. January 24;109(3):387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryan SJ, Carlson CJ, Tesla B, Bonds M, Ngonghala CN, Mordecai EA, et al. Warming temperatures could expose more than 1.3 billion new people to Zika virus risk by 2050. medRxiv. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gloria-Soria A, Soghigian J, Kellner D, Powell JR. Genetic diversity of laboratory strains and implications for research: The case of Aedes aegypti. PLOS Neglected Tropical Diseases. 2019. December 9;13(12):e0007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riahi K, van Vuuren DP, Kriegler E, Edmonds J, O’Neill BC, Fujimori S, et al. The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Global Environmental Change. 2017. January 1;42:153–68. [Google Scholar]
- 54.Jones B, O’Neill B. Spatially explicit global population scenarios consistent with the Shared Socioeconomic Pathways. Environmental Research Letters. 2016;11 (8):084003. [Google Scholar]
- 55.Carpenter SR, Pingali PL, Bennett EM, Zurek MB. Ecosystems and human well-being: scenarios: findings of the Scenarios Working Group, Millennium Ecosystem Assessment, eds. 2005.
- 56.UNEP. geo5 Global Environmental Outlook: Environment for Development. 2012;
- 57.O’Neill BC, Kriegler E, Ebi KL, Kemp-Benedict E, Riahi K, Rothman DS, et al. The roads ahead: Narratives for shared socioeconomic pathways describing world futures in the 21st century. Global Environmental Change. 2017;42:169–80. [Google Scholar]
- 58.O’neill BC, Schweizer V. Mapping the road ahead. Nature Climate Change. 2011;1(7):352–3. [Google Scholar]
- 59.Kriegler E, O’Neill BC, Hallegatte S, Kram T, Lempert RJ, Moss RH, et al. The need for and use of socio-economic scenarios for climate change analysis: a new approach based on shared socio-economic pathways. Global Environmental Change. 2012;22(4):807–22. [Google Scholar]
- 60.Kriegler E, Edmonds J, Hallegatte S, Ebi KL, Kram T, Riahi K, et al. A new scenario framework for climate change research: the concept of shared climate policy assumptions. Climatic Change. 2014;122(3):401–14. [Google Scholar]
- 61.Rohat G, Monaghan A, Hayden MH, Ryan SJ, Charriere E, Wilhelmi OV. Intersecting vulnerabilities: Climatic and demographic contributions to future population exposure to Aedes-borne viruses in the United States. Environmental Research Letters. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swanson DA, Schlottmann A, Schmidt B. Forecasting the population of census tracts by age and sex: An example of the Hamilton–Perry method in action. Population Research and Policy Review. 2010;29(1):47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carlson CJ, Mendenhall E. Preparing for emerging infections means expecting new syndemics. The Lancet. 2019;394(10195):297. [DOI] [PubMed] [Google Scholar]
- 64.Hotez PJ, Murray KO. Dengue, West Nile virus, chikungunya, Zika—and now Mayaro? PLoS neglected tropical diseases. 2017;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barrett AD. The reemergence of yellow fever. Science. 2018;361(6405):847–8. [DOI] [PubMed] [Google Scholar]
- 66.Staples JE, Fischer M. Chikungunya Virus in the Americas — What a Vectorborne Pathogen Can Do. New England Journal of Medicine. 2014. September 3;371(10):887–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mordecai EA, Ryan SJ, Caldwell JM, Shah MM, LaBeaud AD Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planetary Health. in press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takken W, Lindsay S. Increased threat of urban malaria from anopheles stephensi mosquitoes, africa. Emerging infectious diseases. 2019;25(7):1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Villena OC, Ryan SJ, Murdock CC, Johnson LR. Temperature impacts the transmission of malaria parasites by Anopheles gambiae and Anopheles stephensi mosquitoes. bioRxiv. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moran AE, Oliver JT, Mirzaie M, Forouzanfar MH, Chilov M, Anderson L, et al. Assessing the Global Burden of Ischemic Heart Disease: Part 1: Methods for a Systematic Review of the Global Epidemiology of Ischemic Heart Disease in 1990 and 2010. Global Heart. 2012. December 1;7(4):315–29. [DOI] [PMC free article] [PubMed] [Google Scholar]


