Abstract
Aggressive posterior retinopathy of prematurity (AP-ROP) is a severe form of ROP occurring in preterm infants that is characterized by rapid progression and prominent vascularity. We report the use of investigational bedside noninvasive optical coherence tomography angiography to visualize the slow and progressive perifoveal vascular formation in an infant with AP-ROP treated with bevacizumab. We also document extensive vascular shunts and morphological differences between arrested and growing retinal capillaries at the vascular wavefront.
Aggressive posterior retinopathy of prematurity (AP-ROP) is a rapidly progressing, severe form of ROP characterized by posterior disease location and prominence of plus disease without stepwise progression through the classic stages of ROP.1 Treatment of AP-ROP includes laser ablation of avascular retina and anti-vascular endothelial growth factor (VEGF) therapy. One concern over anti-VEGF therapy is that, in addition to promoting involution of pathologic retinal neovascularization, it inhibits physiologic retinal vascular growth, as evident by the persistence of avascular retinal periphery in children with a history of ROP treated with anti-VEGF agents2–4 and findings in animal models of ROP.5 A prominent feature of the macular vascular development in children with history of prematurity is a small or absent foveal avascular zone, yet there does not seem to be a strong correlation between the size of foveal avascular zone and visual acuity.4,6,7 We used investigational handheld optical coherence tomography (OCT) and OCT angiography (OCT-A; investigational UC3 handheld swept source OCT system, Department of Biomedical Engineering, Duke University)8 to document the delayed formation of perifoveal vasculature in an infant who developed AP-ROP and received anti-VEGF treatment.
Case Report
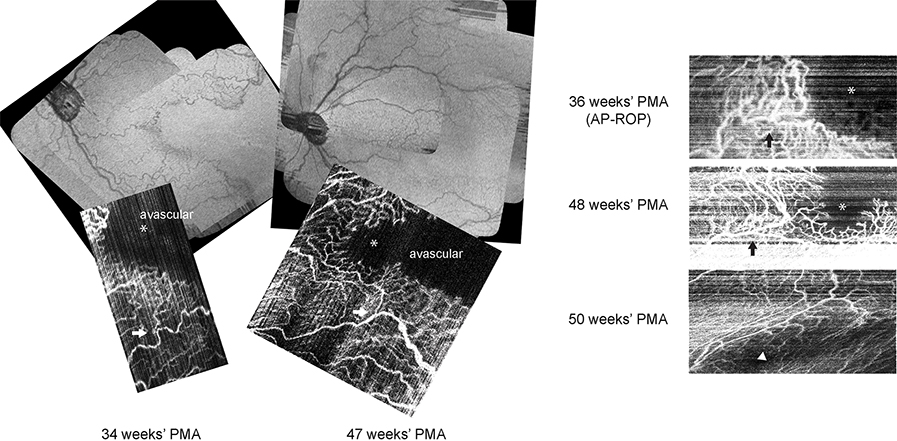
An infant girl born at 24 weeks’ gestational age weighing575 g was enrolled in BabySTEPS, an infant retinal imaging study of retinal microanatomy with ROP and brain development (clinicaltrials.gov NCT02887157). Following written consent from her parents, investigational noncontact handheld OCT/OCT-A imaging was performed at the bedside with or without pharmacological dilation on the same day as ROP screening examinations. From 30 through 34 weeks’ postmenstrual age (PMA), clinical examination showed zone I, stage 0–1 ROP, with no pre-plus or plus in either eye. At 34 weeks’ PMA, posterior pole vessels were tortuous in both eyes. OCT retina view and en face OCT-A of the left eye at 34 weeks’ PMA demonstrated a vascular pattern consistent with temporal notch without closure of the temporal perifoveal vasculature; and at 36 weeks PMA, vascular growth still stalled, with dilated shunt vessels across the nasal macula and at the vascular margin (Figure 1). At this time, both eyes were clinically diagnosed with AP-ROP and treated with intravitreal bevacizumab (0.5 mg in 0.02 mL).
FIG 1.
Optical coherence tomography (OCT) retina views and longitudinal OCT angiography imaging of the perifoveal microvasculature in a preterm infant at 34, 36, 47, 48, and 50 weeks’ postmenstrual age (PMA) who developed aggressive posterior retinopathy of prematurity at 36 weeks’ PMA and received intravitreal bevacizumab injection. At 34 weeks’ PMA, the vascular pattern was consistent with temporal notch without closure of temporal perifoveal vasculature. At 36 weeks’ PMA, vascular growth stalled, with dilated shunt vessels across the nasal macula and at the vascular margin. At 47 and 48 weeks’ PMA, perifoveal vessels and capillaries both were extending beyond the prior vascular-avascular junction, with less dilation and tortuosity than previously seen; the decreased rim of perifoveal shunting allowed growth of capillary branches toward the location of the impending fovea. At 50 weeks’ PMA, complete formation of the perifoveal vasculature (with motion artifact) was seen. Black and white arrows mark the corresponding retinal microvasculature location. Asterisks marks the possible location of impending fovea; the arrowhead marks the fovea.
On follow-up OCT imaging at 47 and 48 weeks’ PMA, the persistent avascular zone was visible on both OCT retina view and OCT-A (Figure 1). Perifoveal vessels and capillaries both extended beyond the prior vascular-avascular junction, with less dilation and tortuosity than previously seen, and decreased rim of perifoveal shunting, which allowed growth of capillary branches toward the location of the impending fovea. At 50 weeks’ PMA, OCT-A (with motion artifact) demonstrated complete formation of the perifoveal vasculature. The infant received peripheral laser ablation at 51 weeks’ PMA for persistent avascular retina. At the most recent clinical visit, at corrected age of 10 months, visual acuity was 3.2 cycles/degree (right eye) and 4.8 cycles/degree (left eye) at 55 cm. The retina remained attached, and no ROP recurrence was noted.
Discussion
The technical advancement of handheld OCT-A technology has enabled us to longitudinally follow retinal capillary development noninvasively in infants screened for ROP. In contrast to what histopathological studies report normally occurs around 26 weeks’ PMA,9 with OCT we observed that perifoveal vascular formation was not complete until 50 weeks’ PMA in an infant who developed AP-ROP and received anti-VEGF treatment.
Although foveal formation appears to be delayed in preterm infants, one report documented continued foveal avascular zone formation following bevacizumab treatment with fluorescein angiography.10 Despite the concerns of inhibition of physiologic retinal vascular growth by anti-VEGF agents, one recent study reports that peripheral retinal vascular findings in infants treated with anti-VEGF therapy on fluorescein angiography are similar to those that had spontaneously regressed ROP.11 The prominent delay in perifoveal vascular development in our case preceded and continued after bevacizumab treatment. Yet, the eventual complete perifoveal vasculature formation offers hope for future visual function.
OCT and OCT-A imaging in this infant who developed AP-ROP revealed extensive shunting in small and large retinal vessels both before and after treatment as well as morphological differences between arrested and growing retinal capillaries at the vascular wavefront. Intravitreal bevacizumab injection resulted in decreased dilation of these shunt vessels without recurrence up to 51 weeks’ PMA. Although prior fluorescein angiography studies in infants with AP-ROP report that capillary nonperfusion within the vascularized retina, shunting, neovascularization, and limited vascular development,12 we did not observe large areas of capillary nonperfusion in the areas imaged. This may be because of the higher image resolution of OCT-A compared with fluorescein angiography as well as the lack of leakage that blurs later interpretation of vascular details on fluorescein angiography. These observations contribute to our understanding of microvascular pathophysiology in infants with AP-ROP.
Acknowledgments
This study was supported by funding from the NIH R01EY025009 (CAT), K23EY028227 (XC), and the Research to Prevent Blindness Career Development Award (XC), and the Stein Innovation award (CAT). The funding agencies did not participate in design, data collection, or interpretation of the study.
Footnotes
Disclosures: CV, CAT, JAI, and Duke University have a patent application pending on the novel handheld probe described in this manuscript.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123:991–9. [DOI] [PubMed] [Google Scholar]
- 2.Lepore D, Quinn GE, Molle F, et al. Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity: report on fluorescein angiographic findings. Ophthalmology 2014;121:2212–19. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz B, Stieger K, Jager M, Mais C, Stieger S, Andrassi-Darida M. Retinal vascular development with 0.312 mg intravitreal bevacizumab to treat severe posterior retinopathy of prematurity: a longitudinal fluorescein angiographic study. Retina 2017;37:97–111. [DOI] [PubMed] [Google Scholar]
- 4.Lepore D, Ji MH, Quinn GE, et al. Functional and morphologic findings at four years after intravitreal bevacizumab or laser for type 1 ROP. Ophthalmic Surg Lasers Imaging Retina 2020;51:180–86. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Yang Z, Jiang Y, et al. Quantitative analyses of retinal vascular area and density after different methods to reduce VEGF in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 2014;55:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mintz-Hittner HA, Knight-Nanan DM, Satriano DR, Kretzer FL. A small foveal avascular zone may be an historic mark of prematurity. Ophthalmology 1999;106:1409–13. [DOI] [PubMed] [Google Scholar]
- 7.Yanni SE, Wang J, Chan M, et al. Foveal avascular zone and foveal pit formation after preterm birth. Br J Ophthalmol 2012;96:961–6. [DOI] [PubMed] [Google Scholar]
- 8.Viehland C, Chen X, Tran-Viet D, et al. Ergonomic handheld OCT angiography probe optimized for pediatric and supine imaging. Biomed Opt Express 2019;10:2623–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes S, Yang H, Chan-Ling T. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci 2000;41:1217–28. [PubMed] [Google Scholar]
- 10.Tiryaki S, Garcia-Gonzalez JM, Zhang DL, Shapiro MJ, Blair MP. Foveal development after use of bevacizumab for aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retina 2019;50:e185–7. [DOI] [PubMed] [Google Scholar]
- 11.Mansukhani SA, Hutchinson AK, Neustein R, Schertzer J, Allen JC, Hubbard GB. Fluorescein angiography in retinopathy of prematurity: comparison of infants treated with bevacizumab to those with spontaneous regression. Ophthalmol Retina 2019;3:436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoi T, Hiraoka M, Miyamoto M, et al. Vascular abnormalities in aggressive posterior retinopathy of prematurity detected by fluorescein angiography. Ophthalmology 2009;116:1377–82. [DOI] [PubMed] [Google Scholar]