Abstract
Dysregulated neutrophil (PMN) transmigration across epithelial surfaces (TEpM) significantly contributes to chronic inflammatory diseases, yet mechanisms defining this process remain poorly understood. In the intestine, uncontrolled PMN TEpM is a hallmark of disease flares in ulcerative colitis. Previous in vitro studies directed at identifying molecular determinants that mediate TEpM have shown that plasma membrane proteins including CD47 and CD11b/CD18 play key roles in regulating PMN TEpM across monolayers of intestinal epithelial cells. Here, we show that CD47 modulates PMN TEpM in vivo using an ileal loop assay. Importantly, using novel tissue-specific CD47 knockout mice and in vitro approaches, we report that PMN-expressed, but not epithelial-expressed CD47 plays a major role in regulating PMN TEpM. We show that CD47 associates with CD11b/CD18 in the plasma membrane of PMN, and that loss of CD47 results in impaired CD11b/CD18 activation. In addition, in vitro and in vivo studies using function blocking antibodies support a role of CD47 in regulating CD11b-dependent PMN TEpM and chemotaxis. Taken together, these findings provide new insights for developing approaches to target dysregulated PMN infiltration in the intestine. Moreover, tissue-specific CD47 knockout mice constitute an important new tool to study contributions of cells expressing CD47 to inflammation in vivo.
Keywords: Neutrophils, Transepithelial migration, CD47, CD11b, Intestinal Inflammation
Introduction
In response to injury or infection, neutrophils (PMN) are essential for host defense against invading pathogens and restoration of tissue homeostasis. However, dysregulated migration of PMN across mucosal surfaces is a prominent feature of chronic inflammatory disorders including inflammatory bowel diseases (IBD). To reach the epithelium, PMN must traffic out of the circulation, a complex multistep process that has been studied in detail elsewhere.1 However, much less is known about mechanisms regulating PMN migration across epithelial surfaces, termed transepithelial migration (TEpM). TEpM of PMN in the intestine requires successful navigation along the lengthy epithelial paracellular space that is sealed by a complex network of intercellular junction proteins. It is thus reasonable to assume that PMN TEpM, like transendothelial migration, is a highly-coordinated multi-step process regulated by adhesive interactions between migrating PMN and intestinal epithelial cells (IECs).
Earlier in vitro studies using blocking antibodies (Abs) combined with transwell-based transmigration models led to the discovery of the role of several proteins expressed on PMN and/or IECs in regulating TEpM.2, 3 Two of these proteins, the leukocyte-specific integrin CD11b/CD18 (also known as αMβ2 or Mac-1)4 and the ubiquitously expressed glycoprotein CD47,5 have been reported as two key regulators of PMN TEpM in vitro. Recently, the role of CD11b/CD18 in regulating PMN TEpM was confirmed in vivo using exteriorized loops of murine intestine.6 Based on this in vivo model, contributions of other plasma membrane proteins have been validated as playing important roles in regulating PMN TEpM including CD44v6,7 intercellular adhesion molecule (ICAM)-1,8 and junctional adhesion molecule (JAM)-A.6 Evaluating the contribution(s) of CD47 in regulating TEpM has been complicated by its ubiquitous expression in all cells, and this has not been studied in vivo in a cell type specific manner. Clearly CD47 plays an important role in regulating PMN recruitment in vivo as global CD47 deficient mice (Cd47−/−) have been shown to display reduced PMN migration into tissues in response to bacterial-induced injury models9, 10 as well as reduced PMN infiltration in sterile inflammation models.11 Despite these reports, the molecular mechanisms and cell specific contributions to CD47 dependent PMN trafficking into tissues, and across mucosal surfaces are not understood. The relative contribution of tissue-specific expression of CD47, particularly in leukocytes, has been hampered by inability to create bone marrow chimera models and the lack of tissue-specific CD47 knockout mice. Recently, we created two transgenic mouse lines with cell-type specific deletion of CD47 in PMN and IECs, respectively. Using an in vivo intestinal loop model of PMN TEpM6, 8 we show that CD47 expressed on PMN but not IECs plays a major role in regulating TEpM. To gain mechanistic insights into how PMN-expressed CD47 modulates TEpM, we extended previous studies linking CD47 to regulation of integrin function.9, 12-15 Since TEpM has been shown to be dependent on CD11b/CD18-mediated adhesive interactions,4 we hypothesized that PMN-expressed CD47 may regulate the function of CD11b/CD18. Using in vivo and in vitro approaches, we show that PMN-expressed CD47 facilitates PMN TEpM by regulating integrin activation through direct interactions with CD11b/CD18.
Results
Loss of CD47 reduces PMN TEpM in vivo
Previous studies using blocking mAbs have shown CD47 regulates human PMN TEpM in vitro, 5, 16 but the relative contributions of PMN- and epithelial-expressed CD47 in regulating this process in vivo is not understood. We employed an exteriorized murine ileal loop assay6-8 to assess the role of CD47 in PMN TEpM in vivo. To induce an inflammatory state, TNF-α and IFN-γ were injected intraperitoneally into WT and Cd47−/− mice 24 hr prior to loop experiments. PMN migration into the intestinal lumen was stimulated by injecting the PMN chemoattractant LTB4 into the intestinal lumen. Cd47−/− mice showed significantly reduced numbers of transmigrated PMN into the intestinal lumen compared to WT mice (Fig. 1a, b). Similarly, we observed reduced PMN TEpM in the colon of Cd47−/− mice by using a proximal colon loop assay, indicating that CD47 regulates PMN TEpM in the ileal and colonic mucosa (Supplemental Fig. 1a-c). In contrast, flow cytometric analysis and immunohistochemical staining of Ly6G/Gr1, showed similar numbers of PMN associated with sub-epithelial space or lamina propria (LP)-enriched fraction from WT and Cd47−/− mice (Fig. 1c, d). We also determined numbers of PMN recruited into the LP after cytokine stimulation prior to ileal loop assay to exclude the possible contribution of a transendothelial migration defect as previously reported in Cd47−/− mice.9-11 As shown in Supplemental Fig. 2a-b, the numbers of PMN accumulated in the sub-epithelial or LP-enriched fraction 24 hr after cytokine stimulation were similar between WT and Cd47−/− mice, indicating that the reduced TEpM observed was not secondary to reduced numbers of leukocytes in the LP of Cd47−/− mice. Overall, these data suggest that reduced PMN TEpM into the intestinal lumen observed in Cd47−/− mice (Fig. 1b) was secondary to impaired TEpM, and demonstrates an important role of CD47 in regulating PMN TEpM in the murine intestine in vivo.
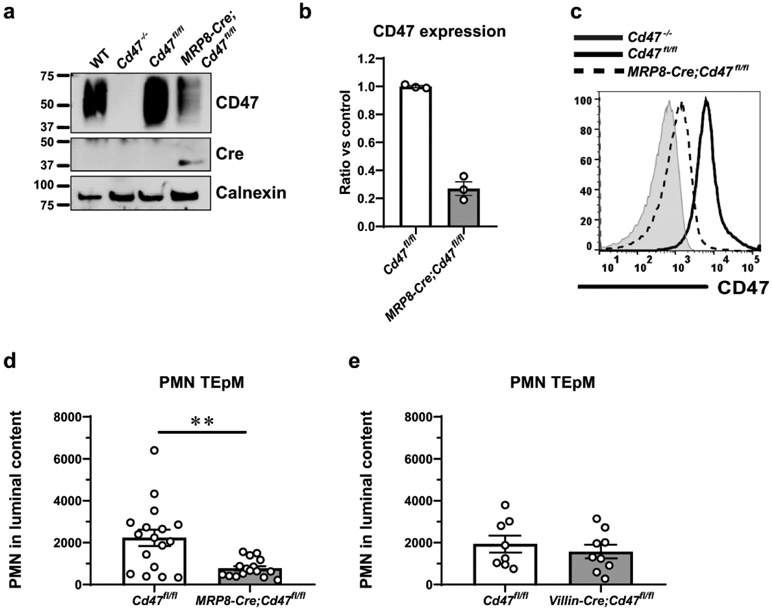
Fig. 1. Loss of CD47 reduces PMN TEpM in vivo.
a Schematic presentation of different fractions collected for neutrophil (PMN) quantification (left) and model of CD47 regulation of CD11b/CD18-dependent PMN transepithelial migration (TEpM) across intestinal mucosa (right). b Analysis of PMN TEpM in vivo using an ileal loop model reveals that Cd47−/− mice have significantly reduced numbers of PMN that migrated to the intestinal lumen 1 hr after intraluminal instillation of leukotriene B4 (LTB4; 1 nM) compared with WT mice. Dots represent individual mice. Data are Means ± SEM of three independent experiments, 14 mice/group (*p ≤ 0.05) as determined by Mann-Whitney U test. c, d After luminal content collection, sections of intestinal loop were enzymatically digested to obtain a Lamina propria (LP)-enriched fraction or fixed and paraffin-embedded for histological analysis. c Quantification of leukocytes by flow cytometry shows comparable numbers of PMN within the LP from WT and Cd47−/− mice. Data are Means ± SEM of 2 independent experiments, 8 mice/group. d Representative images of immunohistochemical staining depicting Ly6G+ PMN. 20x (top) and 40x (bottom) objectives. Scale bars: 100 μm and 50 μm, respectively.
Contribution of tissue-specific expression of CD47 during PMN TEpM in vivo
The relative contribution(s) of PMN- and IEC-expressed CD47 in regulating PMN TEpM in vivo, were explored using newly generated tissue-targeted CD47 deficient mice. To evaluate the role of PMN-expressed CD47, we bred Cd47fl/fl mice with mice expressing Cre recombinase under the granulocyte specific promoter MRP8 (MRP8-Cre-IRES/GFP) to specifically excise CD47 in PMN (MRP8-Cre;Cd47fl/fl). Western blot (WB) quantification and flow cytometry analysis showed a nearly 80% reduction of CD47 on PMN from MRP8-Cre;Cd47fl/fl mice (Fig. 2a-c). MRP8-Cre;Cd47fl/fl mice developed normally, and importantly, no differences were found with littermate controls in the size and appearance of the small and large intestine as well as spleen. Furthermore, MRP8-Cre;Cd47fl/fl mice did not exhibit signs of spontaneous intestinal inflammation and mesenteric lymph nodes were not noticeable. Of interest for this study, we did not find significant differences in the percentage of myeloid cells present in the submucosal space of MRP8-Cre;Cd47fl/fl mice compared to controls, and PMN were barely detectable in the LP as determined by flow cytometric quantification (Supplemental Fig. 3).
Fig. 2. Contributions of tissue-specific expression of CD47 to regulating PMN TEpM in vivo.
a CD47 expression on bone marrow neutrophils (BMN) by western blotting and b densitometry analysis of three experiments reveal that nearly 80% of CD47 is selectively depleted on PMN from mice with specific deletion of CD47 on PMN (MRP8-Cre;Cd47fl/fl) compared to Cd47fl/fl control mice. c Reduced surface expression of CD47 was corroborated by flow cytometry on BMN from MRP8-Cre;Cd47fl/fl compared to Cd47fl/fl mice. d Analysis of PMN TEpM in vivo using a murine ileal loop model reveals significant reduction of transmigrated PMN into the intestinal lumen 1 hr after intraluminal instillation of LTB4 (1 nM) in MRP8-Cre;Cd47fl/fl compared with Cd47fl/fl mice. Data are Means ± SEM of at least three independent experiments, 16-18 mice/group (**p≤0.01) as determined by Mann-Whitney U test. e PMN TEpM in vivo in mice with specific deletion of CD47 on IECs (Villin-Cre;Cd47fl/fl) revealed similar numbers of PMN in the intestinal lumen 1 hr after intraluminal instillation of LTB4 (1 nM) compared with Cd47fl/fl control littermates. Data are Means ± SEM of 2 independent experiments, 6-9 mice/group.
Subsequently, we evaluated the contribution of PMN-expressed CD47 in PMN TEpM in the ileal loop assay. Despite a small amount of residual expression of CD47 observed on PMN, MRP8-Cre;Cd47fl/fl mice displayed significantly reduced numbers of PMN transmigrated into the intestinal lumen compared to that observed in Cd47fl/fl littermate controls (Fig. 2d). The relative contribution of IEC-expressed CD47 was also evaluated using mice with specific loss of CD47 in IECs (Villin-Cre;Cd47fl/fl).17 We recently reported specific deletion of CD47 on IECs by western blot and immunofluorescence staining in the Villin-Cre;Cd47fl/fl mice.17 In contrast to global Cd47−/− and MRP8-Cre;Cd47fl/fl mice, deletion of CD47 on IECs did not result in reduced PMN TEpM, and we observed similar numbers of transmigrated PMN into the intestinal lumen between Villin-Cre;Cd47fl/fl and Cd47fl/fl control mice (Fig. 2e). Overall, these results indicate that CD47 expressed on PMN but not IECs is a major regulator of PMN TEpM in vivo.
CD47 regulates CD11b/CD18-dependent PMN TEpM in vivo
CD47 was originally identified as a regulator of β3 integrin function.14 CD47 has also been reported to interact in cis with integrins belonging to the β1 and β2 family expressed in smooth muscle cells, IECs, reticulocytes and leukocytes.12, 13, 15, 17 We thus investigated whether CD47 may play a role in regulating PMN TEpM through interactions with CD11b/CD18, a leukocyte specific integrin shown to play a major role in regulating PMN TEpM in vivo. Ileal loop assays were performed with WT and Cd47−/− mice in the presence of function blocking mAb against CD11b or isotype control instilled in the intestinal lumen. Consistent with previously reported results,6 intraluminal addition of a blocking mAb against CD11b significantly reduced PMN TEpM in WT mice compared to controls. In addition, PMN TEpM in WT mice treated with anti-CD11b mAb was reduced to similar levels as observed in Cd47−/− mice treated with isotype control. By contrast, CD11b mAb did not further reduce PMN TEpM in CD47−/− mice compared to controls (Fig. 3a). PMN accumulation within the sub-epithelial LP-enriched fraction was comparable between all groups tested (Fig. 3b, c). Collectively, these observations are consistent with a role for CD47 in regulating CD11b/CD18-dependent PMN TEpM in vivo. Further experiments were performed to investigate CD47 regulation of CD11b/CD18 dependent PMN migration.
Fig. 3. CD47 regulates CD11b/18-dependent PMN TEpM in vivo.
a PMN TEpM in vivo in response to LTB4 (1 nM) was determined in ileal loops of WT and Cd47−/− mice after intraluminal instillation of blocking mAb against CD11b (clone M1/70; 20 μg/mL), or rat IgG2b isotype control (20 μg/mL). WT PMN migration was significantly reduced by blocking CD11b in the intestinal lumen compared to mice treated with isotype control. CD47 deficiency also resulted in a significant reduced PMN migration in Cd47−/− mice compared with WT mice. Importantly, blocking CD11b on Cd47−/− mice did not further decrease PMN migration compared to Cd47−/− mice treated with isotype control. Data are Means ± SEM of 3 independent experiments, 12-14 mice/group (**p≤0.01) as determined by two-way ANOVA analysis. b After luminal content collection, intestinal sections were enzymatically digested for quantification of PMN in LP-enriched fraction by flow cytometry, or fixed and paraffin-embedded for histological analysis. Numbers of PMN within the LP fraction were similar between different treatments. Data are Means ± SEM of 2 independent experiments, 8-14 mice/group. c Representative images of immunohistochemical staining including higher magnification insets, depicting Ly6G+ PMN. 40x objective. Scale bars: 50 μm.
CD47 regulates PMN migration across collagen-coated filters
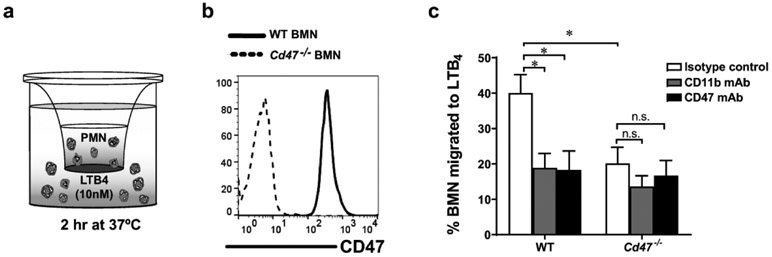
To investigate the role of PMN-expressed CD47 on CD11b/CD18-dependent migration, bone marrow derived neutrophils (BMN) from WT and (Cd47−/− mice were induced to migrate across collagen-coated Transwell filters in response to a 10 nM gradient of LTB4 (Fig. 4a). Complete absence of CD47 was confirmed on global Cd47−/− BMN mice by flow cytometry and western blotting (Fig. 4b and 5a). WT BMN displayed robust migration in response to LTB4 that was significantly reduced by CD11b and CD47 mAbs (20 μg/mL) (Fig. 4c). Similar to the inhibitory effect observed with CD47 mAb, there was significantly reduced PMN migration in Cd47−/− BMN compared to WT BMN. To examine whether CD47 contributes to regulation of CD11b/CD18-dependent PMN chemotaxis, transmigration assays with Cd47−/− BMN were performed in the presence of blocking mAb against CD11b. In contrast to WT BMN, there was no further reduction of migration of CD11b treated Cd47-/~ BMN compared to isotype-control treated Cd47-/~ BMN. As expected, addition of CD47 mAb did not reduce migration of Cd47-/~ BMN. Collectively, these in vitro results corroborate the above in vivo observations, and suggest that CD47 regulates CD11b/CD18-dependent PMN chemotaxis and TEpM.
Fig. 4. CD47 regulates PMN migration across collagen-coated Transwell filters.
a Schematic representation of BMN migration in vitro in response to a chemoattractant gradient of LTB4 (10 nM) for 2 hr at 37°C. b CD47 cell surface expression on BMN isolated from WT and Cd47−/− mice by flow cytometry. c WT and Cd47−/− BMN migration in response to LTB4 in presence of blocking mAb against CD11b, CD47 or isotype control (20 μg/mL in each case). WT BMN migration was significantly reduced by addition of inhibitory CD11b or CD47 mAb. Loss of CD47 on Cd47−/− BMN resulted in a significant reduced migration compared to WT BMN. Addition of blocking mAbs against CD11b or CD47 did not further reduce Cd47−/− BMN migration in comparison to Cd47−/− BMN migration in the presence of isotype mAb. Data are Means ± SEM of three independent experiments. *p ≤ 0.01 as determined by two-way ANOVA analysis.
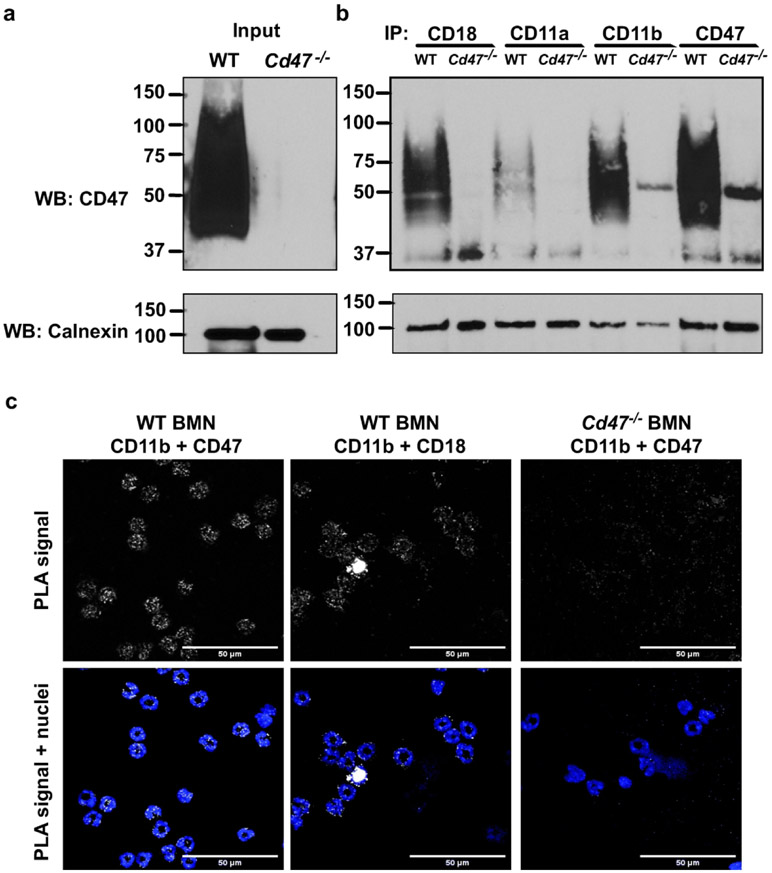
Fig. 5. CD47 physically associates with CD11b/CD18 in BMN.
a, b Murine CD18, CD11a, CD11b and CD47 were immunoprecipitated from non-stimulated WT and Cd47−/− BMN lysates and immunoblotted for CD47 as detailed in the methods. a Representative western blot confirming the expression of CD47 in total lysates from WT but CD47 loss in Cd47−/− BMN. b CD47 was co-immunoprecipitated with CD11b and CD18, and, to a lesser extent, with CD11a on WT BMN. Cd47−/− BMN were used as negative control. c The association between CD47 and CD11b on unstimulated, non-permeabilized BMN was confirmed by in situ proximal ligation assay (PLA) using a combination of CD47 and CD11b Abs as detailed in the methods. The positive fluorescent staining (white dots) indicates close proximity (<40 nm) between CD47 and CD11b targets. Positive controls include use of CD11b-CD18 Abs as a known heterodimeric interaction. Furthermore, as a negative control, CD47-CD11b interaction was probed on BMN from Cd47−/− mice and no signal was detected. Nuclei were counter-stained with Hoechst 33342. 100x objective. Scale bars: 50 μm.
CD47 associates with CD11b/CD18 in neutrophils
Given that CD47 has also been shown to physically associate with integrins to regulate adhesive function in different cell types,9, 12-15 we investigated whether CD47 interacts with CD11b/CD18 in PMN. We performed co-immunoprecipitation assays on non-stimulated murine WT and Cd47−/− BMN. CD47 was probed on total lysates from WT and Cd47−/− BMN revealing a characteristic broad, highly glycosylated ~50kDa band in WT but not in Cd47−/− BMN (Fig. 5a). As shown in Fig. 5b, CD47 co-immunoprecipitated with CD11b and CD18 and to a lesser extent with CD11a. As expected, no co-precipitation was observed using control Cd47−/− BMN. We also examined whether interaction between CD47 and CD11b occurs in the same cell using in situ-proximal ligation assay (PLA), which can be used to detect different proteins that are in close proximity (within 40 nm) and presumably physically associated. A strongly positive fluorescent signal was observed in unstimulated, non-permeabilized BMN when probed with mAbs against CD47 and CD11b (Fig. 5c). As a control, PLA using mAbs against CD11b and CD18 yielded a positive fluorescent signal as would be predictable for the heterodimeric integrin. As expected, no specific fluorescent PLA signal was observed when Cd47−/− BMN were probed with mAbs against CD47 and CD11b. These results are consistent with interaction between CD47 and CD11b in cis within the PMN plasma membrane.
CD47 regulates CD11b/CD18 ligand affinity
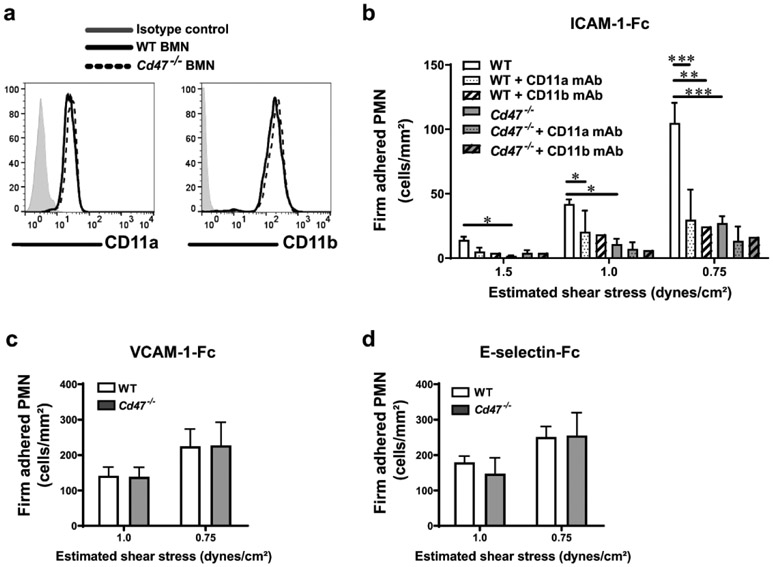
A previous study reported that CD47 regulates CD11a/CD18 ligand affinity on murine CD4+ T cells12. We therefore investigated if CD47 regulates CD11b/CD18-dependent PMN adhesion to ligands under shear flow conditions. Using an in vitro flow chamber system, we assessed Cd47−/− and WT BMN adhesion to immobilized ligands known to regulate PMN adhesion. WT BMN were observed to bind to immobilized ICAM-1, a known ligand for both CD11a/CD18 or CD11b/CD18, across a range of laminar shear stress decreasing from 1.5 to 0.75 dyne/cm2. While deletion of CD47 did not alter surface levels of CD11a and CD11b on BMN (Fig. 6a), adhesion of (Cd47−/− BMN to immobilized ICAM-1 was significantly reduced compared to WT (Fig. 6b). Furthermore, function blocking mAbs against CD11a or CD11b significantly reduced WT BMN adhesion to ICAM-1 to similar levels as observed with Cd47−/− cells, however, antibody mediated inhibition of CD11a or CD11b did not further reduce Cd47−/− BMN adhesion to ICAM-1. By contrast, there were no differences between WT and Cd47−/− BMN adhesion to other non-β2 integrin ligands expressed by vascular endothelium, VCAM-1 or E-selectin (Fig. 6c, d). Consistent with results in Fig. 3 and 4, these observations suggest that loss of CD47 results in a selective defect in β2 integrin affinity for ligands in BMN.
Fig. 6. CD47 regulates CD11b affinity for ligands.
a WT and Cd47−/− BMN express comparable surface levels of CD11a and CD11b integrins. b-d BMN were drawn across coverslips coated with immobilized ICAM-1-Fc, VCAM-1-Fc or E-selectin-Fc adhesion molecules at decreasing levels of shear stress ranging from 1.5 to 0.75 dynes/cm2, and BMN adhesion was measured as described in methods. In some experiments, BMN were pre-treated with function blocking mAbs anti-CD11a (M1/70; 20 μg/mL) or anti-CD11b (M1/70; 20 μg/mL). Data are Means ± SEM of at least three independent experiments. *p≤0.05, **p≤0.01, ***p≤0.001 as measured by ANOVA analysis followed by Tukey’s multiple comparison test.
CD47 regulates CD11b/CD18 integrin activation on PMN
We assessed whether CD47 regulates activation of CD11b/CD18 on PMN using mAbs that have been shown to recognize epitopes that are only displayed on activated integrins that are in a high affinity binding conformation. Since these antibodies are only available for human integrins, we performed experiments using human PMN and the human PMN monomyelocytic cell line HL60 lacking expression of CD47 by CRISPR/Cas9 gene editing. WT HL60 and CD47 null HL60 (HL60/E2) cells were differentiated to neutrophil-like cells. After differentiation, CD47 deletion was confirmed on HL60/E2 cells (Fig. 7a). Neither baseline levels of CD11b on the plasma membrane as measured by flow cytometry, nor total levels of CD11b as measured by WB were significantly altered (Fig. 7b). Using PLA, we confirmed results from murine cells indicating close association between CD47 and CD11b in human PMN and HL60 cells. A strong positive fluorescent signal was elicited by the combination of human CD47-CD11b Abs as well as the positive control using a combination of CD18-CD11b Abs. Conversely, probing CD47 and CD11b Abs on HL60/E2 did not result in any signal (Fig. 7c). The same results were obtained in PLA experiments using PMN from human peripheral blood (Supplemental Fig. 4). With CD47-CD11b association established in human PMN and HL60 cells, we tested whether CD47 regulates CD11b/CD18 activation on these leukocytes. Similar levels of expression of CD11b on HL60 and HL60/E2 were observed under unstimulated conditions. Furthermore, stimulation with 1 μM fMLF over 20 min elicited comparable upregulation of CD11b to the surface of both HL60 and HL60/E2 (Fig. 7d). fMLF stimulation also resulted in activation of CD11b/CD18 in HL60 cells, as shown by increased labeling by activation reporter mAbs against CD11b (clone CBRM1/5) or CD18 (clone m24) (Fig. 7e, f). However, loss of CD47 on HL60/E2 resulted in significant impairment of fMLF-induced integrin activation and is consistent with reduced adhesion of Cd47−/− BMN to ICAM-1 observed in Fig. 6b. Taken together, these findings demonstrate that CD47 associates with and regulates CD11b/CD18 activation that is crucial for guiding efficient PMN trafficking in tissues.
Fig. 7. CD47 associates with CD11b/CD18 and regulates integrin activation in human PMN.
CD47 was knocked down by CRISPR/Cas9 in the human promyelocitic cell line (HL60). HL60 and CD47-null HL60 (HL60/E2) were then differentiated into a neutrophil-like phenotype as described in the methods. a, b CD11b expression on resting, differentiated HL60 was not significantly altered by CD47 knockdown: a cell surface expression of CD11b on differentiated PMN was quantified by flow cytometry and b total CD11b content was determined by western blotting. Image shows a representative western blot and graph represents a densitometry analysis of three different assays. c CD47 and CD11b association in HL60 was assessed by PLA. Positive fluorescent signals were elicited between CD47 and CD11b Abs, as well as between CD11b and CD18 Abs indicating close association of these proteins. CD47 and CD11b Abs did not elicit fluorescent signals on HL60/E2 (CD47 null). Nuclei were counterstained with Hoechst 33342. 100x objective. Scale bars: 50 μm. d Differentiated HL60 and HL60/E2 were stimulated with fMLF (1 μM) for different times and CD11b surface expression was determined by flow cytometry. Increased surface expression of total CD11b after fMLF stimulation was not different between HL60 and HL60/E2. e, f CD11b and CD18 activation on HL60 was determined by using activation reporter mAbs, CBRM1/5 and m24 respectively. fMLF stimulation resulted in activation of CD11b and CD18 on HL60 control cells, but the magnitude was significantly reduced in HL60/E2. Data are Means ± SEM of at least three independent experiments. *p≤0.05, **p≤0.01 as determined by Two-way ANOVA analysis. No Tx (no fMLF treatment).
Discussion
PMN TEpM is an understudied process that involves complex and coordinated PMN adhesive interactions with IECs to efficiently squeeze between epithelial cells that can occur without loss of barrier function.18-21 Relatively few proteins expressed on PMN or IECs have been identified as key participants in this process.2, 3 Here, we show that the ubiquitously expressed glycoprotein CD47 plays an important role in regulating PMN TEpM in vivo. Using the intestinal loop assay as an in vivo model of PMN TEpM6-8 and newly developed tissue-specific knockout mice, we demonstrate that PMN-expressed CD47 is a major determinant in regulating CD11b/CD18-dependent PMN TEpM. We also provide new mechanistic insights into CD47 regulation of CD11b/CD18 function. Since CD47 has been shown to physically associate with some integrins to modulate function,14 and PMN TEpM is regulated by the integrin CD11b/CD18,4 we hypothesized that PMN-expressed CD47 may regulate CD11b/CD18 adhesive function during TEpM. In this study, in vivo and in vitro approaches in combination with function blocking mAbs provide compelling evidence that CD47 physically associates with, and functionally regulates CD11b/CD18 activation during PMN TEpM in the intestine.
The relative contribution(s) of CD47 expressed on individual cell types in regulating PMN trafficking through complex tissues in vivo has not yet been defined. A major challenge has been the lack of available mice with tissue-targeted deletion of CD47. Classical approaches including creation of bone marrow chimeras or adoptive transfer models to study hematopoietic cells versus non-hematopoietic contributions, have failed because of the well described function of CD47 as a self-recognition marker, by inhibiting phagocytosis of CD47 expressing cells through binding to the myeloid ligand Signal-Regulatory Protein α (SIRPα) resulting in a “don’t eat me signal”. Indeed, there are multiple studies reporting that CD47 null cells are actively phagocytosed and cleared from the circulation by splenic macrophages and dendritic cells.22-25 It was thus an unexpected finding to observe that mice with constitutive or inducible deletion of CD47 in the intestinal epithelium had no significant spontaneous immune response against CD47 deficient IECs under resting conditions.17 Here, we report successful creation of mice with robust PMN-specific CD47 deletion, having ~80% reduction in PMN-expressed CD47. Despite having low residual expression of CD47 on PMN, MRP8-Cre;Cd47fl/fl mice showed significantly reduced PMN TEpM in vivo. Previous in vitro studies have suggested that CD47 expressed in human epithelial cell lines may play a role in regulating PMN TEpM by reporting increased migration across an overexpressing transformed cell line 16, however, TEpM studies with polarized natural IECs lacking CD47 have not been reported. By contrast, in the murine in vivo model reported here, there was no significant difference in PMN TEpM between normal controls and mice with CD47 exclusively deleted in IECs (Villin-Cre;Cd4fl/fl) Collectively, these results suggest that PMN-expressed CD47 is the major determinant in regulating PMN TEpM in vivo. Furthermore, the MRP8-Cre;Cd4fl/fl mouse reported here represents the first murine model with successful leukocyte specific deletion of CD47, and will add an important in vivo tool to understand the contributions of CD47 to leukocyte functions.
There are numerous reports linking CD47 to regulation of integrin function. CD47 was first identified as a protein associated with β3 integrins in platelets and placenta.14 Furthermore, CD47 has been shown to interact in cis and modulate function of certain integrins such as αLβ2 in human Jurkat T-cells,12 α4β1 in reticulocytes,13 and α2β1 in human smooth muscle cells.15 Given our interest in understanding basic mechanisms regulating leukocyte migration and the link between CD47 and integrin function, we hypothesized that CD47-dependent regulation of PMN TEpM may be mediated through effects on CD11b/CD18 adhesive functions in PMN. Indeed, we observed that blocking CD11b within the intestinal lumen of WT mice reduced PMN TEpM to comparable levels observed on Cd47−/− mice and blockade of CD11b on Cd47−/− mice did not further reduce PMN TEpM. These findings implicate CD47 in regulating CD11b/CD18-dependent PMN TEpM in vivo. Our in vitro chemotaxis results support these observations.
We previously reported that CD47 physically associates and regulates β1 and β2 (CD11a/CD18) adhesive function in murine effector CD4+ T-cells during transendothelial migration.12 In contrast to T-cells, the major adhesive integrin on PMN is CD11b/CD18. Here, we observed that CD47 co-immunoprecipitates with CD11b, CD11a and CD18 on mouse BMN. Moreover, results from PLA assays demonstrate CD47 and CD11b are in close proximity in the PMN plasma membrane, consistent with interactions in cis. Supportive of our results, there are recent reports suggesting association of CD47 with CD11b in brain mononuclear phagocytes and a macrophage cell line.26, 27 We also report that CD47 association with CD11b/CD18 regulates integrin-dependent PMN adhesion to ligands. CD47 deletion did not affect expression of β2 integrins in PMN but resulted in reduced adhesion of Cd47−/− BMN to the CD11b/CD18 ligand ICAM-1. In contrast to previous studies using CD4+ T-cells,12, 28 PMN adhesion to the α4β1 (VLA4) integrin ligand VCAM-1 was unaffected, indicating CD47 selectively regulates β2 integrin adhesive function in PMN. While precise mechanisms of CD47 regulation of CD11b/CD18-mediated adhesion remain to be determined, our study revealed that loss of CD47 resulted in decreased conformational affinity of CD11b/CD18 upon stimulation of human PMN-like cells as determined by activation reporter mAb CBRM1/529 and mAb 24.30-32 Similarly, in previous work we reported that CD47 null Jurkat T cells had reduced levels of high-affinity conformation of CD11a/CD18 as quantified by m24 and KIM127 reporter mAbs.33 However, CD11a/CD18 avidity, defined as clustering of integrins in the plasma membrane,33 was not affected as determined by a detachment assay using Jurkat T cells under increasing shear rates to evaluate the strengthening of binding to the β2 ligand ICAM-1.12 Interestingly, another group reported that the IgV domain of CD47 linked to GPI was sufficient to promote not only activation but also clustering of αvβ3 in an ovarian cancer cell line.34 These findings strongly suggest a distinct regulation of different integrin families by CD47. To investigate how CD47 regulates CD11b activation, we hypothesized that interaction with CD11b occurs through the extracellular domain of CD47. As observed in figure 4c, the function blocking mAb miap301 that recognizes the IgV domain of CD4735 reduced PMN chemotaxis in vitro, suggesting a blockade of the interaction CD47-CD11b at the extracellular side. Supporting our hypothesis, previous reports have shed light regarding the nature of CD47 and CD11b interaction. Using CD47 chimeric molecules, others have reported that the extracellular IgV and first transmembrane domains of CD47 are required for β1-dependent adhesion of T-cells to VCAM-1 and similarly for αIIbβ3 and αvβ3 activation in platelets and in an ovarian cancer cell line, respectively.28, 34, 36 Furthermore, we hypothesized that lipid and cholesterol composition of the membrane is an important factor for stabilizing CD47-CD11b interactions, as previous work has demonstrated that CD47 engages αvβ3 integrin and Gi proteins in lipid rafts domains to form signaling complexes.34 It is possible that the CD47-CD11b complex is already formed in the membrane of secondary granules as CD11b/CD18 and CD47 are within the membranes of these organelles.16 In addition to in cis interactions, it is well appreciated that PMN-expressed CD47 can also bind in trans with thrombospondins, and SIRPα expressed by other cells. Such complex interactions most certainly play physiologically important roles for immune cell interactions with vascular and lymphatic endothelium, epithelium, and with other immune cells after extravasation into tissues and organs. Thus, future studies are necessary to decipher what specific regions on the extracellular domain of CD47 bind and regulate CD11b/CD18 activation, and to develop biological reagents that selectively interfere with CD47-integrin associations.
In summary, using tissue selective CD47 deficient mice, we report that PMN-expressed CD47 plays a pivotal role in regulating CD11b-dependent PMN TEpM in the gut during inflammation. It is well appreciated that excessive PMN TEpM is a hallmark of disease flares in individuals with IBD. Despite the correlation of PMN infiltration into intestinal mucosa with disease severity, little is known about the regulatory mechanisms controlling this process. The fact that some patients receiving conventional therapies for treating individuals with IBD (i.e corticosteroids, TNF-α antagonists) are unable to attain or maintain remission, reveals the necessity to develop new therapeutic strategies. Our findings provide new insights on the regulatory mechanisms controlling the multi-step cascade of PMN TEpM and support a model where physical association of CD47 with CD11b/CD18 in PMN regulates integrin activation and adhesive function (Fig 1a). Therefore, identification of new ways to selectively inhibit CD47-CD11b interactions may offer potential therapeutic approaches to reduce dysregulated PMN trafficking in the gut during pathologic inflammatory diseases without compromising host defense.
Materials and Methods
Reagents and antibodies
RPMI-1640, Ammonium-Chloride-Potassium (ACK) lysing buffer, EDTA 0.5M, and HEPES 1M were purchased from Lonza (Walkersville, MD). MEM nonessential amino acids (NEAA), L-glutamine, Penicillin/streptomycin, Hanks’ Balanced Salt Solution with Ca2+ and Mg2+ (HBSS+) or HBSS without Ca2+ and Mg2+ (HBSS−), and Phosphate Buffered Saline with (PBS+) or without Ca2+ and Mg2+ (PBS−) were purchased from Corning Cellgro, Mediatech Inc. (Tewksbury, MA). Fetal bovine serum (FBS) was from Atlanta Biologicals (Oakwood, GA). Leukotriene B4 (LTB4) was from Cayman Chemical (Ann Arbor, MI). N-Formylmethionyl-leucyl-phenylalanine (fMLF), DNase I, Histopaques-1077 and −1119, 2,2'-azino-di-(3-ethyl)di-thiazoline-sulfonic-acid (ABTS), DuoLink® In situ-Fluorescence kit, and proteinase inhibitor cocktail (cat#P8340) were from Millipore-Sigma (St. Louis, MO). Recombinant Protein G-Sepharose® 4B, Hoechst 33342, CountBright counting beads, and Prolong antifade mounting agent were from Invitrogen (Carlsbad, CA). Recombinant murine E-selectin-Fc, VCAM-1-Fc and ICAM-1-Fc chimera molecules, and the polyclonal goat anti-mouse CD47 (cat#AF1866) and goat anti-human JAM-A (cat#AF1077) antibodies were from R&D Systems (Minneapolis, MN). Recombinant mouse TNF-α and IFN-γ were purchased from Peprotech (Rocky Hill, NJ). A complete list of antibodies used in this work is provided as supplemental material.
Mice
All mice used in this study were on the C57BL/6 background. WT, total CD47-deficient (Cd47−/−), MRP8-Cre-IRES/GFP, and LysMeGFP/eGFP mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and breeding colonies established in the specific pathogen-free facility at the University of Michigan School of Medicine, Ann Arbor, MI. LysMeGFP/eGFP mice were bred with C57BL/6 WT and Cd47−/− mice to generate LysMeGFP/+ and LysMeGFP/+;Cd47−/− mice. For simplification, we referred to these mice as WT and Cd47−/− respectively, since they phenotypically behave identically to their counterparts. Cd47fl/fl mice17 were bred in-house with mice constitutively expressing Cre under control of the murine intestinal epithelial (Villin-Cre;Cd47fl/fl) or under the granulocyte promoter (MRP8-Cre-IRES/GFP;Cd47 fl/fl Both sexes were used at 8-12 weeks of age. All experiments were approved and conducted in accordance with the guidelines of the Committee of Animal Research at the University of Michigan and the National Institutes of Health animal research guidelines as set forth in the Guide for the Care and Use of Laboratory Animals.
Cell lines and culture conditions
The human myelomonocytic cell line HL60 was obtained from ATCC (clone#CCL-240). CD47 null HL60 (HL60/E2) were created as follows: human CD47 targeting lentivirus was generated using the vector lentiCRISPR v2 (gift from Feng Zhang, Addgene plasmid#52961) expressing the guide sequence AATAGTAGCTGGAGCTGATCC. Transduced HL60 cells were cultured for 5 to 7 days in RPMI 1640 supplemented with 10% fetal calf serum and L-glutamine before depletion of CD47-positive cells using an anti-CD47 mAb (B6H12.2) and magnetic beads coated with sheep anti-mouse IgG (Dynabeads, Life Technologies, Carlsbad, CA). HL60 cells were cultured in RMPI-1640 supplemented with 20% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine and 1% NEAA at 37°C in a 5% CO2 incubator, and differentiated into a neutrophil like phenotype by supplementing growth media with 1.25% DMSO during 5 to 7 days.
Isolation of neutrophils from murine bone marrow
Mouse neutrophils were isolated from bone marrow (BMN) by density gradient centrifugation as previously described.37 Briefly, bone marrow was flushed from femurs and tibias, and red blood cells were lysed with ACK lysis buffer. Single cell suspension was layered over a separation gradient of Histopaque 1119 (1.119 g/mL) overlaid on second Histopaque 1077 (1.007 g/mL), and centrifuged at 2000 rpm for 20 min at 25°C. BMN were located at the interface between the two Histopaques. BMN purity was typically >90% as determined by flow cytometry.
Mouse PMN Chemotaxis Assay
Mouse BMN chemotaxis was evaluated using collagen-coated (10 μg/cm2) 0.33 cm2 polycarbonate Transwells® (5 μm pore size; Costar Corp., Cambridge, MA), as previously described.4, 38 Briefly, migration of 1x106 BMN added to the upper chamber of Transwell inserts was induced by a chemotactic gradient of 10 nM of LTB4 for 2 hr at 37°C. BMN were pretreated with 20 μg/mL of indicated function blocking mAbs. Transmigrated BMN into the bottom chamber were quantified by assaying for myeloperoxidase as published previously.38
Ileal loop
Ileal loop assays were performed as originally described.6, 8 Briefly, mice were pre-treated with TNF-α (100 ng) and IFN-γ (100 ng) administered by intraperitoneal injection 24 hr before surgery. PMN TEpM was assayed in a 4-cm long segment of ileum with uncompromised blood supply, by instilling 200 μL of HBSS+ containing 1 nM of LTB4 into the lumen of the loop for 60 min. In some studies, function blocking mAbs (50 μg per mouse) were injected in the lumen of the ileal loop 30 min prior to administration of LTB4. The luminal content was collected by flushing 500 μL of cold PBS− containing 2 mM EDTA, and the absolute number of PMN (CD45+CD11b+Ly6G+) quantified by flow cytometry. Results are presented as number of PMN per ileal loop. After collection of luminal contents, remaining ileal loop tissues were digested to isolate lamina propria (LP)-enriched fraction, or prepared for immunohistochemical staining.
Collection of sub-epithelial LP-enriched cellular fraction by enzymatic digestion
Ileal loop tissue was opened longitudinally to remove Peyer’s patches and mesenteric vessels, and LP-enriched fraction was enzymatically dissociated at 37°C under mechanical agitation as previously detailed.37 Briefly, mucus was removed by washing tissue in PBS− supplemented with 2% FBS and 5 mM DTT (Fisher BioReagents) for 20 min. The IEC lining was removed by three consecutive washings in chelation buffer (PBS− containing 2% FBS and 5 mM EDTA) for 10 min. Tissue was minced and digested in HBSS+ supplemented with 10 mM HEPES, Liberase TM (37.5 U/mL; Roche Applied Science, Indianapolis, IN) and DNase I (300 Kuntz units/mL) for 30 min. Cell suspension was filtered, washed in a solution of PBS− supplemented with 10% FBS and 2 mM EDTA, and cell number determined. Two-million cells were stained for flow cytometry analysis. Results are presented as number of leukocytes per 2x106 cells in the LP-enriched fraction.
BMN adhesion to immobilized ligands under defined flow conditions
BMN binding to immobilized adhesion molecules was performed under defined laminar shear stress using a parallel-plate flow chamber as previously described.39 Briefly, chimeric adhesion molecules Intercellular Adhesion Molecule-1 (ICAM-1-Fc; 10 μg/mL), Vascular Cell Adhesion Molecule-1 (VCAM-1-Fc; 5 μg/mL), or E-selectin-Fc (10 μg/mL), were immobilized overnight on glass coverslips and then placed in a 37°C pre-warmed parallel-plate flow apparatus. BMN (0.5x106 per mL) were resuspended in PBS containing 0.1% BSA and 20 mM HEPES, pH 7.4. BMN were drawn across the coverslip at estimated shear stress of 1.5, 1.0 and 0.75 dynes/cm2. In some studies, BMN were pre-incubated with function blocking mAbs (20 μg/mL) for 20 min. Live-cell imaging of leukocyte adhesion was recorded by a video camera coupled to a Nikon TE2000 inverted microscope equipped with a 20×/0.75NA phase contrast objective and VideoLab software (Mitov, Moorpark, CA). Accumulation of BMN was determined after 1 minute of flow for each flow rate by counting accumulated cells in six fields as detailed previously.40
Statistical analysis
Data are expressed as mean ± SEM. Statistical analysis was performed by unpaired two-tailed Student’s test followed by Mann-Whitney test for two groups comparisons, or one-way or two-way ANOVA followed by Bonferroni’s post-hoc analysis for multiple groups comparison, using Prism software v8.00 (GraphPad, San Diego, CA). Differences were considered statistically significant atp value ≤ 0.05.
Supplementary Material
Acknowledgments:
We thank Dylan Fink for technical support in this study as well as the Transgenic and Gene Targeting Core at Emory University, Microscopy and Image Analysis Laboratory and Pathology Flow Cytometry Core facilities at the University of Michigan Medical School. This work was supported by Crohn’s and Colitis Foundation Career Development Award CDA454814 to V.A. and NIH grants R01DK59888 and R01DK055679 to A.N, R01HL125780 to F.W.L, and R01DK079392, R01DK072564, and R01DK061379 to C.A.P.
Footnotes
Competing interest: The authors declare no competing interest.
References
- 1.Leick M, Azcutia V, Newton G, Luscinskas FW. Leukocyte recruitment in inflammation: basic concepts and new mechanistic insights based on new models and microscopic imaging technologies. Cell Tissue Res 2014; 355(3): 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brazil JC, Parkos CA. Pathobiology of neutrophil-epithelial interactions. Immunol Rev 2016; 273(1): 94–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkos CA. Neutrophil-Epithelial Interactions: A Double-Edged Sword. Am J Pathol 2016; 186(6): 1404–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest 1991; 88(5): 1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkos CA, Colgan SP, Liang TW, Nusrat A, Bacarra AE, Carnes DK et al. CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. J Cell Biol 1996; 132(3): 437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flemming S, Luissint AC, Nusrat A, Parkos CA. Analysis of leukocyte transepithelial migration using an in vivo murine colonic loop model. JCI Insight 2018; 3(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brazil JC, Liu R, Sumagin R, Kolegraff KN, Nusrat A, Cummings RD et al. alpha3/4 Fucosyltransferase 3-dependent synthesis of Sialyl Lewis A on CD44 variant containing exon 6 mediates polymorphonuclear leukocyte detachment from intestinal epithelium during transepithelial migration. J Immunol 2013; 191(9): 4804–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumagin R, Robin AZ, Nusrat A, Parkos CA. Transmigrated neutrophils in the intestinal lumen engage ICAM-1 to regulate the epithelial barrier and neutrophil recruitment. Mucosal Immunol 2014; 7(4): 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science 1996; 274(5288): 795–798. [DOI] [PubMed] [Google Scholar]
- 10.Su X, Johansen M, Looney MR, Brown EJ, Matthay MA. CD47 deficiency protects mice from lipopolysaccharide-induced acute lung injury and Escherichia coli pneumonia. J Immunol 2008; 180(10): 6947–6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azcutia V, Stefanidakis M, Tsuboi N, Mayadas T, Croce KJ, Fukuda D et al. Endothelial CD47 promotes vascular endothelial-cadherin tyrosine phosphorylation and participates in T cell recruitment at sites of inflammation in vivo. J Immunol 2012; 189(5): 2553–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azcutia V, Routledge M, Williams MR, Newton G, Frazier WA, Manica A et al. CD47 plays a critical role in T-cell recruitment by regulation of LFA-1 and VLA-4 integrin adhesive functions. Mol Biol Cell 2013; 24(21): 3358–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brittain JE, Han J, Ataga KI, Orringer EP, Parise LV. Mechanism of CD47-induced alpha4beta1 integrin activation and adhesion in sickle reticulocytes. J Biol Chem 2004; 279(41): 42393–42402. [DOI] [PubMed] [Google Scholar]
- 14.Brown E, Hooper L, Ho T, Gresham H. Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol 1990; 111(6 Pt 1): 2785–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang XQ, Frazier WA. The thrombospondin receptor CD47 (IAP) modulates and associates with alpha2 beta1 integrin in vascular smooth muscle cells. Mol Biol Cell 1998; 9(4): 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Merlin D, Burst SL, Pochet M, Madara JL, Parkos CA. The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47. J Biol Chem 2001; 276(43): 40156–40166. [DOI] [PubMed] [Google Scholar]
- 17.Reed M, Luissint AC, Azcutia V, Fan S, O'Leary MN, Quiros M et al. Epithelial CD47 is critical for mucosal repair in the murine intestine in vivo. Nat Commun 2019; 10(1): 5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edens HA, Parkos CA. Modulation of epithelial and endothelial paracellular permeability by leukocytes. Adv Drug Deliv Rev 2000; 41(3): 315–328. [DOI] [PubMed] [Google Scholar]
- 19.Martin TR, Pistorese BP, Chi EY, Goodman RB, Matthay MA. Effects of leukotriene B4 in the human lung. Recruitment of neutrophils into the alveolar spaces without a change in protein permeability. J Clin Invest 1989; 84(5): 1609–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nash S, Stafford J, Madara JL. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Invest 1987; 80(4): 1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parsons PE, Sugahara K, Cott GR, Mason RJ, Henson PM. The effect of neutrophil migration and prolonged neutrophil contact on epithelial permeability. Am J Pathol 1987; 129(2): 302–312. [PMC free article] [PubMed] [Google Scholar]
- 22.Blazar BR, Lindberg FP, Ingulli E, Panoskaltsis-Mortari A, Oldenborg PA, Iizuka K et al. CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J Exp Med 2001; 194(4): 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med 2001; 193(7): 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science 2000; 288(5473): 2051–2054. [DOI] [PubMed] [Google Scholar]
- 25.Yi T, Li J, Chen H, Wu J, An J, Xu Y et al. Splenic Dendritic Cells Survey Red Blood Cells for Missing Self-CD47 to Trigger Adaptive Immune Responses. Immunity 2015; 43(4): 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calippe B, Augustin S, Beguier F, Charles-Messance H, Poupel L, Conart JB et al. Complement Factor H Inhibits CD47-Mediated Resolution of Inflammation. Immunity 2017; 46(2): 261–272. [DOI] [PubMed] [Google Scholar]
- 27.Podolnikova NP, Hlavackova M, Wu Y, Yakubenko VP, Faust J, Balabiyev A et al. Interaction between the integrin Mac-1 and signal regulatory protein alpha (SIRPalpha) mediates fusion in heterologous cells. J Biol Chem 2019; 294(19): 7833–7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ticchioni M, Raimondi V, Lamy L, Wijdenes J, Lindberg FP, Brown EJ et al. Integrin-associated protein (CD47/IAP) contributes to T cell arrest on inflammatory vascular endothelium under flow. FASEB J 2001; 15(2): 341–350. [DOI] [PubMed] [Google Scholar]
- 29.Diamond MS, Springer TA. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol 1993; 120(2): 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu C, Shimaoka M, Zang Q, Takagi J, Springer TA. Locking in alternate conformations of the integrin alphaLbeta2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proc Natl Acad Sci U S A 2001; 98(5): 2393–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosetti F, Chen Y, Sen M, Thayer E, Azcutia V, Herter JM et al. A Lupus-Associated Mac-1 Variant Has Defects in Integrin Allostery and Interaction with Ligands under Force. Cell Rep 2015; 10(10): 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salas A, Shimaoka M, Kogan AN, Harwood C, von Andrian UH, Springer TA. Rolling adhesion through an extended conformation of integrin alphaLbeta2 and relation to alpha I and beta I-like domain interaction. Immunity 2004; 20(4): 393–406. [DOI] [PubMed] [Google Scholar]
- 33.Kim M, Carman CV, Yang W, Salas A, Springer TA. The primacy of affinity over clustering in regulation of adhesiveness of the integrin {alpha}L{beta}2. J Cell Biol 2004; 167(6): 1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald JF, Zheleznyak A, Frazier WA. Cholesterol-independent interactions with CD47 enhance alphavbeta3 avidity. J Biol Chem 2004; 279(17): 17301–17311. [DOI] [PubMed] [Google Scholar]
- 35.Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem 1999; 274(2): 559–562. [DOI] [PubMed] [Google Scholar]
- 36.Fujimoto TT, Katsutani S, Shimomura T, Fujimura K. Thrombospondin-bound integrin-associated protein (CD47) physically and functionally modifies integrin alphallbbeta3 by its extracellular domain. J Biol Chem 2003; 278(29): 26655–26665. [DOI] [PubMed] [Google Scholar]
- 37.Luissint AC, Williams HC, Kim W, Flemming S, Azcutia V, Hilgarth RS et al. Macrophage-dependent neutrophil recruitment is impaired under conditions of increased intestinal permeability in JAM-A-deficient mice. Mucosal Immunol 2019; 12(3): 668–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brazil JC, Lee WY, Kolegraff KN, Nusrat A, Parkos CA, Louis NA. Neutrophil migration across intestinal epithelium: evidence for a role of CD44 in regulating detachment of migrating cells from the luminal surface. J Immunol 2010; 185(11): 7026–7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alcaide P, Maganto-Garcia E, Newton G, Travers R, Croce KJ, Bu DX et al. Difference in Th1 and Th17 lymphocyte adhesion to endothelium. J Immunol 2012; 188(3): 1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim YC, Wakelin MW, Henault L, Goetz DJ, Yednock T, Cabanas C et al. Alpha4beta1-integrin activation is necessary for high-efficiency T-cell subset interactions with VCAM-1 under flow. Microcirculation 2000; 7(3): 201–214. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.