Abstract
This study examines how family history-related factors and causal beliefs affect underserved women’s cancer risk perceptions and adherence to mammography. 1,010 patients at a primary care safety net clinic at a large urban hospital completed a survey in 2015. Of the 1,010 patients, 467 women 45 years of age or older were included in this analysis. The majority of participants were African American (68%). We built multivariable linear and logistic regression models to examine the dependent variables of cancer risk perception and mammography screening adherence. According to the results, those with a family history of cancer were significantly more likely to be adherent to mammography. Perceived importance of family health history also significantly predicted their mammography screening adherence. However, cancer risk perceptions did not predict underserved women’s mammography adherence. Significant interaction effects on the associations 1) between family cancer history, cancer risk perceptions, and mammography screening adherence and 2) between race, behavioral causal beliefs, and risk perceptions were found. Findings suggest that implementing different strategies across racial groups and by cancer history may be necessary to promote regular mammography screening.
Keywords: mammography, family health history, causal attribution, cancer risk perception, underserved women
INTRODUCTION
Breast cancer is the second most common cancer among American women, following skin cancer. According to the American Cancer Society (2016), about one in eight (12%) women in the US will develop invasive breast cancer during their lifetime. When breast cancer is diagnosed early using screening mammography, however, treatments are more likely to be successful (American Cancer Society, 2014). These issues are particularly critical to examine among underserved communities. Previous studies have found that socioeconomic factors such as low income and lack of health insurance are frequently identified barriers to use of mammography (Schueler, Chu, & Smith-Bindman, 2008; Alexandraki & Mooradian, 2010; Xu & Jung, 2016). In addition, recent studies have addressed issues related to the accuracy as well as the understandability of perceived risks among underserved populations (LeMasters et al., 2014; Seitz et al, 2016), thereby suggesting the importance of investigating low-income women’s risk perceptions as well as the effects on their screening behaviors. Although the effect of risk perceptions on cancer screening behavior is not clear, scholars have suggested that communicating cancer risks to racially and ethnically diverse female populations may potentially affect their screening (Kim et al., 2008). Therefore, in this study we investigate the integrative influences of race, family health history (hereafter, FHH)-related factors, causal attributions, and cancer risk perceptions (hereafter, CRP) on mammography screening adherence (hereafter, MSA) among a sample of underserved women.
Mammography Screening, Cancer Risk Perception, and Family Health History
While experts try to analyze and evaluate hazards, laypeople mostly depend on their personal risk judgment, which is referred to as “risk perception” (Slovic, 1987). Risk perceptions often refer to an individual’s belief about his or her susceptibility to a particular condition such as breast cancer (Sjoberg, 1998). While risk as analysis involves logic, reason, and scientific deliberation in the process of managing hazards, risk as feelings refers to our fast, instinctive, and intuitive reactions to the hazard (Slovic, Finucane, Peters, & MacGregor, 2004). Since the experienced feelings are used as information in decision-making, risk as feelings may motivate actions that are expected to eliminate the particular affective response (Slovic & Peters, 2006). In many theories of health behavior (e.g., Health Belief Model, Precaution Adoption Model, Protection Motivation Theory, etc.), perceived health risks are important motivators of preventive behaviors (Becker, 1974; Vernon, 1999). For example, as shown in the Extended Parallel Process Model (EPPM, Witte, 1998), appropriate levels of risk perceptions form part of perceived threat, which can motivate individuals to undertake health-protective behaviors. Therefore, theoretically it makes sense that women’s risk perceptions about breast cancer motivate their screening behaviors.
Nevertheless, there has been conflicting evidence with regard to the effect of perceived cancer risks on cancer screening behaviors. In the late 1990s and early 2000s, two meta-analytic reviews based on descriptive studies using population data suggested that greater risk perceptions for breast cancer were positively associated with mammography screening (McCaul, Branstetter, Schroeder, & Glasgow, 1996; Katapodi, Lee, Facione, & Dodd, 2004). Therefore, in an effort to increase early detection of breast cancer, several health interventions attempted to increase women’s perceived risk of developing breast cancer to improve their screening behavior. However, other studies showed conflicting or controversial evidence, particularly with regard to the effect of educational interventions aiming to change risk perceptions to improve subsequent cancer screening (e.g., Vernon, 1999; Calvocoressi et al., 2004). Therefore, researchers started to consider a more complex relationship when developing interventions to improve MSA (Calvocoressi et al., 2004). According to a more recent review article by Walker and colleagues (2013), consistent associations between higher perceived risk and adherence to mammography guidelines have not been observed, although a weak positive association has been found among women with familial breast cancer risk. This finding suggests the important role of FHH in modifying the association between CRP and prevention behaviors. Therefore, family history of cancer, in addition to personal cancer history, may be an essential component in predicting CRP as well as mammography screening.
In particular, FHH is an important risk factor for many common cancers including breast, ovarian, and colon cancers (Acheson et al., 2010; Valdez, Yoon, Qureshi, Green, & Khoury, 2010). FHH communication has been emphasized within the domain of health communication, particularly in the context of minority health (e.g., Ashida et al., 2009; Canary et al., 2019; Hong, 2018; Hong, 2019; Hovick, Yamasaki, Burton-Chase, & Peterson, 2015; Thompson et al., 2015). According to the findings of previous research, having a family history of chronic diseases such as cancer is positively associated with risk perceptions about that disease (Acheson et al., 2010; Kelly et al., 2009; Katapodi, Dodd, Lee, & Facione, 2009). In addition, women with a family history of cancer (Williams et al., 2008) or breast cancer (Khoshravesh, Taymoori, & Roshani, 2015) have been shown to be more likely to adhere to mammography screening recommendations, and CRP of women with a family history of breast cancer had a significant and positive effect on their mammography screening (Laing & Makambi, 2008). As shown in EPPM and other theories of health behavior, it is possible that family health history of cancer, which informs individuals’ CRP, may positively affect their MSA. This issue is more salient for underserved women, because there has been less knowledge about and more limited awareness of issues related to familial disease risks among this population (Kaphingst et al., 2012). Therefore, in this study, we explored the following hypotheses.
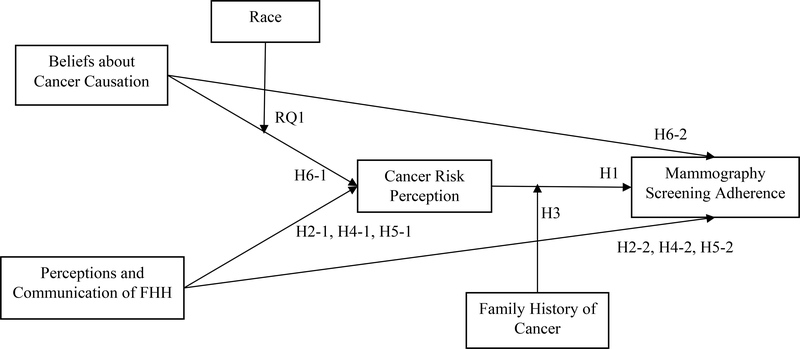
H1: Women’s CRP positively influences their MSA.
H2–1: Women’s perceived knowledge of FHH is positively associated with CRP.
H2–2: Women’s perceived knowledge of FHH is positively associated with MSA.
H3: Family history of cancer moderates the relationship between CRP and MSA such that for women with a family history of cancer, the magnitude of the positive association between CRP and MSA is stronger compared to the association among women without a family history.
Although perceived health risks can be important motivators of preventive behaviors, people may try to find evidence as to why they would not be susceptible to diseases that run in their families (Walter, Emery, Braithwaite, & Marteau, 2004). Therefore, when investigating the effects of family history assessment on disease prevention, it is important to understand the relationship of FHH to people’s perceptions of their own risk and their ability to take actions to reduce the risk (Walker et al., 2013). Given lower levels of education and health literacy, on average, among medically underserved women, it is also even more important to consider these women’s perceptions about the importance of FHH as well as their actual communication of the information with their family and doctors. According to Acheson and colleagues (2010), the majority of people are optimistically biased about their risks for developing common, chronic conditions, and this bias seems to be prevalent even among those with a moderate or strong family history of disease. This suggests that although knowing about FHH is important, it would not be as effective if people don’t believe or understand its significance. Moreover, individuals’ perceptions of FHH can be related to their actual communication with their family and doctors, which can positively influence both CRP and screening behaviors. Therefore, in this study, we investigated how women’s awareness, perceptions, and communication of FHH influence their CRP and MSA.
H4–1: Women’s perceived importance of FHH is positively associated with CRP.
H4–2: Women’s perceived importance of FHH is positively associated with MSA.
H5–1: Women’s communication of FHH with their (a) family and (b) doctors is positively associated with CRP.
H5–2: Women’s communication of FHH with their (a) family and (b) doctors is positively associated with MSA.
Mammography Screening, Cancer Risk Perception, and Causal Attributions
Cameron and Leventhal’s (2003) self-regulation theory describes how information about a health threat is processed within individuals’ pre-existing cognitive schema, and how the cognitive representations within these schemas activate coping procedures for dealing with the perceived threat. This perspective is shaped by the recognition that most common and chronic health conditions are caused by multiple factors. Although health communication interventions have often emphasized specific causal factors (e.g., healthy lifestyles) to reduce cancer-related risks, it is difficult to know to what extent women believe those risk factors as the causes of breast cancer (Wang, Miller, Egleston, Hay, & Weinberg, 2010). Knowing this information is important because causal beliefs and attributions shape subsequent beliefs about disease controllability and efficacy of medical interventions (Parrott, Silk, & Condit, 2003). In fact, the beliefs women hold about the causes of cancer have been shown to influence the preventive actions they undertake to reduce their risk (Costanzo, Lutgendorf, Bradley, Rose, & Anderson 2005; Rabin & Pinto, 2006). This suggests women’s causal attributions reflecting their cognitive schema can be significant predictors of their CRP as well as preventive behaviors such as MSA.
More specifically, the common sense model of self-regulation of health and illness suggests that health risk information activates a cognitive representation of the threat (Cameron & Leventhal, 2003; Marteau & Weinman, 2006). For example, causal attributions may be closely linked to FHH because individuals’ perceptions of familial disease risks can activate their cognitive beliefs in genetic as well as environmental causes of cancer (Kowalkowski, Hart, Du, Baraniuk, & Latini, 2012; Rodríguez et al., 2015). Research has demonstrated the validity of this model, showing that patients’ perceptions about causal attribution and controllability are associated with their positive health practices (Costanzo, Lutgendorf, & Roeder, 2011). Therefore, women’s beliefs about cancer causation may affect women’s CRP as well as MSA. Moreover, each type of causal belief may have different influences on women’s health practice and behaviors. For example, breast cancer survivors who attributed disease to their behaviors (e.g., alcohol use, diet, etc.) were more likely to make positive changes in their behaviors (Rabin & Pinto, 2006). According to a systematic review about breast cancer survivors’ causal attribution, breast cancer patients tend to attribute their illness often to FHH, environmental factors, stress, and chance (Dumalaon-Canaria, Hutchinson, Prichard, & Wilson, 2014). Therefore, given the findings of the systematic review (Dumalaon-Canaria et al., 2014) as well as previous studies (Costanzo et al., 2011; Marteau & Weinman, 2006) suggesting the associations among cancer causal beliefs, CRP, and health practices, we propose the following relationships of underserved women’s causal beliefs related to genes, behavior, stress, and chance to their CRP and MSA.
H6–1: Women’s beliefs about cancer causation are positively associated with CRP.
H6–2: Women’s beliefs about cancer causation are positively associated with MSA.
Racial minorities are often disproportionately at risk due to structural inequalities (Collins, Grineski, Chakraborty, & McDonald, 2011), and risk perceptions have often been shown to vary by race or ethnicity (Rice, Brandt, Hardin, Ingram, & Wilson, 2015). For example, prior studies have shown that African Americans’ beliefs about their risk for cancer tend to be lower than cancer beliefs reported by European Americans (Orom, Kiviniemi, Shavers, Ross, & Underwood, 2010; Rice et al., 2015). Low perceptions of risk among African Americans are of particular concern because this racial group has higher cancer mortality rates and lower screening rates for most cancers, compared to their European American counterparts (Siegel, Jiemin, Zhaohui, & Jemal, 2014). In addition, at all stages of diagnosis, African Americans have poorer survival rates than European Americans for commonly diagnosed cancers (Howlader et al., 2012; Siegel et al., 2014). Racial differences about causal beliefs have been rarely investigated in the cancer context. However, cancer-related beliefs as well as risk perceptions are influenced by a wide variety of demographic factors such as race and socio-economic status (Kim et al., 2008; Rice et al., 2015). For example, according to previous research (Schnittker, Freese, & Powell, 2000; Schostak, Freese, Link, & Phelan, 2009), European American individuals are more likely to endorse genetic explanations of physical illnesses than are their African American counterparts. Therefore, given the pervasiveness of health disparities in the United States, this study investigates how race affects the associations between causal beliefs about cancer development and risk perceptions.
RQ1: How does race moderate the association between women’s causal attributions and CRP?
METHOD
Setting and Procedure
This study was conducted in the primary care clinic of a large urban hospital, the Center for Outpatient Health (COH) at Barnes-Jewish Hospital in St. Louis, Missouri. This clinic serves as the site for ambulatory care training for a large internal medicine residency with about 150 residents. The clinic serves a large and diverse patient population. In one year, the COH saw 16,907 unique patients, 64% African American and 30% European American, 67% female, 59% between 35 and 64 years of age.
Participants were recruited in 2015. Inclusion criteria were: 1) being at least 18 years of age, 2) being a visitor at the COH, and 3) speaking English. Visitors in the waiting rooms were asked to complete a survey by trained data collectors. Participants first completed the informed consent process. Then, after completing a set of verbally administered measures, participants were asked to complete a self-administered written questionnaire. However, verbal administration of written questionnaire items was also possible if requested by participants. This study was approved by the Human Research Protection Office at the Washington University School of Medicine.
Approximately 26% (n=1,110) of those approached were ineligible to participate in the study because they were not patients, did not speak English, or had previously taken the survey. Among eligible participants, 44% (n=1380) agreed to participate in the study and were consented by trained data collectors. Of the 1380 patients who were consented, 1010 (73%) completed the written survey. Of the 1,010 patients, 467 women 45 years of age or older were included in this analysis, because we were interested in CRP among women eligible for screening mammography and the American Cancer Society recommended yearly mammography starting by age 45 when the survey was administered.
Measurements
Mammography screening adherence
MSA was assessed by asking participants’ the number of years since their last mammogram: < 1 year; 1–2 years; 2–3 years; > 3 years; or never. Participants’ responses were recoded as <2 years (41.8%) vs. 2+ years or never for analysis (58.2%) (Calvocoressi, Sun, Kasl, Claus, & Jones, 2008).
Cancer risk perception
CRP was measured by using one item: “Compared to the average person your age, would you say that you are…,” followed by 5 options from A lot less likely to get cancer to A lot more likely to get cancer (M=2.69, SD=1.15) (Hesse et al., 2005; Nelson et al., 2004).
Knowledge about FHH
Perceived knowledge of FHH was assessed by asking “How familiar are you with your family’s health history?” (Kaphingst, Lachance, Gepp, D’Anna, & Rios-Ellis, 2011) Participants responded to this item on a 5-point scale from Very familiar to Not at all familiar. This item was reverse-coded for analysis (M=4.12, SD=1.03).
FHH communication
Communication about FHH was measured using the following items to assess participants’ communication with family as well as doctors; 1) I talk with family members about our FHH (M=3.06, SD=.81); and 2) I talk with a doctor about my FHH (M=2.82, SD=.91). Participants responded to these items on a 4-point scale from Very often to Not at all (Kaphingst et al., 2012). These items were reverse-coded for analysis.
Perceived importance of FHH
Perception of the importance of FHH was assessed by asking, “would you agree or disagree with the following statement: It is important for my own health to know if diseases like cancer, diabetes, stroke, or heart disease run in my family” (Yoon et al., 2004). Participants responded to this item on a 5-point scale from Strongly disagree to Strongly agree (M=2.07, SD=1.61).
Beliefs about cancer causation
Women’s beliefs about cancer causation were measured using four items adapted from the Illness Perception Questionnaire for a study of responses to disease risk information (McBride et al., 2009): 1) How much do you think health habits such as diet, exercise, and smoking determine whether or not a person will get cancer (behavioral)? (M=3.43, SD=1.10); 2) How much do you think genetic make-up, that is characteristics that are passed from one generation to the next, determines whether or not a person will get cancer (genetic)? (M=3.19, SD=1.04); 3) How much do you think stress and worry determine whether or not a person will develop cancer (stress)? (M=2.77, SD=1.31); 4) How much do you think chance determines whether or not a person will get cancer (chance)? (M=2.68, SD=1.20). Participants responded to these items on a 5-point scale from Not at all to Completely. Each type of belief was measured and examined separately.
Family and personal history of cancer
The following two questions were asked to assess family and personal history of cancer (Heeringa & Connor, 1995): 1) As far as you know, does cancer run in your family? and 2) Has a doctor (or other health professional) ever told you that you have cancer? Participants responded to these items by choosing between two options; Yes or No. 164 (35.1%) participants said they had family history of cancer, while 55 (11.8%) said yes to the item asking about personal history of cancer.
Data Analysis
SPSS Version 24 was used for data analysis. To explore the hypotheses and research questions of this study, we built multivariable linear and logistic regression models to examine the dependent variables of CRP and MSA (i.e., less than 2 years since last mammography). The rationale for the modelling was a ‘broad-to-specific’ order where demographic and medical variables were entered first, and then more specific variables regarding FHH-related perceptions and communication, and beliefs about cancer causation were entered. Specifically, in the hierarchical linear regression model employing CRP as the dependent variable, demographic variables (i.e., age, race, income, insurance, and marital status) were entered in block 1, personal and family cancer history in block 2, variables related to FHH (i.e., knowledge about FHH, FHH communication with family and doctors, and opinion on the importance of FHH) in block 3, beliefs about cancer causation (i.e., behavior, gene, stress, and chance) in block 4, and interaction terms in block 5. In the logistic regression model predicting participants’ MSA, the same demographic variables were entered in block 1, personal and family cancer history in block 2, variables related to FHH in block 3, beliefs about cancer causation in block 4, and CRP and interaction terms in block 5. Then, to further investigate interaction effects of race and family cancer history, PROCESS macro for SPSS (Hayes, 2012) was employed. This study used a bootstrapping method to check the consistency of a beta coefficient generated by multiple-regression analysis by repeatedly sampling cases (5,000 times).
RESULTS
Characteristics of Participants
As shown in Table 1, the majority of participants were African American (68%). Most (64%) participants had a household income of less than $19,999 per year, and 48% earned less than $9,999.
Table 1.
Demographic Characteristics of Participants (N = 467)
| Characteristic | N (%) | |
|---|---|---|
| Age | 45–54 | 204 (43.7) |
| 55–64 | 195 (41.8) | |
| 65+ | 68 (14.6) | |
| Race | European American | 127 (27.2) |
| African American | 311 (66.6) | |
| Asian, Pacific Islander | 0 (0) | |
| Native American | 1 (0.2) | |
| Other | 12 (2.6) | |
| Multi-Racial | 7 (1.5) | |
| Hispanic | 1 (0.02) | |
| Unknown | 8 (1.7) | |
| Income | Less than $9,999 | 191 (40.9) |
| $10,000- $19,999 | 106 (22.7) | |
| $20,000-$29,999 | 43 (9.2) | |
| $30,000-$39,999 | 28 (6.0) | |
| $40,000-$49,999 | 16 (3.4) | |
| $50,000-$74,999 | 9 (1.9) | |
| $75,000+ | 8 (1.7) | |
| Not answered | 66 (14.1) | |
| Insurance | Yes | 334 (71.5) |
| No | 109 (23.3) | |
| Not answered | 24 (5.1) | |
| Marital Status | Married | 71 (15.9) |
| Living as married | 7 (1.6) | |
| Widowed | 77 (16.5) | |
| Divorced | 128 (27.4) | |
| Separated | 46 (9.9) | |
| Never been married | 118 (25.3) | |
| Not answered | 20 (4.3) |
Factors Predicting Cancer Risk Perception
Table 3 reports the results of hierarchical linear regression model comparison regarding factors that influence CRP. These factors include FHH-related perceptions and FHH communication (H2–1, H4–1, & H5–1), and beliefs about cancer causation (H6–1). In models 1, 2, 3, and 4, race significantly predicted participants’ CRP (Model1: β=−.23, p<.001; Model2: β=−.19, p<.001; Model3: β =−.19, p<.001; Model4: β =−.19, p<.001). Personal cancer history was also a significant and positive predictor of CRP in models 2, 3, 4, and 5 (Model2: β=.23, p<.001; Model3: β=.23, p<.001; Model4: β=.23, p<.001; Model5: β=.23, p<.001). In model 4, chance causal beliefs (β=.13, p<.01) was positively associated with CRP.
Table 3.
Factors Predicting Cancer Risk Perceptions
| Cancer Risk Perception | ||||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||
| β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | ||
| Demographics | Age a | .03 (.11) | −.01 (.11) | .00 (.11) | −.00 (.11) | −.02 (.11) |
| Race b | −.23 (.12)*** | −.19 (.12)*** | −.19 (.12)*** | −.19 (.11)*** | .00 (.46) | |
| Income c | .10 (.11)+ | .09 (.11)+ | .09 (.11)+ | .09 (.11)+ | .08 (.11)+ | |
| Insurance d | −.05 (.13) | −.03 (.12) | −.04 (.12) | −.03 (.12) | −.04 (.12) | |
| Married e | .02 (.13) | .03 (.15) | .02 (.15) | .03 (.15) | .03 (.15) | |
| Cancer history | Family history of cancer f | .06 (.11) | .06 (.11) | .06 (.11) | .05 (.11) | |
| Personal history of cancer g | .23 (.17)*** | .23 (.16)*** | .23 (.16)*** | .23 (.16)*** | ||
| Family health history | Knowledge about FHH h | −.08 (.06) | −.06 (.06) | −.06 (.06) | ||
| FHH h communication with family | −.04 (.08) | −.05 (.08) | −.06 (.08) | |||
| FHH h communication with doctors | .04 (.06) | .03 (.06) | .04 (.06) | |||
| Perceived importance of FHH h | .03 (.03) | .04 (.03) | .04 (.03) | |||
| Beliefs about cancer causation | Behavior i | −.06 (.05) | .10 (.10) | |||
| Gene i | .06 (.06) | .04 (.11) | ||||
| Stress i | .01 (.04) | −.06 (.08) | ||||
| Chance i | .13 (.05)** | .12 (.08) | ||||
| Interaction terms | Race * Behavior | −.37 (.12)* | ||||
| Race * Gene | .02 (.13) | |||||
| Race * Stress | .12 (.10) | |||||
| Race * Chance | .03 (.10) | |||||
| R2 | .06*** | .12*** | .13*** | .15*** | .16*** | |
Note:
. 55 ore more = 1, less than 55 = 0
. African American = 1, others = 0
. lower than 9,999 = 1, others = 0
. Yes = 1, others = 0
. Married = 1, others = 0
. Yes = 1, others = 0
. Yes = 1, others = 0
= family health history
= causal beliefs.
p < .10
p <. 05
p <.01
p <.001.
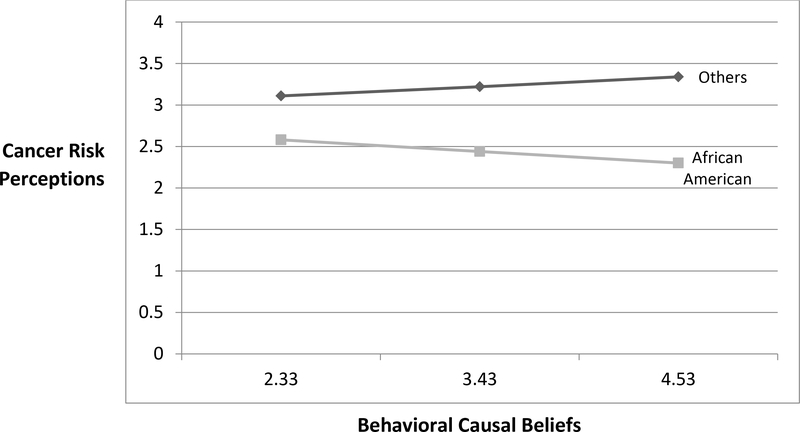
Additionally, in model 5, the interaction effect of race and behavioral causal beliefs on CRP (RQ1) was negatively significant (β=−.37, p<.05). That is, among African American participants (b=−.13, se=.06, p<.05), behavioral causal beliefs have significantly less positive or more negative effects on CRP compared to other ethnicities (b=.10, se=.10, p=.29) (Figure 2).
Figure 2.
Interaction Effect on Cancer Risk Perceptions
Factors Predicting Mammography Screening Adherence
Table 4 reports the results of logistic regression analysis including factors predicting MSA. These factors include CRP (H1), FHH-related perceptions and FHH communication (H2–2, H4–2, & H5–2), and beliefs about cancer causation (H6–2). In models 2, 3, 4, and 5, family history of cancer significantly predicted participants’ MSA (Model2: OR=2.58, p<.001, 95%CI=1.71, 3.96; Model3: OR=2.55, p<.001, 95%CI=1.68, 3.88; Model4: OR=2.57, p<.001, 95%CI=1.68, 3.91; Model5: OR=7.73, p<.001, 95%CI=2.51, 23.78). In models 2, 3, and 4, personal history of cancer also significantly and positively affected MSA (Model2: OR=2.03, p<.05, 95%CI=1.10, 3.74; Model 3: OR=2.17, p<.05, 95%CI=1.05, 3.64; Model4: OR=2.01, p <.05, 95%CI=1.07, 3.75). In addition, in models 3, 4, and 5, participants’ perceived importance of FHH significantly predicted their MSA (Model3: OR=1.15, p<.05, 95%CI=1.02, 1.30; Model4: OR=1.15, p<.05, 95%CI=1.02, 1.30; Model5: OR=1.16, p<.05, 95%CI =1.03, 1.31). However, CRP was not a significant predictor of mammography adherence.
Table 4.
Factors Predicting Mammography Screening Adherence
| Mammography Screening Adherence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Demographics | Age a | 1.37 | .94–2.01 | 1.31 | .88–1.95 | 1.33 | .89–1.99 | 1.33 | .88–2.00 | 1.32 | .87–1.98 |
| Race b | .80 | .53–1.20 | 1.03 | .67–1.59 | 1.01 | .65–1.57 | 1.01 | .65–1.58 | .98 | .62–1.53 | |
| Income c | 1.17 | .79–1.73 | 1.25 | .83–1.88 | 1.21 | .80–1.82 | 1.21 | .79–1.84 | 1.23 | .80–1.88 | |
| Insurance d | .78 | .49–1.23 | .76 | .49–1.25 | .80 | .50–1.29 | .80 | .50–1.30 | .81 | .50–1.30 | |
| Married e | 1.16 | .68–1.97 | 1.25 | .72–2.17 | 1.25 | .72–2.17 | 1.17 | .66–2.04 | 1.17 | .66–2.08 | |
| Cancer history | Family history of cancer f | 2.58*** | 1.71–3.90 | 2.55*** | 1.68–3.88 | 2.57*** | 1.68–3.91 | 7.73*** | 2.51–23.78 | ||
| Personal history of cancer g | 2.03* | 1.10–3.74 | 2.17* | 1.05–3.64 | 2.01* | 1.07–3.75 | 2.88 | .49–16.71 | |||
| Family health history | Knowledge about FHH h | 1.00 | .81–1.24 | 1.00 | .81–1.24 | 1.00 | .80–1.24 | ||||
| FHH h communication with family | 1.13 | .84–1.50 | 1.11 | .83–1.49 | 1.10 | .82–1.47 | |||||
| FHH h communication with doctors | 1.16 | .91–1.48 | 1.18 | .92–1.51 | 1.20 | .94–1.54 | |||||
| Perceived importance of FHH h | 1.15* | 1.02–1.30 | 1.15* | 1.02–1.30 | 1.16* | 1.03–1.31 | |||||
| Beliefs about cancer causation | Behavior i | .89 | .72–1.09 | .89 | .72–1.09 | ||||||
| Gene i | 1.01 | .81–1.27 | 1.03 | .82–1.29 | |||||||
| Stress i | .92 | .78–1.10 | .92 | .77–1.09 | |||||||
| Chance i | 1.04 | .86–1.25 | 1.03 | .86–1.24 | |||||||
| CRP | Cancer risk perception | 1.08 | .86–1.35 | ||||||||
| Interaction terms | FHC * CRP | .67* | .46–.98 | ||||||||
| PHC* CRP | .95 | .58–1.54 | |||||||||
| Nagelkerke R2 | .02 | .10*** | .12*** | .13*** | .14*** | ||||||
Note:
. 55 ore more = 1, less than 55 = 0
. African American = 1, others = 0
. lower than 9,999 = 1, others = 0
. Yes = 1, others = 0
. Married = 1, others = 0
. Yes = 1, others = 0
. Yes = 1, others = 0
= family health history
= causal beliefs.
p < .10
p <. 05
p <.01
p <.001.
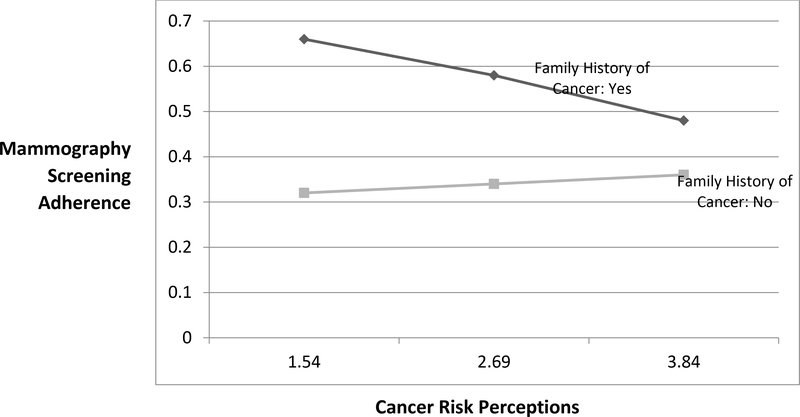
Lastly, the interaction effect between family history of cancer and CRP (H3) was a significant inverse association with participants’ MSA (Model5: OR=.67, p<.05, 95%CI=.46, .98). That is, among participants with family cancer history (b=−.32, se=.17, p=.06), CRP have significantly less positive or more negative effects on mammography adherence compared to those without the cancer history (b=.08, se=.12, p=.51) (Figure 3).
Figure 3.
Interaction Effect on Mammography Screening Adherence
DISCUSSION
The pervasiveness of breast cancer is a serious health problem among American women. Although mammography screening can help through early detection, women’s health-protective behaviors still are significantly influenced by their income level, contributing to health disparities (Anderson et al., 2015; Mays et al, 2012), and it is therefore critical to understand factors impacting screening in underserved groups. In this study, we investigated how CRP as well as factors related to FHH and causal attributions influence underserved women’s MSA. Here, we discuss the theoretical, practice, and research implications of our findings.
First, results suggest the importance of women’s perceptions about FHH in MSA. More specifically, results revealed that women who perceived a greater importance for FHH in disease risk were more likely to be adherent to mammography (H4–2), although perceived importance failed to predict increased risk perception (H4–1). Previous studies have indicated that MSA as well as risk perceptions can be affected by family history of breast cancer (Katapodi et al., 2004; Isaacs et al., 2002). Therefore, when investigating the effects of FHH on disease prevention, researchers have focused on factors affecting individuals’ communication of FHH, communication patterns or contexts, and the diffusion of the risk information (e.g., Ashida et al., 2009; Canary et al., 2019; Hong, 2018; Hovick et al., 2015). However, research is needed to explore people’s perceptions about the importance of FHH and its effects on their health behaviors in the context of cancer. The findings from this study suggest a fundamental change may be needed in those research approaches. Moreover, results suggest that perceived importance of FHH might be more related to risk as analysis than risk as feelings, thereby affecting individuals’ logic, reason, and scientific deliberation in managing cancer risks without increasing perceived susceptibility (Slovic et al., 2004). The results of hypothesis testing FHH-related factors highlight that it is more important for women to understand the importance of FHH than other factors (i.e., knowledge and communication) when making decisions about adhering to mammography recommendations.
Second, although CRP did not predict underserved women’s MSA (H1), a meaningful moderation effect of family cancer history on the association between CRP and MSA was found (H3). The role of CRP has been controversial in public health campaigns. As discussed above, a recent review article (Walker et al., 2013) suggested there was no consistent association between CRP and mammography adherence. Similarly, in this study with a sample of underserved women, CRP did not predict mammography adherence. One reason for this may be because we measured general CRP rather than breast CRP. However, a previous study using data revealed participants’ risk perceptions for cancer in general positively and strongly affected specific risk perceptions for breast, colon, lung, and prostate cancers (Grenen, Ferrer, Klein, & Han, 2016). Therefore, we focused on FHH by hypothesizing that for women with a family history of cancer, the magnitude of the positive association between CRP and MSA is stronger compared to the association among women without a family history (H3). According to our results of interaction analyses, however, although a significant interaction effect was found, the direction of effect was the reverse of our hypothesis. That is, among participants with a family cancer history, CRP had less positive or more negative effects on MSA compared to those without it. This reverse outcome may be explained by EPPM (Witte, 1998) which suggests individuals’ perceived fear and subsequent perceptions of inefficacy can result in defensive motivation and the rejection of promoted behaviors. This suggests that among specific people (i.e., participants with a family cancer history), CRP might not be a significant precursor for screening behaviors. Therefore, although no direct association was observed between risk perceptions and mammography adherence in our study, more complex relationships related to FHH may affect underserved women’s MSA.
Third, although no association was found between women’s beliefs about cancer causation and CRP in the final models predicting CRP and MSA (H6–1 & H6–2), a moderation effect of race was found on the association between women’s behavioral causal attributions and CRP (RQ1). More specifically, African American participants’ behavioral causal beliefs have more negative effects on their CRP compared to other ethnicities. According to self-regulation theory (Cameron & Leventhal, 2003), it can be said that behavioral causal beliefs may play significant roles in women’s risk perceptions as pre-existing cognitive schema. The result is particularly meaningful, because it has been unclear to what extent underserved women endorse modifiable lifestyle behaviors as risk factors or ‘causes’ of breast cancer (Wang et al., 2010). In addition, although there was no significant relationship between causal beliefs and MSA found in this study, given that causal attributions often shape beliefs about self- and response- efficacy (Parrott et al., 2003), as well as subsequent preventive actions (Costanzo et al., 2011), these beliefs should be further investigated.
Lastly, overall, the results of our study suggest the need for further investigating health disparities with regard to minorities’ risk perceptions as well as screening behaviors in the context of cancer communication. In our preliminary analysis, we observed that African American participants had a lower CRP compared to their European American counterparts. These results are consistent with previous findings that suggest 1) risk perceptions vary by race/ethnicity (Rice et al., 2015) as well as 2) individuals from racial and ethnic minority groups are less likely to perceive cancer risks compared to European American counterparts (Kim et al., 2008; Orom, et al., 2010). Specifically, African Americans’ lower risk perception has been a serious concern due to their higher cancer mortality rates as well as lower screening rates compared to their European American counterparts (Siegel et al., 2014; Howlader et al., 2012). The results of this study reveal racial differences in CRP, which might reflect health disparities related to race and socio-economic status. Moreover, as the results of the moderation effect of race (RQ1) suggest, underserved women’s causal beliefs as well as racial difference in CRP would be important in future health interventions. In particular, underserved women’s CRP may also reflect diverse racial as well as economic disparities that affect their health beliefs and behaviors (i.e., beliefs about cancer causation and lifestyles). Therefore, as discussed above, health disparities reflected in racial difference in CRP as well as health beliefs and behaviors among underserved women should be further explored with regard to their mammography adherence in future studies.
Limitations and Future Directions
Although this study provides several important implications for future health interventions towards medically underserved women, it is important to acknowledge its limitations. Since the participants were underserved women living in the Midwest, the results might not be generalizable to other populations of women, and there may be differences by region. Also, we also acknowledge that there is a limitation due to the use of single item measures. Despite the limitations, our study provides several novel findings. More specifically, women’s CRP were positively affected by their race and personal history of cancer, although not their family history of cancer. Family cancer history, as well as beliefs about the importance of FHH, positively predicted MSA. Significant interaction effects on the associations 1) between family cancer history, CRP, and MSA and 2) between race, behavioral causal beliefs, and risk perceptions were found. Although CRP didn’t directly predict women’s MSA, the findings from previous studies as well as the results regarding causal beliefs in this study suggest that CRP should not be ignored in future health interventions. Lastly, these findings suggest that implementing different strategies across racial groups and by cancer history may be necessary to promote regular mammography screening. More specifically, given health disparities reflected in racial difference in CRP and the moderation effect of behavioral causal beliefs in the present study as well as the low level of accurate understandability of cancer risks among underserved women (LeMasters et al., 2014; Seitz et al, 2016), future studies should focus on these women’s accurate CRP based on their FHH, beliefs about cancer causation, and other relevant risk factors.
Figure 1.
Proposed Relationships between Variables
Table 2.
Zero-order Correlations between Study Variables
| Variable | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Mammography screening adherence | 1 | |||||||||
| 2. Cancer risk perception | .04 | 1 | ||||||||
| 3. Knowledge about FHH | −.06 | .06 | 1 | |||||||
| 4. FHH communication with family | −.06 | .08 | .43*** | 1 | ||||||
| 5. FHH communication with doctors | −.07 | −.01 | .30*** | .45*** | 1 | |||||
| 6. Perceived importance of FHH | .13** | .06 | −.05 | −.02 | .08 | 1 | ||||
| 7. Causal belief: Behavior | .04 | −.01 | .02 | −.02 | .05 | −.08 | 1 | |||
| 8. Causal belief: Gene | .02 | −.06 | .05 | .06 | .07 | .00 | .45*** | 1 | ||
| 9. Causal belief: Stress | .07 | −.06 | −.04 | .04 | .06 | .07 | .31*** | .34*** | 1 | |
| 10. Causal belief: Chance | .00 | −.14** | −.03 | .08 | .07 | .07 | .18*** | .31*** | .40*** | 1 |
Note: FHH = family health history
p < .05
p < .01
p < .001
Acknowledgement
The authors would like to thank the patients who participated in this study, data collection and data entry team, Center for Outpatient Health Primary Care Clinic staff, administrators, and residents for their contributions to our work.
Funding
Financial support for the Survey of Center for Outpatient Health Patients and the project team was provided by the Barnes-Jewish Hospital Foundation, Siteman Cancer Center (Grant#: P30CA91842), Washington University School of Medicine (WUSM), and WUSM Faculty Diversity Scholars Program.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Ethical Approval Statement
This study was approved by Washington University IRB (approval no.201212029).
References
- Acheson LS, Wang C, Zyzanski SJ, Lynn A, Ruffin IV MT, Gramling R, … & Nease DE Jr (2010). Family history and perceptions about risk and prevention for chronic diseases in primary care: a report from the Family Healthware™ Impact Trial. Genetics in Medicine, 12, 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandraki I, & Mooradian AD (2010). Barriers related to mammography use for breast cancer screening among minority women. Journal of the National Medical Association, 102, 206–218. [DOI] [PubMed] [Google Scholar]
- American Cancer Society (2016) What are the key statistics about breast cancer? Available from: http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics
- American Cancer Society (2014) Mammography statistics. Available from: http://www.cancer.org/acs/groups/content/documents/image/acspc-043955.pdf
- Anderson EE, Tejeda S, Childers K, Stolley MR, Warnecke RB, & Hoskins KF (2015). Breast cancer risk assessment among low-income women of color in primary care: A pilot study. Journal of Oncology Practice, 11, e460–e467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida S, Koehly LM, Roberts JS, Chen CA, Hiraki S, & Green RC (2009). Disclosing the disclosure: Factors associated with communicating the results of genetic susceptibility testing for Alzheimer’s Disease. Journal of Health Communication, 14, 768–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MH (1974). The health belief model and sick role behavior. Health Education Monographs, 2, 409–419. [Google Scholar]
- Bennett KJ, Pumkam C, Bellinger JD, & Probst JC (2011). Cancer screening delivery in persistent poverty rural counties. Journal of Primary Care & Community Health, 2, 240–249. [DOI] [PubMed] [Google Scholar]
- Calvocoressi L, Kasl SV, Lee CH, Stolar M, Claus EB, & Jones BA (2004). A prospective study of perceived susceptibility to breast cancer and nonadherence to mammography screening guidelines in African American and White women ages 40 to 79 years. Cancer Epidemiology and Prevention Biomarkers, 13, 2096–2105. [PubMed] [Google Scholar]
- Calvocoressi L, Sun A, Kasl SV, Claus EB, & Jones BA (2008). Mammography screening of women in their 40s: impact of changes in screening guidelines. Cancer: Interdisciplinary International Journal of the American Cancer Society, 112, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron L, & Leventhal H (2003). The self-regulation of health and illness behaviour. London: Routledge. [Google Scholar]
- Collins TW, Grineski SE, Chakraborty J, & McDonald YJ (2011). Understanding environmental health inequalities through comparative intracategorical analysis: Racial/ethnic disparities in cancer risks from air toxics in El Paso County, Texas. Health & Place, 17, 335–344. [DOI] [PubMed] [Google Scholar]
- Costanzo ES, Lutgendorf SK, Bradley SL, Rose SL, & Anderson B (2005). Cancer attributions, distress, and health practices among gynecologic cancer survivors. Psychosomatic Medicine, 67, 972–980. [DOI] [PubMed] [Google Scholar]
- Costanzo ES, Lutgendorf SK, & Roeder SL (2011). Common-sense beliefs about cancer and health practices among women completing treatment for breast cancer. Psycho-oncology, 20, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumalaon-Canaria JA, Hutchinson AD, Prichard I, & Wilson C (2014). What causes breast cancer? A systematic review of causal attributions among breast cancer survivors and how these compare to expert-endorsed risk factors. Cancer Causes & Control, 25, 771–785. [DOI] [PubMed] [Google Scholar]
- Canary HE, Elrick A, Pokharel M, Clayton M, Champine M, Sukovic M, … & Kaphingst KA (2019). Family health history tools as communication resources: Perspectives from Caucasian, Hispanic, and Pacific Islander families. Journal of Family Communication, 19, 126–143. [Google Scholar]
- Grenen EG, Ferrer RA, Klein WM, & Han PK (2016). General and specific cancer risk perceptions: how are they related?. Journal of Risk Research, 19, 602–613. [Google Scholar]
- Heeringa SG, & Connor JH (1995). Technical description of the Health and Retirement Survey sample design. Ann Arbor: University of Michigan. [Google Scholar]
- Hesse BW, Nelson DE, Kreps GL, Croyle RT, Arora NK, Rimer BK, & Viswanath K (2005). Trust and sources of health information: the impact of the Internet and its implications for health care providers: findings from the first Health Information National Trends Survey. Archives of Internal Medicine, 165, 2618–2624. [DOI] [PubMed] [Google Scholar]
- Hong SJ (2018). Gendered cultural identities: The influences of family and privacy boundaries, subjective norms, and stigma beliefs on family health history communication. Health Communication, 33, 927–938. [DOI] [PubMed] [Google Scholar]
- Hong SJ (2019). Cross-cultural differences in the influences of spiritual and religious tendencies on beliefs in genetic determinism and family health history communication: A teleological approach. Journal of Religion and Health, 58, 1516–1536. [DOI] [PubMed] [Google Scholar]
- Hovick SR, Yamasaki JS, Burton-Chase AM, & Peterson SK (2015). Patterns of family health history communication among older African American adults. Journal of Health Communication, 20, 80–87. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone A, Krapcho M, Neyman N, Aminou R,… Cronin KA (2012). SEER cancer statistics review, 1975–2009 (vintage 2009 populations). National Cancer Institute; Bethesda, MD: Available from http://seer.cancer.gov/csr/1975_2009_pops09/ [Google Scholar]
- Isaacs C, Peshkin BN, Schwartz M, DeMarco TA, Main D, & Lerman C (2002). Breast and ovarian cancer screening practices in healthy women with a strong family history of breast or ovarian cancer. Breast Cancer Research and Treatment, 71, 103–112. [DOI] [PubMed] [Google Scholar]
- Kaphingst KA, Goodman M, Pandya C, Garg P, Stafford J, & Lachance C (2012). Factors affecting frequency of communication about family health history with family members and doctors in a medically underserved population. Patient Education and Counseling, 88, 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphingst KA, Lachance CR, Gepp A, D’Anna LH, & Rios-Ellis B (2011). Educating underserved Latino communities about family health history using lay health advisors. Public Health Genomics, 14, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katapodi MC, Lee KA, Facione NC, & Dodd MJ (2004). Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Preventive Medicine, 38, 388–402. [DOI] [PubMed] [Google Scholar]
- Katapodi MC, Dodd MJ, Lee KA, & Facione NC (2009). Underestimation of breast cancer risk: influence on screening behavior. Oncology Nursing Forum, 36, 306–314. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Ferketich AK, Sturm AC, Porter K, Sweet K, Kemp K, … & Westman JA (2009). Cancer risk and risk communication in urban, lower-income neighborhoods. Preventive Medicine, 48, 392–396. [DOI] [PubMed] [Google Scholar]
- Khoshravesh S, Taymoori P, & Roshani D (2016). Evaluation of the relationship between family history of breast cancer and risk perception and impacts on repetition of mammography. Asian Pacific Journal of Cancer Prevention, 17, 135–141. [DOI] [PubMed] [Google Scholar]
- Kim SE, Pérez-Stable EJ, Wong S, Gregorich S, Sawaya GF, Walsh JM, & Kaplan CP (2008). Association between cancer risk perception and screening behavior among diverse women. Archives of Internal Medicine, 168, 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalkowski MA, Hart SL, Du XL, Baraniuk S, & Latini DM (2012). Cancer perceptions: Implications from the 2007 Health Information National Trends Survey. Journal of Cancer Survivorship, 6, 287–295. [DOI] [PubMed] [Google Scholar]
- Laing SS, & Makambi K (2008). Predicting regular breast cancer screening in African-American women with a family history of breast cancer. Journal of the National Medical Association, 100, 1309–1317. [DOI] [PubMed] [Google Scholar]
- LeMasters T, Madhavan S, Atkins E, Vyas A, Remick S, & Vona-Davis L (2014). “Don’t know” and accuracy of breast cancer risk perceptions among Appalachian women attending a mobile mammography program: implications for educational interventions and patient empowerment. Journal of Cancer Education, 29, 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau TM, & Weinman J (2006). Self-regulation and the behavioural response to DNA risk information: a theoretical analysis and framework for future research. Social Science & Medicine, 62, 1360–1368. [DOI] [PubMed] [Google Scholar]
- Martin W, & Degner L (2006). Perception of risk and surveillance practices of women with a family history of breast cancer. Cancer Nursing, 29, 227–235. [DOI] [PubMed] [Google Scholar]
- Mays D, Sharff ME, DeMarco TA, Williams B, Beck B, Sheppard VB, … & Tercyak KP (2012). Outcomes of a systems-level intervention offering breast cancer risk assessments to low-income underserved women. Familial Cancer, 11, 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride CM, Alford SH, Reid RJ, Larson EB, Baxevanis AD, & Brody LC (2009). Characteristics of users of online personalized genomic risk assessments: implications for physician-patient interactions. Genetics in Medicine, 11, 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul KD, Branstetter AD, Schroeder DM, & Glasgow RE (1996). What is the relationship between breast cancer risk and mammography screening? A meta-analytic review. Health Psychology, 15, 423–429. [DOI] [PubMed] [Google Scholar]
- Nelson D, Kreps G, Hesse B, Croyle R, Willis G, Arora N, … & Alden S (2004). The health information national trends survey (HINTS): development, design, and dissemination. Journal of Health Communication, 9, 443–460. [DOI] [PubMed] [Google Scholar]
- Orom H, Kiviniemi MT, Underwood W, Ross L, & Shavers VL (2010). Perceived cancer risk: why is it lower among nonwhites than whites?. Cancer Epidemiology and Prevention Biomarkers,19, OF1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott RL, Silk KJ, & Condit C (2003). Diversity in lay perceptions of the sources of human traits: genes, environments, and personal behaviors. Social Science & Medicine, 56, 1099–1109. [DOI] [PubMed] [Google Scholar]
- Price MA, Butow PN, Charles M, Bullen T, Meiser B, McKinley JM, … & Phillips KA (2010). Predictors of breast cancer screening behavior in women with a strong family history of the disease. Breast Cancer Research and Treatment, 124, 509–519. [DOI] [PubMed] [Google Scholar]
- Rabin C, & Pinto B (2006). Cancer-related beliefs and health behavior change among breast cancer survivors and their first-degree relatives. Psycho-Oncology, 15, 701–712. [DOI] [PubMed] [Google Scholar]
- Rice LJ, Brandt HM, Hardin JW, Ingram LA, & Wilson SM (2015). Exploring perceptions of cancer risk, neighborhood environmental risks, and health behaviors of Blacks. Journal of community health, 40, 419–430. [DOI] [PubMed] [Google Scholar]
- Rodríguez VM, Gyure ME, Corona R, Bodurtha JN, Bowen DJ, & Quillin JM (2015). What women think: Cancer causal attributions in a diverse sample of women. Journal of Psychosocial Oncology, 33, 48–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shostak S, Freese J, Link BG, & Phelan JC (2009). The politics of the gene: Social status and beliefs about genetics for individual outcomes. Social Psychology Quarterly, 72, 77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittker J, Freese J, & Powell B (2000). Nature, nurture, neither, nor: Black-White differences in beliefs about the causes and appropriate treatment of mental illness. Social Forces, 78, 1101–1132. [Google Scholar]
- Schueler KM, Chu PW, & Smith-Bindman R (2008). Factors associated with mammography utilization: a systematic quantitative review of the literature. Journal of Women’s Health, 17, 1477–1498. [DOI] [PubMed] [Google Scholar]
- Seitz HH, Gibson L, Skubisz C, Forquer H, Mello S, Schapira MM, … & Cappella JN (2016). Effects of a risk-based online mammography intervention on accuracy of perceived risk and mammography intentions. Patient Education and Counseling, 99, 1647–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Jiemin M, Zhaohui Z, & Jemal A (2014). Cancer statistics. CA: A Cancer Journal for Clinicians, 64, 9–29. [DOI] [PubMed] [Google Scholar]
- Sjöberg L (2000). Factors in risk perception. Risk Analysis, 20, 1–12. [PubMed] [Google Scholar]
- Slovic P (1987). Perception of risk. Science, 236, 280–285. [DOI] [PubMed] [Google Scholar]
- Slovic P, Finucane ML, Peters E, & MacGregor DG (2004). Risk as analysis and risk as feelings: Some thoughts about affect, reason, risk, and rationality. Risk Analysis: An International Journal, 24(2), 311–322. [DOI] [PubMed] [Google Scholar]
- Slovic P, & Peters E (2006). Risk perception and affect. Current Directions in Psychological Science, 15, 322–325. [Google Scholar]
- Thompson T, Seo J, Griffith J, Baxter M, James A, & Kaphingst KA (2015). The context of collecting family health history: Examining definitions of family and family communication about health among African American women. Journal of Health Communication, 20, 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez R, Yoon PW, Qureshi N, Green RF, & Khoury MJ (2010). Family history in public health practice: a genomic tool for disease prevention and health promotion. Annual Review of Public Health, 31, 69–87. [DOI] [PubMed] [Google Scholar]
- Vernon SW (1999). Risk perception and risk communication for cancer screening behaviors: a review. JNCI Monographs, 1999, 101–119. [DOI] [PubMed] [Google Scholar]
- Walker MJ, Chiarelli AM, Knight JA, Mirea L, Glendon G, & Ritvo P (2013). Perceived risk and adherence to breast cancer screening guidelines among women with a familial history of breast cancer: a review of the literature. The Breast, 22, 395–404. [DOI] [PubMed] [Google Scholar]
- Walter FM, Emery J, Braithwaite D, & Marteau TM (2004). Lay understanding of familial risk of common chronic diseases: a systematic review and synthesis of qualitative research. The Annals of Family Medicine, 2, 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Miller SM, Egleston BL, Hay JL, & Weinberg DS (2010). Beliefs about the causes of breast and colorectal cancer among women in the general population. Cancer Causes & Control, 21, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KP, Sheppard VB, Todem D, Mabiso A, Wulu JT Jr, & Hines RD (2008). Family matters in mammography screening among African-American women age≥ 40. Journal of the National Medical Association, 100, 508–520. [DOI] [PubMed] [Google Scholar]
- Xu WY, & Jung JK (2017). Socioeconomic Differences in Use of Low-Value Cancer Screenings and Distributional Effects in Medicare. Health Services Research, 52, 1772–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon PW, Scheuner MY, Gwinn M, Khoury MJ, Jorgensen C, & Hariri S (2004). Awareness of family health history as a risk factor for disease – United States, 2004. Morbidity and Mortality Weekly Report, 53, 1044–1047. [PubMed] [Google Scholar]
- Zhang LR, Chiarelli AM, Glendon G, Mirea L, Edwards S, Knight JA, … & Ritvo P (2011). Influence of perceived breast cancer risk on screening behaviors of female relatives from the Ontario site of the Breast Cancer Family Registry. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP), 20, 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]