Abstract
Background and objectives
We have previously published the characteristics of kidney and liver disease in a cohort of 73 individuals with molecularly confirmed autosomal recessive polycystic kidney disease-congenital hepatic fibrosis, based upon cross-sectional data. Here, we present prospective data on the same cohort.
Design, setting, participants, and measurements
Comprehensive biochemical and imaging data on progression of kidney and liver disease in 60 of the 73 patients were prospectively collected at the NIH Clinical Center on multiple visits between 2003 and 2019.
Results and Conclusions
Of the 73 patients, 23 received a renal allograft at an average age of 17.5 years and 10 underwent liver transplantation at an average age of 20.3 years. Patients who presented perinatally and those who had corticomedullary disease required kidney transplantation significantly earlier. The mean eGFR slope in patients with corticomedullary disease was −1.6 ml/min/1.73 m2/y, in comparison to −0.6 ml/min/1.73 m2/y in those with medullary disease. Kidney size remained the same over time and normalized to the upper limit of normal by 20–25 years of age. The extent of renal disease on ultrasound remained largely unchanged; no patient progressed from the “medullary” to the “corticomedullary” group. There was no correlation between eGFR slope and kidney size. The synthetic function of the liver remained largely intact even in patients with advanced portal hypertension. Based on spleen length/height ratio, two thirds of patients had portal hypertension which remained stable in 39% and worsened in 61%. Patients with portal hypertension had lower platelet counts and relatively higher levels of AST, GGT, direct bilirubin and ammonia. The progression rates of kidney and liver disease were independent of each other. Patients with bi-allelic non-truncating PKHD1 variants had similar progression of kidney and liver disease in comparison to those who were compound heterozygous for a non-truncating and a truncating variant.
Keywords: Autosomal recessive polycystic kidney disease, congenital hepatic fibrosis, chronic kidney disease, Caroli’s syndrome, pediatric kidney transplantation, liver transplantation, noncirrhotic portal hypertension, PKHD1
1. Introduction
Autosomal recessive polycystic kidney disease (ARPKD), the most common hepatorenal fibrocystic disease in childhood, has an estimated incidence of 1 in 20,000 live births (1–10). ARPKD is always associated with congenital hepatic fibrosis (CHF)(11, 12). The disease is caused by bi-allelic pathological variants in PKHD1, which encodes fibrocystin, a membrane protein localized to non-motile cilia and to other parts of the cell(13, 14). Kidney histopathology involves non-obstructive fusiform dilations of the renal collecting ducts. Liver histopathology shows remnants of embryonic forms of bile ducts due to defective remodeling (ductal plate malformation), abnormal branching of the intrahepatic portal vein tree, and portal tract fibrosis that worsens as children grow older(15, 16).
Both kidney and liver disease in ARPKD are progressive in nature with variable degrees of severity; end stage kidney disease and severe portal hypertension are the two major morbidities (2, 3, 6–8, 10). Patients with more severe kidney disease present perinatally with kidney-related manifestations(6) and individuals with milder kidney disease present later in childhood or adulthood, most commonly with liver-related complications (5, 7).
Although current treatments for the kidney and liver disease in ARPKD are symptomatic(9), research trials are underway to evaluate the safety and efficacy of novel, targeted therapies to prevent or attenuate disease progression (17). Accurate understanding of the natural progression of disease enables identification of outcome parameters and informs the design of treatment trials. Currently, the design of disease-changing treatment trials for ARPKD is hampered by the lack of quantitative endpoint measures of therapeutic efficacy. The decline rates of eGFR(18) and platelet count as a marker of portal hypertension(7) may potentially be used as outcome measures in therapeutic trials, but these decline rates have not been prospectively analyzed in a large molecularly confirmed ARPKD cohort. In ADPKD, higher rates of kidney enlargement are associated with a more rapid decrease in renal function(19) and, therefore, kidney volume has been used as a surrogate end point to evaluate response to treatment(20). Imaging data on a small number of patients with ARPKD suggested that kidney size in children with ARPKD does not increase over time (21), however, prospective changes in kidney size have not been previously studied in a large cohort.. Since 2003, we have prospective evaluated a cohort of 73 molecularly confirmed ARPKD patients (4). We have previously published the characteristics of kidney(6) and liver(7) disease in these patients, based on the cross-sectional data collected at their initial NIH evaluations. In this paper, we report prospectively collected biochemical and imaging data on the progression of kidney and liver disease in 60 of these 73 patients in relation to the PKHD1 variants involved.
2. Methods
Detailed methods are provided in supplemental materials.
2.1. Patients
Briefly, all patients were enrolled in the intramural NIH research protocol, “Clinical Investigations into the Kidney and Liver Disease in Autosomal Recessive Polycystic Kidney Disease/Congenital Hepatic Fibrosis and other Ciliopathies” (www.clinicaltrials.gov, trial NCT00068224), approved by the National Human Genome Research Institute (NHGRI) Institutional Review Board. Between 2003 and 2019, we evaluated 90 patients referred with a clinical diagnosis of ARPKD and CHF. Evaluations at NIH included comprehensive biochemical testing, ultrasonography (USG), magnetic resonance imaging (MRI), and PKHD1 gene sequencing. Of the 90 patients, 78 fulfilled the established clinical diagnostic criteria for ARPKD(1). In 73 of these, we confirmed the diagnosis molecularly by finding at least one pathogenic variant in PKHD1 (21); their renal (6) and hepatic (7) disease has been published based on cross sectional data. Two of the 73 patients died after the initial NIH visit. Of the 71 remaining, 60 were prospectively followed for 2–7 visits between 2003 and 2019. In addition, all 73 patients were contacted recently to update information on disease progression (transplantation, infections, variceal bleeding and portosystemic shunt surgery). One patient with Gilbert syndrome was excluded from total and direct bilirubin analysis. One patient who received combined liver-kidney transplantation before the first NIH visit was included only in age at transplantation calculations. Six of the 60 patients with multiple NIH visits were excluded from portal hypertension-related analysis (Table 4) because 2 had surgical portosystemic shunt placed, 3 received liver transplantation after the first NIH visit and 1 had hereditary spherocytosis.
Table 4.
Clinical characteristics of 54 prospectively followed ARPKD patients with respect to portal hypertension.
| No Portal Hypertension | Portal Hypertension | p value | Stable Portal Hypertension | Progressive Portal Hypertension | P value | |
|---|---|---|---|---|---|---|
| Number of patients (%) | 18 of 54 (40%) | 36 of 54 (60) | - | 14 of 36 (39) | 22 of 36 (61) | - |
| Age* | 9.0 ± 5.8 | 11.2 ±11.5 | - | 15.4 ± 15.5 | 8.6 ± 7.4 | - |
| Number of Patients with Truncating£ PKHD1 Variants (%) | 6 of 18 (33) | 14 of 36 (39) | 0.690 | 3 of 14 (21) | 11 of 22 (50) | 0.087 |
| ALT | 23 ± 8 | 29 ± 18 | 0.193 | 22 ± 4 | 34 ± 22 | 0.081 |
| AST | 33 ± 11 | 41 ± 18 | 0.088 | 32 ±11 | 48 ± 19 | 0.013 |
| GGT | 15 ± 6 | 26 ± 20 | 0.030 | 23 ± 17 | 29 ± 22 | 0.467 |
| PT | 12.97 ± 0.58 | 13.53 ± 1.22 | 0.075 | 13.58 ± 1.24 | 13.50 ± 1.06 | 0.858 |
| Direct bilirubin | 0.11 ± 0.03 | 0.16 ± 0.08 | 0.048 | 0.13 ± 0.07 | 0.18 ± 0.09 | 0.197 |
| Albumin | 4.14 ± 0.31 | 3.94 ± 0.40 | 0.086 | 4.01 ± 0.45 | 3.88 ± 0.36 | 0.375 |
| Ammonia | 26 ± 9 | 50 ± 27 | 0.014 | 35 ± 15 | 62 ± 30 | 0.016 |
| Platelet count | 271 ± 61 | 171 ± 84 | <0.0001 | 179 ± 74 | 167 ± 91 | 0.684 |
| APRI | 0.30 ± 0.12 | 0.83 ± 0.84 | 0.018 | 0.53 ± 0.29 | 1.04 ± 1.05 | 0.115 |
| Increased liver echogenicity** | 9 of 18 (50) | 28 of 36 (78) | 0.051 | 11 of 14 (79) | 17 of 22 (77) | 0.943 |
| Number of Patients with Liver cysts (%) | 3 of 18 (17) | 19 of 36 (53) | 0.038 | 4 of 14 (29) | 15 of 22 (68) | 0.065 |
| eGFR slope | −0.91 + 4.05 | −0.43 + 4.4 | 0.743 | −0.15 + 4.4 | −0.63 + 4.6 | 0.809 |
Six of the 60 patients with multiple NIH visits were excluded from portal hypertension-related analysis because 2 had surgical portosystemic shunt placed, 3 received liver transplantation after the first NIH visit and 1 had hereditary spherocytosis.
Age at first NIH evaluation.
Moderate to severe liver echogenicity on ultrasound, ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; PT, prothrombin time; APRI, AST to Platelet Ratio Index; eGFR, estimated glomerular filtration rate. Laboratory values are from the first NIH visits.
Based on the severity of the pathogenic variants in PKHD1, patients were classified into “truncating” (one protein truncating only or one truncating in combination with a missense variants) and “non-truncating” (one or two missense variants) groups.
Patients were classified as having “hypertension” based on review of medical records and use of antihypertensive medications.
2.2. Biochemical methods
Estimated glomerular filtration rate (eGFR) was calculated using pediatric [eGFR =39.8× (height (meters)/Scr)0.456(1.8/cystatin C)0.418(30/BUN)0.0791.076men(height (meters)/1.4)0.179])(22) and adult [eGFR =175× (Scr)−1.154× (age)−0.203×(0.742 if a woman) ×(1.212 if black)] (23) formulas. Serum osmolality and simultaneous urine osmolality were collected while patients had ad lib access to fluids and were presumed to be euvolemic and urine osmolality/serum osmolality ratio was calculated.
2.3. Imaging methods
All USG, MRI and MRCP images were interpreted by a single radiologist (IBT). Kidney, liver and spleen volumes were calculated from MRI images using stereological method (19) and adjusted to patient body surface area [(organ volume (cm3) x 1.73 m2/body surface area (m2)]. Kidney length measured by USG was adjusted to patient’s height [(kidney length (cm) x 1.65 m/height (m)]. When searching for correlations with eGFR slope, kidney length and kidney volume from the first NIH visit were used. Spleen length (mm) was determined by USG. Liver echogenicity was judged to be normal or mildly, moderately or severely increased based on the score card presented (Supplemental Figure 1).
2.4. Classification of patients
Based on the severity of the pathogenic variants in PKHD1(24) patients were classified into “truncating” (one protein truncating or one truncating in combination with a missense) and “non-truncating” (one or bi-allelic missense) groups. Based on the age at presentation, patients who were symptomatic prenatally, or within the first month of life were classified as “perinatal” presenters, and patients who first became symptomatic after the first month of life were classified as “non-perinatal” presenters(6). Patients diagnosed by prenatal USG were classified as “non-perinatal” if they remained asymptomatic during the first month of life. Based on the extent of kidney disease on USG at the time of diagnosis, patients were classified into “corticomedullary” and “medullary-only” disease groups(6, 25); 3 patients could not be classified because kidney USG from the time of diagnosis was not available. The classification of “corticomedullary” or “medullary” disease on ultrasonography was based on review of the actual images by a single radiologist (IBT).
Regarding liver disease, patients were divided into “no portal hypertension”, “stable portal hypertension” and “worsening portal hypertension” groups based on comparison of patient’s spleen length (mm) /height (cm) (SL/H) ratio at each visit to the upper limit of normal for SL/H ratio in healthy children of the same age. Patients with SL/H ratio ≤ 95th percentile for normal were classified as having “no portal hypertension”. For controls for SL/H ratio, we combined normative data on 819 children from two references(26, 27). Patients with SL/H ratios higher than the upper limit of normal defined in these two references were classified as having portal hypertension based on splenomegaly.
2.5. Statistical methods
Data are presented as means ± SD. Mean differences between groups were tested with the two-tailed, two-sample t test. Differences between groups in times to events were investigated by Kaplan–Meier analysis and tested via the log-rank test. One-way ANOVA, Chi-squared tests of independence, Correlation Analysis, and Nonlinear least squares were used. A p value <0.001 (0.05/50) was considered significant after Bonferroni correction for multiple comparisons. Nominal p values are shown.
3. Results
3.1. Patients
The 60 patients prospectively evaluated for 2–7 visits (3.5 ± 1.5 visits) included 34 females and 26 males; ages at the initial visit ranged from 0.8 to 50 years (10.6 ± 10.3 years) and ages at the most recent visit ranged from 3.4 to 54.9 years (17.5 ± 10.7). Duration of prospective follow up (NIH visits) ranged from 1 to 14 years (7 ± 4.2 years). Age at most recent contact for the whole cohort of 73 patients (Table 1) ranged from 6.7 to 67 years (22.2 ± 12.9 years).
Table 1.
History of kidney and liver transplantation and surgical portosystemic shunt placement in 73 ARPKD patients evaluated at the NIH Clinical Center€.
| All cohort | Truncating PKHD1 Variants£ | Non-Truncating PKHD1 Variants£ | P value | Perinatal Presentation | Non-Perinatal Presentation | P value | Corticomedullary Disease | Medullary Disease | P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total patients* | 73 (100) | 28 of 73 (38) | 45 of 73 (62) | - | 31 of 73 (42) | 42 of 73 (58) | - | 47 of 70¥ (67) | 23 of 70 (33) | - |
| Age** | 22.2 ± 12.9 | 25.3 ± 15.2 | 20.3 ± 10.9 | - | 18.0 ± 7.5 | 25.3 ± 15.0 | - | 20.2 ± 10.9 | 22.9 ± 12.9 | - |
| Kidney transplantation | 23 of 73 (32) | 8 of 28 (29) | 15 of 45 (33) | 0.72 | 13 of 31 (42) | 10 of 42 (24) | 0.18 | 19 of 47 (40) | 1 of 23 (4) | 0.01 |
| Age at kidney transplantation¥ | 17.5 ± 13.5 | 13.7 ± 12.5 | 19.6 ± 14.0 | 0.37 | 10.1 ± 7.1 | 27.2 ± 13.9 | 0.0005 | 13.2 ± 8.8 | 27.0 ± 0.0 | 0.002 |
| Liver transplantation | 10 of 73 (14) | 4 of 28 (14) | 6 of 45 (13) | - | 6 of 31 (19) | 4 of 42 (10) | - | 6 of 47 (13) | 3 of 23 (13) | - |
| Age at liver transplantation | 20.3 ± 15.9 | 24.3 ± 6.6 | 17.8 ± 20.2 | - | 15.6 ± 7.4 | 27.5 ± 23.5 | - | 15.6 ± 7.4 | 17.0 ± 3.0 | - |
| Surgical Portosystemic shunt | 7 of 73 (10) | 2 of 28 (7) | 5 of 45 (11) | - | 2 of 31 (7) | 5 of 42 (12) | - | 6 of 47 (13) | 1 of 23 (4) | - |
| Age at surgical portosystemic shunt | 11.7 ± 9.6 | 21.0 ± 15.6 | 8.0 ± 4.1 | - | 4.5 ± 3.5 | 14.6 ± 9.9 | - | 11.5 ± 10.5 | 13.0 ± 0.0 | - |
This table presents data on all patients evaluated under this study (13 with single and 60 with multiple visits).
Results are reported as number (percentage), and mean ± standard deviation.
Age at the time of most recent data collection. Ages reported in years.
Three patients who could not be classified into corticomedullary or medullary groups (because their ultrasound results from the time of diagnosis were not available) received kidney transplantation at ages 32, 33 and 59 years.
Based on the severity of the pathogenic variants in PKHD1, patients were classified into “truncating” (one protein truncating only or one truncating in combination with a missense variants) and “non-truncating” (one or two missense variants) groups.
3.2. Overall progression of disease
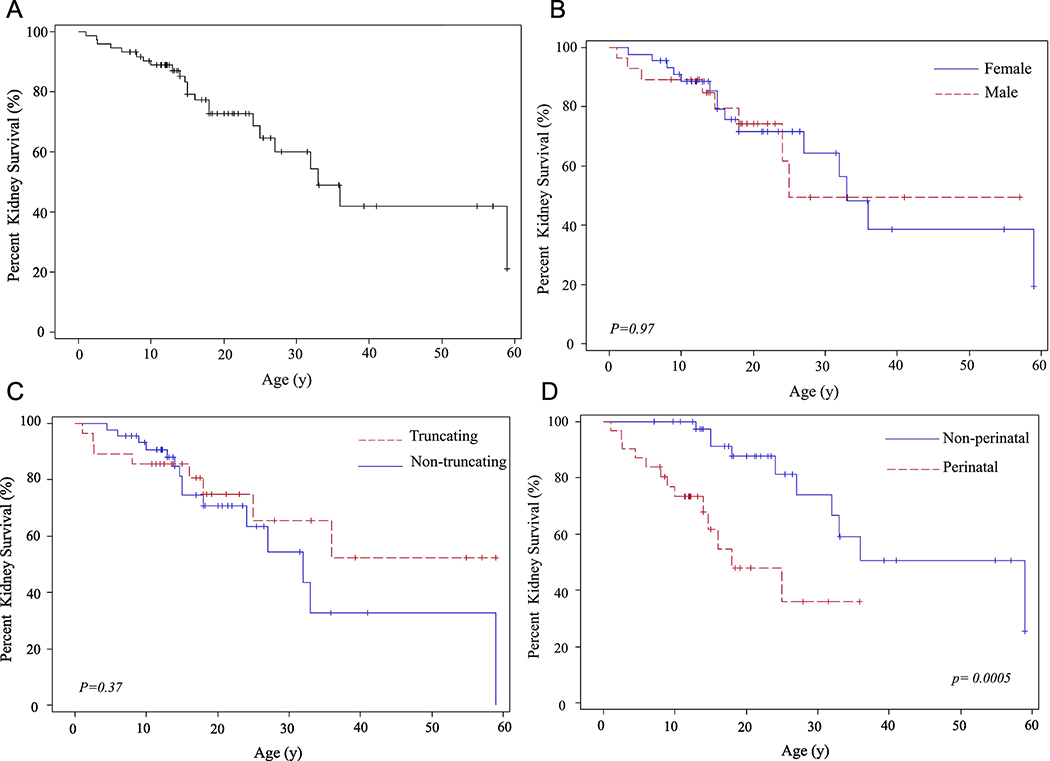
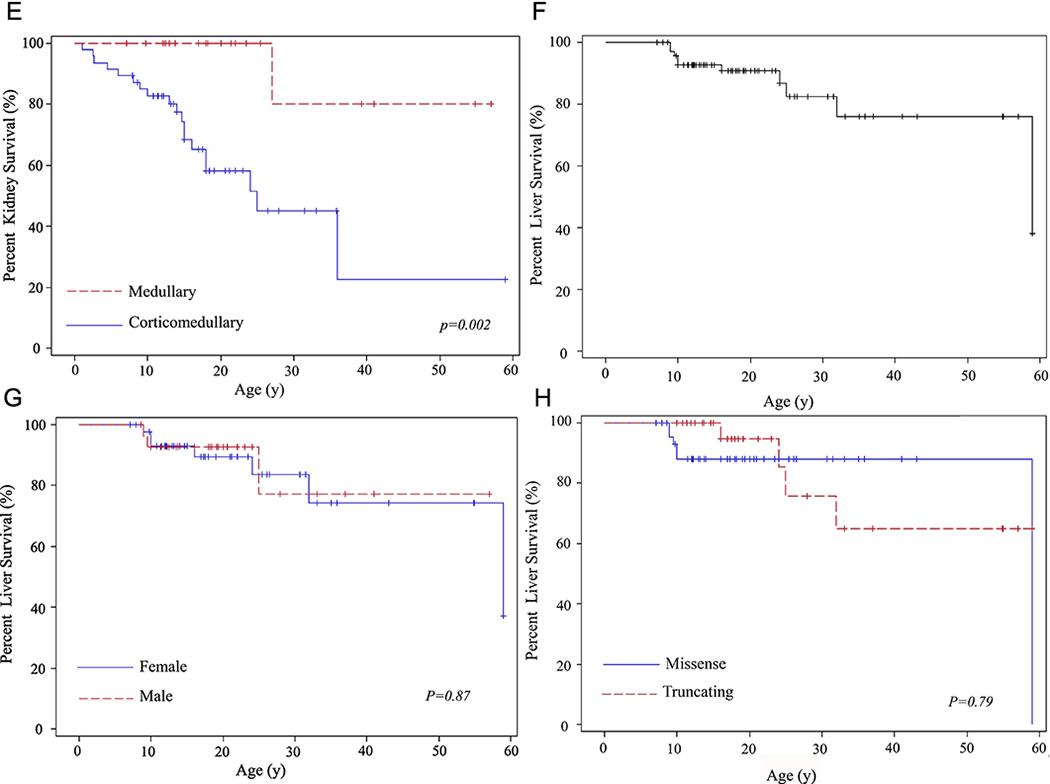
Characteristics of the 73 ARPKD patients are summarized in Table 1. Of the 73 individuals, 23 received kidney and 10 received liver transplants. (Table 1, Figure 1A-H). Age at kidney transplantation ranged from 1.1 to 59 years (17.5 + 13.5 years) (Table 1, Figure 1 A-E). Patients who presented perinatally (“perinatal”) required kidney transplantation significantly earlier (10.1 ± 7.1 years) than those who presented after the first month of life (“non-perinatal”) (27.2 ±13.9 years) (p= 0.0005) (Table 1, Figure 1D). Similarly, patients with “corticomedullary” kidney disease required kidney transplantation at significantly younger ages in comparison to those with medullary-only disease (p=0.002) (Table 1, Figure 1E). Of 47 patients with corticomedullary disease at diagnosis, 19 received kidney transplantation at ages ranging from 1.1 to 36 years (13.2 ± 8.8 years)(Table 1). Of the 23 patients with medullary-only disease, one patient received kidney transplantation at age 27 years (Table 1). Mean ages at kidney transplantation of males and females were not significantly different (Figure 1B) and patients with “truncating” and “non-truncating” variants received renal allografts at similar ages (Table 1, Figure 1C).
Figure 1. Kidney and liver survival rates based on age at organ transplantation.
A) Percent kidney survival of the entire cohort of 73 patients. B) Kidney survival rates in males and females. C) Kidney survival rates in patients with “truncating” and “non-truncating” PKHD1 variants D) Kidney survival rates in patients who became symptomatic perinatally in comparison to those who presented after 1 month of age. E) Kidney survival rates in patients with “corticomedullary” disease compared to those with “medullary” disease. F) Liver survival of the entire cohort of 73 patients. G) Liver survival rates in males and females. H) Liver survival rates in patients with missense (“non-truncating”) and “truncating” PKHD1 variants.
Age at liver transplantation ranged from 9 to 59 years (20.3 ± 15.9 years); no differences were found between males and females or those with truncating and non-truncating variants (Table 1, Figure 1F-H). Four of the 10 liver transplants were combined liver-kidney transplants with the indication of end stage kidney disease associated with moderate-severe portal hypertension including one patient with failed distal spleno-renal shunt.. One patient had an initial kidney transplantation and received combined liver-kidney as the second transplant. Among the remaining 6 patients who required a liver transplantation, severe portal hypertension was the indication in 5 and recurrent cholangitis in one patient. Three of the 10 liver transplant recipients had a renal allograft 4, 8 and 14 years prior to the liver transplant. Seven patients had surgical portosystemic shunt placement at ages ranging from 2 to 32 years (11.7 ± 9.6 years)(Table 1); one of these patients had a shunt placed at age 7 and required liver transplantation at age 9 due to recurrent severe hepatic encephalopathy after shunt placement. One patient had splenectomy. Surgical portosystemic shunt placement improved portal hypertension in the majority of the patients who received this treatment. Of the 7 such patients, one required liver transplantation 2 years later due to recurrent severe hepatic encephalopathy that occurred after shunt placement. The remaining 6 patients, who had portosystemic shunt placed at ages 2, 8, 10, 11, 11, and 32 years, responded well and did not require liver transplantation as of ages 13, 17, 11, 14, 37 and 33 years, respectively.
Of the 73 patients, 52 had hypertension; in 20 patients, hypertension was diagnosed at birth, in 16 during the 1st year of life, and in 13 between 2 and 6 years of age. Recurrent infections were common. Cholangitis was diagnosed in 10 individuals; 4 had recurrent episodes. Urinary tract infections were diagnosed in 25 patients. Severe febrile illness of unknown etiology was diagnosed in 8 patients; 4 of these also had episodes of cholangitis and urinary tract infections, one each had cholangitis and urinary infections.
3.3. Progression of kidney disease
3.3.1. Longitudinal changes in kidney imaging
Throughout the study, the renal pathology on USG remained largely unchanged; no patient progressed from “medullary” to “corticomedullary”. Only 7 of the 54 patients (13%) who had at least 2 kidney USG at NIH prior to transplantation showed progression; in six “corticomedullary” patients, the extent of cystic changes in the cortex increased and in one “medullary” patient, imaging findings advanced from partial to complete medullary involvement.
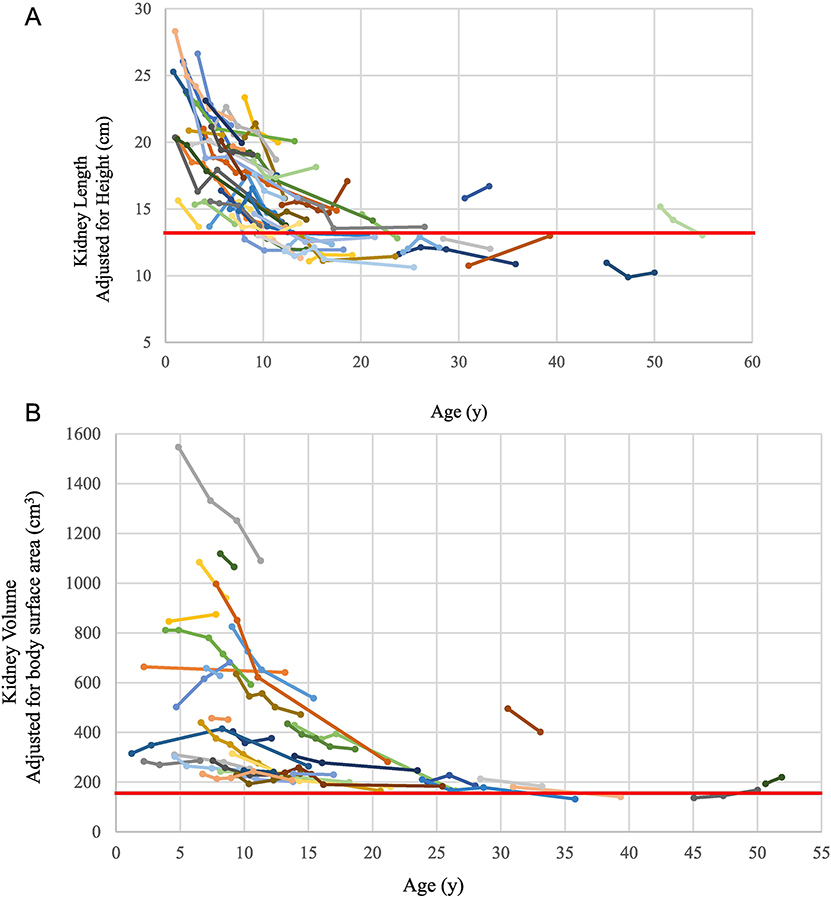
As children grew older, their kidney length and volume remained unchanged (Figure 2 A, B). Therefore, kidney size relative to total body size fell gradually, reaching the upper limit of normal by age 20–25 years.
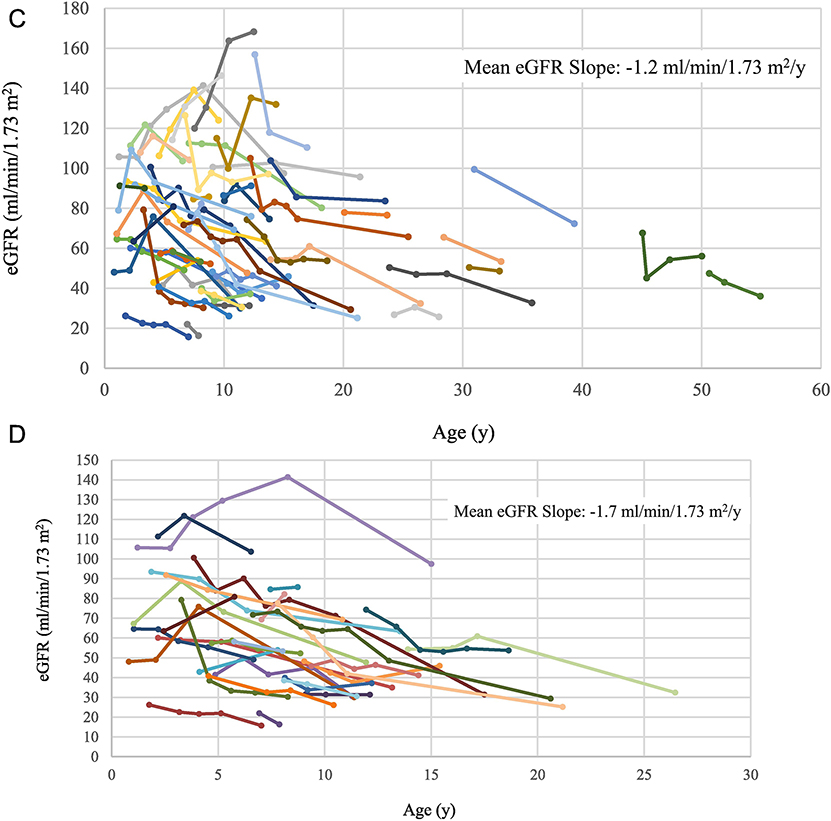
Figure 2. Changes in kidney size and function as ARPKD patients grow older.
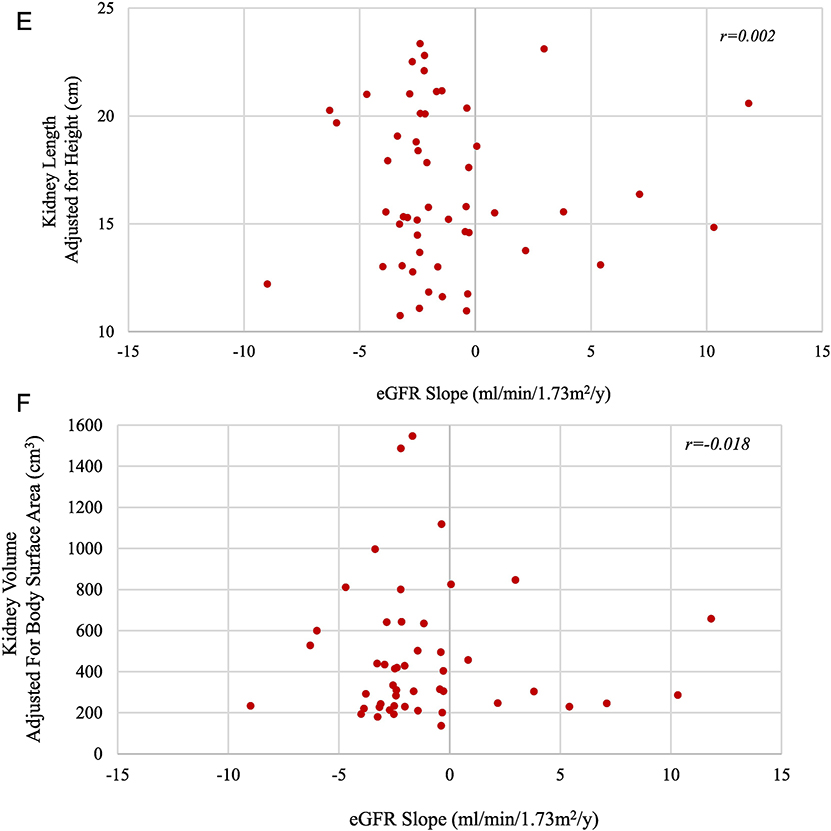
A) Changes in kidney length by age. Each group of dots connected by the same color line represents height-adjusted kidney length measurements of the same patient at multiple NIH visits. The red line represents the upper limit of normal kidney length for an average size adult. B) Changes in kidney volume by age. Each group of dots connected by the same color line represents the body surface area-adjusted kidney volume measurements of the same patient at multiple NIH visits. The red line represents the upper limit of normal kidney volume in an average size adult. C) Changes in eGFR by age of all patients with multiple NIH evaluations. Each group of dots connected by the same color line represents the eGFR values of the same patient calculated at multiple NIH visits. D) Changes in eGFR by age for children with corticomedullary disease. E) Height-adjusted kidney length on USG at first NIH visit plotted against the eGFR slope calculated based on multiple NIH visits. F) Kidney volume adjusted for body surface area based on MRI at first NIH visit plotted against the eGFR slope based on multiple NIH visits.
3.3.2. Longitudinal changes in kidney function
When all patients with multiple NIH evaluations were considered together, the mean eGFR slope was −1.2 ml/min/1.73 m2/y (SD = 3.7, range = − 9.0 to 11.8) (Figure 2C). For “corticomedullary” patients, the slope was more negative (mean= −1.6, SD = 3.0, range – 6.3 to 11.8), but not significantly different from that of patients with only “medullary” involvement (mean= −0.6 ml/min/1.73 m2/y, SD =4.7, range: −9.0 to 10.3) (Table 2). The mean eGFR slope of children (<age 18 at first NIH visit) with corticomedullary disease was −1.7 ml/min/1.73 m2/y (SD =3.3, range: − 6.3 to 11.8) (Figure 2 D).
Table 2.
Characteristics of kidney disease and PKHD1 variants in 60 ARPKD patients prospectively evaluated at the NIH Clinical Center on multiple visits.
| PKHD1 Variant Type | Presentation | Extent of Kidney Disease on USG | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Truncating£ | Non-truncating£ | P Value | Perinatal | Non-perinatal | P Value | Corticomedullary | Medullary | P Value | |
| Number of patients* | 22 of 60 (37) | 38 of 60 (63) | - | 27 of 60 (45) | 33 of 60 (55) | - | 40 of 60 (67) | 20 of 60 (33) | - |
| Age(y)** | 14.5 ±13.8 | 9.1 ± 6.3 | - | 8.9 ± 6.9 | 12.8 ±11.7 | - | 10.1 ± 9.0 | 13.1 ±11.7 | - |
| Patients with truncating PKHD1 mutations | 22 of 22 (100) | 0 of 38 (0) | - | 10 of 27 (37) | 12 of 33 (36) | - | 17 of 40 (43) | 5 of 20 (25) | - |
| Patients with perinatal presentation | 10 of 22 (46) | 17 of 38 (45) | - | 27 of 27 (100) | 0 of 33 (0) | - | 24 of 40 (60) | 3 of 20 (15) | - |
| Patients with corticomedullary involvement | 17 of 22 (77) | 23 of 38 (61) | - | 24 of 27 (89) | 16 of 33 (49) | - | 40 of 40 (100) | 0 of 20 (0) | - |
| Kidney length corrected for height (cm)¥ | 16.3 ± 3.8 | 17.1 ± 3.8 | 0.46 | 18.0 ± 4.1 | 15.9 ± 3.4 | 0.05 | 18.4 ± 3.7 | 14.0 ± 1.7 | < 0.0001 |
| Kidney volume corrected for BSA (ml)¥ | 433 ± 258 | 498 ± 370 | 0.47 | 611 ± 417 | 394 ± 218 | 0.04 | 603 ± 348 | 240 ± 39 | < 0.0001 |
| eGFR slope (ml/mm/1.73m2/y) | −2.2 + 3.9 | −0.6 + 3.4 | 0.14 | −1.6 + 3.7 | −1.0 + 3.7 | 0.60 | −1.6 + 3.1 | −0.6 + 4.7 | 0.46 |
Results are reported as number (percentage), and mean ± standard deviation
Age at the time of first NIH evaluation
Data from first NIH visit
Based on the severity of the pathogenic variants in PKHD1, patients were classified into “truncating” (one protein truncating only or one truncating in combination with a missense variants) and “non-truncating” (one or two missense variants) groups.
There was no correlation between eGFR slope and either kidney length adjusted for height (Figure 2E) or kidney volume adjusted for body surface area (Figure 2F). The results of this analysis remained the same when medullary and corticomedullary patients were evaluated as separate groups.
3.3.3. Correlation of longitudinal changes in kidney function with PKHD1 variants and other parameters
Patients with hypertension exhibited a faster decline in kidney function (mean eGFR slope: − 1.8, SD: 3.4, range: −9.0 to 11.9) compared to those without hypertension (mean: 0.45, SD: 5.0, range: −4.0 to 10.3) but the difference did not reach statistical significance (p=0.09) (data not shown). Similarly, patients with low urine osmolality/serum osmolality ratio (≤ 1), indicating dilute urine, displayed a faster decline in eGFR, but this did not reach statistical significance (p=0.09) (data not shown). There was no significant difference in eGFR slopes between genders, “perinatal” and “non-perinatal” presentation groups, and “truncating” versus “non-truncating” groups (Table 2). Other parameters that showed no correlation with eGFR slope included urine protein, calcium and magnesium excretions, and cholesterol and triglyceride levels (data not shown).
3.4. Progression of liver disease
3.4.1. Longitudinal changes in liver imaging
Characteristics of liver disease are summarized in Table 3. Esophageal varices were diagnosed at ages ranging from 2 to 47 years (16.8 ± 12 years); 17 required banding. Six patients had variceal bleeding at ages ranging from 2 to 51 years (18.7 ± 21 years). Ages of the 14 patients without varices ranged from 4 to 52 years (17.6 ± 11.4 years). At the initial NIH visit, liver echogenicity on USG was normal in 6 of 72 patients (8%), mildly increased in 15 (21%), moderately increased in 37 (51%) and severely increased in 14 patients (19%) (Table 3, Supplemental Figure 1). Based on liver USG, MRI and MRCP, at the initial NIH visit, 29 of 72 patients (40%) had intrahepatic cysts; 18 had numerous tiny cysts in a lacy pattern at the periphery and 11 had larger cysts in addition to the peripheral lacy cysts.
Table 3.
Characteristics of liver disease in 73 ARPKD patients evaluated at the NIH Clinical Center€.
| Number (%) | |
|---|---|
| Portal Hypertension | 51 of 72* (71) |
| Males with portal hypertension | 25 of 51 |
| Females with portal hypertension | 26 of 51 |
| Esophagogastroduodenoscopy performed | 45 of 72 (63) |
| Esophageal Varices | 31 of 45 (69) |
| Variceal Banding | 17 of 31 (55) |
| Variceal Bleeding | 6 of 31 (19) |
| First visit Liver Echogenicity Normal/Mildly Increased | 21 of 72 (29) |
| First Visit Liver Echogenicity Moderately/Severely Increased | 51 of 72 (71) |
| Liver echogenicity progressed during the study | 14 of 72 (19) |
| First visit Intrahepatic Cysts | 29 of 72 (40) |
| First Visit Dilated Common Bile Duct | 38 of 71 (54) |
| Biliary cystic disease progressed during the study | 20 of 57 (35) |
| Cholangitis** | 10 of 73 (14) |
This table presents data on all patients evaluated under this study (13 with single and 60 with multiple visits).
One patient with hereditary spherocytosis was excluded.
Four out of 10 patients had recurrent cholangitis.
3.4.2. Longitudinal changes in liver function and portal hypertension in relationship to liver imaging and liver and spleen size
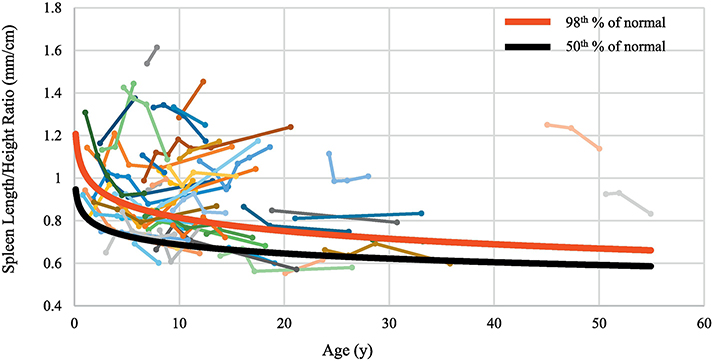
The synthetic function of the liver remained intact in most patients; PT, albumin and direct bilirubin levels remained normal or minimally abnormal (Table 4). Liver enzymes (ALT and AST, and GGT) were either normal or mildly elevated and increased slowly with age (Table 4). To monitor the change in severity of portal hypertension, we compared the slope of the SL/H ratio with age for each patient with the slope for healthy children (Figure 3A). Of the 54 patients who had data from multiple NIH visits, 36 (67%) were classified as having portal hypertension based on SL/H ratio higher than 95th percentile. Among the 36 patients with portal hypertension, 14 (39%) had “stable portal hypertension”(SL/H ratio was high but remained parallel to the normal curve as children grew older) and 22 (61%) had “worsening portal hypertension”(Figure 3A).
Figure 3. Changes in spleen size as patients with ARPKD grew.
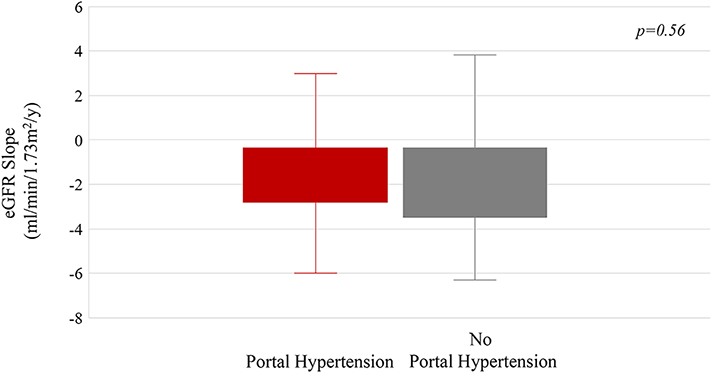
A) Spleen length/height (SL/H) ratio plotted against age. Each group of dots connected by the same color line represents the SL/H ratio of the same patient measured at multiple NIH visits. The red and black lines represent the 98th and 50th percentiles of SL/H ratio in healthy individuals. B) The mean eGFR slopes of patients with and without portal hypertension.
Compared with the 18 patients without portal hypertension, the 36 patients with portal hypertension had nominally increased liver echogenicity and biliary cysts (p=0.05 and 0.03, respectively) (Table 4). GGT, direct bilirubin, ammonia and APRI (aspartate aminotransferase to platelet ratio index) were nominally higher (p = 0.03, 0.05, 0.01, 0.018, respectively) and platelet counts were significantly lower (p< 0.0001) in patients with portal hypertension (Table 4).
Within the group of 36 patients with portal hypertension, the 22 patients with worsening portal hypertension had nominally higher AST (p=0.013) and ammonia (p=0.016) than the 14 with stable portal hypertension (Table 4). The mean difference between the first and last NIH visit AST and ammonia values of patients in the “worsening portal hypertension” group was similar to that of the patients in the “stable portal hypertension” group (data not shown).
3.5. Relationship between longitudinal changes in kidney function and portal hypertension
The rate of decline of kidney function (eGFR slope) was not different among patients with or without portal hypertension or patients with worsening or stable portal hypertension (Table 4, Figure 3B).
4. Discussion
Several targeted therapies for ARPKD and hepatic fibrosis are under investigation(17). Interpretation of the response to such treatments requires accurate and comprehensive understanding of the natural progression of the disease. The main purpose of this study was to define the rate of progression of kidney and liver disease in the context of imaging findings of prospectively followed children and adults with molecularly confirmed ARPKD.
In ADPKD, kidney size correlates negatively with glomerular function and, hence, kidney volume is used as an outcome parameter in treatment trials(19, 20). In ARPKD, previous studies suggested that after the first 3–4 years of life, kidney size in ARPKD remained largely unchanged(21, 28). However, no prospectively collected data have been published on the changes in kidney length and volume of a large cohort of molecularly confirmed ARPKD patients. Our data on kidney length and volume confirmed that kidney size in ARPKD remains unchanged (Figure 2A and B). Retrospective studies had suggested that kidney function in ARPKD did not correlate with kidney size(1, 3), however the relationship between the decline rate of eGFR and kidney size was not prospectively studied to date. We documented that, in contrast to ADPKD, in ARPKD the rate of decline of kidney function, as measured by the slope of eGFR, does not correlate with kidney size (Figure 2E and F), eliminating kidney size as an endpoint measure in therapeutic trials.
On the other hand, the extent of renal USG abnormalities, classified as “corticomedullary” or “medullary”, may help predict the age at kidney transplantation to some extent (Figure 1E, Table 1). Patients with corticomedullary disease require kidney transplantation significantly earlier than those with medullary disease. Hence, the extent of renal USG abnormalities may inform anticipatory guidance for renal transplantation in ARPKD and classification of patients into “corticomedullary” and “medullary” disease groups may be useful in stratifying patients in clinical trials.
We also analyzed the rates of decline in eGFR in the context of renal USG findings, PKHD1 gene variant types and age at presentation (Table 2). When the entire cohort was considered, the mean eGFR slope was −1.2 ml/min/1.73 m2/y, with large variability (range, − 9.0 to 11.8). Variability in the severity of kidney disease is known to occur even among siblings with ARPKD (29). Some of this variability may be due to inaccurate estimation of eGFR; even though we used the best available age/sex/race-specific GFR estimation formulas factoring in multiple parameters including height, serum creatinine, blood urea nitrogen and cystatin-C. Dell et al., identified a comparable annual eGFR decline rate of 1.4 ml/min/1.73 m2 with similar wide variability on 22 children with ARPKD (18). With the caution regarding variability, these mean rates of decline may be useful in counseling families, and determining response to novel therapies. However, given the slow decline rate of eGFR with wide variability, if eGFR slope is used to determine response to therapy in ARPKD clinical trials, a large number of patients have to be followed for many years.
Portal hypertension is the most significant manifestation of liver disease in ARPKD. Our data as well as past publications(2, 12) including the recent report from international ARPKD registry ARegPKD(30), show that approximately half to two thirds of ARPKD patients develop portal hypertension. Progression of portal hypertension in ARPKD was not prospectively studied in a large cohort to date. Our follow up data on ARPKD patients with portal hypertension showed that in a subset of these patients (61%), portal hypertension was progressive with worsening splenomegaly, while 39% had stable portal hypertension. Classification of ARPKD patients into “no splenomegaly”, “stable splenomegaly” and “progressive splenomegaly” groups may be useful in research studies aiming to identify biological markers of liver disease progression. Such stratification of patients may also be useful in design of treatment trials.
Consistent with prior studies(12, 30), liver synthetic function remained largely preserved throughout the study. Elevated ammonia levels correlated somewhat with the presence and the worsening of portal hypertension. As expected, and consistent with our cross sectional data(7), patients with portal hypertension had lower platelet counts and higher APRI; they were also more likely to have increased liver echogenicity and/or liver cysts. These findings may guide clinical monitoring of liver disease in ARPKD patients.
Our data indicated that the progression rates of kidney and liver disease were independent of each other. There were no differences between the eGFR slopes of patients without portal hypertension and those with portal hypertension, consistent with our previously published cross-sectional data(7).
Limitations of this study include wide variation in duration of follow up which was largely due to the fact that the first NIH visits were spread throughout several years as we continued to enroll new patients to the study. All patients were invited back after each visit and travel expenses were covered by the study to minimize bias based on ability to pay for travel. In addition, data points from the first 6 months of life are not included in this study because our inclusion criteria required patients to be older than 6 months. Although a significant number (42%) of patients in our cohort were perinatal onset, the sickest infants who were not well enough to travel may be underrepresented in this cohort. In conclusion, longitudinal data on the kidney and liver disease of ARPKD patients, such as that collected in this prospective study, provide individual and cohort baseline information to evaluate whether therapeutic interventions alter the rates of change of various outcome measures. Kidney size in ARPKD remains unchanged and does not correlate with the decline rate of kidney function and hence it is excluded as an endpoint measure of response to therapeutic trials. The decline rate of eGFR may be used in determining response to treatments but it is of limited value given the very slow rate of decline and wide variability. Similarly, the progression rate of liver disease in ARPKD is highly variable. Therefore, future research is needed for identification of a biological plasma and/or urine marker(s) to measure progression for kidney and liver disease in ARPKD.
Supplementary Material
Supplementary Figure 1. Score card for spectrum of liver echogenicity on USG including normal (A), mildly (B), moderately (C), and severely increased (D) liver echogenicity.
5. Acknowledgments
We thank the ARPKD/CHF Alliance and all patients and their families who generously participated in this investigation. The Intramural Research Programs of the National Human Genome Research Institute and the National Institutes of Health Clinical Center supported this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- 1.Zerres K, Rudnik-Schoneborn S, Deget F, Holtkamp U, Brodehl J, Geisert J, Scharer K: Autosomal recessive polycystic kidney disease in 115 children: clinical presentation, course and influence of gender. Arbeitsgemeinschaft fur Padiatrische, Nephrologie. Acta Paediatr, 85: 437–445, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Roy S, Dillon MJ, Trompeter RS, Barratt TM: Autosomal recessive polycystic kidney disease: long-term outcome of neonatal survivors. Pediatric nephrology, 11: 302–306, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Bergmann C, Senderek J, Windelen E, Kupper F, Middeldorf I, Schneider F, Dornia C, Rudnik-Schoneborn S, Konrad M, Schmitt CP, Seeman T, Neuhaus TJ, Vester U, Kirfel J, Buttner R, Zerres K, Apn: Clinical consequences of PKHD1 mutations in 164 patients with autosomal-recessive polycystic kidney disease (ARPKD). Kidney international, 67: 829–848, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Gunay-Aygun M, Avner ED, Bacallao RL, Choyke PL, Flynn JT, Germino GG, Guay-Woodford L, Harris P, Heller T, Ingelfinger J, Kaskel F, Kleta R, LaRusso NF, Mohan P, Pazour GJ, Shneider BL, Torres VE, Wilson P, Zak C, Zhou J, Gahl WA: Autosomal recessive polycystic kidney disease and congenital hepatic fibrosis: summary statement of a first National Institutes of Health/Office of Rare Diseases conference. The Journal of pediatrics, 149: 159–164, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adeva M, El-Youssef M, Rossetti S, Kamath PS, Kubly V, Consugar MB, Milliner DM, King BF, Torres VE, Harris PC: Clinical and molecular characterization defines a broadened spectrum of autosomal recessive polycystic kidney disease (ARPKD). Medicine (Baltimore), 85: 1–21, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Gunay-Aygun M, Font-Montgomery E, Lukose L, Tuchman M, Graf J, Bryant JC, Kleta R, Garcia A, Edwards H, Piwnica-Worms K, Adams D, Bernardini I, Fischer RE, Krasnewich D, Oden N, Ling A, Quezado Z, Zak C, Daryanani KT, Turkbey B, Choyke P, Guay-Woodford LM, Gahl WA: Correlation of kidney function, volume and imaging findings, and PKHD1 mutations in 73 patients with autosomal recessive polycystic kidney disease. Clinical journal of the American Society of Nephrology : CJASN, 5: 972–984, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunay-Aygun M, Font-Montgomery E, Lukose L, Tuchman Gerstein M, Piwnica-Worms K, Choyke P, Daryanani KT, Turkbey B, Fischer R, Bernardini I, Sincan M, Zhao X, Sandler NG, Roque A, Douek DC, Graf J, Huizing M, Bryant JC, Mohan P, Gahl WA, Heller T: Characteristics of congenital hepatic fibrosis in a large cohort of patients with autosomal recessive polycystic kidney disease. Gastroenterology, 144: 112–121 e112, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweeney WE, Avner ED: Polycystic Kidney Disease, Autosomal Recessive In: GeneReviews((R)). edited by ADAM MP, ARDINGER HH, PAGON RA, WALLACE SE, BEAN LJH., STEPHENS K, AMEMIYA A, Seattle (WA), 1993, [Google Scholar]
- 9.Guay-Woodford LM, Bissler JJ, Braun MC, Bockenhauer D, Cadnapaphornchai MA, Dell KM, Kerecuk L, Liebau MC, Alonso-Peclet MH, Shneider B, Emre S, Heller T, Kamath BM, Murray KF, Moise K, Eichenwald EE, Evans J, Keller RL, Wilkins-Haug L, Bergmann C, Gunay-Aygun M, Hooper SR, Hardy KK, Hartung EA, Streisand R, Perrone R, Moxey-Mims M: Consensus expert recommendations for the diagnosis and management of autosomal recessive polycystic kidney disease: report of an international conference. The Journal of pediatrics, 165: 611–617, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guay-Woodford LM, Desmond RA: Autosomal recessive polycystic kidney disease: the clinical experience in North America. Pediatrics, 111: 1072–1080, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Gunay-Aygun M: Liver and kidney disease in ciliopathies. American journal of medical genetics Part C, Seminars in medical genetics, 151C: 296–306, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinath A, Shneider BL: Congenital hepatic fibrosis and autosomal recessive polycystic kidney disease. Journal of pediatric gastroenterology and nutrition, 54: 580–587, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC: Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Human molecular genetics, 12: 2703–2710, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC: The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet, 30: 259–269, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Desmet VJ: Ludwig symposium on biliary disorders--part I. Pathogenesis of ductal plate abnormalities. Mayo Clin Proc, 73: 80–89, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Gunay-Aygun M, Gahl WA, Heller T: Congenital Hepatic Fibrosis Overview In: GeneReviews(R). edited by PAGON RA, ADAM MP, ARDINGER HH, WALLACE SE, AMEMIYA A, BEAN LJH, BIRD TD, LEDBETTER N, MEFFORD HC, SMITH RJH, STEPHENS K, Seattle (WA), 1993, [PubMed] [Google Scholar]
- 17.Sweeney WE, Jr., Avner ED: Emerging Therapies for Childhood Polycystic Kidney Disease. Front Pediatr, 5: 77, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dell KM, Matheson M, Hartung EA, Warady BA, Furth SL, Chronic Kidney Disease in Children S: Kidney Disease Progression in Autosomal Recessive Polycystic Kidney Disease. J Pediatr, 171: 196–201 e191, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr., Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP, Investigators C: Volume progression in polycystic kidney disease. The New England journal of medicine, 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Grantham JJ, Torres VE: The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nature reviews Nephrology, 12: 667–677, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blickman JG, Bramson RT, Herrin JT: Autosomal recessive polycystic kidney disease: long-term sonographic findings in patients surviving the neonatal period. AJR Am J Roentgenol, 164: 1247–1250, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Munoz A: Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int, 82: 445–453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inker LA, Eckfeldt J, Levey AS, Leiendecker-Foster C, Rynders G, Manzi J, Waheed S, Coresh J: Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis, 58: 682–684, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunay-Aygun M, Tuchman M, Font-Montgomery E, Lukose L, Edwards H, Garcia A, Ausavarat S, Ziegler SG, Piwnica-Worms K, Bryant J, Bernardini I, Fischer R, Huizing M, Guay-Woodford L, Gahl WA: PKHD1 sequence variations in 78 children and adults with autosomal recessive polycystic kidney disease and congenital hepatic fibrosis. Mol Genet Metab, 99: 160–173, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turkbey B, Ocak I, Daryanani K, Font-Montgomery E, Lukose L, Bryant J, Tuchman M, Mohan P, Heller T, Gahl WA, Choyke PL, Gunay-Aygun M: Autosomal recessive polycystic kidney disease and congenital hepatic fibrosis (ARPKD/CHF). Pediatr Radiol, 39: 100–111, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Megremis SD, Vlachonikolis IG, Tsilimigaki AM: Spleen length in childhood with US: normal values based on age, sex, and somatometric parameters. Radiology, 231: 129–134, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Konus OL, Ozdemir A, Akkaya A, Erbas G, Celik H, Isik S: Normal liver, spleen, and kidney dimensions in neonates, infants, and children: evaluation with sonography. AJR Am J Roentgenol, 171: 1693–1698, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Sweeney WE, Avner ED: Polycystic Kidney Disease, Autosomal Recessive In: GeneReviews(R). edited by PAGON RA, ADAM MP, ARDINGER HH, BIRD TD, DOLAN CR, FONG CT, SMITH RJH, STEPHENS K, Seattle (WA), 1993, [Google Scholar]
- 29.Deget F, Rudnik-Schoneborn S, Zerres K: Course of autosomal recessive polycystic kidney disease (ARPKD) in siblings: a clinical comparison of 20 sibships. Clin Genet, 47: 248–253, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Burgmaier K, Kilian S, Bammens B, Benzing T, Billing H, Buscher A, Galiano M, Grundmann F, Klaus G, Mekahli D, Michel-Calemard L, Milosevski-Lomic G, Ranchin B, Sauerstein K, Schaefer S, Shroff R, Sterenborg R, Verbeeck S, Weber LT, Wicher D, Wuhl E, Dotsch J, Schaefer F, Liebau MC: Clinical courses and complications of young adults with Autosomal Recessive Polycystic Kidney Disease (ARPKD). Sci Rep, 9: 7919, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Score card for spectrum of liver echogenicity on USG including normal (A), mildly (B), moderately (C), and severely increased (D) liver echogenicity.