Abstract
This work investigated the effect of poly(L-lysine) (PLL) molecular weight and concentration on chondrogenesis of cocultures of mesenchymal stem cells (MSCs) and articular chondrocytes (ACs) in PLL-loaded hydrogels. An injectable dual-network hydrogel composed of a poly(N-isopropylacrylamide)-based synthetic thermogelling macromer and a chondroitin sulfate-based biological network was leveraged as a model to deliver PLL and encapsulate the two cell populations. Incorporation of PLL into the hydrogel did not affect the hydrogel’s swelling properties and degradation characteristics, nor the viability of encapsulated cells. Coculture groups demonstrated higher type II collagen expression compared to the MSC monoculture group. Expression of hypertrophic phenotype was also limited in the coculture groups. Histological analysis indicated that the ratio of MSCs to ACs was an accurate predictor of the degree of long-term chondrogenesis, while the presence of PLL was shown to have a more substantial short-term effect. Altogether, this study demonstrates that coculturing MSCs with ACs can greatly enhance the chondrogenicity of the overall cell population and offers a platform to further elucidate the short- and long-term effect of polycationic factors on the chondrogenesis of MSC and AC cocultures.
Keywords: poly(L-lysine), mesenchymal stem cell, chondrocyte, coculture, chondrogenesis, hydrogel, hypertrophy, cartilage tissue engineering
1. Introduction
Despite decades of biomedical progress, treatment of articular cartilage defects remains as a largely unmet clinical need due to its complex tissue architecture and lack of nutrient transportation [1,2]. Although many surgical methods have reported varying degrees of success in regenerating the defects, most have the tendency to result in the formation of fibrous cartilage which is mechanically inferior to the native hyaline cartilage [3,4]. In search of alternative treatment options, the field of tissue engineering has been focusing on developing approaches that combine various types of materials, cells, and biomolecules.
One of the most widely sourced cell types for developing cell-laden tissue engineered constructs is mesenchymal stem cells (MSCs). These cells can differentiate in vitro into, among some other cell types, chondrocytes and osteoblasts, making MSCs an attractive candidate for cartilage tissue engineering [5,6]. However, MSCs tend to experience hypertrophy after being induced to undergo chondrogenesis, leading to the degradation of cartilaginous extracellular matrix (ECM), secretion of minerals, and eventual calcification of the construct [7]. Cartilage tissue engineering has thus focused on developing constructs that can guide the differentiation of MSCs into a chondrogenic lineage and, once differentiated, maintain their chondrogenicity for the duration of their culture.
One such approach involves providing MSCs with the environmental and biochemical cues that are present during the skeletal developmental of limbs in utero, otherwise known as endochondral ossification [8]. This process is preceded by mesenchymal condensation, during which the MSCs form clusters and undergo a series of developmental processes that ultimately lead to the formation of long bones. Various studies have shown that recreating the physiological conditions observed during mesenchymal condensation in vitro could lead to enhanced chondrogenesis from the MSCs [9,10]. One class of molecules that has been explored for its potential in stimulating mesenchymal condensation is polycations. In particular, poly(L-lysine) (PLL), a polypeptide with cationic lysine groups as its functional group, has been shown to enhance chondrogenesis in a dose- and molecular weight (MW)-dependent manner from MSC micromass culture in vitro [11–13]. Interestingly, the addition of PLL into the culture medium resulted in an increased expression of N-cadherin, which is a class of cell adhesion molecules that is highly expressed by MSCs in the perichondrium during mesenchymal condensation. Our group has also investigated the effect of directly incorporating PLL into a hydrogel on the chondrogenesis of encapsulated MSCs [14]. Similar to what has been observed in micromass cultures, we observed an early increase in type II collagen (COL2A1) and aggrecan (ACAN) gene expression from MSCs encapsulated in PLL-loaded hydrogels compared to those in blank hydrogels.
Another method that has been implemented to support the chondrogenesis of MSCs and to maintain their chondrogenicity is coculturing with chondrocytes. Chondrocytes tend to undergo dedifferentiation when subjected to prolonged expansion in vitro in two-dimensional culture flasks [15,16]. One of the methods shown to be effective in preventing chondrocyte dedifferentiation is to coculture them with MSCs [17–19]. Coculturing of the two different cell types creates an environment where paracrine signals secreted by chondrocytes induce chondrogenesis from cocultured MSCs without the added effects of exogenous growth factors, while MSCs enhance the proliferation of chondrocytes. Our laboratory has previously investigated the coculture of MSCs and chondrocytes within electrospun poly(ε-caprolactone) scaffolds and found a similar synergistic effect to take place in a three-dimensional environment [20–23].
The objective of this study was to investigate the combined effect of the two approaches discussed above – presence of PLL and coculturing MSCs with chondrocytes – on the chondrogenesis of hydrogel-encapsulated cell populations in vitro. We hypothesized that coculturing MSCs with chondrocytes in the presence of PLL would enhance the chondrogenicity from the overall cell population compared to the MSC-only culture. As a model hydrogel platform, we have chosen a poly(N-isopropylacrylamide) (PNiPAAm)-based, chondroitin sulfate (CS)-containing, thermally responsive hydrogel recently developed in our laboratory [24]. Furthermore, we have established two specific objectives, which were i) to analyze the effect of PLL loading on the physicochemical properties of the hydrogel as well as on the viability and chondrogenic properties of encapsulated MSCs; and ii) to investigate the effect of varying MSC-to-chondrocyte ratios on the overall chondrogenic gene expression of the two cell populations encapsulated in PLL-laden hydrogels. The novelty of our study is in the investigation of the combined effect of introducing a biomolecule known to stimulate mesenchymal condensation and coculturing chondrocytes with MSCs on the chondrogenesis of the overall cell population. Furthermore, our study distinguishes itself from previous studies conducted in our laboratory by utilizing a novel injectable hydrogel as the experimental platform. The TGM-CS dual-network hydrogel described in this study can be simply injected into a mold or a defect site as a precursor solution and be allowed to undergo thermogelation without the need of external catalysts for crosslinking. The epoxides on TGM and NHS esters on CSNHS may also be able to covalently bind to the surface amine groups on the tissue surface, making the hydrogel a promising candidate for potential in vivo applications.
2. Materials and Methods
2.1. Materials
N-isopropylacrylamide (NiPAAm), glycidyl methacrylate (GMA), (R)-α-Acryloyloxy-β,β-dimethyl-γ-butyrolactone (DBA), acrylic acid (AA), 2,2′-azobis(2-methypropionitrile) (AIBN), piperazine, N,N′-methylenebisacrylamide (MBA), chondroitin sulfate type A (sodium salt, from bovine trachea), adipic acid dihydrazide (ADH), hyaluronidase (Type I-S, 400-1000 U/mg, from bovine testes), PLL (average MW 50 kDa and 225 kDa), fluorscein isothiocyanate (FITC)-labeled PLL (average MW 50 kDa), ascorbic acid, dexamethasone, L-proline, HEPES, pepstatin A, iodoacetamide, tris(hydroxymethyl aminomethane) (Tris), ethylenediaminetetraacetic acid (EDTA), 1,9-dimethyl-methylene blue (DMMB) zinc chloride double salt, Alcian Blue staining solution, Nuclear Fast Red staining solution, 1,4-dioxane, diethyl ether, acetone, and xylene were purchased from MilliporeSigma (MilliporeSigma, St. Louis, MO). 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), MES, Dulbecco’s Modified Eagle Medium (DMEM), non-essential amino acids (NEAA), antibiotic-antimycotic, collagenase (type II), ITS+ Premix Universal Culture Supplement, 10% neutral buffered formalin, phosphate buffered saline (PBS), and Quant-iT PicoGreen dsDNA assay kit were purchased from Thermo Fisher (Thermo Fisher Scientific, Waltham, MA). Proteinase K was purchased from Promega (Promega, Madison, WI). Tissue-Tek® O.C.T. Compound and ethanol (170, 190 proof) were purchased from VWR (VWR, Radnor, PA). FBS was purchased from Gemini Bioproducts (Gemini Bioproducts, West Sacramento, CA). All primers were purchased from Integrated DNA Technologies (IDT, Coralville, IA). iScript cDNA synthesis kit and iTaq universal SYBR green supermix were purchased from Bio-Rad (Bio-Rad, Hercules, CA). RNeasy Plus Mini kit and QIAshredder were purchased from Qiagen (Qiagen, Germantown, MD). Ultrapure water from Millipore Super-Q water system (MilliporeSigma) was used to prepare buffers and media.
2.2. Synthesis of CS-based network-forming macromers
CS was modified with ADH and NHS following a previously established protocol [24]. In short, ADH-modified CS was synthesized by reacting excess amounts of ADH with CS using EDC chemistry for 4 h in MES buffer (pH 5.0). The reaction mixture then underwent extensive dialysis, first against 100 mM NaCl and then water/25% ethanol, before undergoing freeze-drying to yield the final product. ADH modification percentage of 80% was used throughout the study. For NHS modification, CS was first digested using hyaluronidase for 48 h to enhance the solubility of NHS-modified CS. Solutions of CS (10% w/v), EDC (67% w/v), and NHS (25% w/v) dissolved in PBS were mixed at a volumetric ratio of 7:1.5:1.5 in a glass scintillation vial and reacted at 37 °C for 10 min. The reaction mixture was purified via a series of ethanol precipitation and allowed to dry completely by being placed under a flow of N2 gas.
2.3. Synthesis of PNiPAAm-based thermogelling macromer (TGM) and its crosslinker
TGM and polyamidoamine (PAMAM) crosslinker were synthesized following protocols that were previously established in our group [24–26]. Briefly, TGM was synthesized by combining four monomers (NiPAAm, GMA, DBA, AA) in N2-purged 1,4-dioxane at a molar feed ratio of 83.5:7.5:5:4 with AIBN as free-radical initiator and reacting at 65 °C for 18 h. The product was purified via ether precipitation and stored in −20 °C under N2 until further use. PAMAM was synthesized by adding piperazine and MBA to N2-purged ultrapure water at a molar feed ratio of 1:0.846 and reacting shielded from light at 30 °C for 48 h. The product was directly precipitated in acetone to yield a fine powder, which was dried and stored in −20 °C until further use.
2.4. Synthesis of TGM/CS dual-network hydrogels
TGM was dissolved in PBS (pH 7.4) at a concentration of 30% w/v for two days at 4 °C, while the other components (40%, 10%, and 30% w/v/ for PAMAM, CSADH, and CSNHS, respectively) were prepared immediately before hydrogel fabrication. The four macromer components were mixed at the ratio of 1.50:2.25:0.125:0.125 TGM:PAMAM:CSADH:CSNHS to fabricate the hydrogels for all studies, where each fraction represents the molar content of the reactive groups in the respective macromer (epoxides in TGM, terminal amines in PAMAM, ADH in CSADH, and NHS in CSNHS). The prepared hydrogel precursor solution was kept on ice to prevent thermogelation prior to being transferred to individual molds. An appropriate volume of PLL (stock solution of 0.5 mg/ml) was added to the hydrogel precursor solution to fabricate PLL-loaded hydrogels. The prepared solution was pipetted into Teflon molds before being transferred to 37 °C to undergo crosslinking for 24 h. 30 µl molds (6 mm diameter × 1 mm height) were used for the swelling, degradation, PLL retention study, and the in vitro study outlined in Table 1. 15 µl molds (3 mm diameter × 2 mm height) were used for the in vitro study outlined in Table 2.
Table 1.
In vitro study design to study the effect of PLL MW and concentration on the viability and sulfated glycosaminoglycan production of encapsulated MSCs.
| Group (n = 4) | PLL MW (kDa) | PLL concentration (µg/ml) | Cell concentration |
|---|---|---|---|
| -PLL | - | - | |
| 50 kDa/10 µg/ml | 50 kDa | 10 | |
| 50 kDa/50 µg/ml | 50 kDa | 50 | 15 million cells/ml |
| 225 kDa/10 µg/ml | 225 kDa | 10 | |
| 225 kDa/50 µg/ml | 225 kDa | 50 |
Hydrogel volume: 30 µl (6 mm diameter × 1 mm height molds)
Table 2.
In vitro study design to study the combined effect of the presence of PLL and varying coculture ratios of MSCs and ACs on chondrogenic and hypertrophic gene expression.
| Group (n = 4) | MSC-to-AC Ratio | PLL MW (kDa) | PLL concentration (µg/ml) | Total cell concentration | |
|---|---|---|---|---|---|
| MSC | AC | ||||
| MSC | 1 | 0 | |||
| 3:1 | 3 | 1 | |||
| 1:1 | 1 | 1 | 0, 225 kDa | 0, 50 µg/ml | 30 million cells/ml |
| 1:3 | 1 | 3 | |||
| AC | 0 | 1 | |||
Hydrogel volume: 15 µl (3 mm diameter × 2 mm height molds)
2.5. Swelling and degradation of PLL-loaded TGM
Swelling and degradation studies were conducted following previously established protocols.[14] For the swelling study, fabricated hydrogels were first weighed at the time of fabrication (i.e., after 24 h of crosslinking) to measure their formation weight, wform. The hydrogels were then placed in 24-well plates, each well containing 2 ml of PBS, and allowed to swell for 24 h, after which they were re-weighed (equilibrium swelling weight, wequil), and freeze-dried to measure their dried weight, wdry. Two swelling ratios, formation (qform) and equilibrium (qequil), were calculated using the following equations: qform = (wform-wdry)/wdry, qequil = (wequil-wdry)/wdry.
The effect of PLL concentration and MW on hydrogel degradation was quantified via an accelerated degradation study. Following their fabrication, hydrogels were frozen and placed in the freeze-dryer for two days, after which their initial dry weights were measured. Individual hydrogels were then placed in 20 ml scintillation vials containing 5 ml of basic PBS (pH 10.5) and placed in a shaker table set to 100 rpm at 37 °C temperature-controlled room for 28 days, with media change occurring every three days. Degradation percentage was calculated at each time point (days 1, 2, 4, 7, 10, 14, 21, 28) by comparing the hydrogel sample’s final dry weight with its initial dry weight.
2.6. The release profiles of PLL from hydrogels
FITC-labeled PLL (FITC-PLL) was utilized to track the retention of loaded PLL. Concentrations of PLL tested for this study (250 and 750 µg/ml) were significantly higher than those that were used for the rest of the studies (10 and 50 µg/ml) to enhance the detection of fluorescence level. Individual hydrogels were placed in 20 ml scintillation vials, each containing 5 ml of PBS (pH 7.4), and the vials were then placed on a shaker table set to 100 rpm in a 37 °C for up to 28 days. The container holding the vials was covered with aluminum foil to prevent photobleaching. At each time point, the amount of FITC-PLL that leached out from the hydrogel was calculated by measuring the fluorescence of the supernatant (excitation/emission wavelength of 485/525 nm) and calculating the concentration of PLL using a standard curve generated with a stock solution of FITC-PLL. The amount of FITC-PLL retained within the hydrogel at each time point was then back-calculated by subtracting the amount of leached FITC-PLL from the initial amount in the hydrogel. In addition, the hydrogels were imaged at each time point using an epifluorescence microscope to qualitatively evaluate the retention of PLL within the bulk of the hydrogel. The same manual exposure settings were used in imaging the samples across different time points to remove any bias from automatic exposure adjustments by the software.
2.7. Cell culture and isolation
The animal protocol describing the usage of animals for this study was approved by Rice University Institutional Animal Care and Usage Committee. Animal studies were performed in agreement with the National Institutes of Health animal care and use guidelines. Following previously established method [27], primary bone marrow MSCs were harvested from the tibiae of six month-old male New Zealand White rabbits and plated on 225 cm2 polystyrene tissue culture flasks. All isolated MSCs were grown to confluency and lifted at passage 1, frozen, and stored under liquid nitrogen vapor until further use. Thawed cells were cultured in expansion medium containing low-glucose DMEM, 10% FBS, and 1% antibiotic/antimycotic, in a 37 °C cell culture incubator. Cells were passaged at 80–90% confluency and expanded until passage 3, at which point the MSCs were lifted using TrypLE cell-dissociation enzyme were used for in vitro studies at passage 4.
Primary articular chondrocytes (ACs) were isolated from the articular joint of female Hampshire pigs donated from the University of Texas McGovern Medical School. All steps were aseptically conducted in a laminar flow cabinet to minimize the risk of contamination. Prior to opening the joint and exposing the cartilage, most of the excess muscle tissues were removed. Cartilage pieces were then shaved from the tibial joint, using sterile razor blades, and placed on ice in sterile PBS containing 2% antibiotic/antimycotic until the harvest was complete. The isolated cartilage pieces were finely minced and digested overnight at 37 °C in a 75 cm2 tissue culture flask containing 10% high-glucose DMEM, 1% antibiotic-antimycotic, and 1% collagenase. ACs were collected by passing the supernatant through a 40 µm cell strainer and centrifuging at 500 × g, and the cell pellet was resuspended in chondrocyte culture medium (10% high-glucose DMEM, 1% NEAA, 50 μg/ml ascorbic acid, 46 μg/ml L-proline, 20 mM HEPES, 1% antibiotic-antimycotic) before being frozen and stored under liquid nitrogen vapor until further use. All ACs used for the in vitro studies were passaged once in 225 cm2 tissue culture flasks and used at passage 1.
2.8. Hydrogel fabrication and culture for the in vitro study of MSC behavior in PLL-loaded hydrogels
Prior to hydrogel fabrication, individual components (TGM, PAMAM, CSADH, CSNHS) were sterilized by being placed under UV light in a laminar flow cabinet for up to 2 h and dissolved in chemically defined chondrogenic medium (CDCM) containing 10% low glucose DMEM, ITS+ Premix (6.25 μg/ml insulin, 6.25 μg/ml transferrin, 6.25 μg/ml selenious acid, 5.35 μg/ml linoleic acid, and 1.25 μg/ml bovine serum albumin), 50 μg/ml ascorbic acid, 0.1 μM dexamethasone, and 1% antibiotic-antimycotic. PLL was dissolved in PBS and filtered using a 0.1 µm syringe filter prior to use. The individual components were then combined on ice and mixed with the cell suspension immediately before being pipetted into the Teflon molds. Combinations of cell concentration and mold size used for the in vitro studies are outlined in Table 1 and Table 2. The molds were then placed in a large container and allowed to crosslink for 3 h in a cell incubator. The crosslinking time was adjusted for cell-laden hydrogels to prevent cell death due to dehydration and lack of nutrient diffusion. The crosslinked hydrogels were then placed in 24-well plates, each well containing 1 ml of CDCM, and cultured for up to 28 days. Media change occurred every three days, with the first change occurring 24 h after crosslinking to remove hydrogel leachables.
2.9. Biochemical analyses
At each time point (2 h, 7, 14, 21, 28 days), individual hydrogels were washed in sterile PBS and placed in 2 ml round-bottom Eppendorf tubes, each containing 250 µl of ultrapure water and a stainless-steel bead. The samples were then disrupted using a Qiagen TissueLyser bead mill (Qiagen) for 5 min at 30 Hz, followed by three freeze-thaw cycles. To further digest the samples, 250 µl of 2× digestion solution (2 mg/mL proteinase K, 0.02 mg/ml pepstatin A, and 0.37 mg/ml iodoacetamide in 50 mM Tris/1 mM EDTA, pH 7.6) was added to each tube, which was then placed in a 60 °C oven overnight.
Cell viability and proliferation were evaluated by quantifying the DNA content in each sample using a PicoGreen dsDNA assay, following the manufacturer’s guideline. In short, cell lysate supernatant was combined with the assay buffer and the reagent solution in an opaque 96-well plate, and the fluorescence was measured using a fluorescence plate reader at excitation/emission wavelengths of 485/525 nm.
Sulfated glycosaminoglycan (sGAG) content, used as an indicator of MSC chondrogenesis, was quantified using a DMMB assay following an established protocol [28]. Briefly, cell lysate supernatant was combined with the reagent buffer (16 µg/ml DMMB, 40 mM glycine, 40 mM NaCl in ultrapure water, pH adjusted with HCl to 1.5) in a transparent 96-well plate, and the absorbance was immediately measured using an absorbance plate reader. Measurements were made at two wavelengths (525 and 595 nm) and their difference (OD525-OD595) was used to calculate the concentration of sGAGs in each sample.
2.10. Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Hydrogels at each time point (days 7, 21, 42) were washed in sterile PBS, flash frozen in liquid N2, and stored at −80 °C until they were ready to be processed. The samples were lysed using a Qiashredder, and the total RNA was isolated using RNeasy Mini Plus kit following the manufacturer’s guideline. Complementary DNA (cDNA) was synthesized from the isolated RNA using an iScript cDNA synthesis kit.
Two sets of primers – one rabbit-specific, and one cross-species – were designed in order to evaluate the individual contribution of MSCs and ACs to the overall gene expression (Table 3). Species specificity of the primer pairs was verified prior to use by conducting conventional PCR and analyzing the products on agarose gels. Gene expressions for type II collagen (COL2A1) and aggrecan (ACAN) were used to evaluate chondrogenicity, while type X collagen (COL10A1) and matrix metallopeptidase 13 (MMP13) were used for hypertrophy. All RT-qPCR experiments were conducted using a Bio-Rad CFX96 real-time system (Bio-Rad).
Table 3.
Primer sequences used for RT-qPCR.
| Gene | Primer Sequence | Amplicon length | |
|---|---|---|---|
| COL2A1 | Rabbit-specific | F: 5'-AACACTGCCAACGTCCAGAT-3' | 95 |
| R: 5'-CTTCATCCAGGTAGGCCACG-3' | |||
| Cross-species | F: 5'-CGCCATGAAGGTTTTCTGCAAC-3' | 103 | |
| R: 5'-TCTTGCTGCTCCACCAGTTC-3' | |||
| ACAN | Rabbit-specific | F: 5'-GGTCTGGACAGGTGCTATGC-3' | 95 |
| R: 5'-GGTAGACGGTTCTCACACCG-3' | |||
| Cross-species | F: 5'-ACTGCGTGGTGATGATCTGG-3' | 112 | |
| R: 5'-GGCCACTGTGCCCTTTTTAC-3' | |||
| COL10A1 | Rabbit-specific | F: 5'-CCGCCTGGGTTAGATGGAAA-3' | 107 |
| R: 5'-CCTCTAACTCCAGCGTCACC-3' | |||
| Cross-species | F: 5'-GCTCCTGGCTTTGGGAAAC-3' | 92 | |
| R: 5'-TGTTCCCCTTTGGCACCTG-3' | |||
| MMP13 | Rabbit-specific | F: 5'-CCTTAGGGCTTGACCACTCC-3' | 109 |
| R: 5'-TGGATTCCTTGCACATCGTCA-3' | |||
| Cross-species | F: 5'-GCCCATGAGTTTGGCCATTC-3' | 149 | |
| R: 5'-TCTCCTGGACCATAGAGAGACTG-3' | |||
| GAPDH | Rabbit-specific | F: 5'-ATCAAGTGGGGTGATGCTGG-3' | 109 |
| R: 5'-TGATGACCCTTTTGGCTCCG-3' | |||
| Cross-species | F: 5'-TGTTTGTGATGGGCGTGAAC-3' | 82 | |
| R: 5'-AAGCAGTTGGTGGTGCAG-3' | |||
2.11. Histology
For sectioning and staining, hydrogels were washed with PBS and fixed in neutral buffered formalin at room temperature overnight. Fixed samples were washed again with PBS to remove excess formalin and soaked in optimal cutting temperature (OCT) compound overnight at 4 °C to prevent ice crystal formation. Samples were placed in cryomolds with OCT compound and allowed to rapidly undergo freezing by being placed on a stainless-steel block chilled with liquid N2. The embedded samples were placed in Ziploc bags and stored at −80 °C until further use. 5–10 µm sections were obtained using a cryotome, and mounted samples were dried overnight at room temperature before being stained using Alcian Blue for GAGs and Nuclear Fast Red as a counterstain.
2.12. Statistics
Biochemical assays and RT-qPCR data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s Honest Significant Difference (HSD) post-hoc test (p < 0.05). All statistical tests were conducted and obtained using JMP Pro 14 (SAS Institute, Cary, NC). Data points are displayed as mean ± standard deviation (with standard deviation being the square root of the variance), unless otherwise noted.
3. Results
3.1. Effects of PLL loading on physicochemical properties of TGM-CS dual-network hydrogels
Two properties – swelling and degradation – of PLL-loaded hydrogels were measured to determine whether the loading of PLL would impact the physicochemical behavior of the hydrogel. From the swelling study, qform and qequil of the hydrogels loaded with different MW (50, 225 kDa) and concentrations (10, 50 µg/ml) of PLL were not significantly different from those of the control (Fig. 2A). In addition, we observed from the degradation study that there was no statistically significant difference between the degradation percentages of all five study groups at day 28 (Fig. 2B).
Figure 2.
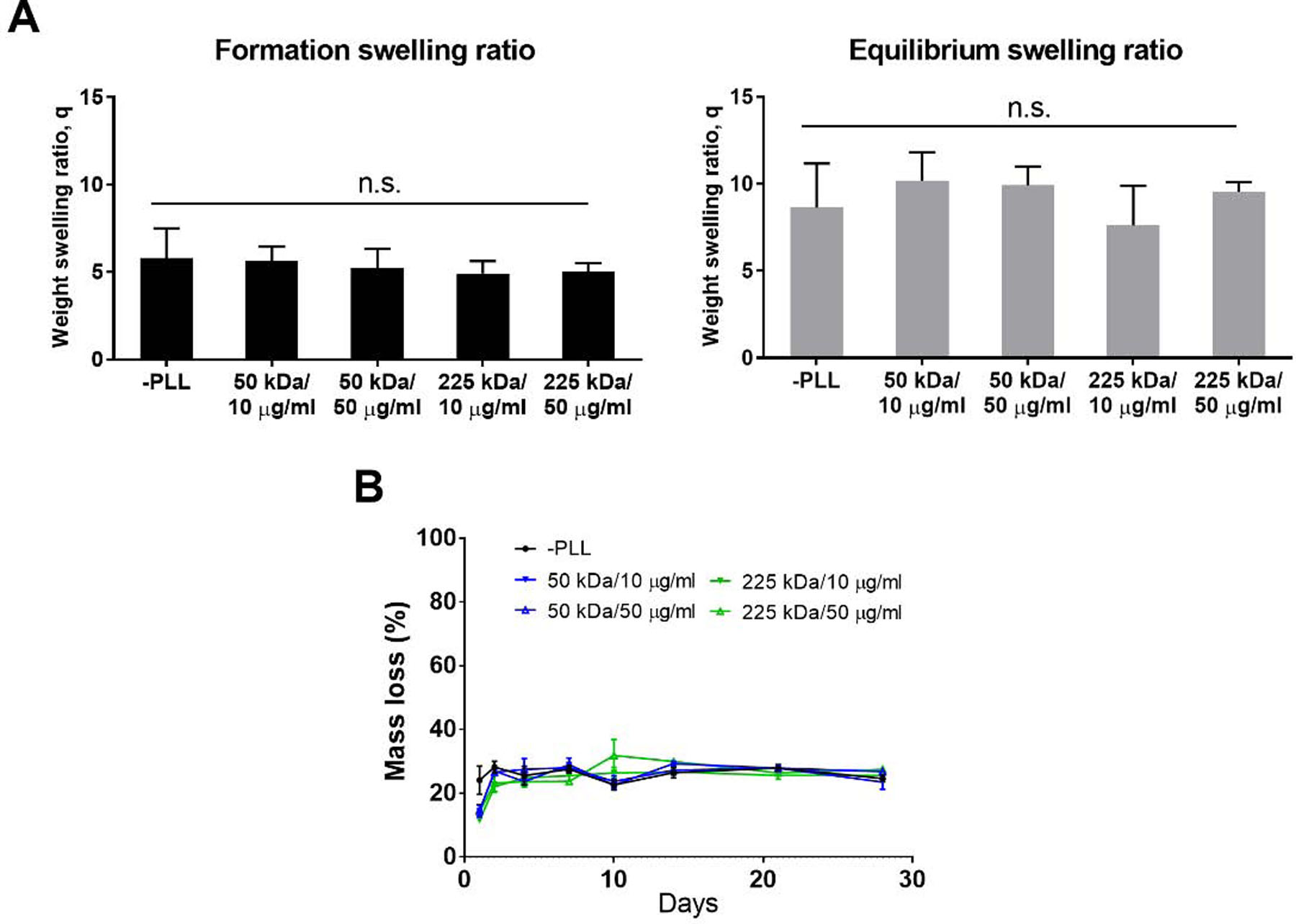
Swelling and degradation profiles of TGM-CS dual-network hydrogels loaded with PLL. (A) Formation and equilibrium swelling ratios of hydrogels loaded with different MW/concentrations of PLL (n = 5 per group, mean ± standard deviation). (B) Degradation profile of PLL-loaded hydrogels (n = 3 per group, mean ± standard deviation).
3.2. Long-term retention of PLL loaded in TGM-CS dual-network hydrogels
Next, we investigated the degree of PLL retention within the hydrogels. From both concentration groups we observed an initial burst release of PLL at day 1 (18.5±2.1% and 15.5±3.6% for 250 and 750 µg/ml, respectively), followed by a slight but gradual increase over the 28-day period (Fig. 3A). By the end of the study, the total amounts of FITC-PLL released from the bulk hydrogel (30.3±4.6% and 25.5±3.6% for 250 and 750 µg/ml, respectively) were significantly higher than the amounts initially released at day 1 for both groups. PLL retention was also qualitatively assessed by imaging the hydrogels at each time point under an epifluorescence microscope (Fig. 3B). The blurriness of the images was caused by the microscope capturing the fluorescence from the full depth of the hydrogel and not from a single focal plane. Regardless, uniform fluorescence was observed throughout the bulk of the hydrogel, with no apparent reduction in fluorescence from day 1 to 28.
Figure 3.

Retention of PLL loaded in TGM-CS dual-network hydrogels. (A) Release profile of FITC-labeled PLL over 28 days (expressed as total amount released at each time point). * represent statistically significant difference (p < 0.05) to the PLL release percentage at day 1 within the same study group (n = 3 per time point, mean ± standard deviation). There were no statistically significant differences (p > 0.05) between the groups (250 and 750 µg/ml) within each time point. (B) Epifluorescence microscopy images of hydrogels loaded with FITC-labeled PLL. Same exposure settings were used within each study group to image hydrogels at all time points. Scale bars represent 100 µm.
3.3. In vitro study to determine the impact of PLL loading on the viability and chondrogenic activity of encapsulated MSCs
MSCs were encapsulated into hydrogels containing different MW and concentrations of PLL, while hydrogels devoid of PLL served as a control (Table 1). No exogenous growth factors were added to CDCM to isolate the effects of PLL and the surrounding hydrogel environment on MSC chondrogenesis. One group (50 µg/ml of 50 kDa PLL) showed significant increase in their DNA content at day 28 compared to the 2 h time point. However, the DNA contents of all four PLL-loaded groups were not significantly different from that of the no PLL group at day 28 (Fig. 4A). The chondrogenic activity of encapsulated MSCs was measured by quantifying the amount of synthesized sGAG using a DMMB assay. All five groups demonstrated significant decreases in sGAG/DNA from 2 h to day 28. At day 28, one study group (50 µg/ml of 225 kDa PLL) demonstrated significantly higher sGAG/DNA content compared to the other four groups (Fig. 4B). Alcian blue/nuclear fast red staining of day 28 hydrogels showed sparse but homogeneous distribution of GAGs in all groups, although we were unable to discern any qualitative difference between the study groups (Fig. 5).
Figure 4.
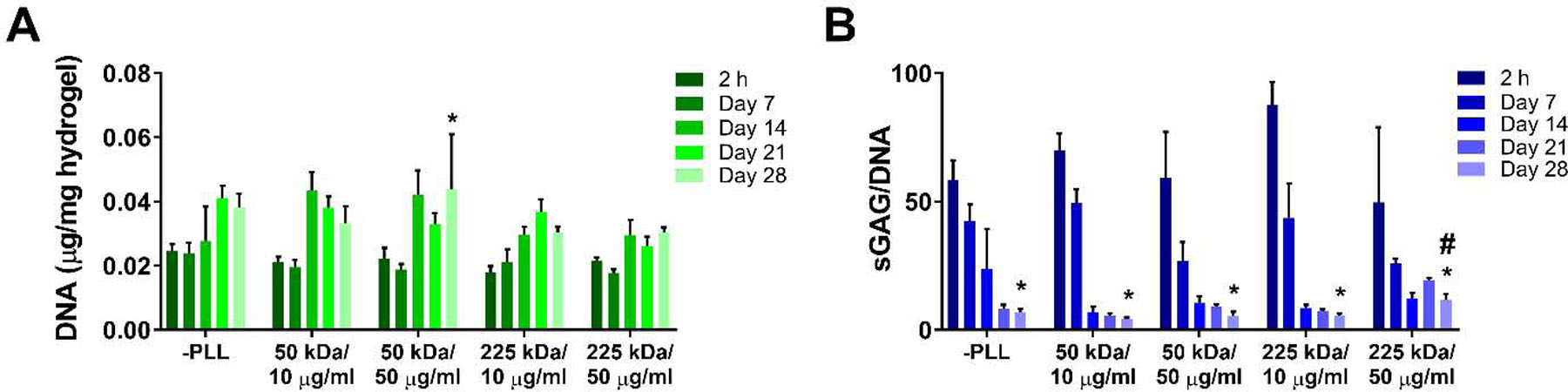
Viability and chondrogenic activity of MSCs encapsulated in PLL-loaded hydrogels. (A) DNA content normalized by the hydrogel weight (wet) at each time point. (B) sGAG content normalized by the DNA content. * represent statistically significant difference (p < 0.05) to the 2 h time point within the same study group. # represent statistically significant difference (p < 0.05) compared to the no PLL group within the same time point (n = 4 per time point, mean ± standard deviation).
Figure 5.

Histological sections of day 28 hydrogels stained for GAG deposition (blue) and cells (red). All images were taken using 20× objective. Scale bars represent 50 µm.
3.4. Impact of PLL loading and MSC:AC ratio on the chondrogenic and hypertrophic gene expression of encapsulated MSC:AC cocultures
A second in vitro study was conducted to evaluate the impact of coculturing MSCs with ACs in hydrogels with or without PLL on the chondrogenesis of the overall cell population (Table 2). For this purpose, cocultures with three different MSC:AC ratios (3:1, 1:1, and 1:3), along with monocultures of MSCs and ACs, were encapsulated in hydrogels with or without 50 µg/ml of 225 kDa PLL.
All three coculture groups demonstrated upregulation of COL2A1 that was comparable to that of the AC monoculture at day 42 (Fig. 6A). Similar results were observed for ACAN expression levels in all coculture groups. The MSC monoculture group showed significantly lower COL2A1 expression in all time points compared to other study groups regardless of the presence of PLL. We did not observe any significant differences in the expression levels of COL2A1 and ACAN between the coculture groups with different MSC:AC ratios. On the other hand, the effect of different coculture ratios was more visible in the expression of hypertrophic genes (Fig. 6B). The highest upregulation of COL10A1 expression at day 42 was observed in the AC monoculture group (both with and without PLL) and decreased as the ratio of MSCs increased in the coculture, with the lowest upregulation observed in the MSC monoculture group. MMP13 expression showed the opposite trend. While its expression at day 42 was similar across the study groups, three groups in the PLL-containing hydrogels with the highest MSC ratio (MSC monoculture, 3:1, and 1:1) showed significantly higher expression of MMP13 at day 7 compared to AC monoculture.
Figure 6.

mRNA expression levels for (A) chondrogenic (COL2A1, ACAN) and (B) hypertrophic (COL10A1, MMP13) genes from study groups cultured in hydrogels with and without PLL. Data are plotted as log-fold change (ΔCt) over AC control at day 0. † represent statistically significant difference (p < 0.05) to the AC monoculture group within the same time point. * represent statistically significant difference (p < 0.05) to the day 7 time point within the same study group. # represent statistically significant difference (p < 0.05) between the +/− PLL group within the same time point/study group (n = 4 per time point, mean ± standard deviation).
The effect of PLL was visible in some study groups during days 7 and 21, but by day 42 there were no differences in expression levels of all four genes tested between the study groups with and without PLL. The 1:1 coculture group was the only coculture group that exhibited significant differences in gene expression, with the group without PLL showing significantly higher upregulation of COL2A1 and ACAN at days 7 and 21, and lower COL10A1 upregulation at day 7 compared to the group with PLL. On the other hand, the expression levels of COL2A1 and ACAN at day 7 in the AC monoculture group were significantly higher for the PLL-containing group. Opposite effects were seen in MSC monoculture encapsulated in PLL-containing hydrogels, where COL10A1 expression levels at day 7 and 21 were significantly higher compared to the same group without PLL.
The ratio of COL2A1 to COL10A1 was calculated to quantify the degree of chondrogenesis versus hypertrophy. The coculture groups showed higher average COL2A1:COL10A1 ratios than that of the AC monoculture group at day 42, although the difference was not statistically significant (Fig. 7). Two groups (1:1 and 1:3 MSC:AC coculture ratios) demonstrated significantly higher COL2A1:COL10A1 ratios at day 7 in hydrogels without PLL compared to the PLL-loaded hydrogels, but the difference was not seen at later time points. The MSC monoculture group had the lowest COL2A1:COL10A1 ratio from all study groups, which closely resembles what was observed in mRNA expression levels for COL2A1 (Fig. 6).
Figure 7.
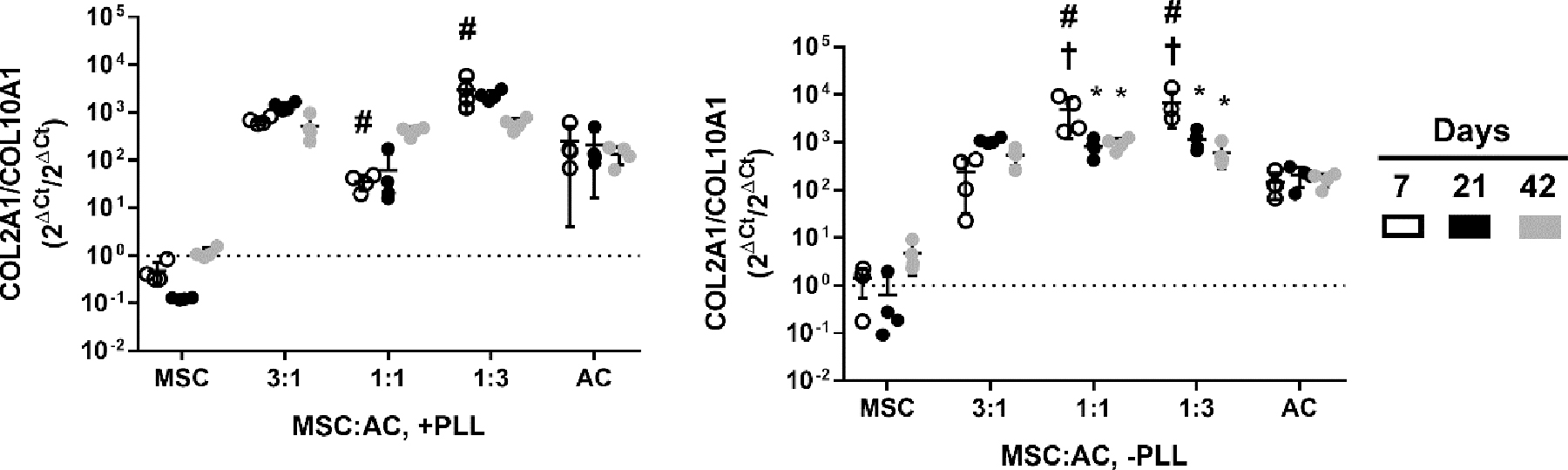
Ratio of the relative mRNA levels of COL2A1 to COL10A1. Data are plotted as relative mRNA ratio of the two genes(2ΔCt/2ΔCt). † represent statistically significant difference (p < 0.05) to the AC monoculture group within the same time point. * represent statistically significant difference (p < 0.05) to the day 7 time point within the same study group. # represent statistically significant difference (p < 0.05) between the +/− PLL group within the same time point/study group (n = 4 per time point, mean ± standard deviation)
Lastly, species-specific gene analysis was conducted to quantify the contribution of MSCs to the overall gene expression in the three coculture groups (Fig. 8). The results indicated that the contribution by MSCs to the chondrogenic gene was not as significant as for the hypertrophic genes. The 1:1 coculture group in PLL-loaded hydrogels demonstrated significantly higher contributions from the MSCs for COL2A1 and ACAN at earlier time points (days 7 and 21 for COL2A1, day 7 for ACAN), but the difference was not observed at day 42 (Fig. 8A). In comparison, the contribution by MSCs to hypertrophic gene expression was partly affected by the coculture ratio. The 1:1 coculture group in hydrogels containing PLL showed significantly higher MSC contribution for both COL10A1 and MMP13 at day 42 than either the 3:1 or 1:3 coculture groups. Similar results were observed from the 1:1 and 1:3 coculture groups in hydrogels without PLL.
Figure 8.
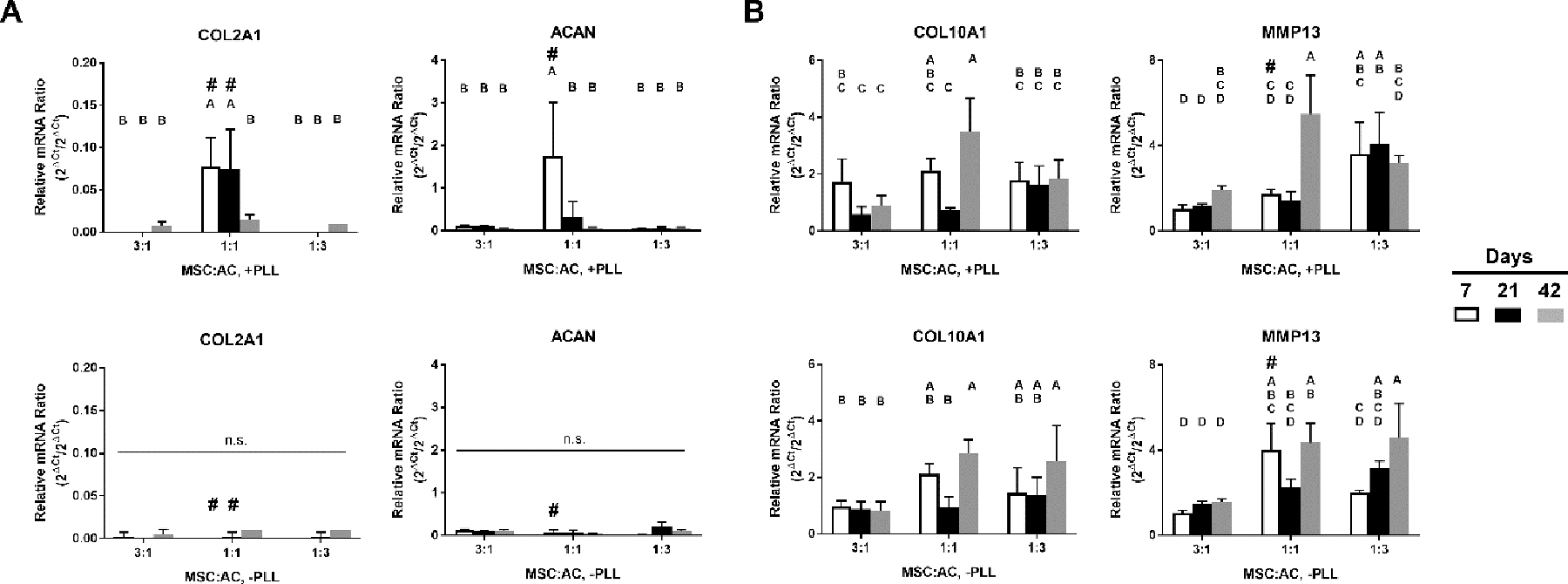
Ratio of rabbit MSC-specific to non-specific mRNA levels of (A) chondrogenic (COL2A1, ACAN) and (B) hypertrophic (COL10A1, MMP13) genes from study groups cultured in hydrogels with and without PLL. Data are plotted as relative mRNA ratio of the MSC-specific gene to the non-specific gene (2ΔCt/2ΔCt). Groups not sharing the same letter are significantly different (p < 0.05). # represents statistically significant difference (p < 0.05) between the +/− PLL group within the same time point/study group (n = 4 per time point, mean ± standard deviation).
Histological analysis corroborated the data obtained from RT-qPCR analyses, where monocultures of MSCs showed the least amount of GAG staining (Fig. 9). The staining intensity, which is indicative of cartilage-like matrix deposition, also increased with increasing ratio of ACs, with AC monocultures showing the strongest staining for GAG. There was no significant difference between the groups with and without PLL.
Figure 9.
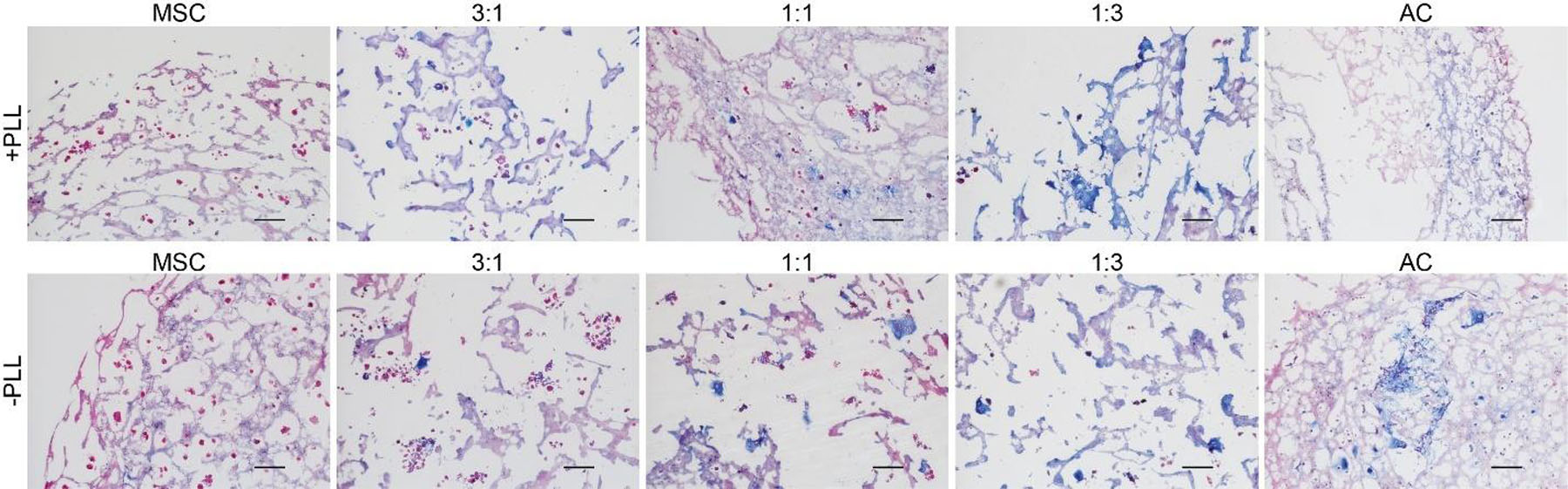
Histological sections of day 42 hydrogels with and without PLL. All sections were 10 µm thick and stained for GAG deposition (blue) and cells (red). All images were taken using 10× objective. Scale bars represent 100 µm.
4. Discussion
One of the biggest challenges associated with cell-based cartilage tissue engineering strategies has been the maintenance of cell chondrogenicity for implantation. Expanding primary chondrocytes on 2D plates prior to implantation, which is often necessary due to their low cell density in harvest of cartilage tissues, has been linked with dedifferentiation and loss of their chondrogenic phenotype [29,30]. MSCs have thus been investigated as an alternative cell source, but directing MSCs to a chondrogenic lineage has been equally challenging due to their tendency to undergo hypertrophic differentiation in the long-term. In this study, we sought to investigate the effect of coculturing chondrocytes with MSCs at different ratios on the chondrogenesis of the overall cell population. We simultaneously sought to investigate the relationship between PLL presentation and chondrogenic/hypertrophic differentiation of MSCs.
As a model hydrogel system, we leveraged a TGM-CS dual network hydrogel system [24] that was chemically crosslinked with PAMAM [25,26] (Figure 1). Chemical crosslinking helped to improve the stability of the cell-laden hydrogel, as the encapsulation of MSCs led to a significant degradation of the hydrogel network within the first few days of in vitro culture (data not shown). By having a TGM-based hydrogel as one of the two components of our hydrogel, we were able to successfully develop a cell delivery system that can be prepared under mild conditions and be injected directly into individual hydrogel molds before undergoing spontaneous gelation. A similar system has been recently explored in our laboratory, which demonstrated favorable results for inducing chondrogenesis and osteogenesis from encapsulated MSCs [31].
Figure 1.
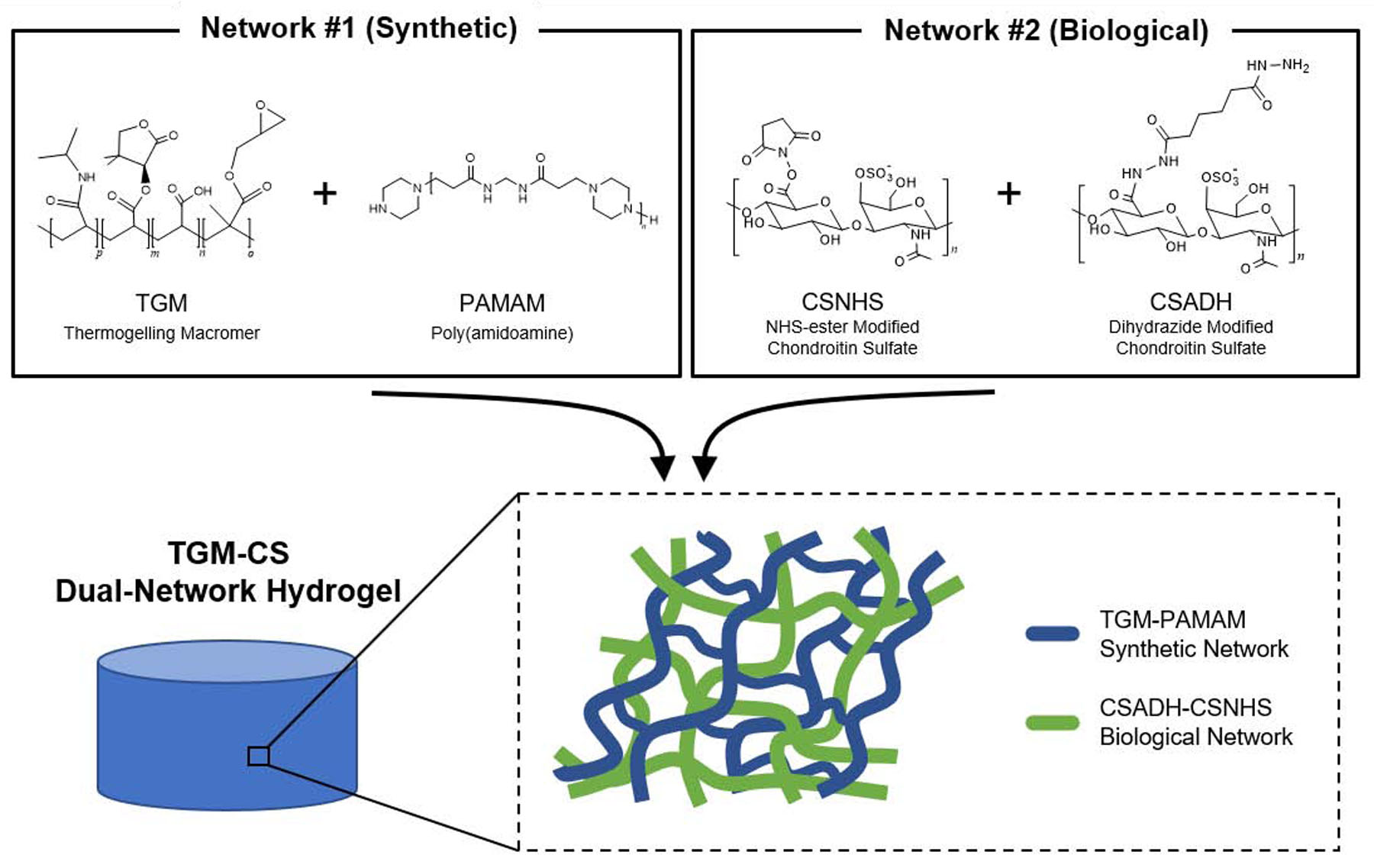
Schematic of the TGM-CS dual network hydrogel. The synthetic portion of the network undergoes epoxy-amine crosslinking, whereas the biological network is formed via hydrazide-NHS ester crosslinking.
One of the prerequisites of adding a bioactive molecule to a scaffold or a hydrogel is that the addition of such molecule does not significantly impact the physicochemical properties of the construct. Since PLL is added to the hydrogel precursor solution prior to gelation, it is possible that a fraction of the amine groups present on PLL are covalently interacting with amine-reactive crosslinking groups in the hydrogel components (e.g., epoxides on TGM and NHS esters on CSNHS). The potential reduction in crosslinking density between the macromers could cause a shift in the hydrogel’s swelling and degradation behavior, where hydrogels with a less dense network will swell more and degrade faster. However, from the swelling and degradation study we were able to show that loading PLL at varying MW and concentrations did not impact the corresponding properties of the hydrogel. We hypothesize that due to the rather small amount of PLL loaded in each hydrogel compared to the concentrations of crosslinking macromers, the effect of undesirable crosslinking between PLL and other macromers was minimal and thus did not impact the swelling and degradation characteristics of the hydrogel. The initial mass loss recorded at day 1 in all study groups is likely due to the loss of sol fraction and is consistent with previous studies conducted in our laboratory with TGM-based hydrogels.[25,26,32]
We confirmed from the PLL retention study that the majority of loaded PLL was confined within the bulk hydrogel throughout 28 days. The initial burst release of PLL observed from both 250 and 750 µg/ml PLL groups (18.5 and 15.5%, respectively) is most likely due to the loss of hydrogel sol fraction. As explained above, it is possible that covalent bonding between PLL and amine-reactive groups on the hydrogel network is partially responsible for the retention of PLL. Another potential contributing factor is the electrostatic interactions between the cationic amines on PLL and the anionic moieties present within the hydrogel (e.g., carboxylates on TGM and CS, sulfates on CS). Although the PLL concentrations tested in the retention study were larger than those in the remaining studies to enable the detection of the fluorescence emitted, the retention of PLL independent of the concentrations tested suggests a high retention also for much lower concentrations.
In a previous study conducted in our laboratory, it was shown that incorporating PLL into an oligo(poly(ethylene) glycol) fumarate) (OPF)-based hydrogel system resulted in an initial increase in swelling compared to non-PLL loaded OPF hydrogels [14]. OPF does not present any charged moieties along its backbone except carbonyl oxygens on esters which are weakly basic [33]. The initial imbalance in charge density caused by the introduction of PLL in OPF hydrogel is thought to have caused the initial swelling of the hydrogel. In the current study, multiple charged groups are present in the TGM-CS hydrogel system and it is likely that the change in the charge density within the hydrogel caused by the introduction of PLL (within the concentration ranges tested in this study) was minimal and thus did not alter the hydrogel’s swelling behavior.
In investigating the effect of PLL MW and concentration on encapsulated MSCs, we initially expected to see a dose-dependent effect on cell viability since the PLL concentrations chosen for this study were higher than those previously tested, both as an encapsulated form in a 3D environment and as a supplement in the culture media [13,14]. However, we discovered that the introduction of polycations, regardless of MW and concentration, did not impact the viability of cells. On the other hand, we saw a gradual decrease in sGAG/DNA from 2h to day 28 from all five study groups. As CS is one of the sulfated GAGs, we were unable to discern the amount of sGAG synthesized purely by the MSCs. In addition, acellular controls for all groups consistently showed higher sGAG content compared to their cell-laden counterparts (data not shown), indicating that the proteolytic activity of encapsulated MSCs was responsible for breaking down the sGAG provided as part of the hydrogel network. Despite the gradual loss of sGAG, one study group (225 kDa/50 µg/ml PLL) showed higher sGAG/DNA compared to all other study groups at day 28 and was chosen for the MSC:AC coculture study. The selection was also supported by the results obtained from a previous study conducted in our laboratory, which demonstrated that higher PLL MW and concentration led to an increase in the expression of chondrogenic genes.[14]
Whereas the effect of PLL on modulating the behavior of MSCs has been studied in 2D and 3D cultures [13,14], its effect on chondrocytes have not yet been studied. We therefore decided to further explore the potential chondrostimulatory effect of PLL in encapsulated MSCs, along with an added variable of coculturing with different ratios of MSCs to ACs. From the gene expression analyses, we found that the presence of ACs was the most contributing factor to the expression levels of both COL2A1 and ACAN at day 42 (Fig. 6A), whereas the ratio of MSCs and ACs did not seem to affect the expression to the same extent. The MSC monoculture group showed low expression levels for both COL2A1 and ACAN at all three time points. This result suggests that the presence of the CS network within the hydrogel was not a sufficient factor in inducing chondrogenesis from the encapsulated MSCs. Although the effect of CS on MSC chondrogenesis has been reported, many studies were conducted in chondrogenic media containing growth factors such as transforming growth factor-β1 and -β3 [34,35]. The effect of CS on the behavior of MSCs in the absence of growth factors therefore merits further investigation.
From analyzing the expression of hypertrophic genes COL10A1 and MMP13, we were able to confirm that the coculturing of MSCs with ACs can sufficiently limit the hypertrophic differentiation of the overall coculture in our TGM-CS hydrogel system, which agrees with previously conducted coculture studies [18,36]. The highest expression of COL10A1 was observed from the AC monoculture group (Fig. 6B). Although COL10A1 is a marker for hypertrophy, it is mostly expressed in chondrocytes that are either prehypertrophic or undergoing hypertrophy [37]. The decrease in COL10A1 expression observed from the coculture groups suggests that the presence of MSCs limited the hypertrophic differentiation of chondrocytes. In contrast to COL10A1, MMP13 expression was highest in the MSC monoculture group and gradually decreased with increasing ratio of ACs. As MMP13 is a marker for terminal chondrocytes and osteoblasts, the result implies that ACs can have a dose-dependent effect on limiting the hypertrophic differentiation of MSCs. Lastly, the degree of hypertrophy was also evaluated by noting the ratio of COL2A1 to COL10A1 (Fig. 7). All coculture groups, on average, showed higher COL2A1:COL10A1 ratios compared to both MSC and AC monoculture groups, which supports our hypothesis that coculturing MSCs with ACs can limit the hypertrophic differentiation of the overall coculture.
The relationship between the presence of PLL and long-term chondrogenesis of MSCs remains to be seen. The chondrostimulatory effect of PLL was only seen minimally across the study groups, and most of the significant differences between the groups with and without PLL were observed at earlier time points. This implies that although the presence of PLL may be affecting the cellular behavior at the beginning of the culture, such modulation might not be resulting in a noticeable long-term difference. Interestingly, PLL seemed to promote the chondrogenesis of the AC monoculture groups more so than the MSCs or any of the coculture groups. For instance, the expression levels of COL2A1 and ACAN at day 7 in the AC monoculture group were significantly higher for the PLL-containing group than the one without. On the other hand, opposite effects were seen in MSC monoculture encapsulated in PLL-containing hydrogels, where COL10A1 expression levels at day 7 and 21 were significantly higher compared to the same group without PLL. Although the differences were no longer noticeable by day 42 in all study groups, the difference in response to PLL between the two cell types warrants further attention.
The highest average expression level of MMP13 detected from the MSC monoculture group could be potentially used to explain the rapid decrease in sGAG content from the bulk hydrogel in our first in vitro study (Fig. 4B). Recently it has been reported that the degradation products of CS could induce hypertrophic changes in chondrocytes [38]. Using hydrogels fabricated using methacrylated CS, the authors demonstrated that the accelerated degradation of CS-based ECM by the addition of chondroitinase ABC led to an upregulation of MMP13. Although we did not quantify the amount of GAG produced by the study groups in our second in vitro study, we did observe the weakest GAG staining from the MSC monoculture group during the histological analysis. Therefore, we hypothesize that the initial lack of sufficient chondroinductive signals guided the encapsulated MSCs to undergo hypertrophic differentiation, leading to an upregulation of MMP13 and eventually to the rapid degradation of the CS network.
From the histological analysis, we observed the strongest GAG staining in the AC monoculture group (Fig. 9). The degree of staining decreased as the ratio of MSCs increased in the coculture groups, which suggests that, although the overall gene expression levels of COL2A1 and ACAN were comparable between the three coculture groups and the AC monoculture group, chondrocytes were the main cell type that was secreting the cartilage-like proteoglycans on the translational level. This was expected, since from the MSC signal ratio analysis we observed that the MSCs did not contribute strongly to the expression of both chondrogenic genes (Fig. 8). In addition, similarly to the RT-qPCR analysis, we did not observe any effect of PLL loading on the histological outcomes. Although we have demonstrated in a previous study that a 3:1 coculture could produce equally robust cartilage-like matrix compared to the AC monoculture group [20], that study was conducted on poly(ε-caprolactone) electrospun scaffolds, unlike the present study where the cells were encapsulated in hydrogels. The different microenvironments might have induced different cellular responses due to differences in the matrix stiffness, rate of nutrient diffusion, or the degree of cell-cell contact.
Overall, the results of this study emphasize the role of ACs in inducing chondrogenesis of MSCs in a 3D hydrogel system. This effect was illustrated most noticeably by the increase in the expression level of COL2A1 in the coculture groups compared to the MSC monoculture group, and the higher average COL2A1/COL10A1 ratios in the coculture groups compared to the AC monoculture group. However, the changes in the gene expression levels did not directly translate to the levels of cartilage-like matrix production. From the histological analysis we observed that the GAG staining increased gradually with the decrease in the MSC-to-AC ratio in the coculture groups. However, although we have initially sought to decipher a combined effect of the presence of PLL and coculturing MSCs with ACs on the chondrogenesis of the overall cell population, we were unable to detect a definitive trend that supported such an effect. It is possible that we were unable to capture the changes in gene expression levels by the presence of PLL because the time frame of our study extended over the course of several weeks. Although PLL was shown to affect MSC chondrogenesis by triggering the genes that participate in skeletal development (e.g., CDH1 for N-cadherin), any changes in the expression levels of such genes occurred early on during the culture. It is also possible that the paracrine signals provided by ACs had a more significant effect in controlling the fate of MSCs compared to the presence of PLL. This is supported by the fact that no significant differences in gene expression levels between study groups with and without PLL were detected at day 42.
5. Conclusions
In this study, we investigated the effect of PLL on the chondrogenesis of MSCs and ACs using a TGM-CS dual network hydrogel system. Incorporating PLL within the hydrogel did not change its physicochemical behavior, and the majority of loaded PLL remained within the hydrogel. Despite its high charge density, PLL did not affect the viability of encapsulated MSCs over 28 days in vitro. MSC chondrogenesis was significantly improved when cocultured with ACs, as demonstrated by an upregulation in COL2A1 expression. The coculture model also successfully limited the hypertrophic gene expression compared to both MSC and AC monocultures. PLL showed transient effects on the chondrogenesis of the study groups at earlier time points, while the long-term chondrogenesis and the secretion of cartilage-like matrix were mainly governed by the ratio of MSCs to ACs present in the culture. In summary, our findings provide a platform to better understand the interplay between MSCs and ACs, and the role of PLL in promoting long-term chondrogenesis.
Highlights.
Coculturing mesenchymal stem cells (MSCs) with chondrocytes is a viable strategy to enhance the chondrogenic differentiation of MSCs in vitro.
The presence of chondrocytes limited the expression of hypertrophic genes from MSCs.
The presence of poly(L-lysine) in the hydrogel transiently affected the gene expression profiles of the encapsulated cell populations.
Ratio of chondrocytes in the culture was the biggest indicator of the degree of cartilage-like matrix secretion.
Acknowledgments
This work was supported by the National Institutes of Health (R01 AR06873, P41 EB023833). E.W. recognizes support by the National Institute of Dental and Craniofacial Research (F31 DE027586). B.T.S. acknowledges support by the and National Institute of Arthritis and Musculoskeletal and Skin Diseases (F30 AR071258). G.L.K. acknowledges the Robert and Janice McNair Foundation MD/PhD Student Scholar Program. H.A.P. and A.M.N. acknowledge support from the National Science Foundation Graduate Research Fellowship Program. Y.K. acknowledges Ang Li and Trenton C. Piepergerdes for helpful discussions on RT-qPCR analysis.
Footnotes
Manuscript submitted for consideration and publication in the special issue of the Journal of Controlled Release in honor of Dr. Jindřich Kopeček
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Detterline AJ, Goldstein JL, Rue JP, Bach BR Jr., Evaluation and treatment of osteochondritis dissecans lesions of the knee, J Knee Surg 21 (2008) 106–115. [DOI] [PubMed] [Google Scholar]
- [2].Pascual-Garrido C, McNickle AG, Cole BJ, Surgical treatment options for osteochondritis dissecans of the knee, Sports Health. 1 (2009) 326–334. 10.1177/1941738109334216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Laprade RF, Botker JC, Donor-site morbidity after osteochondral autograft transfer procedures, Arthroscopy - Journal of Arthroscopic and Related Surgery. 20 (2004) e69–e73. 10.1016/j.arthro.2004.06.022. [DOI] [PubMed] [Google Scholar]
- [4].Kock L, van Donkelaar CC, Ito K, Tissue engineering of functional articular cartilage: The current status, Cell and Tissue Research. 347 (2012) 613–627. 10.1007/s00441-011-1243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Song L, Baksh D, Tuan RS, Mesenchymal stem cell-based cartilage tissue engineering: Cells, scaffold and biology, Cytotherapy. 6 (2004) 596–601. 10.1080/14653240410005276-1. [DOI] [PubMed] [Google Scholar]
- [6].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR, Multilineage potential of adult human mesenchymal stem cells, Science. 284 (1999) 143–147. 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- [7].Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W, Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice, Arthritis & Rheumatism. 54 (2006) 3254–3266. 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- [8].Ortega N, Behonick DJ, Werb Z, Matrix remodeling during endochondral ossification, Elsevier Ltd, 2004. 10.1016/j.tcb.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ghone NV, Grayson WL, Recapitulation of mesenchymal condensation enhances in vitro chondrogenesis of human mesenchymal stem cells, Journal of Cellular Physiology. 227 (2012) 3701–3708. 10.1002/jcp.24078. [DOI] [PubMed] [Google Scholar]
- [10].Ghosh S, Laha M, Mondal S, Sengupta S, Kaplan DL, In vitro model of mesenchymal condensation during chondrogenic development, Biomaterials. 30 (2009) 6530–6540. 10.1016/j.biomaterials.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].San Antonio JD, Tuan RS, Chondrogenesis of limb bud mesenchyme in vitro: Stimulation by cations, Developmental Biology. 115 (1986) 313–324. 10.1016/0012-1606(86)90252-6. [DOI] [PubMed] [Google Scholar]
- [12].San Antonio JD, Jacenko O, Yagami M, Tuan RS, Polyionic regulation of cartilage development: Promotion of chondrogenesis in vitro by polylysine is associated with altered glycosaminoglycan biosynthesis and distribution, Developmental Biology. 152 (1992) 323–335. 10.1016/0012-1606(92)90139-8. [DOI] [PubMed] [Google Scholar]
- [13].Woodward WA, Tuan RS, N-Cadherin expression and signaling in limb mesenchymal chondrogenesis: Stimulation by Poly-l-Lysine, Developmental Genetics. 24 (1999) 178–187. . [DOI] [PubMed] [Google Scholar]
- [14].Lam J, Clark EC, Fong ELS, Lee EJ, Lu S, Tabata Y, Mikos AG, Evaluation of cell-laden polyelectrolyte hydrogels incorporating poly(l-Lysine) for applications in cartilage tissue engineering, Biomaterials. 83 (2016) 332–346. 10.1016/j.biomaterials.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schulze-Tanzil G, Mobasheri A, de Souza P, Johns T, Shakibaei M, Loss of chondrogenic potential in dedifferentiated chondrocytes correlates with deficient Shc-Erk interaction and apoptosis, Osteoarthritis and Cartilage. 12 (2004) 448–458. 10.1016/j.joca.2004.02.007. [DOI] [PubMed] [Google Scholar]
- [16].Von Der Mark K, Gauss V, Von Der Mark H, Müller P, Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture [26], Nature Publishing Group, 1977. 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- [17].Acharya C, Adesida A, Zajac P, Mumme M, Riesle J, Martin I, Barbero A, Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation, Journal of Cellular Physiology. 227 (2012) 88–97. 10.1002/jcp.22706. [DOI] [PubMed] [Google Scholar]
- [18].Bian L, Zhai DY, Mauck RL, Burdick JA, Coculture of Human Mesenchymal Stem Cells and Articular Chondrocytes Reduces Hypertrophy and Enhances Functional Properties of Engineered Cartilage, Tissue Engineering Part A. 17 (2010) 1137–1145. 10.1089/ten.tea.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hubka KM, Dahlin RL, Meretoja VV, Kasper FK, Mikos AG, Enhancing Chondrogenic Phenotype for Cartilage Tissue Engineering: Monoculture and Coculture of Articular Chondrocytes and Mesenchymal Stem Cells, Tissue Engineering Part B: Reviews. 20 (2014) 641–654. 10.1089/ten.teb.2014.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Meretoja VV, Dahlin RL, Kasper FK, Mikos AG, Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells, Biomaterials. 33 (2012) 6362–6369. 10.1016/j.biomaterials.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Levorson EJ, Mountziaris PM, Hu O, Kasper FK, Mikos AG, Cell-Derived Polymer/Extracellular Matrix Composite Scaffolds for Cartilage Regeneration, Part 1: Investigation of Cocultures and Seeding Densities for Improved Extracellular Matrix Deposition, Tissue Engineering Part C: Methods. 20 (2013) 340–357. 10.1089/ten.tec.2013.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Levorson EJ, Hu O, Mountziaris PM, Kasper FK, Mikos AG, Cell-Derived Polymer/Extracellular Matrix Composite Scaffolds for Cartilage Regeneration, Part 2: Construct Devitalization and Determination of Chondroinductive Capacity, Tissue Engineering Part C: Methods. 20 (2013) 358–372. 10.1089/ten.tec.2013.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Levorson EJ, Santoro M, Kurtis Kasper F, Mikos AG, Direct and indirect co-culture of chondrocytes and mesenchymal stem cells for the generation of polymer/extracellular matrix hybrid constructs, Acta Biomaterialia. 10 (2014) 1824–1835. 10.1016/j.actbio.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim YS, Guo JL, Lam J, Grande-Allen KJ, Engel PS, Mikos AG, Synthesis of Injectable, Thermally Responsive, Chondroitin Sulfate-Cross-Linked Poly(N-isopropylacrylamide) Hydrogels, ACS Biomaterials Science and Engineering. 5 (2019) 6405–6413. 10.1021/acsbiomaterials.9b01450. [DOI] [PubMed] [Google Scholar]
- [25].Ekenseair AK, Boere KWM, Tzouanas SN, Vo TN, Kasper FK, Mikos AG, Synthesis and Characterization of Thermally and Chemically Gelling Injectable Hydrogels for Tissue Engineering, Biomacromolecules. 13 (2012) 1908–1915. 10.1021/bm300429e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vo TN, Ekenseair AK, Kasper FK, Mikos AG, Synthesis, physicochemical characterization, and cytocompatibility of bioresorbable, dual-gelling injectable hydrogels, Biomacromolecules. 15 (2014) 132–142. 10.1021/bm401413c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Solchaga LA, Gao J, Dennis JE, Awadallah A, Lundberg M, Caplan AI, Goldberg VM, Treatment of Osteochondral Defects with Autologous Bone Marrow in a Hyaluronan-Based Delivery Vehicle, Tissue Engineering. 8 (2002) 333–347. 10.1089/107632702753725085. [DOI] [PubMed] [Google Scholar]
- [28].Zheng CH, Levenston ME, Fact versus artifact: Avoiding erroneous estimates of sulfated glycosaminoglycan content using the dimethylmethylene blue colorimetric assay for tissue-engineered constructs, European Cells and Materials. 29 (2015) 224–236. 10.22203/eCM.v029a17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Benya PD, Padilla SR, Nimni ME, Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture, Cell. 15 (1978) 1313–1321. 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- [30].Duan L, Ma B, Liang Y, Chen J, Zhu W, Li M, Wang D, Cytokine networking of chondrocyte dedifferentiation in vitro and its implications for cell-based cartilage therapy, Am J Transl Res 7 (2015) 194–208. [PMC free article] [PubMed] [Google Scholar]
- [31].Guo JL, Li A, Kim YS, Xie VY, Smith BT, Watson E, Bao G, Mikos AG, Click functionalized, tissue-specific hydrogels for osteochondral tissue engineering, Journal of Biomedical Materials Research - Part A. 108 (2020) 684–693. 10.1002/jbm.a.36848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim YS, Guo JL, Lam J, Grande-Allen KJ, Engel PS, Mikos AG, Synthesis of Injectable, Thermally Responsive, Chondroitin Sulfate-Cross-Linked Poly(N-isopropylacrylamide) Hydrogels, ACS Biomaterials Science and Engineering. 5 (2019) 6405–6413. 10.1021/acsbiomaterials.9b01450. [DOI] [PubMed] [Google Scholar]
- [33].Kinard LA, Kasper FK, Mikos AG, Synthesis of oligo(poly(ethylene glycol) fumarate), Nat Protoc 7 (2012) 1219–1227. 10.1038/nprot.2012.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Aisenbrey EA, Bryant SJ, The role of chondroitin sulfate in regulating hypertrophy during MSC chondrogenesis in a cartilage mimetic hydrogel under dynamic loading, Biomaterials. 190–191 (2019) 51–62. 10.1016/j.biomaterials.2018.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J, Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells, Matrix Biology. 27 (2008) 12–21. 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- [36].Cooke ME, Allon AA, Cheng T, Kuo AC, Kim HT, Vail TP, Marcucio RS, Schneider RA, Lotz JC, Alliston T, Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy, Osteoarthritis and Cartilage. 19 (2011) 1210–1218. 10.1016/j.joca.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dy P, Wang W, Bhattaram P, Wang Q, Wang L, Ballock RT, Lefebvre V, Sox9 Directs Hypertrophic Maturation and Blocks Osteoblast Differentiation of Growth Plate Chondrocytes, Developmental Cell. 22 (2012) 597–609. 10.1016/j.devcel.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jung Y-K, Park H-R, Cho H-J, Jang J-A, Lee E-J, Han M-S, Kim G-W, Han S, Degrading products of chondroitin sulfate can induce hypertrophy-like changes and MMP-13/ADAMTS5 production in chondrocytes, Scientific Reports. 9 (2019) 15846 10.1038/s41598-019-52358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


