Abstract
Introduction:
Cardiac rehabilitation (CR) significantly reduces secondary cardiovascular events and mortality and is a class 1A recommendation by the American Heart Association (AHA) and American College of Cardiology (ACC). However, it remains an underutilized intervention and many eligible patients fail to enroll or complete CR programs. The aim of this review is to identify barriers to CR attendance and discuss strategies to overcome them.
Areas Covered:
Specific barriers to CR attendance and participation will be reviewed. This will be followed by a discussion of solutions/strategies to help overcome these barriers with a particular focus on home-based CR (HBCR).
Expert Opinion:
HBCR alone or in combination with center-based CR (CBCR) can help overcome many barriers to traditional CBCR participation, such as schedule flexibility, time commitment, travel distance, cost, and patient preference. Using remote coaching with indirect exercise supervision, HBCR has been shown to have comparable benefits to CBCR. At this time however, funding remains the main barrier to universal incorporation of HBCR into health systems, necessitating the need for additional cost benefit analysis and outcome studies. Ultimately the choice for HBCR should be based on patient preference and availability of resources.
Keywords: barriers, cardiac rehabilitation, home-based cardiac rehabilitation, strategies, telerehabilitation
1.0. Introduction
Cardiovascular disease (CVD) is a significant cause of morbidity and mortality and places a large burden on healthcare systems [1]. While improved medical treatments and management of CVD risk factors have decreased mortality, this has paradoxically increased the need for secondary prevention as more patients survive their initial CVD event and live longer.
Cardiac rehabilitation (CR) is a multifaceted and comprehensive outpatient intervention designed to improve the physical and psychological health of patients with CVD. Through a combination of monitored exercise training, health and nutrition education, and psychological support, CR has been shown to lower patients’ risk of CVD mortality by 26%, reduce one-year hospital readmissions by 31%, and decrease patients’ five-year all-cause mortality by up to 34% [2]. A Cochrane review by Anderson et al. showed that exercise-based CR after myocardial infarctions significantly improved cardiovascular mortality, reduced hospital admission and improved quality of life [3]. This mortality benefit from participation in CR extends to all ages, sex, and ethnic groups. However, it is important to note that benefits of CR are variable across different cardiovascular conditions with the strongest mortality and morbidity benefits after acute myocardial infarction (MI) and coronary artery bypass graft surgery (CABG). In those with heart failure with reduced ejection fraction (HFrEF), CR was not associated with reduced mortality or hospital admission, but did improve quality of life [4]. Cardiac rehabilitation has also shown to significantly reduce the severity of depression and cognitive impairment, particularly among the elderly [5].
Due to these significant aggregate benefits, the AHA/ACC give CR a class IA recommendation for secondary prevention after a ST-elevation MI (STEMI)/non‐ST‐elevation MI(NSTEMI)/unstable angina (UA) [6], percutaneous coronary intervention (PCI) [7], coronary artery bypass grafting (CABG) [8], stable angina [9], and symptomatic peripheral arterial disease (PAD) [10,11]. Additionally, CR is also recommended after heart valve surgery [12], cardiac transplantation [13], or in the setting of chronic heart failure (HF) with reduced ejection fraction <35% [4,14].
Unfortunately, despite the well-established benefits and strong endorsement from professional societies, CR remains underutilized by patients with CVD. Fewer than 20% of all eligible patients participate in CR, and of those who are referred, only 34% actually enroll [15]. This is attributed to several barriers including lack of strong physician recommendation, transportation issues, and high out of pocket costs[16,17]. The aim of this paper is to review the current literature and identify barriers to CR utilization and discuss strategies to overcome them.
Cardiac rehabilitation delivery in different countries varies significantly with respect to structure, availability, integration into the healthcare systems, and reimbursement [18-20]. These differences in structure and opinions of CR models around the world raise assorted barriers and require unique solutions, accounting for unique differences in healthcare systems and patient populations. Given the significant heterogeneity in CR programs in different countries, our review will be limited to CR models based in the United States.
2.0. Barriers to CR
Despite being a class IA recommendation by ACC and AHA, referral and participation rates to CR programs after a coronary event remain low. Literature shows that 50–70% of patients eligible for CR do not attend CR and, amongst those that do attend, 30–60% do not complete CR [21-25]. Importantly, CR has a dose response relationship where patients who attend more CR sessions have lower mortality than those that attend fewer sessions [26]. This mortality benefit can be as significant as up to 1% per CR session attended [26,27]. Suaya and colleagues found that CR participation rates were higher after CABG than after MI [28]. This discrepancy may be due to the fact that CR referral rates are higher after cardiac surgery than after PCI [29] and highlights discrepancies in referral practices in the management of post-MI patients.
These statistics represent missed opportunities for patients to receive evidence-based therapy. The reasons behind low CR participation and completion rates are multifactorial and include physician‐, patient‐, and system-related factors. A summary of barriers and solutions to CR barriers can be found in Table 1.
Table 1.
Outline of barriers to CR participation and potential solutions to overcome them. References noted in brackets.
| Barriers to CR participation | Potential Solutions |
|---|---|
| Low CR Referral Rates [28-30] | |
| Inadequate Physician Endorsement [31-35,74,75] | |
| Gender Disparity [36-42,44] | |
| Racial or Ethnic Disparities [30,47-49] | |
| Poor Physical Health [23,36,40,42,54] | |
| Language Barriers [50,51] | |
| Socio-economic factors [28,31,36] | |
| Psychological Factors [55,56,61-65,139] |
|
| Travel Distance to CR Facility [16,67-71] | |
| Cost of CR [17,72] | |
| Fragmented Care [73] |
2.1. Physician Factors
2.1.1. Low Referral Rates
Participation in a CR program must be initiated by a physician referral. Data from the ACC’s Action registry showed that only 62% of eligible patients above age of 65 years were referred for CR [29]. This study also found significant geographic heterogeneity – with referral rates higher in the Midwest and lower in the South (30% vs 10%, respectively) [28]. Aragram et al. noted that on average, only 60% of all patients undergoing PCI were referred for CR. In comparison, prescription rates for preventive medications were significantly higher - 97.5% for aspirin and 89.8% for statins [30]. Study authors also note physicians may be more inclined to refer patients who are presumed likely to participate in and benefit from a CR program, instead of referring all patients as a component of post-procedural care [30]. The EUROASPIRE IV survey revealed that older patients, women, and those of low socioeconomic status (SES) were less likely to be advised to participate in CR [24]. This physician bias can potentially deprive patients of receiving evidence-based therapy.
2.1.2. Physician Endorsement
The corollary to low referral rates is lack of physician endorsement. One study showed that primary care physicians are less likely to refer patients to CR than CVD specialists [31,32]. The lack of physician endorsement results in poor patient understanding of the benefits of CR and decreased motivation to participate. In a systematic review, Elsakr et al. found that this lack of physician endorsement is partly attributed to a lack of physician knowledge on what CR entails, its benefits, and appropriate patients for referral [33]. This is due in part to inadequate education and exposure to CR programs in medical education and training. [34]. In addition, Ghisis et al. cite provider’s lack of awareness of CR availability in the area they practice and how to place CR referrals as critical factors to poor endorsement and low CR referral rates [32]. Other factors related to poor physician endorsement may include professional skepticism about the efficacy of CR and/or the ability or motivation of individual patients to make meaningful lifestyle changes [35]. This skepticism and knowledge gap results in patients receiving inadequate information on what CR entails and their ability to enact meaningful lifestyle changes.
2.2. Patient Factors
2.2.1. Gender and Racial Disparities
Numerous studies have shown that women are less likely than men to be referred to CR programs. While 47% of CVD patients are female, they account for only 20% of all CR participants [36-41]. Referral bias and lack of strong physician endorsement are two of the main reasons why women fail to enroll in CR [31,37,42]. The reasons behind this gender disparity are multifactorial and may be attributable to 1) women’s atypical presentation for MI [37], 2) women’s older age at presentation of MI [31,36,40], 3) lower socioeconomic status of women compared to men [43], 4) insufficient or lack of health insurance [40], and 5) the perceived bias that women with comorbidities are less likely to complete CR programs as compared to their male counterparts [41].A qualitative systematic review by Clark et al. found that that women prioritized the needs of partners and children over their own, often downplaying their own health needs [44]. Furthermore, women often feel out of place in traditional male dominated CR programs and uncomfortable speaking in these groups leading them to avoid joining or to drop out prematurely [45,46].
In addition to gender disparities, racial and ethnic factors also contribute to lower CR referral and completion rates. Several studies have shown lower CR referral rates for non-white individuals as compared to white individuals [47-49]. In a study by Aragam et al., hospitals with high CR referral rates (> 90%), reported no difference in CR referrals between white vs. non-white patients [30]. However, the difference in referral rates for non-white individuals when compared with white individuals was statistically significant in hospitals with low overall CR referral rates (< 10%). This suggests that high overall CR referral rates can overcome racial biases. Analysis of a large US based registry showed that compared with white patients, minorities, particularly Black, Hispanics and Asian patients, were 20%, 36% and 50% less likely to receive CR referral after an MI [40]. Barriers in communication are a likely contributor to this discrepancy [50,51]. The inability to speak English as a native language can lead patients to feel marginalized, excluded, and anxious - resulting in lower enrollment and higher drop-out rates among minorities.
2.2.2. Medical Comorbidities
The presence of multiple medical comorbidities - including stroke, diabetes, HF, chronic kidney disease or chronic pulmonary disease - is another important factor associated with a lower odds of referral to, participation in, and adherence to CR [52,53]. This is in part due to physician perceptions that these populations are too debilitated to meaningfully participate in CR [24]. In addition, it is conceivable that these fragile populations are more likely to suffer repeat setbacks and rehospitalizations, thus limiting their ability to participate and complete CR programs. Borg et al. found that a high burden of comorbidities was associated with CR non-attendance [23]. Paradoxically, while these individuals may benefit the most from CR participation, they are more likely to drop out due to their inability to meet the physical demands of CR participation [54]. It is important to note that women with cardiovascular disease often have greater comorbidities than their male counterparts accounting for some of gender disparities described previously [36,40,42].
2.2.3. Socioeconomic Factors
In general, research has found that patients with lower income, education, and socioeconomic status (SES) have lower CR participation rates than their higher SES counterparts [31]. This has been replicated in several studies where patients living in zip codes with the highest median household income were 23% more likely to participate in CR than those living in zip codes with the lowest median income [28]. Lower SES patient’s often have inadequate health insurance coverage and receive fewer health benefits resulting in higher CR copayments and out of pocket costs of CR attendance [31]. In addition, lower SES patients lack transportation to CR facilities and access to reliable childcare to attend classes [30]. Finally, those of low SES are more likely to carry negative views of health systems in general, leading to mistrust of CR programs which often take place at medical centers [36].
2.2.4. Psychological Factors
In addition to SES factors, psychological factors can also decrease patient CR attendance [55,56]. Literature reveals that about one in five patients meet diagnostic criteria for depression while hospitalized for a cardiac event, and up to one in three experience severe anxiety [57-60]. Patients with depression are three times as likely to be noncompliant with physician recommendations [61]. Additionally, patients with depression are less likely to engage in self-care, adhere to medications, and have increased healthcare utilization, emergency room visits, and hospital readmissions [61-64]. In a retrospective cohort study, patients with moderate depression, anxiety, or stress were significantly less likely to adhere to cardiac rehabilitation compared with those normal to mild symptoms [65]. Ironically, while CR programs are designed to improve the psychological wellbeing of participants [66], those who are most likely to receive these benefits (i.e. those with depression and anxiety) are also less likely to participate.
2.3. Systemic Factors
2.3.1. Travel and Transportation
Distance from CR programs, travel time, cost of travel, and lack of transportation constitute some of the key barriers to CR attendance [67-69]. In rural areas, studies have shown an inverse relationship between distance to a CR center and likelihood of patient enrollment [70]. In a registry-based study, a distance greater than 16 km from a CR center was associated with increased probability of non-attendance [23]. Similarly, Brual et al. showed that a driving time of 60 minutes or more to the nearest CR center was associated with both decreased CR referral and enrollment [71]. Lastly, in a prospective cohort study of 184 patients after CABG, travel time significantly predicted CR attendance[69]. Due to lack of CR availability, patients living in rural areas are less likely to participate in CR than those in urban areas where CR facilities are closer [67].
2.3.2. Cost of Attendance
Costs of attending CR can pose a major barrier to participation [72]. Uninsured or underinsured patients often cannot afford the out-of-pocket cost of CR programs. Medicare and some private insurers cover CR for patients who have had MI, CABG, PCI, HF and several other conditions. Most coverage is for two or three hour-long visits per week, up to 36 sessions. However, many private insurances require patients to pay high co-payments per CR session. Patient copayments are approximately $20 for Medicare beneficiaries and range from $0 to $100 for individuals with employer-sponsored or Medicare Advantage coverage. For insured patients who chose to attend all 36 sessions, total CR costs can range from $720 to $3600. Additionally, indirect costs due to missed employment, transportation, parking, child or eldercare can result in significant financial barriers to CR participation. Moreover, these costs can have immense financial ramifications for patients who are experiencing reductions in salary due to medical illness and whom employment based medical coverage remains uncertain.
2.3.3. Fragmented Care
Care delivery systems in the United States are fragmented such that patients are no longer treated by a single physician, but rather by a wide array of healthcare professionals across multiple specialties and health care settings [73]. Failures in communication and care coordination can negatively impact CR enrollment and participation. Due to the concentration of CR centers in large urban areas, community and rural physicians often have to refer eligible patients outside their network. Referring physicians may be unaware of what CR programs are available and how to initiate a referral to an outside facility [32]. Once a CR referral has been placed, communication between physicians and CR centers can be fragmented - CR centers receive little information on a patient’s medical history and referring physicians receive little information on a patient’s progress or complications experienced while enrolled at a CR center. These failures in communication can erode relationships between physicians and CR centers leading to poor referral chains and ultimately preventing patient from receiving evidence-based care.
3.0. Solutions
3.1. Physician and Patient Education
Strong physician recommendation for CR participation is one of the most powerful predictors for patient enrollment [74]. This effect is even more pronounced amongst women and the elderly [75]. To help overcome inadequate physician endorsement, physicians need to be better educated on the indications and benefits of CR. For physicians in training, this can be accomplished by introducing CR into the curriculum of medicine residencies and fellowships through didactics or first-hand exposure to CR centers. For physicians who have completed their training, this barrier can be addressed by including CR education in medical conferences and continuing medical education credits [44,76,77]. Increasing education and outreach to primary care physicians and cardiologists in the community can lead to more informed discussions of CR with patients and knowledge of what programs are locally available.
Patient education on the benefits of CR can increase enrollment. In a randomized control trial by Lynggaard et al, educating patients on coping strategies significantly improved adherence to CR treatment, especially among those with lower SES and heart failure [78]. Patient education on benefits of CR is not solely the physician’s responsibility. Nurses, physician assistants, dieticians, and physical therapists are routinely involved in the inpatient care of individuals recovering from a CVD event. Nurses are intricately involved in patient care and discharge planning. They are in a crucial position to identify eligible patients and discuss benefits of outpatient CR participation [79]. Similarly, physical therapists routinely assess and help improve a patient’s functional status while recovering from a CVD event. They can play a key role in CR education and help identify and address any physical barriers to exercise [80].
Beyond endorsements for CR by physicians and other healthcare workers, tools such as educational videos have also been efficacious. Demonstration of an educational video on CR, diet, and exercise delivered by a health care professional just prior to hospital discharge has been shown to increase compliance with exercise and dietary recommendations [81]. Other tools such as structured telephone or home visits by a nurse or physical therapist after hospital discharge [81,82], close follow up at CR centers [83,84], and motivational letters [83] have also been shown to be effective in increasing CR participation. These strategies help educate patients on the benefits of CR and reinforce its importance to their cardiac recovery.
3.2. Standardization of CR Referrals
Streamlining or automating CR referrals at discharge for eligible patients can dramatically increase CR participation. Automatic electronic CR referral in combination with patient CR education has been shown to increase CR referral rates from 32% to 86% [35,85]. Automating CR referrals can also prevent referral biases due to sex, race, SES, and existence of comorbid conditions. Cardiac rehabilitation referral and completion rates have been proposed as quality indicators in cardiovascular care [86]. One study demonstrated a significant 8% absolute improvement in CR referral rates in hospitals using CR referral as a quality metric [29]. These measures if considered by accredited organizations such as the ACC, AHA, Agency for Health Care Research and Quality, National Committee for Quality Assurance, and Joint Commission on Accreditation of Health Care Organizations would help increase appropriate CR awareness and enrollment. Furthermore, if CR referral rates were adopted by Medicare in its pay-for-performance initiative, the financial incentives for both the discharging hospital and physician may further motivate clinicians to refer eligible patients [87,88].
3.3. Inclusivity and Representation
As previously discussed, ethnicity, language, and gender can pose significant barriers to CR participation. Prioritizing diversity and hiring CR staff of various ethnic backgrounds and languages can help overcome some of these barriers. Healthcare providers from various ethnic backgrounds may possess culturally specific knowledge, skills and experience that enhance rapport with patients resulting in improved patient compliance and retention. One study by Traylor et al. found that patients were more adherent to medications prescribed by a physician of the same ethnic background [89]. Moreover, when physicians communicate with patients of similar cultural backgrounds, patients are less likely to postpone or delay seeking medical care [90]. Overall, ethnicity and language concordance fosters trust, communication and better patient-provider relationships [91]. Finally, the creation of women-only or women-dominated CR groups has been proposed as a means to increase female participation and retention. The creation of women-only CR groups has been shown to improve diet compliance and decrease symptoms of depression and anxiety leading to improved CR adherence [92-94].
3.4. Home-Based Cardiac Rehabilitation
In order to reach more than 80% of eligible patients in the US who do not participate in CR, alternative care delivery models need to be considered [77]. Home-based CR or alternative site-CR where CR is carried out at home or other non-clinical settings (community centers, health clubs, and parks) increases patient accessibility and circumvents many barriers encountered by CBCR programs. Home-based CR services can be used at any time and at any location, whereas CBCR programs are usually limited to 3–4 hours of scheduled weekly in-person contact. This gives HBCR participants the flexibility to engage in CR on their own time and bypasses driving distance, travel costs, time away from work, or childcare obligations which can increase CR participation and completion rates. Studies have shown that participants limited by time constraints – due to family and work obligations – prefer HBCR to CBCR due to its increased flexibility [95]. Moreover, due to the ongoing coronavirus disease 2019 (COVID-19) pandemic and the closure of many CBCR programs due to concerns of center-based transmission, HBCR may be a safer and readily available alternative or adjunct to traditional CBCR [96].
3.4.1. Home-Based Cardiac Rehabilitation Core Components
In order for HBCR to be effective the core components of HBCR should remain similar to those of CBCR. These components include baseline patient assessment, physical activity, nutrition counselling, risk factor management (blood pressure, weight, lipids, diabetes mellitus, smoking), medication adherence, and psychosocial management. Almost all CR programs begin with an in-person assessment of patient functional status, review of past medical history, and medications. For HBCR, this component can be carried out in the final days of hospitalization, in-person after discharge, or remotely via a video or telephone conference call by physical therapists, nurse practitioners, or physicians. If considering a hybrid center-based and home-based CR model, this can be accomplished on the initial visit at the CR center. At this initial visit, a tailored workout and eating plan can be developed for the patient to follow. As in CBCR, HBCR will also include management of lipids, blood pressure, diabetes mellitus, adherence to cardioprotective medications (such as antiplatelet agents, β-blockers, angiotensin inhibitors, and statins) and assessment of psychological wellbeing. These components can be carried out through regular check-ins via telehealth phone/video calls throughout the enrollment period from a multidisciplinary team. These calls should be completed at structured intervals to ensure patients stay on compliant with prescribed dietary and lifestyle changes.
For physical activity and dietary documentation, smartphones are an appealing healthcare delivery device as they enable personal data collection and delivery of health interventions and reminders. Physical activity can be supervised indirectly through the use of wearable devices while smartphones can remotely transmit data to web-based portals for clinicians to review and provide feedback during their scheduled telehealth calls. These devices can include pedometers, accelerometers, and heart rate monitors that are often incorporated into most smartphones and wearable devices. Furthermore, smartphone cameras and nutritional content applications enable patients to log their dietary intake and receive direct feedback on improvements that can be made at home.
3.4.2. Home-Based Cardiac Rehabilitation Outcomes
While HBCR can potentially expand patient-access and participation, there is concern that lack of direct supervision and physical interaction with healthcare staff will dilute the physical and psychological benefits demonstrated by CBCR. This question was studied in an unblinded randomized control trial by Varnfield et al. comparing the outcomes of patients enrolled in a smartphone-based CR (via CAP-CR platform) vs. traditional CBCR. In the smartphone-based CR group, patients received communications and educational offerings on a regular basis from healthcare professionals via their smartphone and exercised at home or in a local community health center. The study’s investigators showed that smartphone-based CR had significantly higher uptake (80% vs 62%), adherence (94% vs 68%) and completion (80% vs 47%) rates when compared to CBCR [97]. In addition, the smartphone-based CR group had similar improvements in 6-minute walk test compared to CBCR which was maintained at 6 months, and was as effective in improving physiological and psychological health outcomes compared to CBCR [97]. These findings have been replicated by several studies [98-103]. In a Cochrane review comparing HBCR to CBCR, there was no significant difference noted in mortality, exercise capacity, and cardiac events [104]. However, care must be taken when extrapolating these results into long-term outcomes as few trials have studied cardiovascular endpoints beyond 12 months. Trials examining long-term outcomes of HBCR are necessary and forthcoming. Furthermore, it is important to note that self-motivation and self-efficacy are key components to ensuring patients remain adherent to HBCR [105]. Since HBCR lacks direct supervision and relies on high patient motivation, it is only a reasonable alternative or adjunct to CBCR in highly motivated low- to moderate-risk patients [106].
3.4.3. Home-Based Cardiac Rehabilitation Platforms
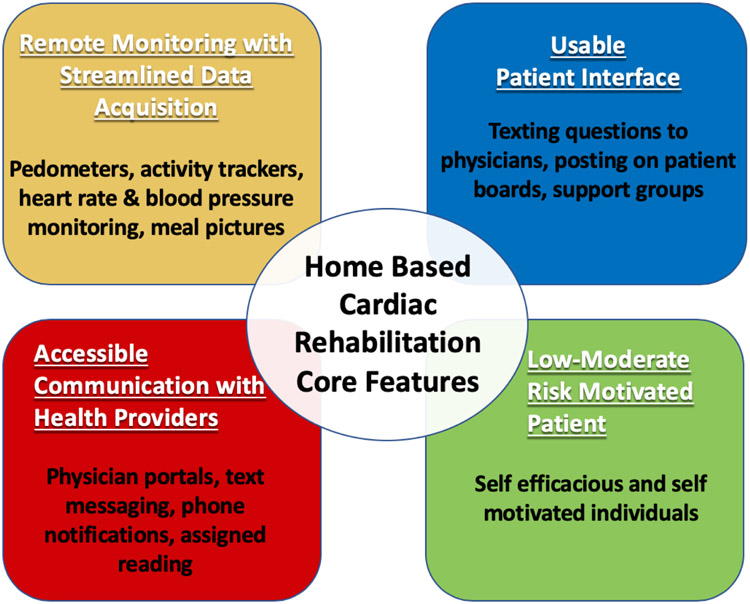
Advancements in wearable devices and mobile technologies make access to HBCR more universal and allow for the remote delivery of clinical expertise, supervision, and coaching that has traditionally been limited to CBCR programs. In order for these platforms to be successful, they need to satisfy four core components as described in Figure 1. First is the ability to remotely monitor, record, and transmit the health data of participants. This can be accomplished via smartphone applications that incorporate measurements of heart rate, steps, distance traveled, medications taken, food groups eaten, etc. Second, as HBCR lacks direct supervision and monitoring of exercise, platforms need to incorporate frequent healthcare provider communication to increase patient accountability. This can be achieved in several formats from phone notifications, text messages, phone calls to participants, reminders to log nutritional intake and exercise followed prompt feedback. Third, patient interfaces need to be tailored to maximize usability. This can be achieved by maximizing the automatic collection of data, such as objectively‐measured physical activity thereby reducing the burden on the individual to track and log data [107]. Patient interfaces also need to incorporate social support and access to healthcare providers when necessary. In order to ensure good adherence to HBCR programs, participants need to be self-motivated [105] and low- to moderate-risk individuals[106]. Lastly, these platforms need to be able to handle patient data securely and confidentially in accordance with Health Insurance Portability and Accountability Act of 1996 (HIPPA) guidelines.
Figure 1.
Key features for home-based cardiac rehabilitation to be successful.
Currently, there are several HBCR platforms being developed to help hospitals easily adopt HBCR into their infrastructure. Two examples of these platforms include REMOTE-CR [108] and Movn Analytics [109]. Both have been shown in literature to be improve functional capacity and overall patient satisfaction [107,108].
REMOTE-CR is a 12-week program of individualized exercise (three exercise sessions per week of 30–60 minutes), monitoring, and coaching with theory-based behavioral strategies to promote physical activity. The REMOTE-CR platform comprises of a smartphone and chest-worn wearable sensor (BioHarness 3, Zephyr Technology, USA), which provides information on heart and respiratory rates, single lead ECG, and accelerometry [108]. The program also incorporates goal setting and behavioral change messages delivered in audio format to participants throughout the program. In a randomized clinical trial, participants using REMOTE-CR had similar maximal oxygen uptake at 12-weeks compared to CBCR participants. Additionally, REMOTE-CR was found to be more cost-effective than CBCR [110].
Movn is another smartphone-based virtual CR program commercially available as Moving Analytics. The 12-month program is based on MULTIFIT (a rehabilitation program developed by Stanford University and Kaiser Permanente). MULTIFIT has been shown in randomized clinical trials to improve outcomes and reduce readmissions for patients with CHD, HF, hypertension, acute coronary syndrome and diabetes [109,111,112]. The program features a smartphone application with daily reminders to follow their exercise prescription, log vitals, and review educational materials. Participants also receive weekly coaching sessions to provide education, emotional support, and counselling on risk factors. Additionally, this platform utilizes social media to provide group support and encouragement for participants.
3.4.4. Home-Based Cardiac Rehabilitation Endorsement
While HBCR programs are still in their infancy, several guidelines have already adopted its use in certain populations. The ACC/AHA consider HBCR “a reasonable option” for eligible low- to moderate-risk patients who cannot attend a traditional center-based CR program [106]. Moreover, Cochrane reviews of combined randomized trials comparing CBCR and HBCR demonstrate similar effects on quality of life and cost among patients with recent MI or PCI/CABG [113].
3.5. Limitations of Home-Based Cardiac Rehabilitation
Despite the numerous advantages of HBCR, there remain significant limitations. Unlike CBCR where patients are embedded in therapeutic groups, HBCR lacks the formal group support and face-to-face interactions with CR staff. In-person group counseling and education sessions are vital components of CBCR programs and provide additional benefit to remote telephone and video sessions. A study comparing the efficacy of cognitive behavioral therapy in patients with depression via telephone-based counseling versus in-person counseling found similar improvements in depression after 18 weeks of therapy and improved adherence in telephone based group compared to in-person group [114]. At 6 months, however, patients who received in-person counseling were more likely to maintain their therapeutic gains than those in the telephone-based group suggesting an added benefit to face-to-face interactions [114].
Home-based cardiac rehabilitation programs also lack the framework for participants to interact with and support one another. The group effect and the belief that “they are not alone” can increase adherence to dietary and lifestyle changes. Using a peer support group, particularly one involving health care worker has been shown to improve adherence to CR [115,116]. Overall, group therapy allows individuals to feel less isolated and better understood. This can result in decreased stress and anxiety, as well as an increased sense of identity and belonging.
3.5.1. Patient Selection and Accountability:
Another potential limitation is that lack of in-person supervision in HBCR can result in decreased patient accountability and adherence. Remote monitoring and frequent healthcare provider feedback and encouragement may help motivate patients to a degree but is less effective than in-person real time feedback [117]. Thus, HBCR participants need to demonstrate both self-efficacy and motivation to derive the maximal benefit from HBCR. Individuals with emotional distress, depression, and anxiety have poorer coping mechanisms and have higher rates of attrition potentially making them less ideal candidates for HBCR[55,56]. Lastly, in HBCR the clinical team’s assessment depends on patient’s subjective reports, which may not accurately reflect their condition. Altogether, group-based dynamics and in-person support provide positive social reinforcements to participants in CBCR, whose effects are difficult to replicate in the HBCR setting. Given these limitations, it is worth considering pre-screening and risk stratifying individuals utilizing scales such as the Cardiac Rehabilitation Barrier scale (CRBS) to appropriately identify individuals best suited for HBCR [118].
3.6. Policy Change and Reimbursement
Home-based CR has been incorporated into the healthcare systems of several countries, including Australia, Canada, and the United Kingdom. Unfortunately, HBCR faces substantial challenges to be fully implemented in the United States. The most notable challenge is lack of reimbursement for HBCR by Centers for Medicare & Medicaid Services and other third-party payers necessitating policy changes [119]. While Medicare and Medicaid Services recently agreed to cover telehealth services due to the COVID-19 pandemic, HBCR services were not among them [96]. As the results of HBCR outcome studies become available, the authors are optimistic that policy changes will support HBCR reimbursement. Cost-analysis studies on HBCR are limited, but emerging studies suggest HBCR to be just as cost-effective as CBCR [120,121]. Two trials, EU-CaRE [122] and eEduHeart I [123], are already ongoing to assess the efficacy of remotely delivered CR. With the growing trend toward shared-risk and bundled payment models, therapeutic HBCR platforms can may be cost-effective alternatives or adjuncts to traditional CBCR programs.
In addition to lobbying for nationwide coverage for HBCR, individuals with insurance also face financial burdens in the form of co-payments, transportation, and parking costs. Arranging for shuttle transportation or simply providing free parking to CR participants can help overcome these barriers. To further encourage adherence to CR programs, financial incentive programs have been proposed as a solution to ensure participants complete all CR sessions. Financial incentive programs have been shown to improve compliance with smoking cessation [124], weight loss [125], and workplace physical activity [126]. Recently, in a randomized clinical trial financial incentives were shown to increase CR attendance [127]. The study randomized 130 Medicaid beneficiaries with CR-qualifying cardiac events 1:1 to receive usual care or financial incentives for completing CR sessions. Patients who received incentives to attend CR completed, on average, seven and a half more sessions than controls (22.4 vs. 14.7, respectively), and were nearly twice as likely to complete the CR program—55.4% vs. 29.2% of controls. Participants who were randomized to the incentive arm earned an average of $716 over the study period, or $32 per session. The base pay for attending a session was $4, and incentives increased $2 per consecutive session attended for a maximum potential sum of $1,238. These findings suggest financial incentives can potentially increase CR completion rates.
While financial incentive programs have been shown to effectively increase CR participation and adherence, caution must be taken as these incentive programs have the potential to coerce behavior, undermine individual’s autonomy, and target a skewed population most likely to benefit from financial incentives. Furthermore, there is a likelihood that patients will revert back to their original behaviors and habits once monetary incentives are discontinued – diluting the cardiovascular benefits of CR over time [128-130].
4.0. Conclusion
Cardiac rehabilitation programs are multidisciplinary outpatient interventions that focus on individualized exercise prescription and lifestyle modification in an effort to support CVD risk reduction and medication adherence. Cardiac rehabilitation has significant mortality and morbidity benefits in wide classes of CVD patients including those with acute coronary syndrome, CABG, PCI, HF, and peripheral arterial disease earning it a class 1A indication by ACC/AHA. Despite these benefits, there continues to be significant underutilization of CR. Barriers to CBCR use are multifactorial and include low referral rates, lack of physician endorsement, patient time constraints, transportation issues, and cost. Beyond automating referrals and empowering physicians to educate patients on the benefits of CR, utilization of HBCR can help overcome many of the barriers encountered by CBCR (Table 1). HBCR employs a patient-centered approach through the use of home-based exercise programs coupled with smartphone applications to track heart rate, blood pressure, glucose, lipids, body weight, and daily activity levels, along with telehealth- or text-based coaching and motivational strategies to keep patients on track. Via remote monitoring and telehealth check-ins, HBCR programs not only increase accessibility, but can also provide comparable health benefits when compared to CBCR in appropriate motivated low-moderate risk patients. This therapeutic platform is still in its infancy, and research in optimizing its delivery and utilization is ongoing. As wearable technologies continue to develop and new communications strategies evolve, HBCR services will expand to include platforms that are capable of overseeing a patient’s recovery continuously. Moreover, the creation of online CR groups and the utilization of social media can partially recreate the benefits of in person group therapy and more research is needed in this area.
Home- based cardiac rehab has been successfully employed in United Kingdom, Canada, and Australia. However, most US healthcare organizations have little to no experience with such programs. Wide-scale adoption of HBCR platforms will require further outcome studies and cost-benefit analyses comparing HBCR to CBCR. As more data on the efficacy of HBCR emerges, policy changes and insurance converge for these alternative delivery systems will surely follow.
With the ongoing Coronavirus (COVID-19) pandemic, there has been an impetus for CR delivery via mobile health platforms and social media [131-134]. Home based cardiac rehab is even more vital now as it maintains social distancing, keeps patients out of hospitals, encourages healthy eating at home, provides virtual mental and emotional support, and reinforces efforts to quit smoking. With closure of CR centers to prevent spread of infection, there is no better time for CR providers to explore and implement HBCR in order to provide continued secondary prevention to patients with CVD. The time has come for patients, clinicians, insurers, and health systems to incorporate what is already digitally possible.
Article highlights:
Cardiac Rehabilitation is an important component in the continuum of care for patients with cardiovascular diseases and provides numerous benefits including increased functional capacity, decreased hospitalizations, secondary cardiovascular events, and mortality.
Despite these benefits and the increasing burden of cardiovascular disease, participation and completion of CR programs by eligible patients remain low.
Physician barriers to CBCR include low referral rates and inadequate physician endorsement.
Patient barriers to CBCR include gender bias, racial, socioeconomic, and psychological factors, language barriers, and poor physical health.
Systemic barriers to CBCR include distance to CR centers, cost of CR, and fragmented care between CBCR programs and referring physicians.
Potential solutions to these barriers include standardized or automated referrals to CR, increasing exposure to CR during medical training, increasing CR staff diversity, travel reimbursements, providing financial incentives for CR completion, and enrolling appropriate patients in HBCR.
HBCR circumvents many barriers to traditional CBCR participation by utilizing remote monitoring and personalized risk factor management to engage patients to improve their cardiovascular health.
The use of HBCR, either alone or in combination with CBCR, represents a potential alternative that may improve the delivery of CR to eligible patients.
Additional research is needed on HBCR outcomes and per-patient cost analysis before Medicare and third-party insurers consider coverage for these interventions.
With the Coronavirus (COVID-19) pandemic and need for social distancing, HBCR provides a unique opportunity for patients to continue receiving CR despite closure of CBCR programs.
Acknowledgments
Funding
This paper was not funded.
Footnotes
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Mozaffarian D, Benjamin EJ, Go AS et al. Executive Summary: Heart Disease and Stroke Statistics−-2016 Update: A Report From the American Heart Association. Circulation, 133(4), 447–454 (2016). [DOI] [PubMed] [Google Scholar]
- 2.McMahon SR, Ades PA, Thompson PD. The role of cardiac rehabilitation in patients with heart disease. Trends Cardiovasc Med, 27(6), 420–425 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson L, Thompson DR, Oldridge N et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev, (1), CD001800 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjarnason-Wehrens B, Nebel R, Jensen K et al. Exercise-based cardiac rehabilitation in patients with reduced left ventricular ejection fraction: The Cardiac Rehabilitation Outcome Study in Heart Failure (CROS-HF): A systematic review and meta-analysis. Eur J Prev Cardiol, 27(9), 929–952 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campo G, Tonet E, Chiaranda G et al. Exercise intervention improves quality of life in older adults after myocardial infarction: randomised clinical trial. Heart, (2020).**A randomized controlled trial demonstrating supervised exercise improved quality of life, reduced anxiety and/or depression, and occurrence of cardiac death and hospitalization in older patients with acute coronary syndrome.
- 6.O’Gara PT, Kushner FG, Ascheim DD et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American College of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv, 82(1), E1–27 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation, 124(23), e574–651 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Hillis LD, Smith PK, Anderson JL et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation, 124(23), 2610–2642 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Smith SC, Allen J, Blair SN et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation, 113(19), 2363–2372 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Drozda J, Messer JV, Spertus J et al. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with coronary artery disease and hypertension: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association-Physician Consortium for Performance Improvement. J Am Coll Cardiol, 58(3), 316–336 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Anderson JL, Adams CD, Antman EM et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 61(23), e179–347 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Butchart EG, Gohlke-Bärwolf C, Antunes MJ et al. Recommendations for the management of patients after heart valve surgery. Eur Heart J, 26(22), 2463–2471 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Nytrøen K, Gullestad L. Effect of exercise in heart transplant recipients. Am J Transplant, 13(2), 527 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Smith SC, Benjamin EJ, Bonow RO et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol, 58(23), 2432–2446 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Sandesara PB, Lambert CT, Gordon NF et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate”. J Am Coll Cardiol, 65(4), 389–395 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Leung YW, Brual J, Macpherson A, Grace SL. Geographic issues in cardiac rehabilitation utilization: a narrative review. Health Place, 16(6), 1196–1205 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moghei M, Pesah E, Turk-Adawi K et al. Funding sources and costs to deliver cardiac rehabilitation around the globe: Drivers and barriers. Int J Cardiol, 276, 278–286 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Abreu A, Pesah E, Supervia M et al. Cardiac rehabilitation availability and delivery in Europe: How does it differ by region and compare with other high-income countries?: Endorsed by the European Association of Preventive Cardiology. Eur J Prev Cardiol, 26(11), 1131–1146 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Supervia M, Turk-Adawi K, Lopez-Jimenez F et al. Nature of Cardiac Rehabilitation Around the Globe. EClinicalMedicine, 13, 46–56 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turk-Adawi K, Supervia M, Lopez-Jimenez F et al. Cardiac Rehabilitation Availability and Density around the Globe. EClinicalMedicine, 13, 31–45 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson JJ, Johnson JA, Franklin BA, VanderLaan RL. Program participation, exercise adherence, cardiovascular outcomes, and program cost of traditional versus modified cardiac rehabilitation. Am J Cardiol, 86(1), 17–23 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Scane K, Alter D, Oh P, Brooks D. Adherence to a cardiac rehabilitation home program model of care: a comparison to a well-established traditional on-site supervised program. Appl Physiol Nutr Metab, 37(2), 206–213 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Borg S, Öberg B, Leosdottir M, Lindolm D, Nilsson L, Bäck M. Factors associated with non-attendance at exercise-based cardiac rehabilitation. BMC Sports Sci Med Rehabil, 11, 13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotseva K, Wood D, De Bacquer D, investigators E. Determinants of participation and risk factor control according to attendance in cardiac rehabilitation programmes in coronary patients in Europe: EUROASPIRE IV survey. Eur J Prev Cardiol, 25(12), 1242–1251 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Tavella R, O’Toole K, Tirimacco R et al. Cardiac rehabilitation referral and completion: results from the South Australian minimum dataset for cardiac rehabilitation programs. (Ed.^(Eds) (Heart Lung and Circulation, 2015) [Google Scholar]
- 26.Martin BJ, Hauer T, Arena R et al. Cardiac rehabilitation attendance and outcomes in coronary artery disease patients. Circulation, 126(6), 677–687 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation, 121(1), 63–70 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation, 116(15), 1653–1662 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Beatty AL, Li S, Thomas L, Amsterdam EA, Alexander KP, Whooley MA. Trends in referral to cardiac rehabilitation after myocardial infarction: data from the National Cardiovascular Data Registry 2007 to 2012. J Am Coll Cardiol, 63(23), 2582–2583 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aragam KG, Dai D, Neely ML et al. Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol, 65(19), 2079–2088 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Ruano-Ravina A, Pena-Gil C, Abu-Assi E et al. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int J Cardiol, 223, 436–443 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Ghisi GL, Polyzotis P, Oh P, Pakosh M, Grace SL. Physician factors affecting cardiac rehabilitation referral and patient enrollment: a systematic review. Clin Cardiol, 36(6), 323–335 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elsakr C, Bulger DA, Roman S, Kirolos I, Khouzam RN. Barriers physicians face when referring patients to cardiac rehabilitation: a narrative review. Ann Transl Med, 7(17), 414 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dahhan A, Maddox WR, Krothapalli S et al. Education of Physicians and Implementation of a Formal Referral System Can Improve Cardiac Rehabilitation Referral and Participation Rates after Percutaneous Coronary Intervention. Heart Lung Circ, 24(8), 806–816 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Grace SL, Gravely-Witte S, Brual J et al. Contribution of patient and physician factors to cardiac rehabilitation enrollment: a prospective multilevel study. Eur J Cardiovasc Prev Rehabil, 15(5), 548–556 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Resurrección DM, Motrico E, Rigabert A et al. Barriers for Nonparticipation and Dropout of Women in Cardiac Rehabilitation Programs: A Systematic Review. J Womens Health (Larchmt), 26(8), 849–859 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Colella TJ, Gravely S, Marzolini S et al. Sex bias in referral of women to outpatient cardiac rehabilitation? A meta-analysis. Eur J Prev Cardiol, 22(4), 423–441 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Supervía M, Medina-Inojosa JR, Yeung C et al. Cardiac Rehabilitation for Women: A Systematic Review of Barriers and Solutions. Mayo Clin Proc, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colbert JD, Martin BJ, Haykowsky MJ et al. Cardiac rehabilitation referral, attendance and mortality in women. Eur J Prev Cardiol, 22(8), 979–986 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Li S, Fonarow GC, Mukamal K et al. Sex and Racial Disparities in Cardiac Rehabilitation Referral at Hospital Discharge and Gaps in Long-Term Mortality. J Am Heart Assoc, 7(8) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cossette S, Maheu-Cadotte MA, Mailhot T et al. Sex- and Gender-Related Factors Associated With Cardiac Rehabilitation Enrollment: A SECONDARY ANALYSIS AMONG SYSTEMATICALLY REFERRED PATIENTS. J Cardiopulm Rehabil Prev, 39(4), 259–265 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Gravely S, Anand SS, Stewart DE, Grace SL, Investigators C. Effect of referral strategies on access to cardiac rehabilitation among women. Eur J Prev Cardiol, 21(8), 1018–1025 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen JK, Scott LB, Stewart KJ, Young DR. Disparities in women’s referral to and enrollment in outpatient cardiac rehabilitation. J Gen Intern Med, 19(7), 747–753 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark AM, King-Shier KM, Thompson DR et al. A qualitative systematic review of influences on attendance at cardiac rehabilitation programs after referral. Am Heart J, 164(6), 835–845.e832 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Farley RL, Wade TD, Birchmore L. Factors influencing attendance at cardiac rehabilitation among coronary heart disease patients. Eur J Cardiovasc Nurs, 2(3), 205–212 (2003). [DOI] [PubMed] [Google Scholar]
- 46.De Vos C, Li X, Van Vlaenderen I et al. Participating or not in a cardiac rehabilitation programme: factors influencing a patient’s decision. Eur J Prev Cardiol, 20(2), 341–348 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Castellanos LR, Viramontes O, Bains NK, Zepeda IA. Disparities in Cardiac Rehabilitation Among Individuals from Racial and Ethnic Groups and Rural Communities-A Systematic Review. J Racial Ethn Health Disparities, 6(1), 1–11 (2019).**A systematic review of 28 studies showing lower CR referral and participation rates among individuals from rural communities, women, and racial and ethnic groups when compared to the general population.
- 48.Mead H, Ramos C, Grantham SC. Drivers of Racial and Ethnic Disparities in Cardiac Rehabilitation Use: Patient and Provider Perspectives. Med Care Res Rev, 73(3), 251–282 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Prince DZ, Sobolev M, Gao J, Taub CC. Racial disparities in cardiac rehabilitation initiation and the effect on survival. PM R, 6(6), 486–492 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Al-Sharifi F, Winther Frederiksen H, Knold Rossau H, Norredam M, Zwisler AD. Access to cardiac rehabilitation and the role of language barriers in the provision of cardiac rehabilitation to migrants. BMC Health Serv Res, 19(1), 223 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chauhan U, Baker D, Lester H, Edwards R. Exploring uptake of cardiac rehabilitation in a minority ethnic population in England: a qualitative study. Eur J Cardiovasc Nurs, 9(1), 68–74 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Brown TM, Hernandez AF, Bittner V et al. Predictors of cardiac rehabilitation referral in coronary artery disease patients: findings from the American Heart Association’s Get With The Guidelines Program. J Am Coll Cardiol, 54(6), 515–521 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Listerman J, Bittner V, Sanderson BK, Brown TM. Cardiac rehabilitation outcomes: impact of comorbidities and age. J Cardiopulm Rehabil Prev, 31(6), 342–348 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yohannes AM, Yalfani A, Doherty P, Bundy C. Predictors of drop-out from an outpatient cardiac rehabilitation programme. Clin Rehabil, 21(3), 222–229 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Jackson JL, Emery CF. Emotional distress, alexithymia, and coping as predictors of cardiac rehabilitation outcomes and attendance. J Cardiopulm Rehabil Prev, 33(1), 26–32 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Glazer KM, Emery CF, Frid DJ, Banyasz RE. Psychological predictors of adherence and outcomes among patients in cardiac rehabilitation. J Cardiopulm Rehabil, 22(1), 40–46 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Murphy B, Le Grande M, Alvarenga M, Worcester M, Jackson A. Anxiety and Depression After a Cardiac Event: Prevalence and Predictors. Front Psychol, 10, 3010 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thombs BD, Bass EB, Ford DE et al. Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med, 21(1), 30–38 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lichtman JH, Bigger JT, Blumenthal JA et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation, 118(17), 1768–1775 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Tully PJ, Baker RA. Depression, anxiety, and cardiac morbidity outcomes after coronary artery bypass surgery: a contemporary and practical review. J Geriatr Cardiol, 9(2), 197–208 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med, 160(14), 2101–2107 (2000). [DOI] [PubMed] [Google Scholar]
- 62.Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul Study. Arch Intern Med, 165(21), 2508–2513 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kronish IM, Rieckmann N, Halm EA et al. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med, 21(11), 1178–1183 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitmarsh A, Koutantji M, Sidell K. Illness perceptions, mood and coping in predicting attendance at cardiac rehabilitation. Br J Health Psychol, 8(Pt 2), 209–221 (2003). [DOI] [PubMed] [Google Scholar]
- 65.Rao A, Zecchin R, Newton PJ et al. The prevalence and impact of depression and anxiety in cardiac rehabilitation: A longitudinal cohort study. Eur J Prev Cardiol, 27(5), 478–489 (2020).** An unblinded randomized control trial in post MI patients comparing smartphone-based CR with traditional center-based CR. They found that home-based CR significantly improved CR participation, adherence and completion when compared to center-based CR.
- 66.Lavie CJ, Menezes AR, De Schutter A, Milani RV, Blumenthal JA. Impact of Cardiac Rehabilitation and Exercise Training on Psychological Risk Factors and Subsequent Prognosis in Patients With Cardiovascular Disease. Can J Cardiol, 32(10 Suppl 2), S365–S373 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Shanmugasegaram S, Oh P, Reid RD, McCumber T, Grace SL. Cardiac rehabilitation barriers by rurality and socioeconomic status: a cross-sectional study. Int J Equity Health, 12, 72 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bakhshayeh S, Sarbaz M, Kimiafar K, Vakilian F, Eslami S. Barriers to participation in center-based cardiac rehabilitation programs and patients’ attitude toward home-based cardiac rehabilitation programs. Physiother Theory Pract, 1–11 (2019).**A cross-sectional study identifying barriers to CR in a low-resource setting from the perspective of health-care administrators, CR providers, and cardiac patients. These barriers included ack of resources and funding, lack of referral or physician encouragement, lack of patient awareness, and poor access for patients (i.e., distance, cost, transportation).
- 69.Higgins RO, Murphy BM, Goble AJ, Le Grande MR, Elliott PC, Worcester MU. Cardiac rehabilitation program attendance after coronary artery bypass surgery: overcoming the barriers. Med J Aust, 188(12), 712–714 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Wingham J, Dalal HM, Sweeney KG, Evans PH. Listening to patients: choice in cardiac rehabilitation. Eur J Cardiovasc Nurs, 5(4), 289–294 (2006). [DOI] [PubMed] [Google Scholar]
- 71.Brual J, Gravely-Witte S, Suskin N, Stewart DE, Macpherson A, Grace SL. Drive time to cardiac rehabilitation: at what point does it affect utilization? Int J Health Geogr, 9, 27 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dunlay SM, Witt BJ, Allison TG et al. Barriers to participation in cardiac rehabilitation. Am Heart J, 158(5), 852–859 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giuliano C, Parmenter BJ, Baker MK et al. Cardiac Rehabilitation for Patients With Coronary Artery Disease: A Practical Guide to Enhance Patient Outcomes Through Continuity of Care. Clin Med Insights Cardiol, 11, 1179546817710028 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ades PA, Waldmann ML, McCann WJ, Weaver SO. Predictors of cardiac rehabilitation participation in older coronary patients. Arch Intern Med, 152(5), 1033–1035 (1992). [PubMed] [Google Scholar]
- 75.Prescott E, Mikkelsen N, Holdgaard A et al. Cardiac rehabilitation in the elderly patient in eight rehabilitation units in Western Europe: Baseline data from the EU-CaRE multicentre observational study. Eur J Prev Cardiol, 26(10), 1052–1063 (2019). [DOI] [PubMed] [Google Scholar]
- 76.Moradi B, Maleki M, Esmaeilzadeh M, Abkenar HB. Physician-related factors affecting cardiac rehabilitation referral. J Tehran Heart Cent, 6(4), 187–192 (2011). [PMC free article] [PubMed] [Google Scholar]
- 77.Arena R, Williams M, Forman DE et al. Increasing referral and participation rates to outpatient cardiac rehabilitation: the valuable role of healthcare professionals in the inpatient and home health settings: a science advisory from the American Heart Association. Circulation, 125(10), 1321–1329 (2012). [DOI] [PubMed] [Google Scholar]
- 78.Lynggaard V, Nielsen CV, Zwisler AD, Taylor RS, May O. The patient education - Learning and Coping Strategies - improves adherence in cardiac rehabilitation (LC-REHAB): A randomised controlled trial. Int J Cardiol, 236, 65–70 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Krantz MJ, Havranek EP, Mehler PS, Haynes DK, Long CS. Impact of a cardiac risk reduction program in vulnerable patients hospitalized with coronary artery disease. Pharmacotherapy, 24(6), 768–775 (2004). [DOI] [PubMed] [Google Scholar]
- 80.Gurewich D, Prottas J, Bhalotra S, Suaya JA, Shepard DS. System-level factors and use of cardiac rehabilitation. J Cardiopulm Rehabil Prev, 28(6), 380–385 (2008). [DOI] [PubMed] [Google Scholar]
- 81.Cossette S, Frasure-Smith N, Dupuis J, Juneau M, Guertin MC. Randomized controlled trial of tailored nursing interventions to improve cardiac rehabilitation enrollment. Nurs Res, 61(2), 111–120 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Jolly K, Bradley F, Sharp S et al. Randomised controlled trial of follow up care in general practice of patients with myocardial infarction and angina: final results of the Southampton heart integrated care project (SHIP). The SHIP Collaborative Group. BMJ, 318(7185), 706–711 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gaalema DE, Savage PD, Rengo JL, Cutler AY, Higgins ST, Ades PA. Financial incentives to promote cardiac rehabilitation participation and adherence among Medicaid patients. Prev Med, 92, 47–50 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frechette KM, Conley SM, Tang A, Welch TD. Cardiac Rehabilitation in a Rural Setting: OPTIMIZATION OF REFERRAL AND PARTICIPATION RATES. J Cardiopulm Rehabil Prev, 39(1), E4–E7 (2019). [DOI] [PubMed] [Google Scholar]
- 85.Liu H, Wilton SB, Southern DA et al. Automated Referral to Cardiac Rehabilitation After Coronary Artery Bypass Grafting Is Associated With Modest Improvement in Program Completion. Can J Cardiol, 35(11), 1491–1498 (2019).**A time series analysis showing that automated referral to CR after CABG led to relative increase of 89% in CR referral rates and associated with a significant 7.2% increase in CR completion.
- 86.Thomas RJ, Witt BJ, Lopez-Jimenez F, King ML, Squires RW. Quality indicators in cardiovascular care: the case for cardiac rehabilitation. J Cardiopulm Rehabil, 25(5), 249–256 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Haffer SC, Bowen SE. Measuring and improving health outcomes in Medicare: the Medicare HOS program. Health Care Financ Rev, 25(4), 1–3 (2004). [PMC free article] [PubMed] [Google Scholar]
- 88.Rosenthal MB, Frank RG, Li Z, Epstein AM. Early experience with pay-for-performance: from concept to practice. JAMA, 294(14), 1788–1793 (2005). [DOI] [PubMed] [Google Scholar]
- 89.Traylor AH, Schmittdiel JA, Uratsu CS, Mangione CM, Subramanian U. Adherence to cardiovascular disease medications: does patient-provider race/ethnicity and language concordance matter? J Gen Intern Med, 25(11), 1172–1177 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.LaVeist TA, Nuru-Jeter A, Jones KE. The association of doctor-patient race concordance with health services utilization. J Public Health Policy, 24(3–4), 312–323 (2003). [PubMed] [Google Scholar]
- 91.Saha S, Arbelaez JJ, Cooper LA. Patient-physician relationships and racial disparities in the quality of health care. Am J Public Health, 93(10), 1713–1719 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Midence L, Arthur HM, Oh P, Stewart DE, Grace SL. Women’s Health Behaviours and Psychosocial Well-Being by Cardiac Rehabilitation Program Model: A Randomized Controlled Trial. Can J Cardiol, 32(8), 956–962 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Forsyth F, Deaton C. Women and cardiac rehabilitation: Moving beyond barriers to solutions? Eur J Prev Cardiol, 2047487320911843 (2020). [DOI] [PubMed] [Google Scholar]
- 94.Davidson P, Digiacomo M, Zecchin R et al. A cardiac rehabilitation program to improve psychosocial outcomes of women with heart disease. J Womens Health (Larchmt), 17(1), 123–134 (2008). [DOI] [PubMed] [Google Scholar]
- 95.Grace SL, McDonald J, Fishman D, Caruso V. Patient preferences for home-based versus hospital-based cardiac rehabilitation. J Cardiopulm Rehabil, 25(1), 24–29 (2005). [DOI] [PubMed] [Google Scholar]
- 96.Besnier F, Gayda M, Nigam A, Juneau M, Bherer L. Cardiac Rehabilitation During Quarantine in COVID-19 Pandemic: Challenges for Center-Based Programs. Arch Phys Med Rehabil, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Varnfield M, Karunanithi M, Lee CK et al. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart, 100(22), 1770–1779 (2014).**An unblinded randomized control trial in post MI patients comparing smartphone-based CR with traditional center-based CR. They found that home-based CR significantly improved CR participation, adherence and completion when compared to center-based CR.
- 98.Kraal JJ, Peek N, Van den Akker-Van Marle ME, Kemps HM. Effects of home-based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation: short-term results of the FIT@Home study. Eur J Prev Cardiol, 21(2 Sbuppl), 26–31 (2014). [DOI] [PubMed] [Google Scholar]
- 99.Lee YH, Hur SH, Sohn J et al. Impact of home-based exercise training with wireless monitoring on patients with acute coronary syndrome undergoing percutaneous coronary intervention. J Korean Med Sci, 28(4), 564–568 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maddison R, Pfaeffli L, Whittaker R et al. A mobile phone intervention increases physical activity in people with cardiovascular disease: Results from the HEART randomized controlled trial. Eur J Prev Cardiol, 22(6), 701–709 (2015).**A single blinded randomized control trial that utilized a mobile phone intervention to improve exercise capacity and physical activity in patients with ischemic heart disease. They showed that while the mobile phone intervention alone did not increase exercise capacity, it did increase physical activity among participants.
- 101.Zutz A, Ignaszewski A, Bates J, Lear SA. Utilization of the internet to deliver cardiac rehabilitation at a distance: a pilot study. Telemed J E Health, 13(3), 323–330 (2007). [DOI] [PubMed] [Google Scholar]
- 102.Reid RD, Morrin LI, Beaton LJ et al. Randomized trial of an internet-based computer-tailored expert system for physical activity in patients with heart disease. Eur J Prev Cardiol, 19(6), 1357–1364 (2012). [DOI] [PubMed] [Google Scholar]
- 103.Batalik L, Filakova K, Batalikova K, Dosbaba F. Remotely monitored telerehabilitation for cardiac patients: A review of the current situation. World J Clin Cases, 8(10), 1818–1831 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anderson L, Sharp GA, Norton RJ et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev, 6, CD007130 (2017).**A Cochrane review of 17 trials of participants undergoing home-based versus center-based CR following MI, revascularization, heart failure showing no significant difference in exercise capacity or cardiac events (mortality, revascularization and hospital readmission) between the 2 groups.
- 105.Ge C, Ma J, Xu Y et al. Predictors of adherence to home-based cardiac rehabilitation program among coronary artery disease outpatients in China. J Geriatr Cardiol, 16(10), 749–755 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thomas RJ, Beatty AL, Beckie TM et al. Home-Based Cardiac Rehabilitation: A Scientific Statement From the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation, 140(1), e69–e89 (2019).**A scientific statement from AHA and ACC highlighting home based CR as a reasonable option for stable low- to moderate-risk patients who are eligible for CR but cannot attend a center-based CR program.
- 107.Rabin C, Bock B. Desired features of smartphone applications promoting physical activity. Telemed J E Health, 17(10), 801–803 (2011). [DOI] [PubMed] [Google Scholar]
- 108.Rawstorn JC, Gant N, Meads A, Warren I, Maddison R. Remotely Delivered Exercise-Based Cardiac Rehabilitation: Design and Content Development of a Novel mHealth Platform. JMIR Mhealth Uhealth, 4(2), e57 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harzand A, Witbrodt B, Davis-Watts ML et al. Feasibility of a Smartphone-enabled Cardiac Rehabilitation Program in Male Veterans With Previous Clinical Evidence of Coronary Heart Disease. Am J Cardiol, 122(9), 1471–1476 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maddison R, Rawstorn JC, Stewart RAH et al. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart, 105(2), 122–129 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.DeBusk RF, Miller NH, Superko HR et al. A case-management system for coronary risk factor modification after acute myocardial infarction. Ann Intern Med, 120(9), 721–729 (1994). [DOI] [PubMed] [Google Scholar]
- 112.Miller NH, Warren D, Myers D. Home-based cardiac rehabilitation and lifestyle modification: the MULTIFIT model. J Cardiovasc Nurs, 11(1), 76–87 (1996). [DOI] [PubMed] [Google Scholar]
- 113.Anderson L, Oldridge N, Thompson DR et al. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J Am Coll Cardiol, 67(1), 1–12 (2016). [DOI] [PubMed] [Google Scholar]
- 114.Mohr DC, Ho J, Duffecy J et al. Effect of telephone-administered vs face-to-face cognitive behavioral therapy on adherence to therapy and depression outcomes among primary care patients: a randomized trial. JAMA, 307(21), 2278–2285 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Focht BC, Brawley LR, Rejeski WJ, Ambrosius WT. Group-mediated activity counseling and traditional exercise therapy programs: effects on health-related quality of life among older adults in cardiac rehabilitation. Ann Behav Med, 28(1), 52–61 (2004). [DOI] [PubMed] [Google Scholar]
- 116.Duncan K, Pozehl B. Effects of an exercise adherence intervention on outcomes in patients with heart failure. Rehabil Nurs, 28(4), 117–122 (2003). [DOI] [PubMed] [Google Scholar]
- 117.Tiefenbeck V, Goette Lorenz et al. Overcoming Salience Bias: How Real-Time Feedback Fosters Resource Conservation. Management Science, 64(3) (2016). [Google Scholar]
- 118.Shanmugasegaram S, Gagliese L, Oh P et al. Psychometric validation of the cardiac rehabilitation barriers scale. Clin Rehabil, 26(2), 152–164 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Babu AS, Lopez-Jimenez F, Thomas RJ et al. Advocacy for outpatient cardiac rehabilitation globally. BMC Health Serv Res, 16, 471 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kraal JJ, Van den Akker-Van Marle ME, Abu-Hanna A, Stut W, Peek N, Kemps HM. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: Results of the FIT@Home study. Eur J Prev Cardiol, 24(12), 1260–1273 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hwang R, Morris NR, Mandrusiak A et al. Cost-Utility Analysis of Home-Based Telerehabilitation Compared With Centre-Based Rehabilitation in Patients With Heart Failure. Heart Lung Circ, 28(12), 1795–1803 (2019). [DOI] [PubMed] [Google Scholar]
- 122.Prescott E, Meindersma EP, van der Velde AE et al. A EUropean study on effectiveness and sustainability of current Cardiac Rehabilitation programmes in the Elderly: Design of the EU-CaRE randomised controlled trial. Eur J Prev Cardiol, 23(2 suppl), 27–40 (2016). [DOI] [PubMed] [Google Scholar]
- 123.Frederix I, Vandenberk T, Janssen L, Geurden A, Vandervoort P, Dendale P. eEduHeart I: A Multicenter, Randomized, Controlled Trial Investigating the Effectiveness of a Cardiac Web-Based eLearning Platform - Rationale and Study Design. Cardiology, 136(3), 157–163 (2017). [DOI] [PubMed] [Google Scholar]
- 124.Volpp KG, Troxel AB, Pauly MV et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med, 360(7), 699–709 (2009). [DOI] [PubMed] [Google Scholar]
- 125.Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA, 300(22), 2631–2637 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Robison JI, Rogers MA, Carlson JJ et al. Effects of a 6-month incentive-based exercise program on adherence and work capacity. Med Sci Sports Exerc, 24(1), 85–93 (1992). [PubMed] [Google Scholar]
- 127.Gaalema DE, Elliott RJ, Savage PD et al. Financial Incentives to Increase Cardiac Rehabilitation Participation Among Low-Socioeconomic Status Patients: A Randomized Clinical Trial. JACC Heart Fail, 7(7), 537–546 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wall J, Mhurchu CN, Blakely T, Rodgers A, Wilton J. Effectiveness of monetary incentives in modifying dietary behavior:a review of randomized, controlled trials. Nutr Rev, 64(12), 518–531 (2006). [DOI] [PubMed] [Google Scholar]
- 129.Paul-Ebhohimhen V, Avenell A. Systematic review of the use of financial incentives in treatments for obesity and overweight. Obes Rev, 9(4), 355–367 (2008). [DOI] [PubMed] [Google Scholar]
- 130.Burns RJ, Donovan AS, Ackermann RT, Finch EA, Rothman AJ, Jeffery RW. A theoretically grounded systematic review of material incentives for weight loss: implications for interventions. Ann Behav Med, 44(3), 375–388 (2012). [DOI] [PubMed] [Google Scholar]
- 131.Babu AS, Arena R, Ozemek C, Lavie CJ. COVID-19: A Time for Alternate Models in Cardiac Rehabilitation to Take Centre Stage. Can J Cardiol, (2020).**A perspective piece highlighting importance of considering and implementing alternative care delivery models, specifically technology driven CR in the COVID 19 pandemic.
- 132.Thomas E, Gallagher R, Grace SL. Future-proofing cardiac rehabilitation: Transitioning services to telehealth during COVID-19. Eur J Prev Cardiol, 2047487320922926 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Drwal KR, Forman DE, Wakefield BJ, El Accaoui RN. Cardiac Rehabilitation During COVID-19 Pandemic: Highlighting the Value of Home-Based Programs. Telemed J E Health, (2020). [DOI] [PubMed] [Google Scholar]
- 134.Dalal H, Taylor RS, Greaves C et al. Correspondence to the. Eur J Prev Cardiol, 2047487320923053 (2020). [Google Scholar]
- 135.Anderson L, Brown JP, Clark AM et al. Patient education in the management of coronary heart disease. Cochrane Database Syst Rev, 6, CD008895 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Johnson N, Inder K, Nagle A, Wiggers J. Attendance at outpatient cardiac rehabilitation: is it enhanced by specialist nurse referral? In: Australian journal of advanced nursing : a quarterly publication of the Royal Australian Nursing Federation , The. (Ed.^(Eds) (2010) 31–37. [Google Scholar]
- 137.Grace SL, Midence L, Oh P et al. Cardiac Rehabilitation Program Adherence and Functional Capacity Among Women: A Randomized Controlled Trial. Mayo Clin Proc, 91(2), 140–148 (2016). [DOI] [PubMed] [Google Scholar]
- 138.Balady GJ, Ades PA, Bittner VA et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation, 124(25), 2951–2960 (2011). [DOI] [PubMed] [Google Scholar]
- 139.Lavie CJ, Bennett A, Arena R. Enhancing Cardiac Rehabilitation in Women. J Womens Health (Larchmt), 26(8), 817–819 (2017). [DOI] [PubMed] [Google Scholar]
- 140.Doll JA, Kaltenbach LA, Anstrom KJ et al. Impact of a Copayment Reduction Intervention on Medication Persistence and Cardiovascular Events in Hospitals With and Without Prior Medication Financial Assistance Programs. J Am Heart Assoc, 9(8), e014975 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]