Abstract
Sjögren-Larsson syndrome (SLS) is an inherited metabolic disease characterized by ichthyosis, spasticity, intellectual disability and deficient oxidation and accumulation of of fatty aldehydes and alcohols. We investigated whether excess fatty alcohols in SLS are diverted into biosynthesis of ether glycerolipids (eGLs) by measuring the 1-O-alkylglycerol (AG) backbone of eGLs in stratum corneum, plasma and red blood cells (RBCs). In all tissues, saturated and monounsaturated AGs were detected. In stratum corneum from SLS patients, saturated AGs (C15-C20) were increased 97-fold (range: 86- to 169-fold) compared to controls. AGs were largely (67±9%) derived from neutral esterified eGLs (i.e. alkyl-diacylglyerol) and free non-esterified AGs (28±10%), but very little from plasmalogens (3±5%). Plasma from SLS patients had 2-fold more C18:0-AG (p<0.005) and 40% less C16:1-AG (p<0.01) than controls but the total concentration of AGs was not increased, and the AG profile in RBCs from SLS subjects was normal. All AGs were profoundly reduced in plasma and RBCs from patients with Zellweger spectrum disorder, who have impaired eGL ( i.e. plasmalogen) synthesis. The striking accumulation of AGs in stratum corneum of SLS patients constitutes a novel lipid biomarker for this disease, and may contribute to the pathogenesis of the ichthyosis. Measurement of AGs is a simple and convenient method to assess global synthesis of eGLs and potentially identify patients with defects in their metabolism.
Keywords: plasmalogen, ether lipid, metabolism, peroxisome, skin, stratum corneum, ichthyosis
1. Introduction
Sjögren-Larsson syndrome (SLS) is an inborn error of metabolism caused by mutations in ALDH3A2 which results in deficiency of fatty aldehyde dehydrogenase (FALDH; EC 1.2.1.3) and impaired oxidation of fatty aldehydes and alcohols to fatty acids [1]. SLS patients accumulate fatty alcohols in plasma and cultured cells [2]. They exhibit neurocutaneous symptoms of ichthyosis, spasticity, intellectual disability and a distinctive maculopathy [3].
Fatty alcohol metabolism in SLS is closely linked to biosynthesis of ether glycerolipids (eGLs), a biochemical pathway that is initiated in peroxisomes and requires fatty alcohol substrates. Consequently, disorders with abnormal peroxisome biogenesis have deficient eGL biosynthesis. Those patients with Zellweger spectrum disorder (ZSD) are unable to utilize fatty alcohols to synthesize eGLs (i.e. plasmalogens) due to impaired peroxisomal import of two key enzymes: glyceronephosphate O-acyltransferase (GNPAT, EC 2.3.1.42), which produces 1-acyl-dihydroxyacetone phosphate (1-acyl-DHAP), and alkylglycerone phosphate synthase (AGPS, EC 2.5.1.26) that incorporates fatty alcohol into the sn-1 position of 1-acyl-DHAP to form the ether bond characteristic of eGLs [5]. Similarly, rhizomelic chondrodysplasia punctata (RCDP) type 1 is associated with defective peroxisomal import of AGPS and deficient plasmalogens [4]. RCDP type 1 patients typically exhibit a greater deficit in plasmalogen biosynthesis than those with ZSD and, like SLS, accumulate fatty alcohols in plasma [6]. Clinical features of RCDP type 1 include rhizomelic limb shortening, developmental delay, intellectual disability, growth failure, cataracts and facial dysmorphisms. This RCDP phenotype is also caused by isolated genetic deficiencies of GNPAT (RCDP type 2) and AGPS (RCDP type 3), which likewise prevent utilization of fatty alcohol for eGL synthesis [7]. In contrast, genetic deficiency of fatty acyl-CoA-reductase-1 (FAR1, EC 1.2.1.84), clinically referred to as RCDP type 4, results in reduced synthesis of fatty alcohols and impaired eGL synthesis [8]. Finally, RCDP type 5 is caused by certain mutations in PEX5 that disrupt peroxisomal import of AGPS and result in decreased plasmalogens [9]. All of these genetic diseases are associated with deficient eGL biosynthesis.
eGLs are defined by the presence of an ether-linked alkyl group at the sn-1 position of glycerol (see Fig 1). Neutral eGLs, such as 1-O-alkyl-diacylglycerol, typically have fatty acids attached to the sn-2 and/or sn-3 carbon position of glycerol. They are minor lipids in most tissues. A more abundant and well characterized subgroup of eGLs are plasmalogen phospholipids that have an alkyl chain with an unsaturated vinyl ether bond (-C-O-C=C-) at the sn-1 position of glycerol and the sn-3 position is occupied by a phosphate group linked to ethanolamine, choline, serine or inositol. For lipid analysis, the vinyl ether bond can be readily cleaved by strong acid to generate fatty aldehyde, which in the presence of methanol forms dimethyl acetal (DMA) derivatives. In contrast, saturated 1-O-alkanyl chains in neutral eGLs are not susceptible to acid hydrolysis and therefore not captured as DMAs. The ether bond in both types of eGLs, however, is resistant to alkaline hydrolysis, which cleaves fatty acid ester bonds resulting in saturated and monounsaturated 1-O-alkylglycerols (AGs) that are predominantly derived from neutral eGLs and plasmalogen eGLs, respectively (Fig 1).
Figure 1.

Alkylglycerol (AG) lipids derived from alkaline hydrolysis of eGLs. Structures are the plasmalogen form of phosphatidylethanolamine (PE) and 1-O-alkanyl-2,3-diacylglycerol, which are hydrolyzed to monounsaturated AG and saturated AG, respectively.
In an effort to characterize in vivo metabolic derangements in SLS and identify potential biomarkers, we hypothesized that elevated fatty alcohols in tissues of SLS patients would be diverted into eGL biosynthesis [10], but initial studies of a few SLS patients provided no convincing evidence that RBC plasmalogens, measured as DMAs, were significantly increased [11]. Fatty alcohols in cultured SLS keratinocytes, however, are largely diverted into formation of neutral eGLs instead of plasmalogens [12]. We therefore used an alkaline hydrolysis method that generates AGs from all eGLs to assess these lipids in stratum corneum, plasma and RBCs from SLS patients, and in plasma and RBCs from ZSD controls.
2. Materials and Methods
2.1. Materials:
1-O-palmitoyl-rac-glycerol (C16:0-AG), 1-O-octadecanyl-rac-glycerol (C18:0-AG), 1-O-palmitoy-2,3-dipalmitoylglycerol and heptadecanol were obtained from Sigma-Aldrich. 1-O-heptadecylglycerol (C17:0-AG) was synthesized as described (12). Solvents were of HPLC-grade or analytical-grade and obtained from Fisher Chemical. N,O-Bis(trimethylsilyl)trifluoroacetamide with trimethylchlorosilane (BSTFA/TMCS) was obtained from Sigma-Aldrich. All other chemicals were from Sigma-Aldrich.
2.2. Patients:
SLS subjects were recruited into a longitudinal natural history study within the Sterol and Isoprenoid Research Consortium. All SLS subjects showed the typical symptoms of ichthyosis, spastic diplegia and intellectual disability, and carried pathogenic mutations in ALDH3A2. All ZSD subjects demonstrated biochemical features of peroxisomal dysfunction (accumulation of very long chain fatty acids and deficiency of RBC plasmalogens) and had mutations in PEX genes. Controls were healthy volunteers who provided blood specimens and stratum corneum. This research was approved by the Institutional Review Board at the University of Nebraska Medical Center.
2.3. Stratum corneum AG analysis:
All subjects were instructed to refrain from use of topical lotions on their leg for 7 days. Samples of stratum corneum scales were collected from SLS subjects by scraping their legs with a disposable plastic knife. Stratum corneum from controls was obtained from the lower heel using a dermaplane. The samples (2-10 mg weight) were suspended in 1 ml chloroform/methanol (1/1) and homogenized by hand in a 1 ml ground-glass homogenizer. Lipids were extracted overnight. After addition of 1 μg C17:0-AG as internal standard, the samples were centrifuged at 3,000 g for 5 min, and the solvent was removed and dried under a stream of nitrogen. After addition of 1.25 ml chloroform and 0.75 ml water, the lipids were washed according to Folch et al [13] using 2 ml theoretical upper phase. The lower chloroform phases containing lipids were dried under a stream of nitrogen and hydrolyzed with 2 ml of 1N NaOH in 95% methanol at 120° C for 4 hours. After addition of 2 ml water, AGs were extracted twice into 2 ml hexane, combined, dried under nitrogen and treated with 50 μl BSTFA/TCMS at 70° C for 1 hour to produce trimethylsilyl (TMS) derivatives. The AGs were analyzed by gas chromatography-mass spectrometry (GC-MS) using an Agilent Technologies 6890N gas chromatograph equipped with a 30-m x 0.25 mm internal diameter HP-5MS column and Agilent 5973 inert mass selective detector. Samples were injected in splitless mode. The column flow rate was 1 ml helium/min, initial oven temperature was 100° C and injection temperature 280° C. After 2 min, the oven temperature was increased at 6° C per min to 324° C. AGs were identified according to their retention times, mass spectra and characteristic m/z 205 fragment using appropriate standards. AGs were quantitated using C17:0-AG as internal standard and normalizing to mg dry weight of stratum corneum.
2.4. Plasma and RBC AG analysis:
Plasma was prepared from fasting venous blood by centrifugation at 3,000 g and the RBC pellet was washed twice with phosphate buffered saline to provide packed RBCs. Specimens were stored at −70° C until use. Plasmas (0.2 ml) were extracted with 2 ml chloroform/methanol (1/1), whereas packed RBCs (0.2 ml) were first brought up to 1 ml by addition of water and sonicated 10 sec with a microprobe tip at 50% maximum power and 50% duty cycle. An aliquot (0.25 ml) of RBC sonicate was removed for lipid extraction with 2 ml chloroform/methanol (1/1). To each sample was added 0.1 μg of C17:0-AG as internal standard. Plasmas or RBCs were mixed by vortexing and extracted overnight. Samples were subsequently centrifuged at 3,000 g and the upper aqueous phase was discarded. The lower organic phase was dried under nitrogen, washed according to Folch et al [13] and submitted to alkaline hydrolysis as described above (Section 2.3). AGs were analyzed by GC-MS using single ion monitoring of m/z 205 as described above. The AGs were quantitated using C17:0-AG as internal standard and expressed as μg/ml plasma or packed RBCs.
2.5. RBC plasmalogen (DMA) measurement:
RBC lipid extracts were transmethylated with 1 M methanolic-HCl for determination of DMA composition by GC-MS [11]. Data were expressed as the ratio of 16:0-DMA and C18:0-DMA to their corresponding fatty acid methyl esters.
2.6. Isolation of stratum corneum 1-O-alkyl-2,3-diacylglycerol, AG, and plasmalogens
To separate 1-O-2,3-diacylglycerol, AG and plasmalogen lipids, stratum corneum lipid extracts were spotted on silica gel thin-layer chromatography plates and developed sequentially in the following solvent systems: 1) petroleum ether/diethyl ether/acetic acid (80/20/1); 2) diethyl ether to 60% up the plate; 3) methanol to 25% up the plate. Lipid standards were chromatographed on adjacent channels. After drying, the standard lanes were sprayed with rhodamine G and lipids were visualized under UV light. Silica regions of stratum corneum lipids corresponding to 1-O-2,3-diacylglycerol (Rf 0.88), AG (Rf 0.28) and plasmalogen phospholipids (Rf 0.19) were collected by scraping, and lipids were eluted from the silica with chloroform/methanol (1/1). The AG contents of the separated lipids were measured as described in Section 2.3.
2.7. Statistical analysis:
Data were analyzed with Prism 8 software (GraphPad Software, LLC) using unpaired Student t-tests comparing SLS or ZSD to controls, p values < 0.05 denoted statistical significance.
3. Results
Lipid extracts were treated with 1 N NaOH to hydrolyze eGLs and generate AGs (Fig 1), which were subsequently converted to TMS derivatives and analyzed by GC-MS. The AGs were identified by their chromatographic retention time compared to authentic standards and distinct ion spectra with a major common fragment with m/z 205, together with ions that were specific for the alkyl chain length and unsaturation (Table 1) [14]. AGs were quantitated using the m/z 205 ion and C17:0-AG as internal standard.
Table 1.
Prominent fragmentation ions of alkylglycerol-TMS derivatives seen on GC-MS.
| Alkylglycerol | Major Ion (m/z) |
M-(73+74) Ion (m/z) |
|---|---|---|
| C15:0 | 205 | 299 |
| C16:0 | 205 | 313 |
| C16:1 | 205 | 311 |
| C17:0 | 205 | 327 |
| C18:0 | 205 | 341 |
| C18:1 | 205 | 339 |
| C20:0 | 205 | 369 |
| C22:0 | 205 | 397 |
3.1. AGs in Stratum Corneum
In stratum corneum from SLS subjects, AGs with 15- to 20-carbons long were readily detected (Fig 2). Both saturated and monounsaturated AGs were present. Longer chain AGs were not seen. In contrast to SLS, stratum corneum from control subjects had far less amounts of AGs and some of these were barely detectable (Fig 2).
Figure 2.
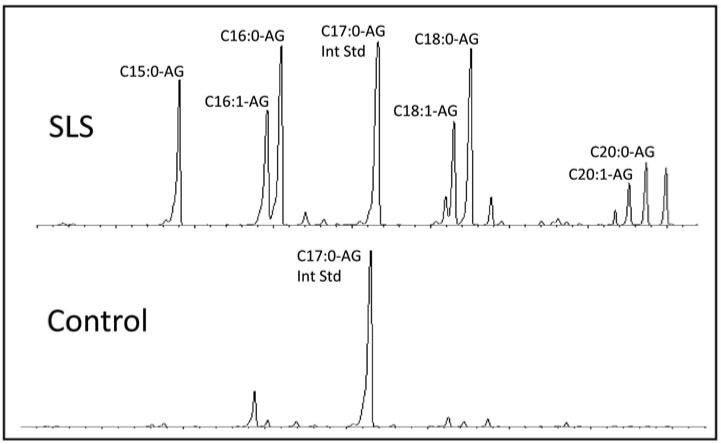
Chromatogram of alkylglycerol lipids in stratum corneum using single ion monitoring for m/z 205. C17:0-AG was used as internal standard.
Saturated AGs (C15:0-, C16:0-, C18:0- and C20-AG) showed the greatest relative accumulation in SLS (n=8) compared to controls (n=6) (Fig 3). Their total sum was 97-fold greater than controls; the mean ranges for these individual AGs varied between 86-fold to 169-fold greater than the mean for controls, with no overlap (Fig 3). Except for C18:1-AG, monounsaturated AGs in SLS tended to accumulate but did not reach statistical significance due to greater variation.
Figure 3.
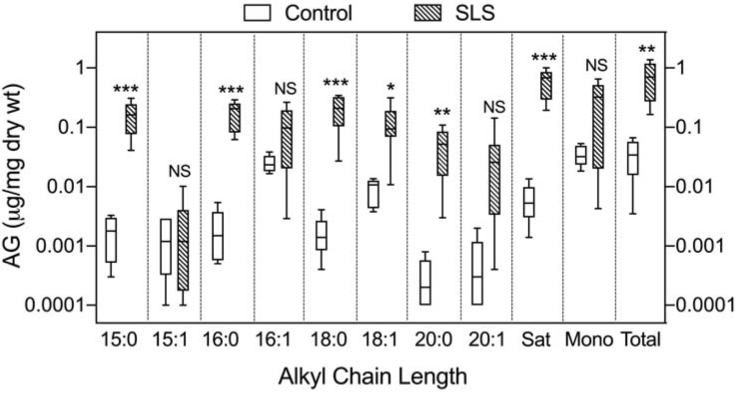
Alkylglycerol lipids are elevated in SLS stratum corneum. Note the logarithmic scale of the Y-axis. Controls: open boxes; SLS: cross-hatched boxes. Horizontal lines represent means; error bars correspond to highest and lowest values. SLS (n=8) and controls (n=6) were compared using Students t -Test for unpaired samples. Compared to controls, mean elevation of AGs in SLS are increased by 94-fold (C15:0-AG), 86-fold (C16:0-AG), 120-fold (C18:0-AG), 169-fold (C20:0-AG), and 97-fold (total saturated AGs). Asterisks indicate statistically significant differences in SLS compared to controls using Student’s t test: * p<0.05; ** p<0.05; *** p<0.001.
To determine which eGLs in SLS stratum corneum contributed AGs to the total lipid extract, we separated the lipids into 3 fractions: nonesterified AGs, neutral esterified AGs (1-O-alkyl-diacylglycerol) and phospholipids (plasmalogens), and then measured the AG composition. In SLS (n=3), AGs were largely derived from 1-O-alkyl-diacylglycerol (68±9%) and free non-esterified AG (29±10%) fractions, but little from plasmalogens (3±5%).
3.2. AGs in SLS Plasma
To determine whether the large excess of AGs seen in SLS stratum corneum was also present in other accessible tissues, we measured AGs in plasma. Saturated and monounsaturated AGs with C16-C18 alkyl chain lengths were detected in plasma from control subjects (Fig 4). Saturated AGs (C16:0 + C18:0) together comprised 63±6% (SD) of the total AGs in plasma; the remainder consisted of monounsaturated AGs.
Figure 4.

The plasma AG profile is abnormal in SLS and ZSD. Subjects with SLS (n=10) and ZSD (n=8) were compared to controls (n=7) using Students t-test. Asterisks indicate statistically significant differences in SLS compared to controls using Student’s t test: * p<0.01; ** p<0.005; *** p<0.001. Data shown are mean ± SD.
In plasma, SLS subjects (n=10) had a two-fold increase in C18:0-AG compared to controls (n=7) (SLS: 1.72±0.60 μg/ml; controls: 0.82±0.46; p<0.005) and a 40% reduction in C16:1-AG (SLS: 0.14±0.06 μg/ml; controls: 0.26±0.08; p<0.01), but no difference was seen in total C16+C18 AGs (Fig 4). The ratio of C18:0-AG to C16:1- AG was increased 4-fold in SLS subjects compared to controls (SLS: 11.83±5.37; controls: 3.04±1.30; p<0.001).
3.3. AGs in SLS RBCs
In RBCs from SLS patients and controls, C16-C18 AGs were the major species detected; saturated AGs accounted for 66±20% of the total AGs in controls (n=10) and 70±20% in SLS (n=9). In contrast to stratum corneum and plasma, RBC AGs were not abnormal in SLS (Table 2). Furthermore, DMA analysis of RBC lipids revealed no significant differences between SLS and controls (data not shown).
Table 2.
RBC Alkylglycerols in SLS and ZSD. Data are expressed as μg AG/ml packed RBCs. Mean ± SD.
| Alkylglycerol | Control (n=10) | SLS (n=9) | ZSD (n=5) |
|---|---|---|---|
| C16:1 | 0.47 ± 0.13 | 0.39 ± 0.15 (NS)* |
0.01± 0.01 (p<0.0001) |
| C16:0 | 0.72 ± 0.25 | 0.81 ± 0.23 (NS) |
0.08 ± 0.08 (p=0.0003) |
| C18:1 | 0.87 ± 0.40 | 0.66 ± 0.23 (NS) |
0.06 ± 0.06 (p=0.003) |
| C18:0 | 1.98 ± 0.58 | 1.78 ± 0.50 (NS) |
0.26 ± 0.16 (p<0.0001) |
| Total | 4.04 ± 1.34 | 3.64 ± 1.09 (NS) |
0.33 ± 0.21 (p<0.0001) |
Numbers in parentheses indicate statistical significance compared to control values. NS, not significant.
3.4. AGs in ZSD Plasma and RBCs
In ZSD subjects (n=5), all plasma AG species were profoundly deficient (Fig 4). Total plasma AGs were decreased to mean 19% of controls (ZSD: 0.73±0.52 μg/ml; controls: 3.86±1.91; p<0.005) (Fig 4). Unlike SLS, however, the ratio of C18:0-AG/C16:1-AG in plasma was normal in ZSD subjects. In RBCs from ZSD subjects, total AG species were also severely reduced to a mean 8% of controls (Table 2).
4. Discussion
Our results demonstrate a striking accumulation of AGs in stratum corneum of SLS patients. SLS is marked by impaired fatty aldehyde oxidation due to deficient FALDH activity, which is also involved in the fatty alcohol:NAD oxidoreductase (FAO) enzyme complex that oxidizes fatty alcohols to fatty acids [10]. FALDH and FAO act on C6-C24 aliphatic substrates with a preference for long chain C16-C18 aldehydes and alcohols [15]. Fatty alcohols, mainly C16-C18, are increased in plasma [2], cultured keratinocytes [12] and cultured fibroblasts from SLS subjects [2,16]. These fatty alcohols are substrates for two biosynthetic pathways: incorporation into the ether bond of eGLs, or into the ester bond of waxes [10]. In cultured keratinocytes, fatty alcohols are largely used for synthesis of neutral eGL (1-O-alkyl-diacylglycerol) and wax esters, which both accumulate in SLS cells, whereas in cultured fibroblasts the plasmalogen pathway is most active and neutral eGLs or wax esters are not made [12]. eGLs include plasmalogen forms of phosphatidylcholine and phosphatidylethanolamine (PE), which are especially prominent lipids in brain, myelin and RBC membranes [17]. Neutral eGLs are usually considered very minor constituents in most tissues, although rodent skin produces significant amounts of 1-O-alkyl-diacylglycerols [18]. In stratum corneum of control humans, we found very little neutral eGLs.
The AG accumulation in SLS stratum corneum is consistent with diversion of excess fatty alcohols into synthesis of neutral eGLs (i.e. 1-O-alkyl-diacylglycerol), as previously demonstrated in cultured SLS keratinocytes [12]. Keratinocytes undergo terminal differentiation to produce the stratum corneum, which is pathologically hyperkeratotic in SLS resulting in clinically apparent ichthyosis. In contrast to the skin, we found that the total AG content of SLS plasma was normal, but the AG profile showed increased C18:0-AG and commensurately reduced C16:1-AG. RBCs from SLS patients did not show any AG abnormalities. The lack of increase in monounsaturated AGs in plasma and RBCs, together with normal RBC DMAs, argues against a widespread diversion of fatty alcohols into plasmalogens in SLS.
The large excess of AGs and eGLs in the stratum corneum of SLS subjects may contribute to the cutaneous disease. Most types of ichthyosis are characterized by a defective epidermal water barrier, which is dependent on the presence of multilamellar stacked membranes in the stratum corneum [19]. These membranes are delivered to the stratum corneum via exocytosis of membrane-containing lamellar body vesicles in the keratinocytes of the underlying stratum granulosum. Along with membranes, lamellar bodies deliver lipid-modifying hydrolytic enzymes that generate a unique membrane composition consisting of cholesterol, free fatty acids and ceramide s, which is critical for a functional epidermal water barrier [20]. The ichthyosis in SLS is characterized by ultrastructurally abnormal lamellar bodies lacking cargo membranes in the stratum granulosum and evidence for impaired lamellar body exocytosis in keratinocytes at the stratum granulosum-stratum corneum interface [21]. Consequently, the epidermal water barrier is functionally leaky and allows the skin to dry out. Alterations in the lipid composition of the stratum corneum membranes by pathological accumulation of eGLs, AGs and fatty alcohols probably contribute to the defective water barrier in SLS and induces a hyper-proliferative state in the skin in an attempt to restore the barrier by making more stratum corneum, leading to hyperkeratosis [21]. The finding of nonesterified AGs in the stratum corneum of SLS patients suggests that eGL metabolism may be abnormal beyond an increased synthesis rate. The SLS keratinocytes may be unable to degrade the excess ether bonds in eGLs due to insufficient hydrolytic enzyme activity (i.e. alkylglycerol monooxygenase or lyso-plasmalogenase). Future studies will be needed to investigate AG metabolism in SLS skin.
Our results also underscore the regulatory mechanisms for eGL synthesis. In normal cultured cells, plasmalogen synthesis is regulated by activity of FAR1, which catalyzes the synthesis of fatty alcohols [22,23]. Increases in AG, or one of its downstream metabolite(s) (i.e. plasmalogens) decreases FAR1 activity by enhanced degradation of the protein and thereby reduces fatty alcohol synthesis. In this fashion, plasmalogen synthesis is coordinately regulated with fatty alcohol availability. When plasmalogens cannot be made, for example in RCDP type 1 fibroblasts, FAR1 activity is excessively high and fatty alcohols accumulate [6]. Whether neutral eGL synthesis is similarly regulated through FAR1 activity has not yet been investigated, but the accumulation of neutral eGLs rather than plasmalogens in SLS skin and keratinocytes points to a funneling of AGs away from synthesis of plasmalogens and towards neutral eGLs.
In addition to SLS, we measured AGs in ZSD owing to the well-known deficiency in plasmalogen synthesis [24] and tissue plasmalogen content [25,26]. A reduction in RBC plasmalogens is commonly used as a diagnostic test for ZSD and RCDP by measuring C16:0-DMA and C18:0-DMA, typically expressed as their ratios to fatty acids C16:0 and C18:0, respectively [27,28]. However, this test only captures lipids with vinyl ether bonds (largely plasmalogens) rather than the total eGL family that we measured here and therefore misses a majority of eGLs in RBCs and plasma, which are otherwise revealed by AG analysis.
As clinical therapies are developed for SLS, the need for reliable biomarkers for this disease becomes paramount [29]. To date, the only in vivo biochemical abnormalities detected in SLS patients are accumulation of plasma fatty alcohols [2] and increased urinary leukotriene B4, which requires FALDH and FAO for omega-oxidation [30]. Measurement of these lipids in plasma or urine are technically challenging due to their low amounts. Our results suggest that AG accumulation in stratum corneum or an abnormal plasma AG profile may be useful biomarkers for SLS. Further studies to correlate the AG profile with disease severity will be needed to establish its value for therapeutic trials of SLS. Nevertheless, detecting AG accumulation in stratum corneum might be a simple and non-invasive approach for screening ichthyosis patients for SLS.
In conclusion, the abnormal AG profile in SLS is consistent with diversion of fatty alcohols into neutral eGLs. Moreover, measurement of AGs is a relatively simple technique and affords a convenient alternative to DMAs for detecting an abnormal eGL profile in ZSD and possibly other disorders of eGL metabolism.
5. Acknowledgements
This work was supported by the Sterol and Isoprenoid Research Consortium of the Rare Disease Clinical Research Network (grant U54 HD061939) from the Eunice Kennedy Shriver National Institutes of Child Health & Human Development and National Center for Advancing Translational Sciences, NIH. Funding was also provided by the Child Health Research Institute of the University of Nebraska Medical Center and Children’s Hospital & Medical Center.
Abbreviations:
- 1-acyl-DHAP
1-acyl-dihydroxyacetone phosphate
- AG
1-O-alkylglycerol
- AGPS
alkylglycerone phosphate synthase
- BSTFA/TMCS
N,O-Bis(trimethylsilyl)trifluoroacetamide with trimethylchlorosilane
- C15:0-AG
1-O-pentadecanylglycerol
- C15:1-AG
1-O-pentadecenylglycerol
- C16:0-AG
1-O-hexadecanylglycerol
- C16:1-AG
1-O-hexadecenylglycerol
- C17:0-AG
1-O-heptadecanylglycerol
- C18:0-AG
1-O-octadecanylglycerol
- C18:1-AG
1-O-octadecenylglycerol
- C20:0-AG
1-O-docosanoylglycerol
- C20:1-AG
1-O-docosenoylglycerol
- DHAP
dihydroxyacetonephosphate
- DMA
dimethyl acetal
- eGL
ether glycerolipids
- FALDH
fatty aldehyde dehydrogenase
- FAR1
fatty acyl-CoA reductase-1
- FAO
fatty alcohol
- NAD
oxidoreductase
- GC-MS
gas chromatography-mass spectrometry
- GNPAT
glyceronephosphate O-acyltransferase
- PE
phosphatidylethanolamine
- RBC
red blood cell
- RCDP
rhizomelic chondrodysplasia
- SLS
Sjögren-Larsson syndrome
- TMS
trimethylsilyl
- ZSD
Zellweger spectrum disorder
Footnotes
Conflict of Interest: All authors have no conflicts of interest in this research. The funders of this work had no involvement in its design, conduct, writing of the manuscript or decision to submit it.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rizzo WB, Sjögren-Larsson syndrome: molecular genetics and biochemical pathogenesis of fatty aldehyde dehydrogenase deficiency., Mol Genet Metab 90 (2007) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rizzo WB, Craft DA, Sjögren-Larsson syndrome: accumulation of free fatty alcohols in cultured fibroblasts and plasma., J Lipid Res 41 (2000) 1077–1081. [PubMed] [Google Scholar]
- [3].Fuijkschot J, Theelen T, Seyger MMB, van der Graaf M, de Groot IJM, Wevers RA, Wanders RJA, Waterham HR, Willemsen MAAP, Sjögren-Larsson syndrome in clinical practice., J Inherit Metab Dis 35 (2012) 955–962. [DOI] [PubMed] [Google Scholar]
- [4].Hoefler G, Hoefler S, Watkins PA, Chen WW, Moser A, Baldwin V, McGillivary B, Charrow J, Friedman JM, Rutledge L, Biochemical abnormalities in rhizomelic chondrodysplasia punctata., J Pediatr 112 (1988) 726–733. [DOI] [PubMed] [Google Scholar]
- [5].de Vet EC, Ijlst L, Oostheim W, Wanders RJ, van den Bosch H, Alkyl-dihydroxyacetonephosphate synthase. Fate in peroxisome biogenesis disorders and identification of the point mutation underlying a single enzyme deficiency., J Biol Chem 273 (1998) 10296–10301. [DOI] [PubMed] [Google Scholar]
- [6].Rizzo WB, Craft DA, Judd LL, Moser HW, Moser AB, Fatty alcohol accumulation in the autosomal recessive form of rhizomelic chondrodysplasia punctata., Biochem Med Metab Biol 50 (1993) 93–102. [DOI] [PubMed] [Google Scholar]
- [7].Itzkovitz B, Jiralerspong S, Nimmo G, Loscalzo M, Horovitz DD, Snowden A, Moser A, Steinberg S, Braverman N, Functional characterization of nov el mutations in GNPAT and AGPS, causing rhizomelic chondrodysplasia punctata (RCDP) types 2 and 3., Hum Mutat 33 (2012) 189–197. [DOI] [PubMed] [Google Scholar]
- [8].Buchert R, Tawamie H, Smith C, Uebe S, Innes AM, Al Hallak B, Ekici AB, Sticht H, Schwarze B, Lamont RE, Parboosingh JS, Bernier FP, Abou Jamra R, A peroxisomal disorder of severe intellectual disability, epilepsy, and cataracts due to fatty acyl-CoA reductase 1 deficiency., Am J Hum Genet 95 (2014) 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barøy T, Koster J, Stromme P, Ebberink MS, Misceo D, Ferdinandusse S, Holmgren A, Hughes T, Merckoll E, Westvik J, Woldseth B, Walter J, Wood N, Tvedt B, Stadskleiv K, Wanders RJA, Waterham HR, Frengen E, A novel type of rhizomelic chondrodysplasia punctate, RCDP5, is caused by loss of the PEX5 long isoform., Hum Mol Genet 24 (2015) 5845–5854. [DOI] [PubMed] [Google Scholar]
- [10].Rizzo WB, Fatty aldehyde and fatty alcohol metabolism: review and importance for epidermal structure and function., Biochim Biophys Acta 1841 (2014) 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rizzo WB, Dammann AL, Craft DA, Black SH, Tilton AH, Africk D, Chaves-Carballo E, Holmgren G, Jagell S, Sjögren-Larsson syndrome: inherited defect in the fatty alcohol cycle., J Pediatr 115 (1989) 228–234. [DOI] [PubMed] [Google Scholar]
- [12].Rizzo WB, Craft DA, Somer T, Carney G, Trafrova J, Simon M, Abnormal fatty alcohol metabolism in cultured keratinocytes from patients with Sjögren-Larsson syndrome., J Lipid Res 49 (2008) 410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Folch J, Lees M, Sloane Stanley GH, A simple method for the isolation and purification of total lipides from animal tissues., J Biol Chem 226 (1957) 497–509. [PubMed] [Google Scholar]
- [14].Myher JJ, Marai L, Kuksis A, Identification of monoacyl- and monoalkylglycerols by gas-liquid chromatography-mass spectrometry., J Lipid Res 15 (1974) 586–592. [PubMed] [Google Scholar]
- [15].Kelson TL, Secor McVoy JR, Rizzo WB, Human liver fatty aldehyde dehydrogenase: microsomal localization, purification, and biochemical characterization., Biochim Biophys Acta 1335 (1997) 99–110. [DOI] [PubMed] [Google Scholar]
- [16].Rizzo WB, Dammann AL, Craft DA, Sjögren-Larsson syndrome. Impaired fatty alcohol oxidation in cultured fibroblasts due to deficient fatty alcohol:nicotinamide adenine dinucleotide oxidoreductase activity., J Clin Invest 81 (1988) 738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Braverman NE, Moser AB, Functions of plasmalogen lipids in health and disease., Biochim Biophys Acta 1822 (2012) 1442–1452. [DOI] [PubMed] [Google Scholar]
- [18].Oku H, Shudo J, Mimura K, Haratake A, Nagata J, Chinen I, 1-O-alkyl-2,3-diacylglycerols in the skin surface lipids of the hairless mouse., Lipids 30 (1995) 169–172. [DOI] [PubMed] [Google Scholar]
- [19].Elias PM, Williams ML, Holleran WM, Jiang YJ, Schmuth M, Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism., J Lipid Res 49 (2008) 697–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Feingold KR, The outer frontier: the importance of lipid metabolism in the skin., J Lipid Res 50 Suppl (2009) S417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rizzo WB, S’Aulis D, Jennings MA, Crumrine DA, Williams ML, Elias PM, Ichthyosis in Sjögren-Larsson syndrome reflects defective barrier function due to abnormal lamellar body structure and secretion., Arch Dermatol Res 302 (2010) 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Honsho M, Asaoku FS, Fujiki Y, Posttranslational regulation of fatty acyl-CoA reductase, Farl, controls ether glycerophospholipid synthes., J Biol Chem 285 (2010) 8537–8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Honsho M, Fujiki Y, Plasmalogen homeostasis – regulation of plasmalogen biosynthesis and its physiological consequence in mammals., FEBS Letters 591 (2017) 2720–2729. [DOI] [PubMed] [Google Scholar]
- [24].Wanders RJ, Metabolic functions of peroxisomes in health and disease., Biochimie 98 (2014) 36–44. [DOI] [PubMed] [Google Scholar]
- [25].Datta NS, Wilson GN, Hajra AK, Deficiency of enzymes catalyzing the biosynthesis of glycerol-ether lipids in Zellweger syndrome. A new category of metabolic disease involving the absence of peroxisomes., N Engl J Med 311 (1984) 1080–1083. [DOI] [PubMed] [Google Scholar]
- [26].Heymans HS, vd Bosch H, Schutgens RB, Tegelaers WH, Walther JU, Muller-Hocker J, Borst P, Deficiency of plasmalogens in the cerebro-hepato-renal (Zellweger) syndrome., Eur J Pediatr 142 (1984) 10–15. [DOI] [PubMed] [Google Scholar]
- [27].Bjorkhem I, Sisfontes L, Bostrom B, Kase BF, Blomstrand R, Simple diagnosis of the Zellweger syndrome by gas-liquid chromatography of dimethylacetals., J Lipid Res 27 (1986) 786–791. [PubMed] [Google Scholar]
- [28].De Biase I, Tortorelli S, Kratz L, Steinberg SJ, Cusmano-Ozog K, Braverman N, L.Q.A. ACMG, Committee, Laboratory diagnosis of disorders of peroxisomal biogenesis and function: a technical standard of the American College of Medical Genetics and Genomics (ACMG)., Genet Med (2019). [DOI] [PubMed] [Google Scholar]
- [29].Rizzo WB, Genetics and prospective therapeutic targets for Sjögren-Larsson Syndrome., Expert Opin Orphan Drugs 4 (2016) 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Willemsen MA, de Jong JG, van Domburg PH, Rotteveel JJ, Wanders RJ, Mayatepek E, Defective inactivation of leukotriene B4 in patients with Sjögren-Larsson syndrome., J Pediatr 136 (2000) 258–260. [DOI] [PubMed] [Google Scholar]


