Abstract
The hippocampus is known to play a critical role in a variety of complex abilities, including visual-spatial reasoning, social functioning, and math. Nonverbal Learning Disability (NVLD) is a neurodevelopmental disorder characterized by deficits in visual-spatial reasoning that are accompanied by impairment in social function or mathematics, as well as motor or executive function skills. Despite the overlap between behaviors supported by the hippocampus and impairments in NVLD, the structure and function of the hippocampus in NVLD has not been studied. To address this gap in the literature, we first compared hippocampal volume and resting-state functional connectivity in children with NVLD (n = 24) and typically developing (TD) children (n = 20). We then explored associations between hippocampal structure, connectivity, and performance on measures of spatial, social, and mathematical ability. Relative to TD children, those with NVLD showed significant reductions in left hippocampal volume and greater hippocampal-cerebellar connectivity. In children with NVLD, reduced hippocampal volume associated with worse mathematical problem solving. Although children with NVLD exhibited more social problems (Social Responsiveness Scale [SRS]) and higher hippocampal-cerebellar connectivity relative to TD children, greater connectivity was associated with fewer social problems among children with NVLD but not TD children. Such an effect may suggest a compensatory mechanism. These structural and functional alterations of the hippocampus may disrupt its putative role in organizing conceptual frameworks through cognitive mapping, thus contributing to the cross-domain difficulties that characterize NVLD.
Keywords: hippocampus, resting-state fMRI, structural MRI, nonverbal learning disability, social behavior
Introduction
In addition to its critical role in declarative memory (DeMaster, Coughlin, & Ghetti, 2016; Eichenbaum, 2014; Giovanello, Schnyer, & Verfaellie, 2009; Rubin, Watson, Duff, & Cohen, 2014; Tavares et al., 2015; Tulving & Markowitsch, 1998), the hippocampus supports a variety of complex abilities including visual-spatial reasoning (Brown, Whiteman, Aselcioglu, & Stern, 2014; Guderian et al., 2015; Lee, Brodersen, & Rudebeck, 2013; Eleanor A. Maguire, Woollett, & Spiers, 2006; Morgan, Macevoy, Aguirre, & Epstein, 2011; O’Keefe & Dostrovsky, 1971), social functioning (Davidson, Drouin, Kwan, Moscovitch, & Rosenbaum, 2012; Montagrin, Saiote, & Schiller, 2018; Rubin et al., 2014; Tavares et al., 2015; Trinkler, King, Doeller, Rugg, & Burgess, 2009), and math (Qin et al., 2014; Rosenberg-Lee et al., 2018; Supekar et al., 2013; Wilkey, Cutting, & Price, 2018). Altered hippocampal structure and function is associated with marked deficits in these domains, and is implicated in numerous psychiatric disorders (Chen & Etkin, 2013; Cooper et al., 2017; Posner et al., 2014). Nonverbal Learning Disability (NVLD), a neurodevelopmental disorder with estimated prevalence of 3–4%, is characterized by a primary deficit in visual-spatial reasoning that is often accompanied by impairments in social functioning or math ability (Cornoldi, Mammarella, & Fine, 2016; Fine, Semrud-Clikeman, Bledsoe, & Musielak, 2013; Mammarella & Cornoldi, 2014; Margolis et al., 2020). Despite the overlap between behaviors supported by the hippocampus and impairments in NVLD, little is known about the structure and function of the hippocampus in NVLD.
Structural and functional studies of the hippocampus point to its integral role in visual-spatial reasoning. Electrophysiological evidence from rodents suggests that the hippocampus plays a key role in spatial navigation (O’Keefe & Dostrovsky, 1971). Indeed, multiple cells types that encode spatial information have been discovered in the rodent hippocampus, including place cells, head direction cells, grid cells, and boundary cells (Hartley, Lever, Burgess, & O’Keefe, 2013). In humans, larger hippocampal volume is associated with greater navigation experience in taxi drivers and more flexible navigation in healthy adults (Brown et al., 2014; Eleanor A. Maguire et al., 2006), and individuals with hippocampal damage have impaired visual-spatial recall (Guderian et al., 2015). Task-based functional magnetic resonance (fMRI) studies suggest that hippocampal activity is linked to spatial processing. Specifically, the hippocampus has been shown to encode information essential to spatial navigation including distances between real-world locations, details of scenes, and maps of abstract locations (Lee et al., 2013; Morgan et al., 2011). Furthermore, resting-state functional connectivity of the hippocampus predicts performance on spatial memory tasks (Persson, Stening, Nordin, & Soderlund, 2018; Woolley et al., 2015).
In addition to its well-established role in visual-spatial reasoning, the hippocampus supports social and mathematical processing, capacities that are frequently impaired in NVLD. Decreased hippocampal volume in preterm female infants is associated with increased peer problems by age 5 (Rogers et al., 2012) and individuals with hippocampal damage show smaller social networks, including a reduced number of close relationships (Davidson et al., 2012). FMRI studies have shown that increased hippocampal activity is associated with processing familiar versus novel faces (Trinkler et al., 2009), and that, during a social role-playing game, the hippocampus tracks evolving social relationships as characters move through a 2D “social space” with dimensions of power and affiliation (Tavares et al., 2015). Greater hippocampal volume is associated with improved math skills following tutoring and with performance on math standardized tests (Supekar et al., 2013; Wilkey et al., 2018); greater improvement was also observed in individuals with greater hippocampal resting-state functional connectivity with the dorsolateral and ventrolateral prefrontal cortices (Supekar et al., 2013). Finally, hippocampal activity and cortical connectivity during math problem solving increased following arithmetic training (Qin et al., 2014; Rosenberg-Lee et al., 2018). Together, these findings suggest that decreases in volume and activation of the hippocampus index decrements in social and mathematical skills.
The hippocampus is theorized to support diverse abilities through multidimensional cognitive mapping, a process that involves the organization of relational information in a manner that supports flexible behavior (Eichenbaum & Cohen, 2014; O’Keefe & Nadel, 1978; Tolman, 1948b). Though most commonly conceptualizing the hippocampus as supporting visual-spatial processing, the cognitive map theory has been expanded to include a variety of more abstract domains, including social relationships, which can be mapped onto a relational space (Schafer & Schiller, 2018). The role of cognitive mapping in the mathematical domain is less well studied. However, mathematical processing inherently requires creating and maintaining mental representations and is likely supported by cognitive mapping (Varma & Schwartz, 2011). Within this theoretical framework, many of the symptoms seen in NVLD could derive from impairments in the ability to generate cognitive maps. Together, the data suggest that deficits in hippocampal function may underlie many of the symptoms associated with NVLD.
The current study examined both the structure and resting-state functional connectivity of the hippocampus in children with NVLD. Given the role of the hippocampus in spatial, social, and mathematical processing, and given that children with NVLD often have difficulties in all three of these domains, we hypothesized that children with NVLD would show altered hippocampal structure and functional connectivity. Although no prior studies have examined hippocampal volume in children with NVLD versus typically developing (TD) children directly, one previous study reported increased hippocampal volume in children with ASD compared to children with NVLD and TD children (Semrud-Clikeman, Fine, Bledsoe, & Zhu, 2013). We expected that children with NVLD would have reduced hippocampal volume relative to their TD peers, consistent with findings from healthy and hippocampus-damaged individuals (Brown et al., 2014; Guderian et al., 2015; Eleanor A. Maguire et al., 2006; Rogers et al., 2012; Supekar et al., 2013; Wilkey et al., 2018). Furthermore, we expected that children with NVLD would have altered resting-state functional connectivity of the hippocampus relative to their TD peers. While our prior work documents reduced cortico-cortico resting state functional connectivity of non-hippocampal regions that associated with both social (Margolis, Pagliaccio, Thomas, Banker, & Marsh, 2019) and spatial (Margolis et al., 2019) functioning in children with NVLD, the sparsity of functional hippocampal findings in this area limited our ability to make directional hypotheses. Finally, we hypothesized that NVLD-related differences in hippocampal structure and function would be associated with behavioral measures of spatial, social, and mathematical ability.
Methods
Participants
Seventy-two children (7–15 years old) enrolled in the current study and were screened for inclusion/exclusion at the New York State Psychiatric Institute. This included two groups of children (n = 50 children with suspected NVLD and n = 22 typically developing children) recruited through announcements posted at local schools and clinics, on social media, and in the newsletter of The NVLD Project, a non-profit aimed at developing resources for families of children with NVLD. All children were monolingual English speakers. The institutional review board at New York State Psychiatric Institute approved the study; children and their guardians provided written informed assent and consent, respectively. All research was performed in accordance with the relevant guidelines and regulations.
Of the 50 children with suspected NVLD (see Table 1 for specific criteria), 15 did not meet diagnostic criteria, and five others did not successfully complete an MRI scan (refused to scan, aborted during scan, and/or fell asleep), leaving 30 children with NVLD who completed the structural and resting-state scans. Of the 22 TD children recruited, 21 TD children successfully completed the structural and resting-state scans. Six children with NVLD and one TD child were then excluded from imaging analyses due to head motion (described below). One TD participant was subsequently excluded from structural analyses due to missing socioeconomic status data, and one NVLD participant had structural data that could not be segmented due to motion (described below). Thus, 23 children with NVLD and 19 TD children had useable structural data, and 24 children with NVLD and 20 TD children had useable resting-state data.
Table 1.
Diagnostic Criteria for Nonverbal Learning Disability
| Criterion | Assessment Measure |
|---|---|
| Child must have: | |
| Perceptual deficit OR a discrepancy between VIQ and PIQ (>15 points) | WISC or WASI: Block Design or Matrix Reasoning ≤16th%ile |
| Intact single word reading abilities | WJ-III Letter Word Identification ≥16th%ile |
| Absence of autistic traits | ADI-R Interests and Behaviors Module ≤4 |
| Child must also have 2 of the following: | |
| Fine motor difficulties | Perdue Pegboard ≤16th%ile |
| Math calculation difficulties | WJ-III Calculation ≤16th%ile |
| Visual executive functioning difficulties | Rey Osterrieth Complex Figure Test Copy ≤16th%ile |
| Social difficulties | Vineland-II Socialization domain ≤16th%ile or CBCL Social Problems ≥ 95th%ile |
ADI-R = Autism Diagnostic Interview – Revised; CBCL = Child Behavior Checklist; NVLD = Nonverbal Learning Disability; WJ = Woodcock Johnson; WASI = Wechsler Abbreviated Scale of Intelligence; WISC = Wechsler Intelligence Scale for Children
Diagnostic Criteria for NVLD
A diagnosis of NVLD was established in accord with prior research criteria (Table 1; (Fine, Musielak, & Semrud-Clikeman, 2014; Margolis et al., 2019; Semrud-Clikeman et al., 2013) Children were included in the NVLD group if they had visual-spatial deficits, intact reading abilities, and deficits in two of the following domains: fine motor, math calculation, visual executive functioning, or social skills. Visual-spatial deficits included either a perceptual deficit, as measured by a score below the 16th percentile on Block Design or Matrix Reasoning on either the Wechsler Abbreviated Scale of Intelligence (WASI-I) or Wechsler Intelligence Scale for Children (WISC-IV), or a discrepancy between the Verbal Intelligence Quotient (VIQ) and Performance Intelligence Quotient (PIQ) of at least 15 points, as measured from the WASI-I; (A. S. Kaufman, Raiford, & Coalson, 2015; Wechsler, 2003). Additionally, they could not have significant levels of Autism Spectrum Disorder (ASD) features. Typically developing children had no current or lifetime diagnoses as determined by the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS; (J. Kaufman et al., 1997). Children were excluded if they had a Full-Scale Intelligence Quotient (FSIQ) < 80 based on the WASI ((Wechsler, 2003), any history of major medical conditions, or MRI contraindication. To determine NVLD status, a neuropsychological assessment was administered by a certified school psychologist (Ed.M.) who had formal Autism Diagnosis Interview-Revised (ADI-R; (Rutter, Le Couteur, & Lord, 2003) and KSADS clinical training.
Outcome Measures
The outcomes of interest in the current study included measures of visual-spatial, social, and mathematical ability. Full details of the neuropsychological assessment are presented in the supplement.
Visual-spatial ability was measured by the Performance Intelligence Quotient (PIQ), from the WASI (Wechsler, 2003). The PIQ averages performance on the Matrix Reasoning and Block Design subtests.
Children’s socioemotional functioning was measured by means of parent report on the Social Responsiveness Scale (SRS; Total score) and the Child Behavior Checklist (CBCL; Total Problems T-score). These scales are widely used, clinically validated measures. The SRS provides a measure of specific aspects of social function (Constantino & Gruber, 2005). The CBCL Total Problems score provides a measure of overall socioemotional difficulties (Achenbach & Rescorla, 2001). Among children with NVLD, social function was additionally measured by child performance on the Child and Adolescent Social Perception (CASP) test (Magill-Evans, Koning, Cameron-Sadava, & Manyk, 1995). During the CASP test, a participant is tasked with using social cues to identify the emotions displayed by an on-screen character.
Mathematics ability was measured in children with NVLD using the Woodcock-Johnson Tests of Achievement 3rd edition (WJ-III) Calculation subtest (Woodcock, McGrew, & Mather, 2001).
Neuroimaging Acquisition
Functional and anatomical images were acquired on a 3T GE 750 scanner with a 32-channel head coil. Two structural T1 images were collected for each participant with an 8-channel head coil using a 3D FSPGR sequence (flip angle=11, TE=2.588ms, TR=6.412ms, 180 slices, 1mm isotropic resolution). Two runs of resting-state data were acquired using an echo planar imaging (EPI) sequence (flip angle=77, TE=30ms, TR=2000ms, 34 slices, 3.5mm isotropic resolution, 140 acquisition frames, 4 minutes and 40 seconds long). During the two resting-state runs, participants were instructed to rest quietly with their eyes open without falling asleep. The examiner monitored that participants kept their eyes open and stayed awake during these scans using an in-scanner eye-tracking camera.
Structural Analyses
Preprocessing:
Structural data were processed using the standard FreeSurfer v6.0 pipeline (recon-all;(Fischl et al., 2002; Fischl et al., 2004). Both T1 scans were averaged for analysis except when participants had only one useable scan. Processing included intensity normalization, subcortical segmentation, and cortical parcellation. Output images were visually inspected for quality. To correct for errors in cortical segmentation, manual control point edits were used for seven image files (FreeSurfer was re-run with these manual edits and results were re-checked). Hippocampal volume estimates were extracted from the subcortical segmentations and all structural analyses covaried for estimates of total intracranial volume.
Resting-State Functional Connectivity
Preprocessing:
Analysis was performed in the CONN toolbox v17.f (www.nitrc.org/projects/conn(Whitfield-Gabrieli & Nieto-Castanon, 2012) for SPM 12. Preprocessing followed a previously published pipeline and included realignment, unwarping, centering, slice timing correction, Artifact Detection Tools outlier detection, segmentation of cerebral spinal fluid (CSF), gray, and white matter, normalization to the Montreal Neurological Institute (MNI) template, and 8mm full-width half-maximum smoothing for functional images. Structural images were centered, segmented, and normalized to the MNI template. BOLD signal was band-pass filtered (0.008–0.09Hz). Denoising was completed with anatomical component-based noise correction (aCompCor(Behzadi, Restom, Liau, & Liu, 2007), specifically regressing ten white matter and ten CSF components (detrended and despiked).
Motion Correction:
To minimize effects of head motion, frames exceeding 0.5mm frame-wise displacement or frame-to-frame change in global signal z>3 were regressed from the data in the first level models. In addition, 24 head motion parameters (motion + first-order derivatives + quadradic effects) were included as regressors in the first level models. To further adjust for potential effects of motion on functional connectivity measures, mean head motion was included as a second level covariate. Participants with less than 3 minutes of useable data (<81 useable frames, or 30% of total frames), were excluded from the analyses (n = 6 NVLD, n = 1 TD).
Statistical Analyses
T-tests and chi-square tests evaluated group differences (TD vs. NVLD; Table 2) in demographics (age, sex, socioeconomic status [SES; as measured by the Hollingshead Scale ranging from 8–66 (Hollingshead, 1975)]), outcome measures (PIQ, SRS Total, CBCL Total Problems) and in-scanner motion (mean framewise displacement). Means and ranges of all outcome measures, including those only available in children with NVLD (CASP Emotions and Cues, WJ Calculation), are also presented in Table 2.
Table 2:
Clinical and Demographic Characteristics
| TD mean/count (SD/%) | NVLD mean/count (SD/%) | t/χ2 | |
|---|---|---|---|
| n=20 | n=24 | ||
| Age (months) | 116.7 (15.06) | 140.8 (29.9) | −3.28*** |
| 86 – 139 | 87 – 185 | ||
| Sex (female) | 10 (50%) | 10 (41.7%) | 0.306 |
| - | - | ||
| VIQ | 125.0 (11.7) | 105.3 (11.3) | 5.66*** |
| 95 – 144 | 82 – 122 | ||
| PIQ | 120.3 (13.3) | 88.3 (10.1) | 9.08*** |
| 84 – 143 | 70 – 114 | ||
| SES | 59.4 (5.0) | 58.17 (6.1) | 0.742 |
| 45.0 – 66.0 | 41.0 – 66.0 | ||
| SRS Total | 43.9 (6.7) | 71.8 (16.0) | −7.30*** |
| 35 – 64 | 45 – 105 | ||
| CBCL Total Problems T-score | 44.96 (8.4) | 64.1 (8.4) | −7.43*** |
| 34 – 69 | 43 – 82 | ||
| CASP Emotions (PC) | - | 42.6% (13.1%) | - |
| 23.8% – 69.0% | |||
| CASP Cues (PC) | - | 22.8% (8.6%) | - |
| 9.8% – 41.5% | |||
| WJ Calculation | - | 92.6 (15.1) | - |
| 65 – 131 | |||
| Valid Frames | 214.3 (59.2) | 186.9 (55.4) | 1.58 |
| 106 – 273 | 98 – 276 | ||
| Mean Motion | .22 (.21) | .40 (.30) | −2.14* |
| 0.06 – 0.94 | 0.08 – 1.37 |
VIQ=Verbal IQ, PIQ=Performance IQ, SES=Socioeconomic status (Hollingshead, 1975), SRS=Social Responsiveness Scale, CBCL=Child Behavior Checklist, CASP=Child and Adolescent Social Perception Test, PC = Percent Correct, WJ=Woodcock Johnson. Ranges are included for continuous variables.
p<.05,
p<.01,
p<.001.
Linear regressions tested differences between groups (NVLD, TD) in hippocampal volume, controlling for age, sex, SES, and total intracranial volume. Hippocampal volumes greater than three standard deviations from the mean were considered outliers and were winsorized (two participants). Hippocampal volume results without winsorizing are presented in supplemental materials. SES was included as a covariate in these models because of its well-documented association with brain structure, particularly in the hippocampus (Brito & Noble, 2014; Merz et al., 2019; Noble et al., 2015).
To investigate connectivity from left and right hippocampus to the rest of the brain, seed-to-voxel functional connectivity maps were generated in CONN using the Harvard-Oxford atlas defined left and right hippocampal seeds (Figure S1). Second-level voxel-wise regression coefficient maps compared hippocampal connectivity of children with NVLD to TD children, controlling for age, sex, and mean motion. Maps were thresholded at voxel-level significance p<.001 and a false discovery rate (FDR) corrected cluster size threshold q<.05.
We tested whether brain-behavior associations varied by group in linear regression models with group, volume/connectivity, and their interaction as predictors of behavioral outcomes. The interaction term was dropped from models if it was not significant. P-values were Bonferroni-corrected for six tests (SRS, CBCL, PIQ, WJ Calculation, CASP Emotions, and CASP Cues; p<0.0083). Significant interaction effects were followed up by post-hoc analyses within each group (NVLD, TD). Direct measures that were only available in children with NVLD (CASP, WJ Calculation) were only assessed within the clinical group. All structural models included age, sex, SES, and total intracranial volume; all models of functional connectivity included age, sex, and mean head motion.
For parent report of social functioning (SRS, CBCL), significant total score associations were followed up by exploratory analyses of subscales to further parse effects. Such exploratory analyses were performed for informational purposes to generate hypotheses for future studies. Supplementary analyses included VIQ as well as the interaction between VIQ and Group as covariates in structural and functional connectivity models. In additional supplemental analyses, we tested for associations between behavior and hippocampal connectivity with the whole-brain using brain connectivity as the dependent variable (supplemental materials). Rather than limit the behavioral associations to areas that showed significant group differences, this supplemental analysis offers a broader test of brain-behavior associations.
Results
Clinical and Demographic Characteristics of Participants
Participants with useable MRI data ranged in age from 7 to 15 years old; children with NVLD were older on average than TD children (Table 2). As defined by the diagnostic criteria, relative to TD children, those with NVLD had lower PIQ, more parent-reported social problems (SRS Total) and more general socioemotional problems (CBCL Total Problems). Groups also differed in VIQ, with children with NVLD placing in the average range and TD children placing above the average range.
Hippocampal Volume
Distributions of hippocampal volumes are presented in Figure S2. Children with NVLD had significantly smaller left hippocampal volumes relative to TD children with a medium effect size (b= −0.67, t(36) = −2.34, p = .025, Cohen’s d = .78; Figure 1); differences in right hippocampal were at trend level with a medium effect size (b=−0.53, t(36) = −1.80, p = .080, Cohen’s d = .60; Figure 1; covariate-adjusted Cohen’s D calculated according to equation 10 in Nakagawa and Cuthill (Nakagawa & Cuthill, 2007)). Results were largely the same with non-winsorized hippocampal volumes (supplemental materials).
Figure 1.
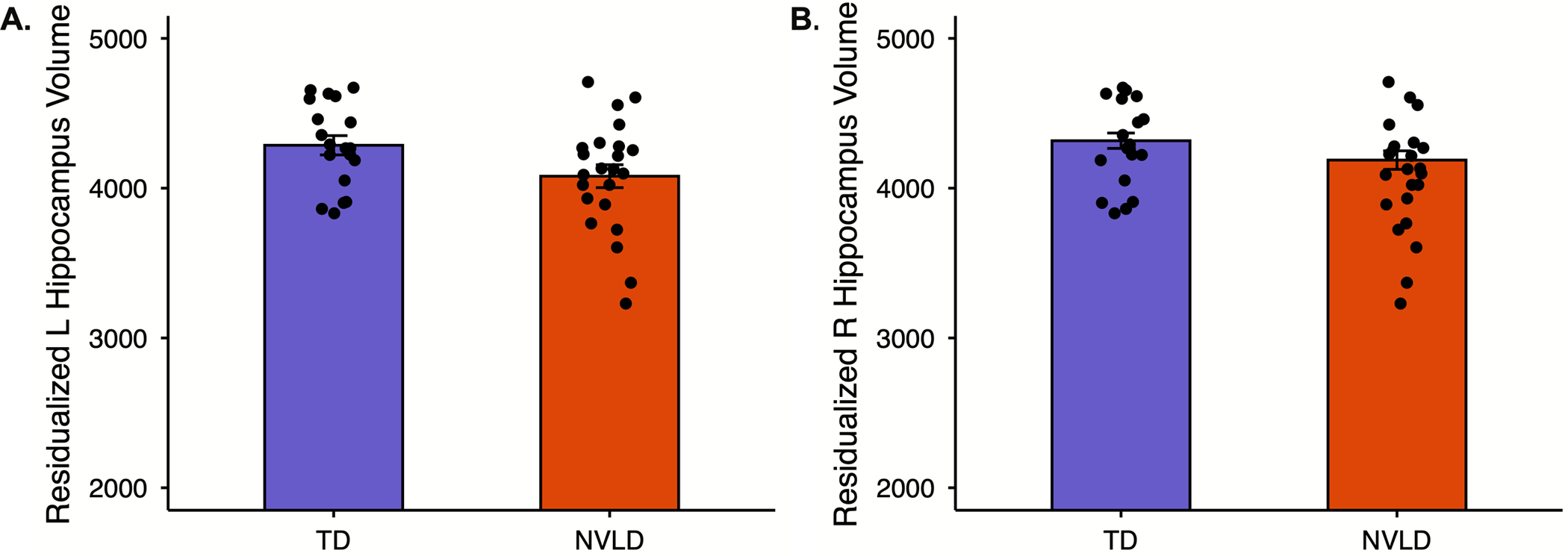
Children with Nonverbal Learning Disability (NVLD) had reduced hippocampus volume relative to typically developing (TD) children controlling for age, sex, socioeconomic status, and intracranial volume.
2Hippocampal Resting-State Functional Connectivity
Relative to TD children, those with NVLD showed significantly increased functional connectivity between right hippocampus and a cluster in lobule VI of the right cerebellum (peak located at x=30, y=−60, z=−32 in MNI coordinates; k = 72; p-FDR = 0.008, Figure 3). No significant clusters were detected using the left hippocampal seed. Although group differences in age and motion were detected, neither was associated with connectivity (age: b=.16, p=.32; motion: b=−.06, p=.64).
Figure 3.
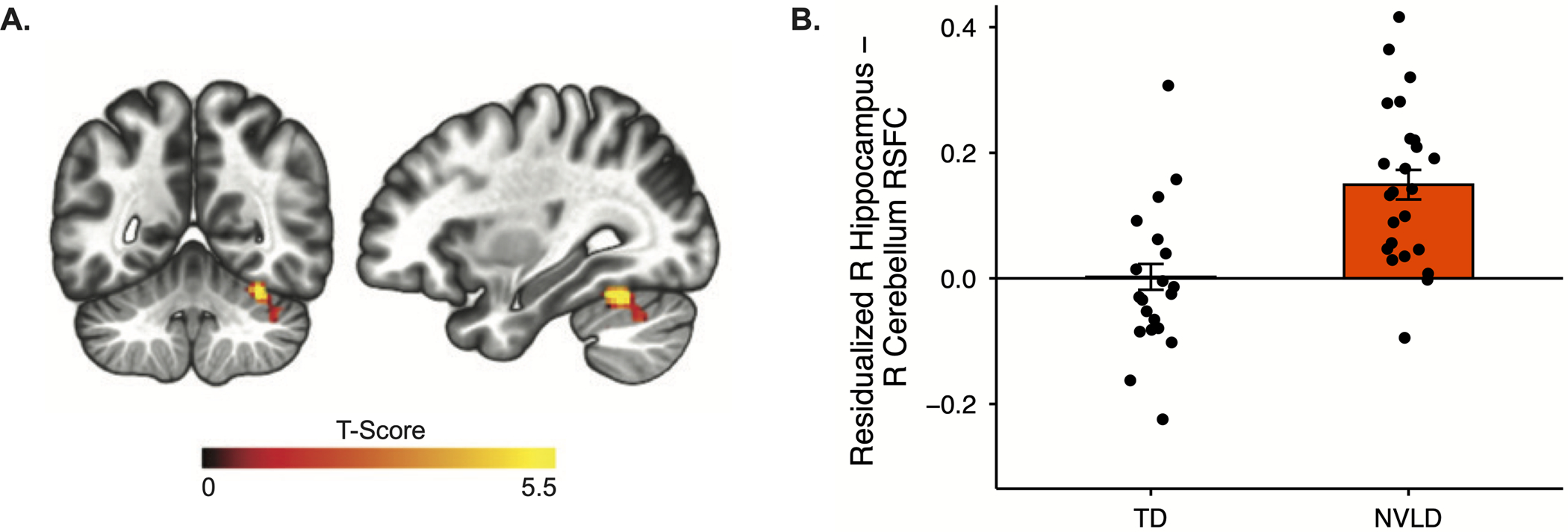
Resting-state functional connectivity (RSFC) between the right hippocampus and the A) right cerebellum B) differs between healthy controls and children with NVLD, controlling for age, sex, and mean head motion.
Brain-Behavior Associations
No associations were detected between visual-spatial ability and hippocampal volume or hippocampal-cerebellar connectivity. Among children with NVLD, worse performance on WJ Calculation was associated with reduced left hippocampal volume (b= 0.92, t(17)=3.42, p=.004; Figure 2; Table S1) but not with hippocampal-cerebellar connectivity (Table S2).
Figure 2.
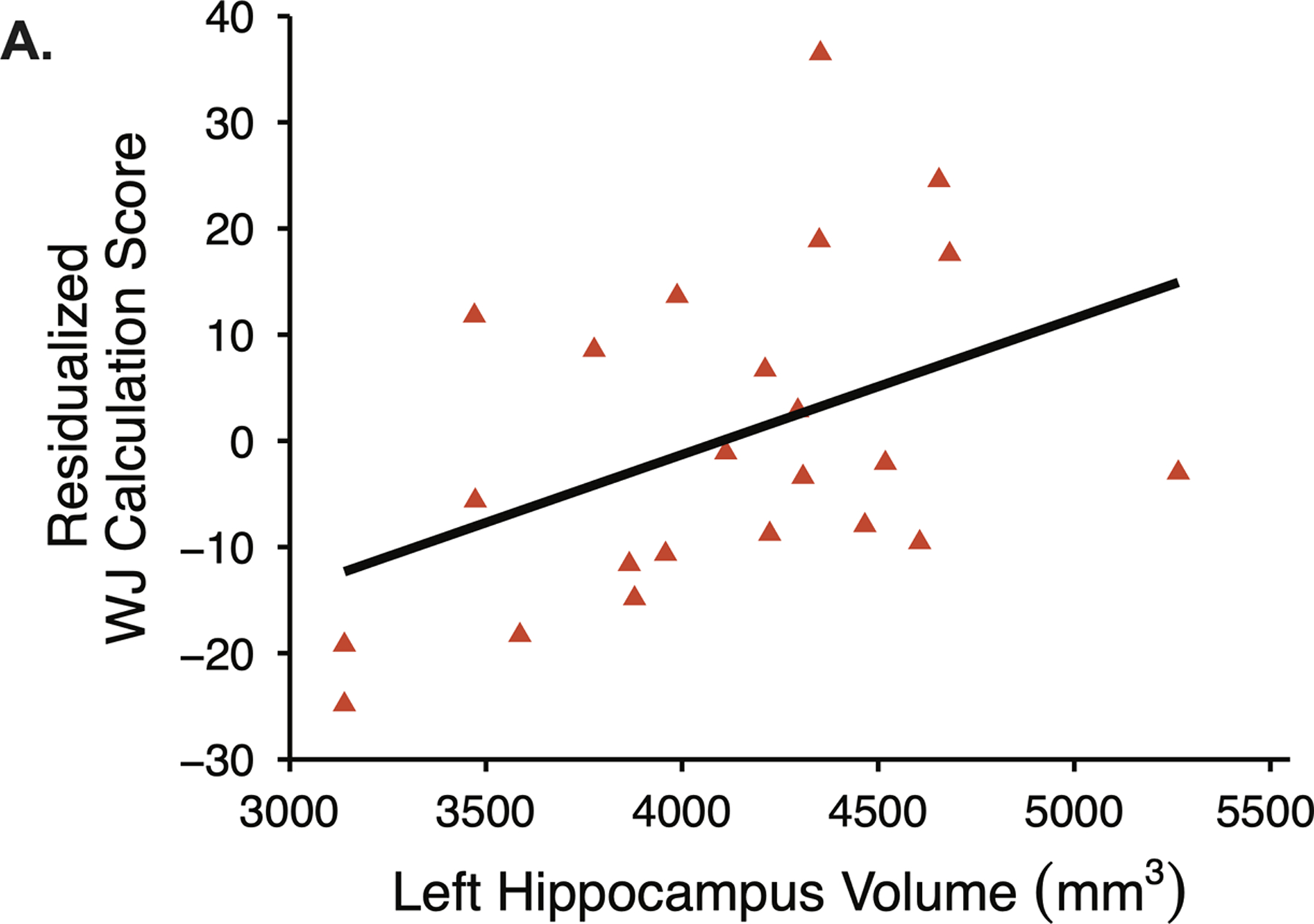
Among children with NVLD, smaller left hippocampal volume was associated with impaired math ability as measured by the Woodcock Johnson (WJ) Calculation subscore, controlling for age, sex, socioeconomic status, and intracranial volume.
Across all participants, social problems as measured by the SRS Total score were significantly associated with the group by hippocampal-cerebellar connectivity interaction (b=−0.72, t(37)=−2.89, p=.006, Figure 4). A significant group by hippocampal-cerebellar connectivity interaction that did not pass Bonferroni correction was detected for socioemotional problems as measured by the CBCL Total Problems score (b=−0.65, t(36)=2.41, p=.02, Figure 4). Hippocampal-cerebellar connectivity was inversely associated with socioemotional problems in children with NVLD (SRS Total Score: b=−0.67, t(19)=−3.70, p=0.002; CBCL Total Problems: b=−0.60, t(19)=−3.04, p=0.007), whereas there was no association between connectivity and behavior in TD children (Table S2). No significant associations were detected between hippocampal-cerebellar connectivity and CASP scores (Table S2), nor between hippocampal volume and measures of social function (Table S1). A significant group-by-VIQ interaction showed a positive association between VIQ and hippocampal volume in children with NVLD but not TD children (supplemental results).
Figure 4.
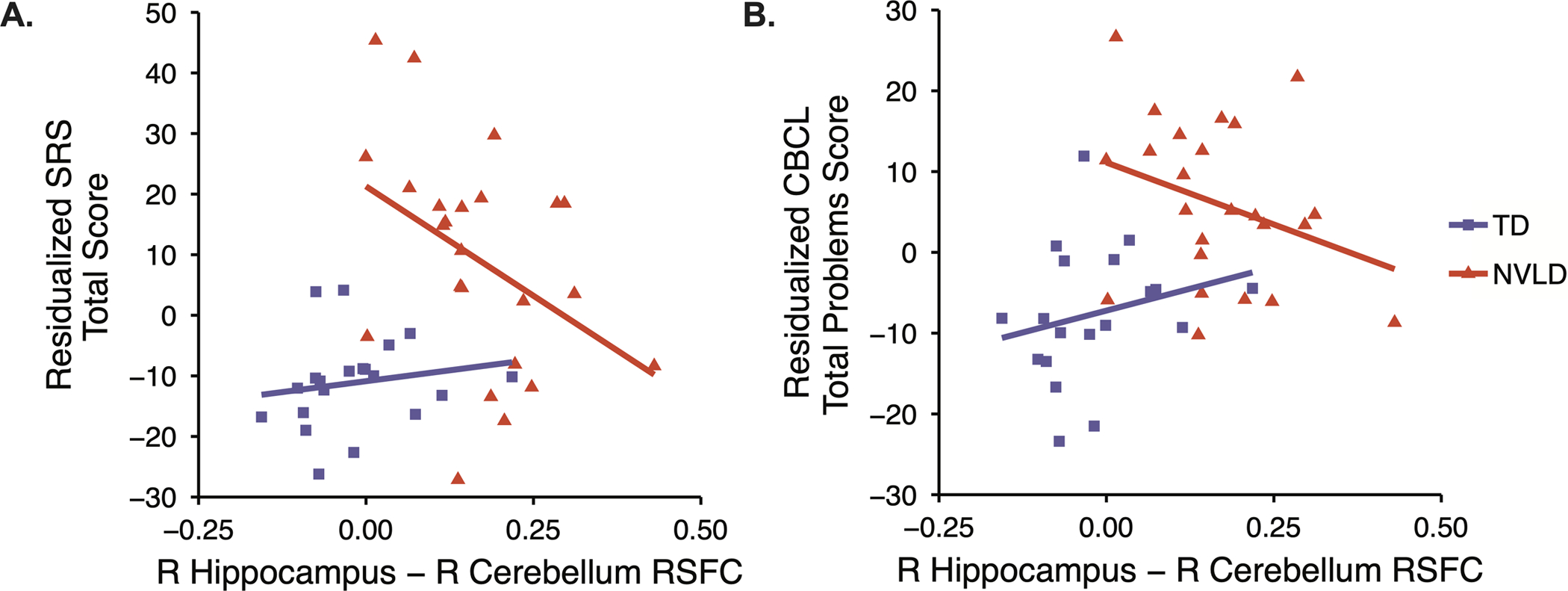
NVLD diagnosis moderates the association between right hippocampus – right cerebellum resting-state functional connectivity (RSFC) and both A) social responsiveness (SRS Total Score) and B) socioemotional wellbeing (CBCL Total Problems) Among typically developing children (TD), connectivity was unrelated to these behaviors. Among children with NVLD, increased connectivity was associated with less socioemotional and social impairment.
To understand whether altered hippocampal-cerebellar connectivity was associated with specific aspects of social function in children with NVLD, we explored associations between connectivity and subscales of the SRS and CBCL. The group by hippocampal-cerebellar connectivity interaction was significant for SRS communication, cognition, and awareness, as well as CBCL externalizing, and at trend level for CBCL social problems (Table S2). Among children with NVLD, hippocampal-cerebellar connectivity was inversely associated with the communication (b=−0.74, t(19)=−4.65, p=0.0002), cognition (b=−0.62, t(19)=−3.20, p=0.005), and awareness (b=−0.55, t(19)=−2.65, p=0.02; Table S2) SRS subscales, and the externalizing (b=−0.49, t(19)=−2.39, p=0.03), and social problems (b=−0.50, t(19)=−3.39, p=0.03; Table S2) CBCL subscales.
Discussion
Our study provides multimodal evidence that hippocampal abnormalities may underlie several domains of dysfunction in NVLD. Compared to TD children, those with NVLD showed smaller left hippocampal volumes that associated with worse mathematical calculation skills. In addition, children with NVLD had more parent reported social problems and greater right hippocampal-cerebellar connectivity than TD children. Among children with NVLD, greater hippocampal-cerebellar connectivity was associated with fewer social problems, suggesting a compensatory mechanism. As impairments in mathematical ability and social functioning are key features of NVLD, our findings suggest that hippocampal alterations may characterize the pathophysiology of the disorder.
Left hippocampal volume was significantly reduced in children with NVLD relative to TD children, and right hippocampal volume showed trend level differences in the same direction, both with medium effect sizes. One prior study showed no differences in hippocampal volumes between children with NVLD and TD children (Semrud-Clikeman et al., 2013), however, methodological differences such as the use of voxel-based morphometry and exclusion of covariates such as intracranial volume may contribute to this discrepancy. In our study, the observed reduction in left hippocampal volume was associated with impairments in mathematical ability in children with NVLD, consistent with prior imaging findings that hippocampal volume indexes arithmetic skills (Supekar et al., 2013; Wilkey et al., 2018). We also observed a significant group-by-VIQ interaction on hippocampal volume, such that VIQ was associated with hippocampal volume only in children with NVLD and not in TD children. Genetic or environmental factors that contribute to a higher VIQ may protect against hippocampal decrements in NVLD. Future studies with larger samples are required to better understand these differences.
Relative to typically developing children, children with NVLD had greater functional connectivity between right hippocampus and lobule VI of the cerebellum as well as more parent reported social problems. A significant group by connectivity interaction on social problems was driven by greater hippocampal-cerebellar connectivity indexing better social function in children with NVLD, but a lack of association in TD children. Taken together, these findings suggest that increased hippocampal-cerebellar connectivity may operate as a compensatory mechanism in children with NVLD. Supporting this theory, studies have implicated both the cerebellum and the hippocampus in social processes. Lobule VI and neighboring cerebellar regions are involved in interpreting emotional intonation (Schmahmann & Caplan, 2006; Van Overwalle, Baetens, Marien, & Vandekerckhove, 2014; Wildgruber et al., 2005), and the hippocampus is involved in the organization of social information and the maintenance of social networks (Tavares et al., 2015; Davidson et al., 2012).
Despite known associations between spatial navigation and hippocampal structure and connectivity with the cerebellum (Babayan et al., 2017; Doeller, Barry, & Burgess, 2010; Morgan et al., 2011; O’Keefe & Dostrovsky, 1971; Watson et al., 2019), neither was associated with visual-spatial performance on the perceptual tasks of the Wechsler IQ test in our study. This discrepancy may reflect the fact that hippocampal functional connectivity with the cerebellum is more strongly associated with dynamic, navigation-based aspects of visual-spatial reasoning (Babayan et al., 2017; Berthoz et al., 2014) than the static, perceptual reasoning aspects indexed by performance IQ. Nevertheless, our results add to a growing literature by showing differences in hippocampal volume in children and adolescents who anecdotally have trouble with route finding and spatial navigation (Cornoldi et al., 2016). This is consistent with prior work reporting associations between spatial navigation and hippocampal volume among individuals with either expertise or deficits in spatial navigation e.g., London taxi drivers or Alzheimer’s patients (Convit et al., 1997; Eleanor A Maguire et al., 2000). Future studies of NVLD should include a range of spatial navigation tasks to better understand the nature of the perceptual processing deficits and hippocampal abnormalities that characterize NVLD.
The specific ways in which structural and functional changes in the hippocampus interact to support human spatial navigation remain largely unknown. A recent theoretical model puts forth the retrosplenial cortex as the critical hub for navigation in dynamic circuit that includes the hippocampus (Ekstrom, Huffman, & Starrett, 2017). This circuit-based account can be reconciled with data showing that, although associations between hippocampal volume and spatial navigation are detected in individuals at the extremes of performance (those with expertise or deficits), such associations are not detected in individuals in the midrange of ability (Weisberg & Newcombe, 2018). At the extremes of behavior, detectable changes in hippocampal volume may disrupt circuit function. For individuals in the midrange, slight fluctuations in hippocampal volume may not result in changes in navigation ability due to the more central role of the retrosplenial cortex. Our prior findings that children with NVLD show reduced retrosplenial connectivity which associates with their PIQ further support this circuit-based view (Banker et al., 2020).
Our study has several limitations. The sample size is small, potentially limiting the generalizability of findings, and the study therefore requires replication in a larger independent sample. Additionally, the lack of a spatial navigation measure limited our ability to comprehensively assess the many aspects visual-spatial reasoning and made it difficult to align our findings with prior work in rodents. Furthermore, group differences in age and motion indicate that these factors could be cofounding the association between group membership and functional connectivity. However, given the lack of association between age or motion with functional connectivity, and that both effects are statistically controlled, such a possibility is unlikely. Finally, exploratory correlations between sub-scores of the SRS and CBCL and hippocampal structure and connectivity values were not corrected for multiple comparisons and therefore should be interpreted with caution and require replication.
To our knowledge, this is the first study to examine both hippocampal structure and functional connectivity in children with NVLD. Our data suggest that the pathophysiology of NVLD derives in part from altered structure of the hippocampus, which associated with impairments in math, and its functional connectivity with cerebellum, which associated with social problems. One theory posits that the hippocampus supports a variety of functions through cognitive mapping, which represents the capacity to organize conceptual relationships within a spatial framework (Epstein, Patai, Julian, & Spiers, 2017; O’Keefe & Nadel, 1978; Tolman, 1948a). Similarly, the theory of a retrosplenial-centered circuit for spatial navigation (Ekstrom et al., 2017) supports a broadened role for the hippocampus that is not confined spatial reasoning. In line with these ideas and with our results, NVLD may in part be related to an impairment in hippocampal cognitive mapping, leading to the cross-domain difficulties that characterize the disorder. Our findings present the hippocampus as a critical region for future studies in NVLD.
Supplementary Material
Acknowledgments:
This work was supported by funding from NIEHS K23ES026239 (AEM) and the NVLD Project (AEM).
Footnotes
Data availability: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Achenbach T, & Rescorla L (2001). Manual for the ASEBA school-age forms & profiles: an integrated system of mult-informant assessment. Burlington, VT: University of Vermont, Research Center for Children, Youth & Families. [Google Scholar]
- Babayan BM, Watilliaux A, Viejo G, Paradis A-L, Girard B, & Rondi-Reig L (2017). A hippocampo-cerebellar centred network for the learning and execution of sequence-based navigation. Scientific Reports, 7(1), 17812. doi: 10.1038/s41598-017-18004-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker SM, Ramphal B, Pagliaccio D, Thomas L, Rosen E, Sigel AN, … Margolis AE (2020). Spatial Network Connectivity and Spatial Reasoning Ability in Children with Nonverbal Learning Disability. Scientific Reports, 10(1), 561–561. doi: 10.1038/s41598-019-56003-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37(1), 90–101. doi: 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoz A, Rondi-Reig L, Iglói K, Paradis A-L, Benchenane K, Doeller CF, & Burgess N (2014). Interaction Between Hippocampus and Cerebellum Crus I in Sequence-Based but not Place-Based Navigation. Cerebral Cortex, 25(11), 4146–4154. doi: 10.1093/cercor/bhu1327JCerebralCortex [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NH, & Noble KG (2014). Socioeconomic status and structural brain development. 8(276). doi: 10.3389/fnins.2014.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TI, Whiteman AS, Aselcioglu I, & Stern CE (2014). Structural Differences in Hippocampal and Prefrontal Gray Matter Volume Support Flexible Context-Dependent Navigation Ability. The Journal of Neuroscience, 34(6), 2314. doi: 10.1523/JNEUROSCI.2202-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, & Etkin A (2013). Hippocampal Network Connectivity and Activation Differentiates Post-Traumatic Stress Disorder From Generalized Anxiety Disorder. Neuropsychopharmacology, 38, 1889. doi:10.1038/npp.2013.12210.1038/npp.2013.122https://www.nature.com/articles/npp2013122#supplementary-informationhttps://www.nature.com/articles/npp2013122#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, & Gruber CP (2005). The Social Responsiveness Scale Manual. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Convit A, De Leon M, Tarshish C, De Santi S, Tsui W, Rusinek H, & George A (1997). Specific hippocampal volume reductions in individuals at risk for Alzheimer’s disease. Neurobiology of aging, 18(2), 131–138. [DOI] [PubMed] [Google Scholar]
- Cooper RA, Richter FR, Bays PM, Plaisted-Grant KC, Baron-Cohen S, & Simons JS (2017). Reduced Hippocampal Functional Connectivity During Episodic Memory Retrieval in Autism. Cerebral Cortex, 27(2), 888–902. doi: 10.1093/cercor/bhw417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornoldi C, Mammarella I, & Fine J (2016). Nonverbal Learning Disabilities: Guilford Press. [Google Scholar]
- Davidson P, Drouin H, Kwan D, Moscovitch M, & Rosenbaum RS (2012). Memory as Social Glue: Close Interpersonal Relationships in Amnesic Patients. Frontiers in Psychology, 3(531). doi: 10.3389/fpsyg.2012.00531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaster D, Coughlin C, & Ghetti S (2016). Retrieval flexibility and reinstatement in the developing hippocampus. Hippocampus, 26(4), 492–501. doi:doi: 10.1002/hipo.22538 [DOI] [PubMed] [Google Scholar]
- Doeller CF, Barry C, & Burgess N (2010). Evidence for grid cells in a human memory network. Nature, 463(7281), 657–661. doi: 10.1038/nature08704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2014). Time cells in the hippocampus: a new dimension for mapping memories. Nature reviews. Neuroscience, 15(11), 732–744. doi: 10.1038/nrn3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, & Cohen NJ (2014). Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron, 83(4), 764–770. doi: 10.1016/j.neuron.2014.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Huffman DJ, & Starrett M (2017). Interacting networks of brain regions underlie human spatial navigation: a review and novel synthesis of the literature. Journal of neurophysiology, 118(6), 3328–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Patai EZ, Julian JB, & Spiers HJ (2017). The cognitive map in humans: spatial navigation and beyond. Nature Neuroscience, 20, 1504. doi: 10.1038/nn.4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine JG, Musielak KA, & Semrud-Clikeman M (2014). Smaller splenium in children with nonverbal learning disability compared to controls, high-functioning autism and ADHD. Child Neuropsychol, 20(6), 641–661. doi: 10.1080/09297049.2013.854763 [DOI] [PubMed] [Google Scholar]
- Fine JG, Semrud-Clikeman M, Bledsoe JC, & Musielak KA (2013). A critical review of the literature on NLD as a developmental disorder. Child Neuropsychol, 19(2), 190–223. doi: 10.1080/09297049.2011.648923 [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Dale AM (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, & Dale AM (2004). Sequence-independent segmentation of magnetic resonance images. Neuroimage, 23 Suppl 1, S69–84. doi: 10.1016/j.neuroimage.2004.07.016 [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer D, & Verfaellie M (2009). Distinct hippocampal regions make unique contributions to relational memory. Hippocampus, 19(2), 111–117. doi:doi: 10.1002/hipo.20491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guderian S, Dzieciol AM, Gadian DG, Jentschke S, Doeller CF, Burgess N, … Vargha-Khadem F (2015). Hippocampal Volume Reduction in Humans Predicts Impaired Allocentric Spatial Memory in Virtual-Reality Navigation. The Journal of Neuroscience, 35(42), 14123. doi: 10.1523/JNEUROSCI.0801-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Lever C, Burgess N, & O’Keefe J (2013). Space in the brain: how the hippocampal formation supports spatial cognition. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 369(1635), 20120510–20120510. doi: 10.1098/rstb.2012.0510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB (1975). Four-factor index of social status. New Haven, CT: Yale University Press. [Google Scholar]
- Kaufman AS, Raiford SE, & Coalson DL (2015). Intelligent testing with the WISC-V: John Wiley & Sons. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry, 36(7), 980–988. doi: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Lee AC, Brodersen KH, & Rudebeck SR (2013). Disentangling spatial perception and spatial memory in the hippocampus: a univariate and multivariate pattern analysis fMRI study. J Cogn Neurosci, 25(4), 534–546. doi: 10.1162/jocn_a_00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill-Evans J, Koning C, Cameron-Sadava A, & Manyk K (1995). The child and adolescent social perception measure. Journal of Nonverbal Behavior, 19(3), 151–169. doi: 10.1007/BF02175502 [DOI] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, & Frith CD (2000). Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences, 97(8), 4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Woollett K, & Spiers HJ (2006). London taxi drivers and bus drivers: A structural MRI and neuropsychological analysis. Hippocampus, 16(12), 1091–1101. doi:doi: 10.1002/hipo.20233 [DOI] [PubMed] [Google Scholar]
- Mammarella IC, & Cornoldi C (2014). An analysis of the criteria used to diagnose children with Nonverbal Learning Disability (NLD). Child Neuropsychol, 20(3), 255–280. doi: 10.1080/09297049.2013.796920 [DOI] [PubMed] [Google Scholar]
- Margolis AE, Broitman J, Davis JM, Alexander L, Hamilton A, Liao Z, … Milham MP (2020). Estimated Prevalence of Nonverbal Learning Disability Among North American Children and Adolescents. JAMA Network Open, 3(4), e202551–e202551. doi: 10.1001/jamanetworkopen.2020.2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis AE, Pagliaccio D, Thomas L, Banker S, & Marsh R (2019). Salience network connectivity and social processing in children with nonverbal learning disability or autism spectrum disorder. Neuropsychology, 33(1), 135–143. doi: 10.1037/neu0000494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, Desai PM, Maskus EA, Melvin SA, Rehman R, Torres SD, … Noble KG (2019). Socioeconomic Disparities in Chronic Physiologic Stress Are Associated With Brain Structure in Children. Biol Psychiatry. doi: 10.1016/j.biopsych.2019.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagrin A, Saiote C, & Schiller D (2018). The social hippocampus. Hippocampus, 28(9), 672–679. doi: 10.1002/hipo.22797 [DOI] [PubMed] [Google Scholar]
- Morgan LK, Macevoy SP, Aguirre GK, & Epstein RA (2011). Distances between real-world locations are represented in the human hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience, 31(4), 1238–1245. doi: 10.1523/JNEUROSCI.4667-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, & Cuthill IC (2007). Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological Reviews, 82(4), 591–605. doi: 10.1111/j.1469-185X.2007.00027.x [DOI] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, … Sowell ER (2015). Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18, 773. doi:10.1038/nn.398310.1038/nn.3983https://www.nature.com/articles/nn.3983#supplementary-informationhttps://www.nature.com/articles/nn.3983#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, & Dostrovsky J (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research, 34(1), 171–175. doi: 10.1016/0006-8993(71)90358-1 [DOI] [PubMed] [Google Scholar]
- O’Keefe J, & Nadel L (1978). The Hippocampus as a Cognitive Map: Oxford: Clarendon Press. [Google Scholar]
- Persson J, Stening E, Nordin K, & Soderlund H (2018). Predicting episodic and spatial memory performance from hippocampal resting-state functional connectivity: Evidence for an anterior-posterior division of function. Hippocampus, 28(1), 53–66. doi: 10.1002/hipo.22807 [DOI] [PubMed] [Google Scholar]
- Posner J, Siciliano F, Wang Z, Liu J, Sonuga-Barke E, & Greenhill L (2014). A multimodal MRI study of the hippocampus in medication-naive children with ADHD: what connects ADHD and depression? Psychiatry research, 224(2), 112–118. doi: 10.1016/j.pscychresns.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Cho S, Chen T, Rosenberg-Lee M, Geary DC, & Menon V (2014). Hippocampal-neocortical functional reorganization underlies children’s cognitive development. Nature Neuroscience, 17, 1263. doi:10.1038/nn.378810.1038/nn.3788https://www.nature.com/articles/nn.3788#supplementary-informationhttps://www.nature.com/articles/nn.3788#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CE, Anderson PJ, Thompson DK, Kidokoro H, Wallendorf M, Treyvaud K, … Inder TE (2012). Regional cerebral development at term relates to school-age social-emotional development in very preterm children. Journal of the American Academy of Child and Adolescent Psychiatry, 51(2), 181–191. doi: 10.1016/j.jaac.2011.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg-Lee M, Iuculano T, Bae SR, Richardson J, Qin S, Jolles D, & Menon V (2018). Short-term cognitive training recapitulates hippocampal functional changes associated with one year of longitudinal skill development. Trends in Neuroscience and Education, 10, 19–29. doi: 10.1016/j.tine.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RD, Watson PD, Duff MC, & Cohen NJ (2014). The role of the hippocampus in flexible cognition and social behavior. Frontiers in human neuroscience, 8(742). doi: 10.3389/fnhum.2014.00742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, & Lord C (2003). Autism diagnostic interview-revised. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Schafer M, & Schiller D (2018). Navigating Social Space. Neuron, 100(2), 476–489. doi: 10.1016/j.neuron.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, & Caplan D (2006). Cognition, emotion and the cerebellum. Brain, 129(Pt 2), 290–292. doi: 10.1093/brain/awh729 [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Fine JG, Bledsoe J, & Zhu DC (2013). Magnetic resonance imaging volumetric findings in children with Asperger syndrome, nonverbal learning disability, or healthy controls. J Clin Exp Neuropsychol, 35(5), 540–550. doi: 10.1080/13803395.2013.795528 [DOI] [PubMed] [Google Scholar]
- Supekar K, Swigart AG, Tenison C, Jolles DD, Rosenberg-Lee M, Fuchs L, & Menon V (2013). Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proceedings of the National Academy of Sciences of the United States of America, 110(20), 8230–8235. doi: 10.1073/pnas.1222154110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares Rita M., Mendelsohn A, Grossman Y, Williams Christian H., Shapiro M, Trope Y, & Schiller D (2015). A Map for Social Navigation in the Human Brain. Neuron, 87(1), 231–243. doi: 10.1016/j.neuron.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman EC (1948a). Cognitive maps in rats and men. Psychological review, 55. [DOI] [PubMed] [Google Scholar]
- Tolman EC (1948b). Cognitive maps in rats and men. Psychological review, 55(4), 189–208. doi: 10.1037/h0061626 [DOI] [PubMed] [Google Scholar]
- Trinkler I, King JA, Doeller CF, Rugg MD, & Burgess N (2009). Neural bases of autobiographical support for episodic recollection of faces. Hippocampus, 19(8), 718–730. doi: 10.1002/hipo.20556 [DOI] [PubMed] [Google Scholar]
- Tulving E, & Markowitsch HJ (1998). Episodic and declarative memory: role of the hippocampus. Hippocampus, 8(3), 198–204. doi: [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K, Marien P, & Vandekerckhove M (2014). Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. Neuroimage, 86, 554–572. doi: 10.1016/j.neuroimage.2013.09.033 [DOI] [PubMed] [Google Scholar]
- Varma S, & Schwartz DL (2011). The mental representation of integers: an abstract-to-concrete shift in the understanding of mathematical concepts. Cognition, 121(3), 363–385. doi: 10.1016/j.cognition.2011.08.005 [DOI] [PubMed] [Google Scholar]
- Watson TC, Obiang P, Torres-Herraez A, Watilliaux A, Coulon P, Rochefort C, & Rondi-Reig L (2019). Anatomical and physiological foundations of cerebello-hippocampal interaction. Elife, 8. doi: 10.7554/eLife.41896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler DTPC (2003). Wechsler intelligence scale for children–Fourth Edition (WISC-IV). The Psychological Corporation. [Google Scholar]
- Weisberg SM, & Newcombe NS (2018). Cognitive Maps: Some People Make Them, Some People Struggle. Current Directions in Psychological Science, 27(4), 220–226. doi: 10.1177/0963721417744521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect, 2(3), 125–141. doi: 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Riecker A, Hertrich I, Erb M, Grodd W, Ethofer T, & Ackermann H (2005). Identification of emotional intonation evaluated by fMRI. Neuroimage, 24(4), 1233–1241. doi: 10.1016/j.neuroimage.2004.10.034 [DOI] [PubMed] [Google Scholar]
- Wilkey ED, Cutting LE, & Price GR (2018). Neuroanatomical correlates of performance in a state-wide test of math achievement. Developmental Science, 21(2), e12545. doi:doi: 10.1111/desc.12545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock R, McGrew K, & Mather N (2001). Woodcock-Johnson III. Itasca, IL: Riverside Publishing. [Google Scholar]
- Woolley DG, Mantini D, Coxon JP, D’Hooge R, Swinnen SP, & Wenderoth N (2015). Virtual water maze learning in human increases functional connectivity between posterior hippocampus and dorsal caudate. Hum Brain Mapp, 36(4), 1265–1277. doi: 10.1002/hbm.22700 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


