Abstract
Background:
Varus thrust during walking, visualized as excessive frontal plane knee motion during weight acceptance, is a modifiable risk factor for progression of knee osteoarthritis. However, visual assessment does not capture thrust severity and quantification with optical motion capture is often not feasible. Inertial sensors may provide a convenient alternative to optical motion capture. This proof-of-concept study sought to compare wearable inertial sensors to optical motion capture for the quantification of varus thrust.
Methods:
Twenty-six participants with medial knee osteoarthritis underwent gait analysis at self-selected and fast speeds. Linear regression with generalized estimating equations assessed associations between peak knee adduction velocity or knee adduction excursion from optical motion capture and peak thigh or shank adduction velocity from two inertial sensors on the lower limb. Relationships between inertial measures and peak external knee adduction moment were assessed as a secondary aim.
Findings:
Both thigh and shank inertial sensor measures were associated with the optical motion capture measures for both speeds (P < 0.001 to P = 0.020), with the thigh measures having less variability than the shank. After accounting for age, sex, body mass index, radiographic severity, and limb alignment, thigh adduction velocity was also associated with knee adduction moment at both speeds (both P < 0.001).
Interpretation:
An inertial sensor placed on the mid-thigh can quantify varus thrust in people with medial knee osteoarthritis without the need for optical motion capture. This single sensor may be useful for risk screening or evaluating the effects of interventions in large samples.
Keywords: angular velocity, gyroscope, motion capture, adduction
1. Introduction
Knee osteoarthritis (OA) is a leading cause of disability among older adults1 and most commonly affects the medial tibiofemoral compartment of the knee joint2. While age, sex, genetics, and other non-modifiable factors have been implicated in OA pathogenesis, gait patterns leading to increased or abnormal biomechanical joint loading also play a role and are frequently targeted in interventions3. A common gait abnormality in people with medial knee OA is varus thrust, an excessive ‘bowing-out’ knee motion in the frontal-plane during ambulation as the limb accepts weight with a return towards a more neutral alignment in late stance and swing4,5. Varus thrust has been reported to be present in 12% to 46% of individuals with medial knee OA and has been associated with radiographic disease severity6 and progression5. Cross-sectionally, those with varus thrust have a five-times greater odds for higher pain during walking and standing than those without it7. Individuals with knee OA who exhibit varus thrust also exhibit greater peak external knee adduction moments (EKAM) during gait5, an indication of medial tibiofemoral load8 which has been reported to be a risk factor for future OA progression9. Thus, interventions to reduce varus thrust may lead to reduced pain and slow structural worsening in individuals with medial compartment knee OA.
To aid in the development of effective interventions, it is important to accurately and reliably identify the presence of varus thrust. Typically, varus thrust is assessed through a subjective, visual evaluation of walking5–7,10–14. While these assessments are used clinically, they only provide a dichotomous categorization (present/absent) without any indication of severity. To overcome this limitation, optical motion capture has been used to objectively quantify biomechanical parameters as surrogate measures of varus thrust5,6,15–21, including knee adduction velocity6 and knee adduction angular excursion21. However, while optical motion capture provides detailed information on joint kinematics and kinetics, these systems require expensive equipment, time-consuming data collections run by skilled technicians, and a large calibrated measurement volume, making their clinical use infeasible. Additionally, analyses conducted in a laboratory environment do not always reflect typical walking in real-world settings22. In contrast, small, low-cost wearable inertial sensors have become increasingly popular for collecting biomechanical data in free-living conditions and may provide a convenient alternative to optical motion capture systems for quantifying varus thrust23.
The primary aim of this study was to compare data from a single wearable inertial sensor to surrogate measures of varus thrust captured using optical motion capture technology during self-selected and fast speed walking in individuals with medial compartment knee osteoarthritis. We hypothesized that measures of frontal plane segment velocity from single inertial sensors placed on the thigh or shank would be significantly associated with measures from optical motion capture based on previously reported agreement between inertial sensor and optical motion capture kinematic and kinetic measures24,25. For a secondary aim, we hypothesized that the inertial sensor measures would be associated with EKAM after adjusting for confounders.
2. Methods
2.1. Participants
Participants were recruited using advertisements online and in local newspapers from October 2017 to May 2019. Inclusion criteria were age between 45–80 years, body mass index (BMI) ≤ 40 kg/m2, and at least one knee meeting the American College of Rheumatology clinical or radiographic criteria for knee OA26 with primarily medial tibiofemoral compartment involvement (medial joint space narrowing identified from weight bearing knee radiographs). Exclusion criteria were regular use of a walking aid, inflammatory arthritis, lower limb total joint replacement, neurological conditions, muscular disease, or other conditions/treatments affecting gait. This study was approved by the Boston University Institutional Review Board, Boston, USA, and all individuals provided written informed consent prior to radiographic screening and data collection.
2.2. Radiographs and Assessment of Symptoms
All participants underwent three radiographs: (1) bilateral weight-bearing posterior-anterior flexed knee radiograph using a Synaflexer positioning frame (BioClinica, Princeton, NJ, USA) for assessment of Kellgren-Lawrence grade (KLG) and assessment of medial and lateral tibiofemoral compartment involvement based on the Osteoarthritis Research Society International (OARSI) atlas, (2) bilateral knee sunrise view for assessment of patellofemoral involvement, and (3) bilateral standing long limb view for measurement of static mechanical axis alignment. An experienced clinician assessed OA severity (KLG) and determined the most involved compartment (medial or lateral) for each knee. Inter-reader reliability for KLG of 0.79 has been reported previously27. Static alignment was calculated using OsiriX open-source software (www.osirixviewer.com)28 as the angle formed by the intersection of the line between the center of the femoral head and midpoint of the femoral epicondyles, and the line between the midpoint of the femoral epicondyles and midpoint of the malleoli. All participants also completed the Knee Injury and Osteoarthritis Outcome Score (KOOS)29. All knees with KLG < 2 were excluded from the analyses.
2.3. Optical motion capture
Ground reaction force (GRF) and kinematic data were collected from both legs for all participants while walking along a 15-meter walkway at self-selected and fast speeds. A passive optical motion capture system with 12 infrared cameras and one video camera (Qualisys Medical, Gothenburg, Sweden) was used to capture kinematic data at 250 Hz. Twenty-six spherical retroreflective markers were attached to bony landmarks of the trunk (manubrium, C7 spinous process, T8 spinous process, and the right and left acromion), pelvis (right and left anterior superior iliac spines, superior aspects of iliac crests, right and left posterior superior iliac spines, and sacrum), and lower extremities (greater trochanters, lateral and medial femoral epicondyles, lateral and medial malleoli, first and fifth metatarsal heads) which were used to identify joint centers during a static standing trial (Figure 1A). Rigid clusters of four markers were placed on the shank and thigh segments, and markers were placed on the posterior aspect of the heel, medial and lateral mid-foot, 2nd metatarsal head, and lateral aspect of 5th metatarsal head for segment tracking (Figure 1A). Three force platforms (AMTI, Watertown, MA, USA) were used to collect GRF data at 2000 Hz synchronously with the motion data. Walking speed was measured using a timing system (Brower Timing Systems, Draper, UT, USA). Self-selected and fast walking target speeds were determined after 3–4 practice trials. For self-selected speed trials, participants were instructed to “walk across the room towards the door with a purposeful pace.” For fast walking trials, participants were instructed to “walk as quickly as you comfortably can without running and without going faster than what you feel is safe for you.” Any trials that were outside ±5% of the target walking speeds were excluded. Four to six clean force plate foot strikes were collected for each foot. Each participant wore laboratory-provided shoes (Gel-Cumulus 19, ASICS, Kobe, Japan).
Figure 1.
(A) Optical motion capture markers (red markers used for static trial only) and (B) inertial sensors, showing placement of the mid-thigh and mid-shank inertial sensors on the optical motion capture marker clusters for the thigh and shank segments, respectively, and distal shank inertial sensor directly on the skin on the lateral aspect of the distal tibia, along with the coordinate systems for the knee (optical system) and inertial sensors.
Within the motion capture software, marker trajectories were identified and any gaps in data were filled using polynomial splines for gaps of less than 10 frames and trajectory matching for larger gaps. All data were then imported into Visual3D (C-Motion, Germantown, MD, USA). Marker and GRF data were filtered using low-pass, 4th order Butterworth filters with cut-off frequencies of 6 Hz and 12 Hz, respectively, as the majority of movement during walking occurs below these frequencies. Joint kinematics were calculated from the marker data using Euler angles (x-y-z) and right-handed coordinate systems. Initial and final contact for each clean foot strike were identified from the GRF using a threshold of 20 N to define stance phase.
The primary measure from the optical motion capture system was peak knee adduction velocity and the secondary measure was knee adduction excursion. Chang et al. suggested peak knee adduction velocity as an appropriate biomechanical index to quantify varus thrust as it closely corresponded with visual assessments and captures both the direction and speed of movement6. As the majority of the varus thrust movement occurs in early stance6, peak knee adduction velocity during the first half of stance phase was recorded (Figure 2B). It should be noted that because the joint angle (Euler angle) is a vector quantity, it is not possible to compute the joint angular velocity by taking the first derivative of the joint angle30. We used X-Y-Z cardan sequence to calculate the knee joint angle (shank relative to thigh); the knee joint angular velocity was calculated as the angular velocity of the shank relative to the thigh30,31. The secondary motion capture varus thrust measure, knee adduction excursion, was calculated as the difference between the knee adduction angle at initial contact and the maximum knee adduction angle during the first half of stance6 (Figure 2C). Inverse dynamics were used to calculate the EKAM, normalized to body weight and height (% bodyweight-height), and the peak EKAM was extracted from the first half of stance (Figure 2D). All variables were averaged across all clean foot strikes for each leg.
Figure 2.
Representative waveforms from self-selected speed walking showing (A) inertial sensor segment adduction velocity from the mid-thigh and mid-shank sensors, (B) knee adduction velocity, (C) knee adduction angle and knee adduction excursion, and (D) external knee adduction moment (EKAM).
2.4. Inertial motion capture
Small, lightweight inertial sensors (Trigno™ IM Sensor, Delsys, Inc., Natick, MA, USA) were used concurrently with the optical motion capture system. Each sensor measured 37mm x 26mm x 15mm, weighed 14.7g, and consisted of a triaxial accelerometer (±16g), a triaxial gyroscope (±2000 degree/s), and a triaxial magnetometer (±1000μT). Initially, three sensor locations were tested for each limb (Figure 1B): 1) lateral mid-thigh (attached to the thigh segment optical motion capture marker cluster), 2) lateral mid-shank (attached to the shank segment optical motion capture marker cluster), and 3) lateral distal shank (attached directly to the skin on the lateral aspect of the distal tibia proximal to the lateral malleolus). The distal shank placement was included with the assumption that it would be more convenient for use in a non-laboratory environment. However, interim analyses showed poor association between optical motion capture data and data from the distal sensor, particularly during fast speed walking, and this sensor was perceived as uncomfortable by a few participants. For these reasons, use of the distal sensor placement was discontinued for the final 11 participants (22 knees) enrolled in this study and the data from this sensor are not presented here. For all sensor placements, while a general orientation of the sensors was specified (i.e. arrow side ‘up’), the placement along the length of the leg and anterior-posterior position were not constrained, nor were any calibration procedures performed, in order to better replicate placement of the sensors in a clinical setting and/or by untrained individuals (e.g. participants).
Data were recorded from each sensor at 148 Hz, upsampled to 2000 Hz, and time synchronized with the optical motion capture system. The frontal plane component of the raw gyroscope data was used as a measure of segment adduction velocity. The gyroscope component was chosen as it captures angular velocity as compared to accelerometer components which capture linear acceleration. Peak segment adduction velocity in degrees per second, which was calculated as the peak value between initial contact and midstance, was extracted for each trial for each sensor (Figure 2A) and averaged across trials for each leg. Outcomes used in the analyses included peak thigh adduction velocity from the mid-thigh sensor (mid-thigh adduction velocity) and peak shank adduction velocity from the mid-shank sensor (mid-shank adduction velocity) for each walking speed. It should be noted that the thigh adduction velocity is recorded as positive and shank adduction velocity is recorded as negative (Figure 2, Table 2) given the nature of segmental motion and orientation of the sensor coordinate systems (Figure 1B).
Table 2:
Inertial and optical motion capture outcome average values in knees with radiographic OA (KLG ≥ 2)
| Inertial motion capture | |
| Mid-thigh adduction velocity (degree/s) (n = 39 legs): Self-selected speed walking |
45.2 (25.2) |
| Fast speed walking | 58.9 (28.1) |
| Mid-shank adduction velocity (degree/s) (n = 41 legs): Self-selected speed walking |
−80.4 (36.9) |
| Fast speed walking | −97.3 (43.4) |
| Optical motion capture | |
| Knee adduction velocity (degree/s) (n = 45 legs): Self-selected speed walking |
63.9 (24.8) |
| Fast speed walking | 75.6 (28.6) |
| Knee adduction excursion (degree) (n = 45 legs): Self-selected speed walking |
3.8 (2.0) |
| Fast speed walking 4.0 (2.1) External knee adduction moment (% bodyweight-height) (n = 45 legs): Self-selected speed walking 3.25 (1.22) Fast speed walking 3.71 (1.43) | |
Data presented as mean (standard deviation)
2.5. Statistical Analyses
For the primary aim, univariate regression models with generalized estimating equations (GEE) were used to assess the relationships between the inertial sensor measures and the optical motion capture measures, separately for self-selected and fast speed walking. The GEEs allowed us to account the correlation between knees within each person. For the secondary aim, multivariate regression models with GEE were used to assess the relationships between EKAM and the inertial sensor measures, separately for each speed, while adjusting for a number of confounders (specifically age, sex, BMI, KLG, and static alignment) that may affect both varus thrust13 and EKAM32–34. While pain can also affect EKAM32, we hypothesized that it acts as a mediator rather than a confounder on the causal pathway (i.e. varus thrust causes pain rather than the other way around), and thus did not include it in the models. All models were constructed including a term for leg and an interaction term between exposure and leg to determine whether the association of the exposure and the outcome differed by leg. If there was no interaction between exposure and leg (i.e. this interaction term was not significant), the model was re-run without the exposure by leg interaction term. All analyses were performed using IBM SPSS (Armonk, NY, USA) in all knees with KLG ≥ 2. Significance was set at α = 0.05.
3. Results
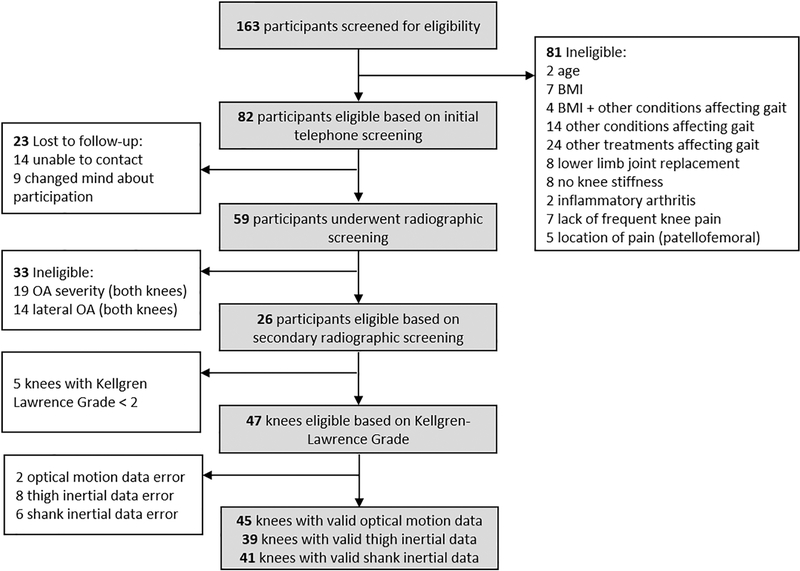
One hundred and sixty-three individuals underwent telephone screening, 82 passed the initial screening process, 59 underwent a radiographic screening visit, and 26 individuals (16 female) were deemed eligible for this study (Figure 3, Table 1). The number of knees across analyses differed depending on useable data available for each inertial sensor (Figure 3). Average values for each inertial and optical motion capture measure are reported in Table 2.
Figure 3.
Study recruitment and final study sample.
Table 1.
Sample characteristics
| Clinical & Demographic Characteristics | n = 26 participants† |
|---|---|
| Sex | n = 16 [62%] female |
| Age (years) | 64.5 (8.4) |
| Body mass index (kg/m2) | 28.6 (4.7) |
| Height (m) | 1.67 (0.12) |
| Gait speed (m/s): | |
| Self-selected | 1.32 (0.22) |
| Fast | 1.62 (0.32) |
| Knee Injury and Osteoarthritis Outcome Score - Pain (/100*) | 59.2 (11.1) |
| Kellgren-Lawrence Grade (KLG): | |
| KLG = 2 | n = 13 legs |
| KLG = 3 | n = 26 legs |
| KLG = 4 | n = 8 legs |
| Static limb alignment (degrees)** | 175.9 (3.6) |
Data presented as mean (standard deviation) except where noted
n = 47 knees from these participants had KLG ≥ 2. KOOS, KLG, & alignment values are reported for these knees only.
Lower scores represent greater pain
Angles < 180 degrees indicate static varus alignment
Both mid-thigh and mid-shank adduction velocity were associated with knee adduction velocity and excursion during self-selected and fast speed walking. An increase of 10.0°/s in mid-thigh adduction velocity was associated with an increase in knee adduction velocity of 6.1°/s during self-selected speed walking (P < 0.001, Figure 4A) and 5.3°/s during fast walking (P = 0.001, Figure 4B), and an increase in knee adduction excursion of 0.35° during self-selected speed walking (P = 0.005, Figure 4C) and 0.33° during fast walking (P = 0.020, Figure 4D). Similarly, an increase of 10.0°/s in mid-shank adduction velocity was associated with an increase in knee adduction velocity of 3.4°/s during self-selected speed walking (P < 0.001, Figure 4A) and 2.2°/s during fast walking (P = 0.005, Figure 4B), and an increase in knee adduction excursion of 0.20° during both self-selected (P = 0.004, Figure 4C) and fast (P < 0.001, Figure 4D) speed walking.
Figure 4.
Relationships between segment adduction velocity (captured by the inertial sensors) and (A) knee adduction velocity during self-selected speed walking, (B) knee adduction velocity during fast speed walking, (C) knee adduction excursion during self-selected speed walking, and (D) knee adduction excursion during fast speed walking in knees with radiographic osteoarthritis.
After accounting for age, sex, BMI, KLG, and static alignment, an increase of 10.0°/s in mid-thigh adduction velocity was associated with an increase in EKAM of 0.16 % bodyweight-height (P < 0.001) during self-selected speed walking and an increase of 0.17 % bodyweight-height (P <0.001) during fast speed walking. For the models investigating the relationship between mid-shank adduction velocity and EKAM during self-selected and fast speed walking, the interaction term between leg and mid-shank adduction velocity was significant, thus separate models were run for left and right legs. For left legs (n = 18), an increase in 10.0°/s in mid-shank adduction velocity was associated with an increase in EKAM of 0.10 % bodyweight-height (P = 0.010) during self-selected speed walking and an increase in 0.17 % bodyweight-height (P < 0.001) during fast speed walking, after accounting for confounders. For right legs (n = 23), mid-shank adduction velocity was not associated with EKAM at either self-selected speed (P = 0.88) or fast speed (P = 0.21).
4. Discussion
This proof-of-concept study showed that the measures from single inertial sensors were associated with surrogate measures of varus thrust obtained using optical motion capture. Furthermore, supporting our secondary hypothesis, mid-thigh adduction velocity was significantly associated with peak EKAM after adjusting for confounders. These results suggest that inertial sensors should be further investigated as a tool to objectively quantify varus thrust in clinical settings where optical motion capture is not feasible and visual assessment is insufficient. The ability to quickly and accurately quantify varus thrust in clinical or other real-world settings could lead to better identification and treatment of those at risk of OA progression due to varus thrust.
Both inertial sensor metrics – mid-thigh adduction velocity and mid-shank adduction velocity – were associated with both optical motion capture thrust measures at both walking speeds. The mid-shank data, however, had greater variability than the mid-thigh data, e.g. an adduction velocity range of 165°/s for the mid-shank versus 99°/s for the mid-thigh during self-selected speed walking (Figure 4, Table 2), suggesting that the mid-thigh sensor placement is superior. These results are supported by previous research that found data from a single mid-thigh inertial sensor were a better predictor of peak knee extensor moment and power absorption during a single limb task than a mid-shank sensor35 and that data from a single thigh accelerometer, but not a single shank accelerometer, were predictive of between-limb differences in knee power absorption during running36.
In the current study, both of the mid-segment inertial sensors were placed directly on the rigid optical motion capture marker clusters used for segment tracking. However, it is the anatomical coordinate system defined by markers placed on bony landmarks, rather than these rigid clusters, that define the segment coordinate systems for the motion capture measures. Orientation of the inertial sensors relative to the anatomical coordinate system is a key factor in accuracy of joint angles measured by inertial sensors37. A varying degree of curvature on the lateral aspect of the shank due to the shape of the lateral head of the gastrocnemius muscle could have resulted in less consistent placement of the mid-shank inertial sensor among legs, thus affecting the relationship between the inertial sensor coordinate system and the anatomical coordinate system of the shank. In contrast, the lateral thigh has less variation in curvature along its length, which may have ensured more consistent placement of the mid-thigh inertial sensor across legs. Thus, the mid-thigh sensor placement may be preferable to the mid-shank placement for assessing varus thrust in individuals with knee OA, particularly in cases where limited time or experience prevent more standardized placement.
The regression models for EKAM also provide support for the mid-thigh versus mid-shank placement as the association between segment angular velocity and EKAM was not consistent across legs for the mid-shank sensor, i.e. it was significant for the left but not the right leg. While the current dataset did not provide enough power for a thorough comparison between left and right legs, these groups were similar in terms of KLG and HKA, thus no difference between legs was expected. Despite this discrepancy between legs for the mid-shank sensor, the novel finding that mid-thigh adduction velocity was significantly associated with EKAM, even after adjusting for confounders, is promising as it suggests that an estimation of EKAM may be possible without the need for extensive laboratory equipment or analysis of multiple inertial sensors. This finding also supports previous studies that have shown associations between various quantitative measures of varus thrust and EKAM17,20,21.
In the current study, only single-sensor inertial measures were examined with the idea that large-scale screening in a clinical setting would require a quick set-up with minimal data processing. However, the lack of an exact 1:1 increase in adduction velocity between these inertial sensor and optical motion captures measures may be attributed to the fact that the inertial sensor segment adduction velocity measures only describe movement of a single segment (thigh or shank), while knee adduction velocity describes the relative movement between the thigh and shank segments. Integration of multiple inertial sensors or sensor components may result in better estimation of knee adduction velocity, however, this typically requires a series of functional calibration exercises, modeling assumptions, and data filtering to address issues such as drift and sensor alignment38. The finding in the current study of a significant association between single inertial sensor measures and optical motion capture measures suggests a single sensor may be sufficient as a quick screening tool for severity of varus thrust in knee OA populations where more extensive data collection is not feasible.
We observed significant associations of mid-thigh and mid-shank sensors with both knee adduction velocity and knee adduction excursion measures at both walking speeds. As noted by Chang et al.6, knee adduction velocity captures the speed of the movement and can be used as a reliable measure of varus thrust in people with knee OA. Knee adduction excursion has also been used as a quantitative metric of varus thrust and has been associated with both OA severity (KLG) and EKAM in individuals with medial knee OA21. Associations would be expected between measures of thigh/shank adduction velocity from the inertial sensors and knee adduction velocity from optical motion capture given the similar constructs being measured. However, significant associations of metrics from thigh/shank inertial sensors and knee adduction excursion further support the use of inertial sensors to assess varus thrust. Furthermore, the similarity of results across the two speeds for the analyses in the current study suggests that a single sensor could be used to quantify varus thrust across different walking speeds in individuals with knee OA.
There are a number of limitations of the current study that should be acknowledged. Given that this was a proof of concept study, the sample size was small. Data error resulted in the loss of inertial sensor data for some legs due to signal clipping. While this appeared to be an issue with software presets, rather than the inertial sensors themselves, the loss of this data and small sample size overall did not provide enough power for a definitive comparison across sensor locations or speeds. The lack of standardized placement of the inertial sensors on the leg, e.g. a specified percent distance along a given segment, may have increased variability in the measurement of segment adduction velocities from the inertial sensors, resulting in misalignment of coordinate systems between the inertial and optical motion capture systems and an inability to provide a definitive recommendation on sensor placement. The attachment of sensors in a clinical setting would likely also be done without standardized placement or calibration and thus these results may be a good representation of how clinical inertial sensor data would correspond to optical motion capture data. It should be noted, however, that the sensors in the current study were placed on top of the rigid plate containing optical motion capture markers and it is unclear whether a rigid sensor alone versus the rigid sensor/plate combination would produce similar data. In this study, gait events identified by the optical motion capture system were used for calculation of the inertial measures. In a clinical setting where optical motion capture data are not available, gait events calculated from the inertial sensors, as has been done previously39, would need to be utilized. Reliability of the thigh inertial sensor measurement will also need to be established before it can be used in clinical settings, particularly given the limited number of studies investigating reliability of inertial sensors in knee OA populations40,41. It should also be noted that while the sample in the current study is similar to that of larger studies of individuals with medial knee OA in terms of age, sex, and BMI13, the results may not generalize to other sub-groups of the OA population such as individuals with BMI > 40.
5. Conclusions
In this proof-of-concept study, we demonstrated a significant association between increases in thigh angular velocity derived from the gyroscope signal of a single inertial sensor and increases in surrogate varus thrust measures derived from an optical motion capture system. Furthermore, increased thigh angular velocity from this single inertial sensor was associated with increased peak EKAM after adjusting for confounders. These results highlight the potential of inertial sensors for quantifying varus thrust without the need for an optical motion capture system.
Highlights.
Frontal plane gyroscope data were extracted from a single triaxial inertial sensor
Inertial data were associated with optical motion capture varus thrust measures
Mid-thigh inertial sensor data was associated with knee adduction moment
A single mid-thigh inertial sensor may be used to monitor outcomes in large studies
Acknowledgments
We would like to acknowledge the staff and students of the Movement & Applied Imaging Laboratory for their contributions to data collection and processing. We would also like to thank the participants for their contributions to this study and ASICS for donating the shoes used in this study.
Research reported in this publication was supported by the National Institutes of Health, under award numbers K01AR069720 and T32AR007598. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declarations of interest: Ali Guermazi is Shareholder of BICL, LLC and Consultant to AstraZeneca, MerckSerono, TissueGene, Pfizer, Roche and Galapagos. Other authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guccione A, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham study. Am J Public Health. 1994;84(3):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: The importance of the patellofemoral joint. Ann Rheum Dis. 1992;51:844–849. doi: 10.1136/ard.51.7.844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: New insights - Part 1: The disease and its risk factors. Ann Intern Med. 2000. doi: 10.7326/0003-4819-133-8-200010170-00016 [DOI] [PubMed] [Google Scholar]
- 4.Wink AE, Gross KD, Brown CA, et al. Varus thrust during walking and the risk of incident and worsening medial tibiofemoral MRI lesions: the Multicenter Osteoarthritis Study. Osteoarthr Cartil. 2017;25(6):839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang A, Hayes K, Dunlop D, et al. Thrust during ambulation and the progression of knee osteoarthritis. Arthritis Rheum. 2004;50(12):3897–3903. doi: 10.1002/art.20657 [DOI] [PubMed] [Google Scholar]
- 6.Chang AH, Chmiel JS, Moisio KC, et al. Varus thrust and knee frontal plane dynamic motion in persons with knee osteoarthritis. Osteoarthr Cartil. 2013;21(11):1668–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo GH, Harvey WF, McAlindon TE. Associations of varus thrust and alignment with pain in knee osteoarthritis. Arthritis Rheum. 2012;64(7):2252–2259. doi: 10.1002/art.34422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurwitz DE, Sumner DR, Andriacchi TP, Sugar DA. Dynamic knee loads during gait predict proximal tibial bone distribution. J Biomech. 1998;31(5):423–430. doi: 10.1016/S0021-9290(98)00028-1 [DOI] [PubMed] [Google Scholar]
- 9.Chang AH, Moisio KC, Chmiel JS, et al. External knee adduction and flexion moments during gait and medial tibiofemoral disease progression in knee osteoarthritis. Osteoarthr Cartil 2015;23(7):1099–1106. doi: 10.1016/j.joca.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iijima H, Fukutani N, Aoyama T, et al. Clinical phenotype classifications based on static varus alignment and varus thrust in Japanese patients with medial knee osteoarthritis. Arthritis Rheumatol. 2015;67(9):2354–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iijima H, Fukutani N, Yamamoto Y, et al. Association of varus thrust with prevalent patellofemoral osteoarthritis: A cross-sectional study. Gait Posture 2017;58:394–400. doi: 10.1016/j.gaitpost.2017.08.033 [DOI] [PubMed] [Google Scholar]
- 12.Fukutani N, Iijima H, Fukumoto T, et al. Association of varus thrust with pain and stiffness and activities of daily living in patients with medial knee osteoarthritis. Phys Ther. 2016;96(2):167–175. doi: 10.2522/ptj.20140441 [DOI] [PubMed] [Google Scholar]
- 13.Chang A, Hochberg M, Song J, et al. Frequency of varus and valgus thrust and factors associated with thrust presence in persons with or at higher risk of developing knee osteoarthritis. Arthritis Rheum. 2010;62(5):1403–1411. doi: 10.1002/art.27377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma L, Chang AH, Jackson RD, et al. Varus thrust and incident and progressive knee osteoarthritis. Arthritis Rheumatol 2017. doi: 10.1002/art.40224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukaya T, Mutsuzaki H, Wadano Y. Kinematic analysis of knee varus and rotation movements at the initial stance phase with severe osteoarthritis of the knee. Knee. 2015;22(3):213–216. doi: 10.1016/j.knee.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 16.Kakihana W, Akai M, Nakazawa K, Naito K, Torii S. Inconsistent knee varus moment reduction caused by a lateral wedge in knee osteoarthritis. Am J Phys Med Rehabil. 2007;86(6):446–454. doi: 10.1097/PHM.0b013e31805bfff5 [DOI] [PubMed] [Google Scholar]
- 17.Mahmoudian A, van Dieen JH, Bruijn SM, et al. Varus thrust in women with early medial knee osteoarthritis and its relation with the external knee adduction moment. Clin Biomech. 2016;39:109–114. doi: 10.1016/j.clinbiomech.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 18.Brown TN, Kaplan JT, Cameron SE, Seymore KD, Ramsay JW. Individuals with varus thrust do not increase knee adduction when running with body borne load. Journal of Biomechanics. 2018. [DOI] [PubMed] [Google Scholar]
- 19.Hunt MA, Schache AG, Hinman RS, Crossley KM. Varus thrust in medial knee osteoarthritis: Quantification and effects of different gait-related interventions using a single case study. Arthritis Care Res. 2011;63(2):293–297. doi: 10.1002/acr.20341 [DOI] [PubMed] [Google Scholar]
- 20.Sosdian L, Hinman RS, Wrigley T V., et al. Quantifying varus and valgus thrust in individuals with severe knee osteoarthritis. Clin Biomech. 2016;39:44–51. doi: 10.1016/j.clinbiomech.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 21.Kuroyanagi Y, Nagura T, Kiriyama Y, et al. A quantitative assessment of varus thrust in patients with medial knee osteoarthritis. Knee. 2012;19(2):130–134. doi: 10.1016/j.knee.2010.12.007 [DOI] [PubMed] [Google Scholar]
- 22.Brodie MAD, Coppens MJM, Lord SR, et al. Wearable pendant device monitoring using new wavelet-based methods shows daily life and laboratory gaits are different. Med Biol Eng Comput. 2016;54(4):663–674. doi: 10.1007/s11517-015-1357-9 [DOI] [PubMed] [Google Scholar]
- 23.Tao W, Liu T, Zheng R, Feng H. Gait analysis using wearable sensors. Sensors. 2012;12(2):2255–2283. doi: 10.3390/s120202255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konrath JM, Karatsidis A, Schepers HM, Bellusci G, de Zee M, Andersen MS. Estimation of the Knee Adduction Moment and Joint Contact Force during Daily Living Activities Using Inertial Motion Capture. Sensors. 2019;19. doi: 10.3390/s19071681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zügner R, Tranberg R, Timperley J, Hodgins D, Mohaddes M, Kärrholm J . Validation of inertial measurement units with optical tracking system in patients operated with Total hip arthroplasty. BMC Musculoskelet Disord 2019;20(1). doi: 10.1186/s12891-019-2416-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: Classification of osteoarthritis of the knee. Arthritis Rheumatol. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816 [DOI] [PubMed] [Google Scholar]
- 27.Guermazi A, Hayashi D, Roemer F, et al. Severe radiographic knee osteoarthritis - does Kellgren and Lawrence grade 4 represent end stage disease? - the MOST study. Osteoarthr Cartil. 2015;23(9):1499–1505. doi: 10.1016/j.joca.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosset A, Spadola L, Ratib O. OsiriX: An open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17(3):205–216. doi: 10.1007/s10278-004-1014-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—Development of a self-administered outcome measure. J Orthop Sport Phys Ther. 1998;28(2):88–96. doi: 10.2519/jospt.1998.28.2.88 [DOI] [PubMed] [Google Scholar]
- 30.Hamill J, Selbie W, Kepple T. Three-dimensional kinematics In: Robertson DGE, Caldwell GE, Hamill J, Kamen G, Whittlesey SN, eds. Research Methods in Biomechanics 2nd ed. Champaign, IL, USA; 2013:35–60. [Google Scholar]
- 31.C-Motion. Joint Velocity. https://c-motion.com/v3dwiki/index.php/Joint_Velocity. Accessed October 13, 2020.
- 32.Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J Orthop Res. 2002;20(1):101–107. doi: 10.1016/S0736-0266(01)00081-X [DOI] [PubMed] [Google Scholar]
- 33.Blazek K, Asay JL, Erhart-Hledik J, Andriacchi T. Adduction moment increases with age in healthy obese individuals. J Orthop Res. 2013;31(9):1414–1422. doi: 10.1002/jor.22390 [DOI] [PubMed] [Google Scholar]
- 34.Sims EL, Carland JM, Keefe FJ, Kraus VB, Guilak F, Schmitt D. Sex differences in biomechanics associated with knee osteoarthritis. J Women Aging. 2009;21(3):159–170. doi: 10.1080/08952840903054856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pratt KA, Sigward SM. Inertial sensor angular velocities reflect dynamic knee loading during single limb loading in individuals following anterior cruciate ligament reconstruction. Sensors (Switzerland). 2018;18(10). doi: 10.3390/s18103460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Havens KL, Cohen SC, Pratt KA, Sigward SM. Accelerations from wearable accelerometers reflect knee loading during running after anterior cruciate ligament reconstruction. Clin Biomech 2018;58(July):57–61. doi: 10.1016/j.clinbiomech.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 37.Kianifar R, Joukov V, Lee A, Raina S, Kulid D. Inertial measurement unit-based pose estimation: Analyzing and reducing sensitivity to sensor placement and body measures. J Rehabil Assist Technol Eng. 2019;6:205566831881345. doi: 10.1177/2055668318813455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seel T, Raisch J, Schauer T. IMU-based joint angle measurement for gait analysis. Sensors. 2014;14(4):6891–6909. doi: 10.3390/s140406891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aminian K, Najafi B, Büla C, Leyvraz P-F, Robert P. Spatio-temporal parameters of gait measured by an ambulatory system using miniature gyroscopes. J Biomech. 2002;35(5):689–699. doi: 10.1016/S0021-9290(02)00008-8 [DOI] [PubMed] [Google Scholar]
- 40.Kobsar D, Osis ST, Phinyomark A, Boyd JE, Ferber R. Reliability of gait analysis using wearable sensors in patients with knee osteoarthritis. J Biomech. 2016;49(16):3977–3982. doi: 10.1016/j.jbiomech.2016.11.047 [DOI] [PubMed] [Google Scholar]
- 41.Turcot K, Aissaoui R, Boivin K, Hagemeister N, Pelletier M, Guis JA d. Test-retest reliability and minimal clinical change determination for 3-dimensional tibial and femoral accelerations during treadmill walking in knee osteoarthritis patients. Arch Phys Med Rehabil. 2008;89(4):732–737. doi: 10.1016/j.apmr.2007.09.033 [DOI] [PubMed] [Google Scholar]